Abstract
Objective:
This study compared baseline metabolite levels in components of the brain reward system among individuals who remained abstinent and those who resumed hazardous alcohol consumption after treatment for alcohol dependence.
Method:
Fifty-one treatment-seeking alcohol-dependent individuals (abstinent for approximately 7 days [SD = 3]) and 26 light-drinking nonsmoking controls completed 1.5-T proton magnetic resonance spectroscopic imaging, yielding regional concentrations of N-acetylaspartate, choline-containing compounds, creatine-containing compounds, and myoinositol. Metabolite levels were obtained in the following component of the brain reward system: dorsolateral prefrontal cortex, anterior cingulate cortex, insula, superior corona radiata, and cerebellar vermis. Alcohol-dependent participants were followed over a 12-month period after baseline study (i.e., at 7 days of abstinence [SD = 3]) and were classified as abstainers (no alcohol consumption; n = 18) and resumers (any alcohol consumption; n = 33) at follow-up. Baseline metabolite levels in abstainers and resumers and light-drinking nonsmoking controls were compared in the above regions of interest.
Results:
Resumers demonstrated significantly lower baseline N-acetylaspartate concentrations than light-drinking nonsmoking controls and abstainers in all regions of interest. Resumers also exhibited lower creatine-containing-compound concentrations than abstainers in the dorsolateral prefrontal cortex, superior corona radiata, and cerebellar vermis. Abstainers did not differ from light-drinking nonsmoking controls on baseline metabolite concentrations in any region of interest.
Conclusions:
The significantly decreased N-acetylaspartate and creatine-containing-compound concentrations in resumers suggest compromised neuronal integrity and abnormalities in cellular bioenergetics in major neocortical components and white-matter interconnectivity of the brain reward pathway. The lack of metabolite differences between abstainers and light-drinking nonsmoking controls suggests premorbid factors potentially contributed to the baseline brain metabolite abnormalities observed in resumers.
Alcohol-use disorders (AUDs; i.e., alcohol dependence and abuse) are characterized by a chronically relapsing/remitting course over the lifetime (Dawson et al., 2007; Jin et al., 1998; Miller et al., 2001; Zywiak et al., 2006). The resumption of hazardous levels of alcohol consumption after treatment is common (Donovan, 1996; Maisto and Connors, 2006; Miller et al., 2001; Monahan and Finney, 1996), and appears to be mediated by a complex interplay among genetic, neurobiological, neurocognitive, psychological/psychiatric, and sociodemographic factors (Adinoff et al., 2005; Baler and Volkow, 2006; Bottlender and Soyka, 2005; Bradizza et al., 2006; Glenn and Parsons, 1991; Goodman, 2008; Heinz et al., 2003; Jin et al., 1998; Koob, 2003; Moos and Moos, 2006; Parsons et al., 1990; Sher et al., 2005; Weiss and Porrino, 2002; Zywiak et al., 2006). A considerable amount of research has investigated the psychological, psychiatric, sociodemographic, and behavioral correlates of relapse following treatment; however, the neurobiological mechanisms underlying relapse in humans have only recently begun to be delineated, largely because of advances in in-vivo magnetic resonance and positron emission tomography neuroimaging methods (Volkow et al., 2004).
