Abstract
Sensory neurons in aging mammals undergo changes in anatomy, physiology and gene expression that correlate with reduced sensory perception. In this study we compared young and aged mice to identify proteins that might contribute to this loss of sensation. We first show using behavioral testing that thermal sensitivity in aged male and female mice is reduced. Expression of sodium channel (Nav1.8 and Nav1.9) and transient receptor potential vanilloid (TRPV) channels in DRG and peripheral nerves of young and old male mice was then examined. Immunoblotting and RT-PCR assays showed reduced Nav1.8 levels in aged mice. No change was measured in TRPV1 mRNA levels in DRG though TRPV1 protein appeared reduced in the DRG and peripheral nerves. The GFRα3 receptor, which binds the growth factor artemin and is expressed by TRPV1-positive neurons, was also decreased in the DRG of aged animals. These findings indicate that loss of thermal sensitivity in aging animals may result from a decreased level of TRPV1 and Nav1.8 and decreased trophic support that inhibits efficient transport of channel proteins to peripheral afferents.
Keywords: Sensory neuron, Vanilloid receptor, TRPV1, Sodium channel, Behavior, Thermal sensitivity
1. Introduction
The cutaneous sensory system, comprised of sensory ganglia, cutaneous nerves and sensory receptors in the skin, detects tactile and thermal pain sensations. Functional studies in several species have shown that impairment of cutaneous sensitivity occurs with aging [13,37]. This age-related decrease in sensitivity can reduce tactile perception, detection of noxious stimuli and tissue injury. To identify how these degenerative changes occur, several studies of human and rodent have compared electrophysiological and anatomical properties of young and aged neurons, peripheral nerves and target tissues. In humans, electrophysiological measures have found lower nerve conduction velocity in older individuals, suggesting impairment in axon structure and function [9,37]. Analysis of nerve fibers in aging mice from 20 months of age onward, showed both myelinated and unmyelinated fibers to be reduced in number, which in the very old mouse (33 months) approaches 50% loss [13]. In contrast, mice between 12 and 20 months of age show only mild age-related changes (fiber reduction, myelin loops, membrane irregularity). In the peripheral target, measures in human of intraepidermal nerve fiber density showed no significant change in epidermal fiber density with age, except when comparison of aged samples was made to the highest values in the youngest subjects [25,27] (though see [7,14]). In 18-month old mice, innervation to the epidermis showed only a moderate loss (10–15%) in nerve fiber density [37]. Interestingly, this loss of peripheral nerve fibers in very old rodents does not appear to reflect a loss of neurons in cervical and lumbar DRG. Analysis of neuron number using a dissector counting methodology showed no significant change in sensory neurons of very old (30 months) rats [6]. Collectively, these data suggest age-related sensory deficits are not a result of neuronal death, but rather reflect a gradual alteration of both afferent structure and sensitivity.
Little is known of the cellular and molecular changes that occur in aging sensory neurons that lead to impaired perception. It is clear however, that afferent sensitivity is highly dependent on the expression of several classes of membrane channel proteins that regulate ion flow in response to a given stimulus. With this in mind, we investigated in this study whether an age-related decline in afferent sensitivity correlates with altered expression of membrane channel proteins. We focused on the thermosensitive ion channels in the transient receptor potential (TRP) family and the tetrototoxin resistant sodium channels since these channels are involved in the generation and transmission of impulse trains in response to mechanical and thermal stimuli [19,23]. Effects of aging on electrical membrane properties (EMP) of mouse DRG neurons in culture have indicated that neurons of aged animals (22–23-month old) exhibit decreased electrical excitability and increased action potential duration compared to younger animals (2–3.5-month old) [33]. This pattern of altered EMP is consistent with an age-induced shift from voltage-sensitive sodium channels to less excitable voltage-dependent calcium channels [24]. Tetrodotoxin-resistant voltage-dependent sodium channels Nav1.8 (SNS) and Nav1.9 (NaN or SNS2) are selectively expressed in small/medium-sized nociceptive neurons and contribute to the production of an action potential in these neurons. In addition, functional studies reveal that Nav1.8 and Nav1.9 have a specialized role in mediating pain [1,2,15-17]. Thus, the level of gene expression and/or distribution of these channels may be altered in aging neurons that have lowered sensitivity.
Members of the TRP channel family may also play a role in the age-related decline in cutaneous sensitivity [20,22]. The TRPV1 (vanilloid receptor 1) channel was first identified as a receptor for capsaicin that was activated by noxious heat [12]. TRPV1 is predominantly expressed in small, unmyelinated neurons and has a thermal activation threshold of ~43 °C [10,29,36]. The TRPV2 channel is expressed in medium and large myelinated neurons and mediates high threshold heat response with a threshold of ~52 °C [11]. TRPV3 is also heat sensitive with an activation threshold between 31 and 39 °C [34,38]. TRPV4 (VRL-2 or VROAC) is expressed in small and medium sized neurons and appears to mediate changes in osmoregulation, mechanical nociception and response to temperatures greater than 25 °C [26,35]. Whether age-related changes in expression and/or function of these ion channels contributes to altered neuronal sensitivity and excitability with aging is unclear.
To test whether changes occur in channel expression and distribution in the aging sensory system, we compared expression of the Nav1.8 and TRP channels in ganglia and nerves of young, middle-aged and old mice. To examine the relationship between trophic factor signaling and the measured neuronal properties, the relative expression level of receptor proteins in the glial cell line-derived neurotrophic factor (GDNF) family were also assayed.