Abnormalities of neurotransmission, metabolism, and/or morphology in the brain reward system (also known as the mesocorticolimbic reward circuit) are implicated as major contributors to the development and maintenance of all forms of substance-use disorders (Bowirrat and Oscar-Berman, 2005; Kalivas and O'Brien, 2008; Kalivas and Volkow, 2005; Koob, 2003; Lubman et al., 2004; Makris et al., 2008; Pierce and Kumaresan, 2006; Volkow et al., 2004, 2008; Wrase et al., 2008). Major components of the brain reward system include the dorsolateral prefrontal cortex (DLPFC), orbitofrontal cortex, insula, anterior cingulate cortex (ACC), hippocampus, amygdala, thalamus, nucleus accumbens, ventral tegmental area, and other nuclei of the basal forebrain and ventral pallidum (Kalivas and Volkow, 2005; Makris et al., 2008; Volkow et al., 2008). Although the cerebellum and corona radiata are not considered to be core components of the brain reward system, there is a clear rationale for their inclusion as constituents of the reward system. The cerebellum is involved in aspects of learning and memory, working memory, executive skills, and reward processing (Anderson et al., 2006; Martin-Solch et al., 2001; Olbrich et al., 2006; Paul et al., 2009; Sullivan, 2003; Sullivan et al., 2003). The corona radiata is comprised of projection bundles (e.g., corticothalamic, corticostriatal, corticopontine) and reciprocal fibers that link frontal, parietal, and temporal neocortical regions and subcortical nuclei involved in executive skills, impulse control, emotional regulation, and reward processing (Aralasmak et al., 2006; Cummings, 1995, 1998; Makris et al., 1999; Mega and Cummings, 1994; Saint-Cyr, 2003; Schmahmann et al., 2007). AUDs are associated with morphological and metabolite abnormalities of the cerebellum, particularly the vermis, as well as in the reciprocal connections between the cerebellum and frontal lobes (Bendszus et al., 2001; Martin et al., 1995; Parks et al., 2002; Sullivan, 2000; Sullivan and Pfefferbaum, 2005; Sullivan et al., 2003). Additionally, numerous studies have reported regional white-matter morphological and metabolite abnormalities in AUDs, particularly in the frontal white matter that includes the corona radiata (Durazzo and Meyerhoff, 2007; Pfefferbaum and Sullivan, 2005; Sullivan, 2000). Therefore, compromised integrity of the cerebellum, cerebellothalamocortical, corticopontocerebellar circuits, and/or pathways comprising the corona radiata may contribute to initiation and/or maintenance of AUDs.
Makris and colleagues (2008) employed resonance-based techniques to specifically investigate the morphology of several major components of the brain reward system in AUDs. They observed significantly decreased volumes in the right DLPFC, right anterior insula, right nucleus accumbens, and left amygdala in long-term abstinent alcohol-use-disordered individuals relative to controls. Greater length of abstinence was related to larger anterior insula and nucleus accumbens volumes, and the total brain reward network and amygdala volumes were positively related to memory in the AUD sample. In treatment-seeking alcohol-use-disordered individuals, initially studied after 1–12 weeks of abstinence (Wrase et al., 2008), those who relapsed within 6 months following treatment demonstrated significantly lower amygdala volume compared with those who abstained over the same interval and relative to controls. Relapsers had smaller ventral striatal and hippocampal volumes than controls but were not significantly different from abstainers in these regions. Abstainers exhibited a lower volume than controls only in the ventral striatum. In the AUD group as a whole, smaller amygdala volume was related to greater alcohol craving, which appeared to be primarily driven by the relapsers.
Our group previously combined multimodality proton magnetic resonance, neurocognitive, psychiatric, and so-ciodemographic measures to predict resumption of hazardous levels of alcohol consumption following outpatient treatment for AUDs (Durazzo et al., 2008). In addition to comorbid unipolar mood disorders and neurocognitive measures of processing speed, decreased concentrations of N-acetylaspartate (NAA; a surrogate marker of neuronal integrity; Moffett et al., 2007) in the temporal gray matter and frontal white matter, and decreased levels of frontal gray-matter choline-containing compounds (Cho; a marker of cell membrane turnover and/or synthesis; Ross and Bluml, 2001) were independent predictors of resumption of hazardous drinking levels within 12 months of treatment. However, our proton magnetic resonance spectroscopic imaging (1H MRSI) results reflected metabolite concentrations for the entire lobar regions of interest (e.g., total frontal lobe gray matter and white matter) and did not specifically quantitate metabolite levels in components of the brain reward system.
The purpose of the present study was to examine metabolite levels in multiple components of the brain reward system in alcohol-dependent individuals near the inception of outpatient treatment (i.e., baseline) for AUDs. We predicted that those who resumed hazardous levels of alcohol consumption within 12 months following treatment demonstrate lower baseline concentrations of NAA and Cho, compared with baseline levels of those who remained abstinent and healthy light drinking controls in the DLPFC, insula, ACC, cerebellar vermis, and superior corona radiata (SCR). We also predicted that lower regional levels of NAA would be related to greater levels of hazardous alcohol consumption in those who resumed drinking after outpatient treatment.
Method
Participants
Fifty-one outpatient participants (three women) were recruited from the Veterans Affairs Medical Center Substance Abuse Day Hospital and the Kaiser Permanente Chemical Dependence Recovery Program in San Francisco. All treatment-seeking participants met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), criteria for alcohol dependence (all with physiological dependence) at study enrollment. Primary inclusion criteria for the alcohol-dependent participants were fluency in English, DSM-IV diagnosis of alcohol dependence or alcohol abuse at the time of enrollment, consumption of greater than 150 standard alcoholic drinks (i.e., 13.6 g of ethanol) per month for at least 8 years before enrollment for men, or consumption of greater than 80 drinks per month for at least 6 years before enrollment for women. Light-drinking nonsmoking controls (n = 26; 3 women) were recruited from the local community. Participants were between 28 and 66 years of age. (See Table 1 for group demographic data.) All participants provided written informed consent before the study, which was approved by the University of California San Francisco and the San Francisco Veterans Affairs Medical Center.
Table 1.
Group demographics, alcohol and cigarette consumption, and mood and anxiety measures
| Variable | nsLD (n = 26) M (SD) or % | Abstainers (n = 18) M (SD) or % | Resumers (n = 33) M (SD) or % | |
| Age | 45.5 (8.5) | 50.7 (9.3) | 47.5 (8.9) | |
| Education | 16.2 (2.6) | 14.6 (2.3) | 13.6(1.8) | |
| AMNART | 118 (7) | 111 (10) | 112 (8) | |
| 1 -year drinks/month | 16 (18) | 345 (173) | 415 (171) | |
| 8-year drinks/month | 16 (18) | 276 (153) | 351 (131) | |
| Lifetime drinks/month | 17 (15) | 184 (98) | 260 (162) | |
| Months of heavy drinking | na | 229 (116) | 274 (112) | |
| Smokers | 0% | 50% | 55% | |
| FTND total | na | 5.5 (1.7) | 5.1 (2.4) | |
| Smoking duration | na | 29 (12) | 28 (15) | |
| Cigarette pack years | na | 29 (18) | 28 (20) | |
| Beck Depression Inventory | 4 (4) | 13 (9) | 16 (10) | |
| STAI-trait | 38 (7) | 48 (10) | 49 (13) | |
| Comorbid psychiatric disorder | na | 33% | 42% | |
| Comorbid medical condition | na | 44% | 46% | |
| Comorbid substance-use disorder | na | 12% | 18% | |
| Body mass index, kg/m2 | 25.8 (3.1) | 26.2 (3.8) | 26.5 (3.9) | |
| History of previous treatment for AUD | na | 75% | 80% |
Notes: nsLD = light-drinking nonsmoking controls; AMNART = American National Adult Reading Test; FTND = Fagerstrom Test for Nicotine Dependence; na = not applicable; STAI = State-Trait Anxiety Inventory; AUD = alcohol-use disorder.