2. Materials and methods
2.1. Animals
Young (6–8 weeks), middle-aged (15 months) and aged (2 years) male and female C57BL/6NIA (B6) mice were obtained from the aging rodent colony supported by the National Institute on Aging at Harlan (Indianapolis, IN, USA). Upon arrival at the University of Pittsburgh Animal Facility, mice were housed in microisolator caging and maintained on a 12-h light/dark cycle in a temperature controlled environment (20.5 °C) with free access to food and water ad libitum. These studies were carried out in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Pittsburgh and the NIH Guide for the Care and Use of Laboratory Animals.
2.2. Behavioral testing
Mice were placed in individual chambers (10.0 cm long × 10.0 cm wide × 13.0 cm high) of a 16-chamber plexiglas container that was placed on top of a 6.0 mm thick glass surface (Model 390; IITC Inc., Woodland Hills, CA). Mice were acclimated to this environment for 1–2 h prior to testing. Focused radiant heat (setting at 20) was applied to the plantar surface of the mid-hind paw of the mouse and the latency of withdrawal measured to the nearest 0.1 s. The left and right hind paw was tested on each mouse once a day for three consecutive days. Data were analyzed using an analysis of variance test (ANOVA). Mechanical responsiveness was tested by applying von Frey filaments (Stoelting, Wood Dale, IL) of varying thickness to the dorsum of the foot and recording the force needed to elicit a response, e.g., hindpaw withdrawal, biting of the filament. Testing was done twice a day for three consecutive days.
2.3. Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
RNA was extracted from either pooled (from cervical, thoracic and lumbar levels) or lumbar (L3/L4/L5) dorsal root ganglia. Mice were deeply anesthetized by injection of Avertin (2-2-2 tribromoethanol in tert-amyl alcohol) anesthesic and then killed by transcardial perfusion with approximately 75 ml ice-cold phosphate buffer. DRGs were collected on dry ice and frozen at minus 80 °C until RNA extraction. RNA was isolated by homogenizing frozen tissue in 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA) followed by isopropanol precipitation. Pellets were washed with 70% ethanol, suspended in RNase-free water and the concentration determined using a GeneQuant RNA/DNA calculator (Amersham Biosciences, Piscataway, NJ). Five micrograms of RNA was treated with DNase (Invitrogen) to remove genomic DNA and then 1 μg was reverse-transcribed using Superscript II reverse transcriptase (Invitrogen). Routine control reactions included PCR reactions on DNased RNA (without RT) and reactions run without templates to test for contamination. Two methods of PCR amplification were used: Method 1: PCR reactions were done in the presence of 32P-dCTP and aliquots of the reaction run on 8% polyacrylamide gels in Tris borate EDTA buffer. Gels were dried, placed against a phosphorimager screen and the relative level of incorporated label determined using a BioRad phosphorimager. The cycle number was optimized for each set of primers by first running PCR reactions at different cycle numbers to establish the mid-phase of the reaction. Values were normalized to either actin or GAPDH. Similar values were obtained using either actin or GAPDH for normalization. In Method 2, SYBR Green labeled PCR amplification was performed using a real-time thermal cycler (Applied Biosystems, Foster City, CA) controlled by a Dell Latitude laptop computer running ABI Prism 7000 SDS software. Twenty nanograms of cDNA template were added to 50 μl reaction mixtures provided in the SYBR Green reagent kit (Applied Biosystems). The amplification protocol included 2 min at 50 °C to activate the AmpErase UNG to prevent the reamplification of any carryover PCR products, 12 min at 95 °C to activate the Amplitaq polymerase, 40 cycles of 15 s at 95 °C for denaturation and 1 min at 60 °C for annealing and extension. After amplification, a dissociation curve was plotted against melting temperature to ensure amplification of a single product and to test for primer dimers. All samples were run in duplicate. Controls were run with water replacing the template (to further test for primer dimers). The CT values for each reaction were obtained and the ΔCT was calculated by subtraction of control (GAPDH) CT from the experimental value. ΔΔCT = Mean ΔCT young – MeanΔCTold and fold change = 2−ΔΔCT. An unpaired t-test (p ≤ 0.05) was used to determine significance of expression. PCR primers were generated using Primer Express software (Applied Biosystems, Foster City, CA) using parameters optimized by the manufacturer.
2.4. Western immunoblotting
Isolated nerves and DRG (either pooled from all levels or pooled from lumbar levels L3/L4/L5) were analyzed. Tissues from young and aged animals were homogenized in lysis buffer containing 1% sodium dodecyl sulfate (SDS), 10 mM Tris-HCl (pH 7.4), 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mM sodium orthovanadate and 100 μg/ml phenylmethylsulfonyl fluoride (Sigma Biochemicals). Homogenates were spun 10 min at 10,000 rpm at 4 °C, the supernatant recovered and protein concentration determined using a Bio-Rad protein assay. Samples (10–20 μg of protein) were boiled 10 min in denaturing buffer containing β-mercaptoethanol and SDS, separated on either 7.5 or 10% polyacrylamide SDS-page gels and transferred to Hybond-P PVDF membrane (Amersham Life Sciences) that was blocked for 1 h in TBS solution containing 5% powdered milk, 0.01% Tween-20, pH 7.6. Membranes were incubated with primary antibodies overnight at 4 °C. Antibodies used were: rabbit anti-TRPV1 (Oncogene Research Products; 1:500) and rabbit anti-Nav1.8 [8]. Antibody binding was visualized using a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:10,000) and ECL plus detection (Amersham Life Sciences). Immunoreactive bands were analyzed by densitometry and their intensity quantified using NIH Image J software. Immunoblot band intensity was normalized to either tubulin (saphenous nerve samples) or protein bands from each sample visualized on Coomassie blue stained gels. Bands at the approximate molecular weight of the protein of interest were chosen for comparative measure.