Medical exclusion criteria for all participants were history of any of the following: intrinsic cerebral masses, HIV/AIDS, cerebrovascular accident, brain aneurysm, arteriovenous malformations, peripheral vascular disease, myocardial infarction, uncontrolled chronic hypertension (systolic blood pressure > 180 mm Hg and/or diastolic blood pressure > 120 mm Hg), Type 1 diabetes, moderate or severe chronic obstructive pulmonary disease, non-alcohol-related seizures, significant exposure to known neurotoxins (e.g., toluene and carbon tetrachloride), demyelinating and neurodegenerative diseases, clinically documented Wernicke-Korsakoff syndrome, alcohol-induced persisting dementia, penetrating head trauma, and closed-head injury resulting in loss of consciousness for more than 10 minutes. Psychiatric exclusion criteria included dependence on any substance other than alcohol or nicotine in the 5 years immediately before enrollment, any intravenous drug use in the 5 years immediately before enrollment in the study, current opioid agonist therapy, history of schizophrenia-spectrum disorders, bipolar disorder, dissociative disorders, posttraumatic stress disorder, obsessive-compulsive disorder, panic disorder (with or without agoraphobia), and major depression with mood-incongruent psychotic symptoms. Hepatitis C, Type 2 diabetes, hypertension, and unipolar mood disorder (major depression and/or substance-induced mood disorder) were permitted in the alcohol-dependent cohort given their high prevalence in AUDs (Hasin et al., 2007; Mertens et al., 2005; Parekh and Klag, 2001; Stinson et al., 2005). Light drinking nonsmoking controls had no history of DSM-IV Axis I diagnoses. Participants were urine-tested for illicit substances immediately before all assessments (i.e., cannabinoids, opiates, phencyclidine, cocaine, and amphetamines) and did not test positive for these substances at any assessment.
Baseline assessment
For the alcohol-dependent cohort, baseline clinical and magnetic-resonance procedures were conducted a mean of 7 days (SD = 3) after last drink. All alcohol-dependent participants were actively involved in outpatient treatment at the time of the baseline assessment, and durations for these programs typically ranged from 14 to 28 days.
Clinical measures
At the baseline assessment, participants completed the Structured Clinical Interview for DSM-IV Axis I Disorders, Version 2.0 (First et al., 1998), and semistructured interviews for lifetime alcohol consumption (Lifetime Drinking History; Sobell and Sobell, 1992; Sobell et al., 1988) and substance use (in-house questionnaire assessing substance type, and quantity and frequency of use). From the Lifetime Drinking History, average number of alcoholic drinks per month over 1, 3, and 8 years before enrollment, average number of drinks per month over lifetime, lifetime years of regular drinking (i.e., years in which the participant consumed at least one alcoholic drink per month), age at onset, and duration of heavy drinking (defined as drinking more than 100 drinks per month for men and 80 drinks per month for women) were calculated. Premorbid verbal intelligence was estimated with the American National Adult Reading Test (Grober and Sliwinski, 1991). Participants also completed standardized questionnaires assessing depressive (Beck Depression Inventory; Beck, 1978) and anxiety symptomatology (State-Trait Anxiety Inventory, form Y-2; Spielberger et al., 1977) and nicotine dependence via the Fagerstrom Tolerance Test for Nicotine Dependence (Fagerstrom et al., 1991). These measures were typically completed within 1 day of the magnetic resonance study described below.
Magnetic resonance acquisition and analyses
At baseline, participants were studied with three-dimensional magnetic resonance imaging (magnetization-prepared rapid acquisition with gradient echo, TR/TE/TI = 9.7/4/300 ms) and double-spin echo (TR/TE1/TE2 = 5,000/20/80 ms) sequences, with 1 × 1 mm2 in-plane resolution. Regional gray-matter, white-matter, and cerebrospinal-fluid volumetry was performed using automated probabilistic segmentation, combined with automated atlas-based region labeling of the four lobes, cerebellum, and subcortical structures (Cardenas et al., 2005; Meyerhoff et al., 2004). Values obtained were normalized to intracranial volume for each participant. In the same scanning session at baseline, participants completed multislice 1H MRSI (TR/TI/TE = 1,800/165/25 ms) to obtain absolute concentrations for NAA, Cho, creatine-containing compounds (Cr), and myoinositol (mI). Cr is characterized as a marker of bioenergetics of neuronal and glial tissue (Ferguson et al., 2002), and mI is characterized as an astrocyte marker and as an osmolyte (Brand et al., 1993; Schweinsburg et al., 2000). Metabolite concentrations were derived from atrophy-corrected, region-averaged metabolite spectra obtained in three parallel planes through the centrum semiovale, basal ganglia, and cerebellar vermis. The three slices of the 1H MRSI data set were spatially aligned with a T2-weighted oblique axial magnetic resonance imaging data set (on which the specific smaller regions of interest in the reward pathway were selected; see below). Each 1H MRSI voxel (spectrum) for a particular plane was coded by coordinates on a 64 × 64 grid that corresponded to the field of view and contained information about anatomical location and tissue type amount (i.e., lobar gray matter, white matter, and cerebrospinal fluid) that contributed to each of the voxels. Nominal voxel size was 0.8 × 0.8 × 1.5 cm3 or approximately 1 ml (see Meyerhoff et al., 2004, for details).