2.5. Immunocytochemistry
Tissues (DRGs, nerve and skin) were removed from young, aged and TRPV1 knockout animals that were perfused with saline. Samples were placed in 25% sucrose made in 0.1 M phosphate buffer (PB) overnight at 4 °C and then embedded in Optimal Cutting Temperature (OCT; Tissue Tek) compound on dry ice. Twenty-micron cryostat sections were mounted on Superfrost/Plus slides (Fisher), fixed in 4% paraformaldehyde for 10 min, blocked in 5% NGS, 2% BSA and 0.25% Triton X-100 for 1 h and then incubated in primary antibody (rabbit anti-TRPV1; Oncogene Research) overnight at room temperature. Antibody binding was visualized by avidin–biotin–peroxidase complex formation (Vector Laboratories, Burlingame, CA). The percentage of TRPV1-positive neurons was determined by first capturing entire, nonconsecutive labeled sections of the L4/L5 ganglia using a camera mounted on a microscope and Photoshop software. NIH Image software was then used to circle both labeled and unlabeled neurons to determine size and density of immunolabeled cells.
3. Results
3.1. Aged mice are less sensitive than younger mice to thermal stimuli applied to the foot
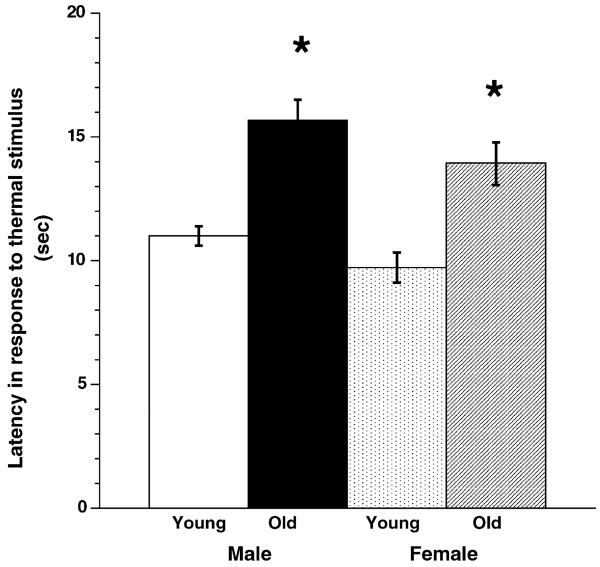
We began our analysis of age-related changes in sensory neurons by comparing the behavioral response properties of young and old male and female C57Blk6 mice. Measures of response latency to an applied noxious thermal stimulus showed that, for both males and females (n = 11–12 animals/group), a longer latency occurred in aged animals (p < 0.0001) (Fig. 1). Comparison of all females (young and old) to all males (young and old) showed that as a group, females had a shorter latency than males, i.e., they were more sensitive to thermal stimuli (p < 0.05). To determine if differences in mechanical sensitivity were present in aged animals, von Frey filaments of varying thickness were applied to the hindpaw and the amount of force that evoked a response (lifting or licking) recorded. No significant difference was measured between young and old mice nor male and female mice (Komolgorov–Smirnov test, p > 0.05, n = 11–12 per group). Thus, using the described assays, 24-month old mice were less sensitive to thermal but not mechanical stimuli.
Fig. 1.
Behavioral analysis of heat sensitivity in young and old Blk6 male and female mice. Young (6–8-week old) and old (2-year old) male and female mice were compared to determine their latency to noxious heat applied to the glabrous skin of the hindfoot. Both age and sex were found to influence latency. Young mice (n = 11–12) responded to thermal noxious stimulation with shorter latencies than old mice (n = 11–12; p < 0.0001; ANOVA). In addition, female mice (in combined young and old groups) had lower thresholds to noxious heat than males (combined young and old groups) did (p < 0.050). Asterisk indicates significance.
3.2. Expression of TTX-resistant sodium channels is reduced in DRG of aging mice
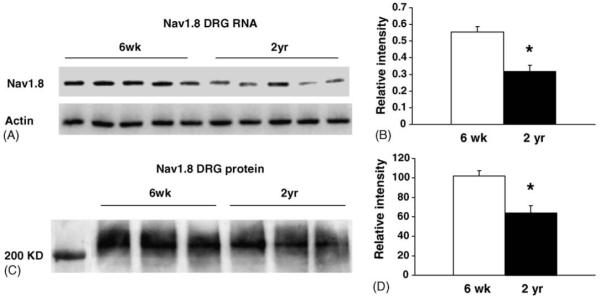
Voltage-gated sodium channels are responsible for the rising phase of the action potential (AP) and play a key role, with potassium channels, in determining the excitability of sensory neurons. The neuron-specific TTXr sodium channels Nav1.8 and Nav1.9 are predominantly expressed in small neurons, many of which are heat sensitive. To determine whether Nav1.8 and Nav1.9 expression are altered in aging sensory neurons, we first examined the abundance of the mRNA encoding each gene using reverse transcriptase-PCR assays. In pooled RNA from cervical, thoracic and lumbar ganglia, a slight reduction (18%; p < 0.05) in Nav1.9 mRNA was measured in DRG from 2-year old animals (n = 5 young, n = 5 aged; Table 1). However, the level of Nav1.8 transcript was reduced 43% in ganglia from 2-year old mice (p < 0.05) (Fig. 2A and B). To examine expression on the protein level, proteins extracted from pooled ganglia were analyzed using western immunoblotting with an antibody made to the rat Nav1.8 channel (Fig. 2C and D). A significant decrease in Nav1.8 abundance was detected (37% decrease, p < 0.05).
Table 1.
Percent change in mRNA abundance in sensory ganglia of young (6–8 weeks) and old (1.5 or 2 year) Blk6 male mice
| Gene assayed | L3/L4/L5 DRG (% change) | Pooled DRG (% change) |
|---|---|---|
| Nav1.9 | n.d. | ↓18* |
| Nav1.8 | n.d. | ↓43* |
| TRPV1 | 6 | 8 |
| TRPV2 | 23 | 5 |
| TRPV3 | 13 | n.d. |
| TRPV4 | 12 | 12 |
| GFRα1 | 23 | n.d. |
| GFRα2 | ↓13* | n.d. |
| GFRα3 | ↓32* | ↓29* |
| C-Ret | ↑35** | n.d. |
Values are intensity readings obtained from RT-PCR assays run for each transcript. N.d.: not done; N = 5–6 for each group.
p ≤ 0.05.
p ≤ 0.01.
Fig. 2.
Expression of the Nav1.8 sodium channel is decreased in DRG of old mice. (A) Reverse transcribed RNA isolated from pooled DRG was placed in a PCR reaction containing 32P-dCTP nucleotide and DNA primers made to either Nav1.8 or actin. Phosphorimager scan shows a decrease in Nav1.8 abundance in DRG from aged animals. (B) Intensity of bands from young (n = 5) and aged (n = 5) samples was quantified using a phosphorimager. Actin expression was used to verify the integrity of the samples and normalize expression. The abundance of Nav1.8 transcripts decreased 43% in old mice (p < 0.05). This decrease was confirmed using SYBR green labeling in real time PCR assays (not shown), which showed a 50% reduction (p < 0.05) in Nav1.8 abundance. (C) Relative amount of Nav1.8 protein measured using a Western immunoblot show a reduced level in pooled DRG from aged animals (n = 3 animals/age group). (D) Densitometric measure of Nav1.8 bands (in panel C) show a 37% decrease in Nav1.8 protein (p < 0.05) in pooled DRG samples from 2-year old mice. Band intensity was normalized to values obtained from Coomassie stained protein bands measured on a gel run in parallel with the samples analyzed.
3.3. The amount of the TRPV1 channel protein is reduced in the aging sensory system
The reduced behavioral sensitivity exhibited by aged Blk6 mice indicated that aged animals are less sensitive to thermal stimuli applied to the foot (Fig. 1). To begin to identify the cellular level changes that could underlie this reduction in thermal sensitivity, we analyzed the expression of genes in the thermosensitive TRP channel family in young and aged DRG. We focused on the TRPV (TRPV1, TRPV2, TRPV3 and TRPV4) members known to be heat responsive [22]. Expression in pooled ganglia and from the L3/L4/L5 lumbar ganglia was analyzed using RT-PCR. Lumbar ganglia were analyzed separately to enrich for neurons that innervate the hindfoot, the site of behavioral testing. Both pooled and lumbar DRG samples showed no difference in TRP expression on the transcriptional level (Table 1).
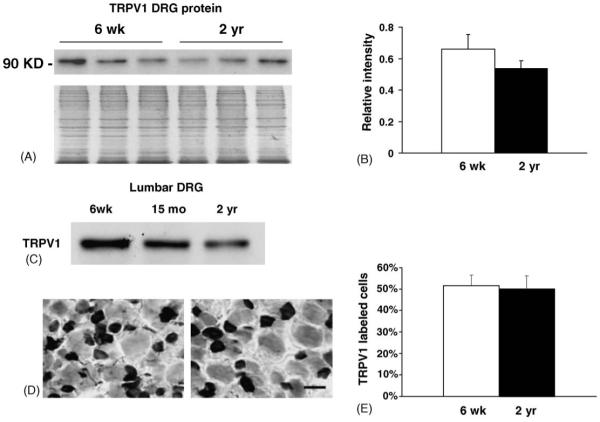
To determine whether an age-related reduction occurred for TRP channels on the translational level, we analyzed TRPV1 expression in protein extracts obtained from pooled DRG (Fig. 3A and B) and lumbar DRG (Fig. 3C) using western immunoblotting. Analysis of pooled DRG showed no statistically significant change in TRPV1 (p = 0.06) (Fig. 3A and B), whereas in lumbar DRG, a reduction in TRPV1 in aging ganglia appears to occur by 15 months of age (Fig. 3C). These results suggest that the amount of TRPV1 protein steadily declines in aged neurons despite the lack of change on the RNA level. In addition, this decline may be greater in neurons that project to distal targets in the leg and foot.
Fig. 3.