Region segmentation and proton magnetic resonance spectroscopic imaging spectra extraction
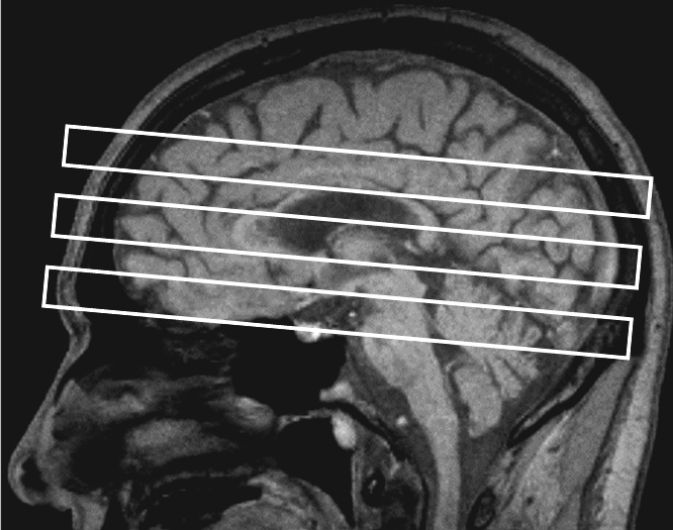
The 1H MRSI data (metabolite concentration maps) were displayed together with corresponding brain anatomy on a Proton density-weighted magnetic resonance imaging in SITOOLS (Soher et al., 1998). This software program has been extensively used to process 1H MRSI data in our laboratory (e.g., Durazzo et al., 2004; Gazdzinski et al., 2008; Meyerhoff et al., 2004). For the ACC, DLPFC, cerebellar vermis, and SCR, a rectangular region encompassing the volume of the specific region of interest was circumscribed on the co-aligned magnetic-resonance images, and the corresponding 1H MRSI voxel coordinates for each region of interest were recorded. Given the winding morphology of the insula, voxels were selected by noting the coordinates of the individual 1H MRSI voxels that contained the targeted tissue. The following conventions were applied to position the volume for the regions of interest (see Figure 1 for approximate placement of dorsal boundary of each region of interest).
Figure 1.
Representative example of proton magnetic resonance spectroscopic imaging multislice spatial positioning on sagittal T1-weighted magnetic resonance imaging
Dorsolateral prefrontal cortex region of interest.
This region generally corresponds to Brodmann Areas 6 (inferior aspect), 8, 9, and 46 (posterior and superior aspects). In the axial plane, the posterior boundary was the inferior frontal sulcus. The ventral boundary was the ventral aspect of the frontal horns of the lateral ventricles.
Anterior cingulate cortex region of interest.
This region generally corresponds to Brodmann Areas 24 and 33, the anterior boundary was the anterior margin of cingulate sulcus and the posterior boundary was a line parallel to the origin of the precentral sulcus in the axial plane. The ventral boundary was the ventral aspect of the frontal horns of the lateral ventricles.
Insular region of interest.
The anterior boundary was the anterior margin of the circular sulcus, and the posterior border was the posterior margin of the circular sulcus in the axial plane. The ventral boundary was the ventral aspect of the thalamus.