Expression of TRPV1 in DRG of young and aged mice. (A) Relative amount of TRPV1 protein in pooled DRG of young (n = 3) and old (n = 3) animals was determined using western immunoblotting. An equal amount of protein (20 μg) was loaded per lane. Coomassie stained gel of DRG samples is shown beneath blot to show loading. (B) Plot shows relative amount of protein in pooled DRG determined from band densitometry normalized to samples run on a Coomassie stained gel. No significant decrease in TRPV1 level was found (p = 0.06). C. Immunoblot of protein from lumbar (L3/L4/L5) DRG shows reduction in TRPV1 in 15 months (9% decrease) and 2-year old (27% decrease) DRG samples. Each lane represents lumbar DRG from three animals (total of nine animals). D. TRPV1 immunolabeling of L4/L5 ganglia of young (left panel) and 2-year old (right panel) ganglia shows prominent expression in smaller neurons. Lighter labeling was also found in some neurons. Both types of labeled cells were counted as TRPV1-positive. F. Plot shows the percentage of TRPV1-labeled neurons does not change in L4/L5 DRG with age. Bar in D = 40 μm.
To examine TRPV1 expression on a per cell level, L4/L5 DRG were immunolabeled with TRPV1 antibody to determine the percentage of neurons that express TRPV1 in young and aged ganglia (Fig. 3D and E). The percentage of TRPV1-positive neurons in DRG from 2–3-month old mice was not different from the percent of TRPV1-positive neurons in aged (2-year old) ganglia (Fig. 3E; young, 51.4±5% versus old, 50.3±5.8%, p = 0.893). Coupled with the reduction of TRPV1 protein detected by immunoblotting, these data suggest that on a per cell level, the decrease in TRPV1 protein may be related to translational processing.
3.4. There are fewer TRPV1-positive fibers in peripheral nerves of aged mice
The reduced level of TRPV1 protein in DRG of aged mice led us to question whether the terminals of these sensory neurons would also exhibit deficiency in TRPV1 level. Tibial nerves from young and old animals were immunolabeled using the anti-TRPV1 antibody to test this possibility (Fig. 4A). Immunolabeling shows an apparent reduction in the number of TRPV1 positive fibers in nerves from aged animals. To verify this decrease we isolated protein from the tibial and saphenous nerve. Whereas the tibial nerve primarily contains nerve fibers innervating muscle and skin, the saphenous nerve is a pure cutaneous nerve that innervates the skin of the leg and foot. A significant reduction in the level of TRPV1 protein in tibial (52%, p < 0.05; Fig. 4B and C) and saphenous nerve samples (25%, Fig. 4D) was measured.
Fig. 4.
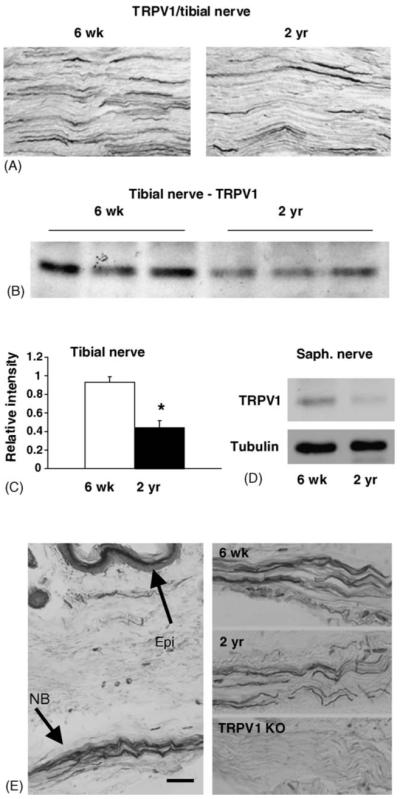
Peripheral nerves contain fewer TRPV1–positive afferents. (A) Immunolabeling of longitudinal sections of tibial nerve form young and aged animals. A reduction of TRPV1 fibers appears in nerve from 2-year old animal. B. Western blot of TRPV1 protein in tibial nerve of young (n = 3) and old (n = 3) animals indicate TRPV1 reduction in aged samples. (C) Plot of band intensity measured from immunoblot shown in (B). (D) Decreased TRPV1 protein was also found in cutaneous saphenous nerves of aged mice. Each lane represents saphenous nerve protein pooled from three young and aged animals. (E) Plantar skin of young animal labeled with TRPV1 antibody to illustrate deep dermal location of nerve bundles that were used to compare TRPV1 afferent density. Comparison of young, aged and TRPV1 knockout nerves are shown in right side panels. Note reduction in TRPV1 immunoreactivity in aged mice and complete lack of TRPV1-immunoreactivity in nerve from TRPV1 knockout animal. Asterisk in C indicates significance. Significance could not be calculated for saphenous samples since three samples were pooled to obtain sufficient amounts of protein. NB, nerve bundle; Epi, epidermis. Scale bar in panel E = 80 μm.
To determine if TRPV1 was reduced in sensory afferents in the skin of aged animals, we compared the density of TRPV1-positive fibers in the footpad of young and aged animals (Fig. 4E). Although the anatomical variation in the footpad hindered the quantitative measure of TRPV1-positive fibers, a general decrease in TRPV1-positive fibers was apparent when comparing the overall number of TRPV1-positive fibers coursing through the nerve bundles in the deep dermal tissue of the foot (Fig. 4E). TRPV1 afferents appeared fewer in number in the aged animals compared to younger animals, which is consistent with the reduction in TRPV1-labeled fibers in the tibial and saphenous nerves.
3.5. The reduction in TRPV1 may result from decreased trophic factor support
Accumulating evidence suggests that the maintenance and sensitivity of sensory neurons is modulated by the level of trophic support provided by cells in peripheral and ganglionic tissues. This support appears to decline in aging systems, as evidenced by the reduction in mRNAs encoding the trk neurotrophin receptors in sensory neurons of the aging rat [4]. A decline in growth factor support and signaling may impede synthesis and transport of neuron specific proteins (e.g., TRPV1 channels) and thereby reduce neuronal sensitivity, leading to higher response thresholds to thermal stimuli (see Fig. 1). With this possibility in mind, we examined expression of receptors for artemin, a neurotrophic factor that supports a nociceptor neuron population that expresses high levels of TRPV1 [31]. We examined the relative expression of the artemin specific GPI-linked binding component GFRα3 and its associated signaling component, the tyrosine kinase receptor Ret in lumbar DRG using RT-PCR and immunoblot assays (Table 1; Fig. 5A). Relative to young animals, GFRα3 mRNA was reduced 32% in lumbar DRG in 15-month old animals (relative intensity young, 0.298±0.022 versus aged, 0.202±0.021; p < 0.05) (Table 1). Protein level expression of GFRα3 was also reduced as shown by immunoblot assay of lumbar DRG pooled from three animals of each age group (total of nine animals) (Fig. 5B). A 24% reduction was measured in the level of GFRα3 in the lumbar ganglia of 15-month old animals relative to 6-week-old animals. Thus both transcriptional and translational downregulation of GFRα3 occurred in aged ganglia.
Fig. 5.
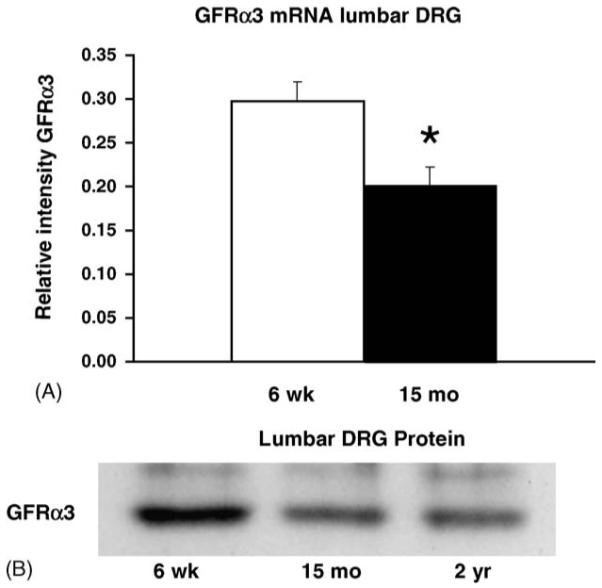
The GFRα3 receptor for the artemin growth factor is decreased in DRG of aging animals. (A) RT-PCR analysis of the level of GFRα3 mRNA in lumbar DRG isolated from young (n = 5) and 15-month old (n = 5) animals. GFRα3 mRNA in aged lumbar DRG is reduced 32%. (B) Immunoblot analysis of protein from lumbar DRG of different aged animals. The amount of GFRα3 receptor declined in 15 months (24%) and 2-year old (23%) age groups. Each lane represents protein pooled from three animals at each age analyzed.
We also examined the relative expression level of other GDNF-ligand binding molecules. Measure of GFRα1, which binds GDNF, showed no significant change in expression in aging DRG whereas GFRα2, which binds neurturin, was slightly decreased (13%, p < 0.05). Interestingly, the Ret tyrosine kinase receptor, which is the signaling component for all GDNF-family ligands, was increased 35% in the aged DRG (Table 1, p < 0.01).
4. Discussion
In this study we examined whether reduced detection of noxious heat in aging mice correlated with changes in ion channel and growth factor receptor expression in DRG and nerves that have projections to the skin. Our findings support the hypothesis that reduced expression of the Nav1.8 and the TRPV1 channel proteins contribute to the decrease in thermal sensitivity we observed in aging mice. This reduction in expression appeared to be more prominent in neurons at lumbar levels that project to the limbs, supporting the notion that long axonal length is a hindrance to anterograde and retrograde transport of substances important for normal neuron function in aged animals [12].
In our behavioral study we show that a decrease in thermosensitive behavior occurs in aged mice in both gender groups. Prior studies of age-associated thermal sensitivity in rodents have focused on the rat. Outcomes of these studies are quite varied, with some reporting reduced thresholds in aged rats and others showing no change [18]. These different outcomes could reflect the rat strain tested or variability in the test used (tail flick and hot plate) to measure thermal responsiveness. In this study we took great care to minimize possible environmental variables, i.e., animals were acclimated to the testing environment, they were tested at the same time of day by the same investigators and we used a radiant heat source applied to a discrete area of the foot with responses timed within 0.1 s (i.e., the Hargreave’s test). As in all behavioral studies of aged animals, a potential caveat is whether motor abilities are impaired in older animals that could slow an avoidance reflex. Although this remains a possibility, the 2-year old animals used in this study showed no discernable impairment in mobility or motor control.