Superior corona radiata region of interest.
The anterior boundary was a line perpendicular to the inception of the precentral sulcus, and the posterior boundary was a line perpendicular to the inception of the central sulcus in the axial plane. The medial boundary was adjacent to the central portion of the lateral ventricle. The lateral boundary was approximately three contiguous voxels from the medial boundary in the axial plane. The ventral boundary was the rostral aspect of the central body of the lateral ventricle.
Cerebellar vermis region of interest.
Anterior boundary was the anterior aspect of the anterior lobe, and the posterior boundary was the posterior aspect of the posterior lobe. The region of interest spanned the length of the vermis of the left and right hemispheres and was approximately two to three contiguous voxels wide in the axial plane. The ventral boundary was the rostral margin of the fourth ventricle.
For the DLPFC, insula, and SCR regions of interest, voxel coordinates were separately recorded from the left and right hemispheres. Voxel coordinates for the ACC represent those for the combined left and right hemispheres. For each region of interest, the spatially corresponding spectra for each metabolite were extracted from the database, and the concentrations of metabolites for each region of interest represented the average of all extracted spectra. The database also contained information about tissue composition for each MRSI voxel, based on the co-aligned magnetic resonance imaging data set that was previously segmented into tissue types and major anatomical subdivisions. The DLPFC, ACC, insula, and vermis voxels were selected according to the gray-matter tissue fraction (volume of gray matter in the voxel divided by total tissue volume that comprised the voxel). All spectra with more than 33% cerebrospinal fluid were excluded because of a relatively low signal-to-noise ratio. DLPFC, ACC, insula, and vermis voxels contained greater than 45%, 40%, 40%, and 50% gray matter, respectively. For the SCR, voxels were selected according to the white matter tissue fraction (volume of white matter in the voxel divided by total tissue volume that comprised the voxel). All spectra with greater than 33% cerebrospinal fluid were excluded. SCR voxels contained greater than 70% white matter. These percentages were chosen because they yielded the maximum number of voxels passing quality control (see Meyerhoff et al., 2004). There were no hemispheric differences within groups for metabolite concentrations for the DLPFC, insula, and SCR; therefore, the metabolite concentrations for these regions are based on the average of the left and right hemispheres. The mean number of voxels used to calculate the average metabolite concentrations in each of the regions of interest were not significantly different among abstainers, resumers, and light-drinking nonsmoking controls and were as follows for all groups combined: DLPFC (M = 26.2, SD = 16.3), ACC (M = 21.8, SD = 8.5), insula (M = 26.3, SD = 16.2), SCR (M = 56.9, SD = 14.1), and cerebellar vermis (M = 16.0, SD = 4.8).
Follow-up assessment for alcohol-dependent cohort
Follow-up for the alcohol-dependent participants occurred 1–12 months following the baseline studies. It involved face-to-face and telephone contact with participants, review of available medical records, and/or telephone interview of collateral sources. Thirty-four of 51 participants were reevaluated an average of 265 days (SD = 56) after baseline assessment with all magnetic-resonance, psychiatric, and behavioral measures administered at the baseline assessment. Alcohol consumption during this interval was evaluated with the Timeline Followback interview (Sobell and Sobell, 1992), and the quantity/frequency of any other substance use was recorded during follow-up interviews when possible. The disposition of the remaining 17 participants was obtained via brief face-to-face or telephone interview (n = 7), review of medical records (confined to entries from mental health professionals providing outpatient substance-use disorder treatment for the participant; n = 8), or telephone interview of collateral sources (i.e., family or friends; n = 2).