To understand the cellular and molecular basis for the reduction in heat sensitivity, we measured relative levels of the Nav1.8 and Nav1.9 sodium channels in young and old mice. These channels are preferentially expressed by small, peptidergic neurons, many of which are responsive to heat. They also express the tyrosine kinase TrkA and are therefore responsive to NGF, a major mediator of inflammatory pain released from tissues following injury [28]. Although the Nav channels are not directly activated by heat, they are essential for generation and propagation of the action potential that follows a heat stimulus. Nav1.8 channels, in particular, contribute more than 50% of the inward current underlying the depolarizing phase of the action potential in cells in which they are present, and endow cells with the capability to generate sustained trains of action potentials in response to long-lasting stimuli [32]. Thus, the reduction in Nav1.8 levels in the DRG, though modest, could impair nerve function. Likewise, modest reduction of TRPV channel proteins in the DRG cell bodies and afferents could alter the heat threshold of firing. Interestingly, our results indicate that the reduction in TRPV1 in the DRG occurs only at the translational level. This mode of TRPV1 regulation is not unique since translational regulation of TRPV1 expression has also been reported in rat following experimentally induced inflammation of the footpad [21].
Mice at 15 months of age exhibited reduced levels of TRPV1 protein in DRG neurons, though no change in the overall number of TRPV1-positive neurons occurred. This suggests that changes in protein expression that contribute to sensory perception begin at midlife. This decrease in TRPV1 was greater in the 2-year old group indicating a progressive decline. Reduced levels of TRPV1 were also found in peripheral nerves of aged animals suggesting TRPV1 transport is also less efficient. Coincident with these processes, it is also possible that some TRPV1-positive fibers are lost with age due to neuronal death or degeneration of afferents in the periphery. Indeed, the lower density of immunolabeled TRPV1-positive fibers in tibial and cutaneous nerves would support this possibility. However, anatomical studies have indicated that loss of afferents in the skin becomes most prominent only in very old animals [13]. Only a modest afferent loss would therefore be expected at 15 months of age, the time at which a reduction in TRPV1 protein was measured in this study. In addition, in rat, DRG cell counts indicate no significant difference in neuron number between 2-year old DRG and young DRG [6]. Even so, it remains a possibility that afferent or neuron loss contributes to the reduction in thermal sensation measured in this study.
Our analysis of the aging mouse ganglia and peripheral nerves suggest that translation of TRPV1 protein and its transport to the periphery is reduced in the aging animal. A factor that may influence the production and transport of TRPV1 and Nav1.8 channel expression may relate to neurotrophic support provided to aging neurons from peripheral as well as ganglionic sources. Trophic reduction could compromise maintenance of the neuronal phenotype, i.e., channel and peptide expression, and thereby cause loss of functional sensitivity. Trophic signaling may decline due to structural changes in axons that impair retrograde (and anterograde) transport of growth factors and their receptors. In the ganglia, paracrine and autocrine trophic signaling may also be affected. Indeed, decreases in trk tyrosine kinase receptors (which bind the neurotrophins NGF, NT3, NT4 and BDNF) have been reported in sensory neurons of the 30-month old rat, supporting the notion that trophic signaling declines with age [4]. Although an age-related reduction may not be sufficient to cause neuronal death, it may compromise regulation of gene expression. With this in mind, we examined trophic support in the aged ganglia by measuring the relative expression of receptors that bind the GDNF family of ligands, with particular interest in the GPI-anchored GFRα3 receptor. Neurons that express GFRα3, which binds the growth factor artemin, comprise approximately 20% of the lumbar mouse DRG population, show 99% colocalization with TRPV1 [31] and express the tyrosine kinase Ret [3]. Results show the relative abundance of GFRα3 is decreased in aged ganglia on both the transcriptional and translational level in lumbar DRG suggesting this TRPV1 enriched population is preferentially affected in aging systems.
Though a decrease was found in GFRα3, our RT-PCR measures showed an increase in Ret receptor expression and a trend toward increased GFRα1 expression. These results are consistent with previous studies of Ret and GFR expression in the lumbar (L4/L5) DRG of the very old (30 months) rat [5,30]. These investigators also reported an increase in GFRα2, which differs from our finding of a slight decrease in GFRα2 in the DRG. This difference in GFRα2 may be related to differences in the animal model used and/or age of the animals. In Ming et al., female rats were used in comparison to the male mice used in this study. In addition, our measures were made on 15–24-month old animals whereas the rat analysis was done using 30-month old animals that exhibited severe behavioral sensorimotor disturbances.
As mentioned, the Ret receptor component of the artemin signaling complex was increased in the aged ganglia. This increase could reflect an attempt to compensate for the decrease in GFRα3 (and GFRα2), which may occur in response to decreased production of trophic factors or decreased retrograde transport due to degenerative changes in peripheral afferents. Thus, autocrine and paracrine Ret signaling in the aging ganglia may increase to compensate for loss of target-derived signaling. Future studies that examine transcriptional, translational and phosphorylation states of the GFRα3 and Ret receptors will address these possibilities.
Acknowledgement
This work was supported by grants from the NIH to KMA (AG18134 and AG20576). We thank Beth Knapick, Christopher Sullivan and Jessica Lindsay for their excellent technical assistance, Dr. Michael Caterina for providing TRPV1 knockout mice and Dr. Patrick Crumrine for his statistical expertise.
References
- [1].Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, et al. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat Neurosci. 1999;2(6):541–8. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- [2].Amaya F, Decosterd I, Samad TA, Plumpton C, Tate S, Mannion RJ, et al. Diversity of expression of the sensory neuron-specific TTX-resistant voltage-gated sodium ion channels SNS and SNS2. Mol Cell Neurosci. 2000;15(4):331–42. doi: 10.1006/mcne.1999.0828. [DOI] [PubMed] [Google Scholar]
- [3].Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, et al. Artemin a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21(6):1291–302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- [4].Bergman E, Fundin BT, Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons. J Comp Neurol. 1999;410(3):368–86. doi: 10.1002/(sici)1096-9861(19990802)410:3<368::aid-cne2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- [5].Bergman E, Kullberg S, Ming Y, Ulfhake B. Upregulation of GFRalpha-1 and c-ret in primary sensory neurons and spinal motoneurons of aged rats. J Neurosci Res. 1999;57(2):153–65. doi: 10.1002/(SICI)1097-4547(19990715)57:2<153::AID-JNR1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [6].Bergman E, Ulfhake B. Loss of primary sensory neurons in the very old rat: neuron number estimates using the disector method and confocal optical sectioning. J Comp Neurol. 1998;396(2):211–22. [PubMed] [Google Scholar]
- [7].Besne I, Descombes C, Breton L. Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch Dermatol. 2002;138(11):1445–50. doi: 10.1001/archderm.138.11.1445. [DOI] [PubMed] [Google Scholar]
- [8].Black JA, Fjell J, Dib-Hajj S, Duncan ID, O’Connor LT, Fried K, et al. Abnormal expression of SNS/PN3 sodium channel in cerebellar Purkinje cells following loss of myelin in the taiep rat. Neuroreport. 1999;10(5):913–8. doi: 10.1097/00001756-199904060-00004. [DOI] [PubMed] [Google Scholar]
- [9].Bouche P, Cattelin F, Saint-Jean O, Leger JM, Queslati S, Guez D, et al. Clinical and electrophysiological study of the peripheral nervous system in the elderly. J Neurol. 1993;240(5):263–8. doi: 10.1007/BF00838158. [DOI] [PubMed] [Google Scholar]
- [10].Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- [11].Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398(6726):436–41. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- [12].Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- [13].Ceballos D, Cuadras J, Verdu E, Navarro X. Morphometric and ultra-structural changes with ageing in mouse peripheral nerve. J Anat. 1999;195(Pt 4):563–76. doi: 10.1046/j.1469-7580.1999.19540563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chang YC, Lin WM, Hsieh ST. Effects of aging on human skin innervation. Neuroreport. 2004;15(1):149–53. doi: 10.1097/00001756-200401190-00029. [DOI] [PubMed] [Google Scholar]
- [15].Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA. 1998;95(15):8963–8. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol. 2003;550(Pt 3):739–52. doi: 10.1113/jphysiol.2003.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fang X, Djouhri L, Black JA, Dib-Hajj SD, Waxman SG, Lawson SN. The presence and role of the tetrodotoxin-resistant sodium channel Na(v)1.9 (NaN) in nociceptive primary afferent neurons. J Neurosci. 2002;22(17):7425–33. doi: 10.1523/JNEUROSCI.22-17-07425.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gagliese L, Melzack R. Age differences in nociception and pain behaviours in the rat. Neurosci Biobehav Rev. 2000;24(8):843–54. doi: 10.1016/s0149-7634(00)00041-5. [DOI] [PubMed] [Google Scholar]
- [19].Gillespie PG, Walker RG. Molecular basis of mechanosensory transduction. Nature. 2001;413(6852):194–202. doi: 10.1038/35093011. [DOI] [PubMed] [Google Scholar]
- [20].Gunthorpe MJ, Benham CD, Randall A, Davis JB. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 2002;23(4):183–91. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- [21].Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- [22].Jordt SE, McKemy DD, Julius D. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 2003;13(4):487–92. doi: 10.1016/s0959-4388(03)00101-6. [DOI] [PubMed] [Google Scholar]
- [23].Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413(6852):203–10. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- [24].Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226(4678):1089–92. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- [25].Lauria G, Holland N, Hauer P, Cornblath DR, Griffin JW, McArthur JC. Epidermal innervation: changes with aging, topographic location, and in sensory neuropathy. J Neurol Sci. 1999;164(2):172–8. doi: 10.1016/s0022-510x(99)00063-5. [DOI] [PubMed] [Google Scholar]
- [26].Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VROAC), a candidate vertebrate osmoreceptor. Cell. 2000;103(3):525–35. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55(12):1513–20. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- [28].Mendell LM, Albers KM, Davis BM. Neurotrophins, nociceptors, and pain. Microsc Res Tech. 1999;45(45):252–61. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<252::AID-JEMT9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [29].Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. J Neurosci. 1999;19(5):1844–54. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ming Y, Bergman E, Edstrom E, Ulfhake B. Evidence for increased GDNF signaling in aged sensory and motor neurons. Neuroreport. 1999;10(7):1529–35. doi: 10.1097/00001756-199905140-00025. [DOI] [PubMed] [Google Scholar]
- [31].Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRal-pha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13(11):2177–82. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- [32].Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol. 2001;86(2):629–40. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- [33].Scott B, Leu J, Cinader B. Effects of aging on neuronal electrical membrane properties. Mech Ageing Dev. 1988;44(3):203–14. doi: 10.1016/0047-6374(88)90022-x. [DOI] [PubMed] [Google Scholar]
- [34].Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418(6894):186–90. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- [35].Suzuki M, Watanabe Y, Oyama Y, Mizuno A, Kusano E, Hirao A, et al. Localization of mechanosensitive channel TRPV4 in mouse skin. Neurosci Lett. 2003;353(3):189–92. doi: 10.1016/j.neulet.2003.09.041. [DOI] [PubMed] [Google Scholar]
- [36].Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–43. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- [37].Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5(4):191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- [38].Xu H, Ramsey IS, Kotecha SA, Moran MM, Chong JA, Lawson D, et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418(6894):181–6. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]