Participants were designated as abstainers (n = 18) if they met all the following criteria: (a) self-reported no alcohol consumption between the baseline assessment and follow-up; (b) there was no report of alcohol consumption between the baseline and follow-up in available medical records; and (c) available laboratory indicators of alcohol consumption (e.g., gamma-glutamyltransferase) were within normal limits at follow-up. Participants were designated as resumers of alcohol consumption (resumers; n = 33) if they met any of the following criteria: (a) self-reported any alcohol consumption at any time between the baseline assessment and follow up via telephone or in-person interview, (b) use of alcohol was indicated in medical records by mental health professionals providing outpatient substance abuse and dependence treatment for the participant or other medical professionals, or (c) report of alcohol use by a relative or close friend of the participant via telephone or in-person interview. To assist in characterizing the severity of the drinking episode(s) for resumers, we identified the number of participants who met Project MATCH (Matching Alcoholism Treatments to Client Heterogeneity) criteria for an alcohol relapse (i.e., men: ≥3 consecutive days of consumption of six or more drinks per day; women: ≥3 consecutive days of consumption of four or more drinks per day). These criteria were applied only to those resumers who had specific quantity/frequency information regarding their drinking episodes after the baseline assessment (see Table 2).
Table 2.
Alcohol-consumption characteristics of resumers following baseline assessment
| Variable | M (SD) or % | Min. | Max. |
| Duration of abstinence, days | 166 (75) | 24 | 337 |
| Duration of drinking episode(s),days | 59 (60) | 3 | 226 |
| Drinks per day during drinking episode(s) | 14 (8) | 3 | 24 |
| Total drinks during drinking episode(s) | 708 (755) | 9 | 3.024 |
| % consuming ≥6 drinks per drinking day | 90% | ||
| % meeting Project MATCH relapse criteria | 90% |
Notes: Min. = minimum; max. = maximum; duration of abstinence = number of consecutive abstinent days from baseline assessment to first drink; duration of drinking episode(s) = total number of days where at least one alcoholic beverage was consumed; MATCH = Matching Alcoholism Treatments to Client Heterogeneity. All correlation coefficients are Spearman's rho.
The 18 abstainers were initially re-assessed a mean of 216 days (SD = 33), and the 33 resumers were initially reassessed a mean of 243 days (SD = 79) after the baseline assessment. All 18 of the abstainers were again successfully recontacted in person or via telephone after the initial follow-up assessment, at different intervals, to obtain self-reports on drinking status. At the longest follow-up interval, abstainers self-reported a mean of 917 days (SD = 502; range: 265-2,184) of continuous sobriety following their baseline assessment.
Data analyses
Group comparisons among abstainers, resumers, and light-drinking nonsmoking controls on baseline NAA, Cho, Cr, and mI levels were conducted via univariate analyses (generalized linear model) for the following regions: DLPFC, ACC, insula, SCR, and cerebellar vermis. Age was included as a covariate because light-drinking nonsmoking controls were significantly younger than abstainers. Significant univariate analyses were followed-up with pairwise t tests. Effect sizes for pairwise comparisons were calculated via Cohen's d (Cohen, 1988). Alpha levels (.05) for the univariate omnibus tests for metabolites in each region were adjusted for multiple comparisons according to the number of metabolites measured (i.e., 4) and the average intercorrelations among the four metabolites for all groups combined in the region of interest (ACC: r = .72; DLPFC: r = .63; insula: r = .61; SCR: r = .61; vermis: r = .60) (see Sankoh et al., 1997). Alpha levels for pairwise t tests were adjusted for multiple comparisons according to the number of pairwise tests (i.e., 3) and the average intercorrelations among the metabolites for all groups combined in the region of interest (see Sankoh et al., 1997). In exploratory analyses, relationships between metabolite concentrations in the regions of interest, relapse severity variables for resumers (e.g., duration of relapse, number of drinks consumed during relapse), and prestudy enrollment alcohol and cigarette consumption variables in the alcohol-dependent cohort were examined with Spearman's rho; alpha levels were not adjusted for multiplicity of tests in these exploratory analyses All analyses were conducted with SPSS, Version 16 (SPSS Inc., Chicago, IL).
Results
Demographic, alcohol, and cigarette consumption variables
Sixty-eight percent of the light-drinking nonsmoking controls and 74% of the alcohol-dependent cohorts were Caucasian. Of the 51 alcohol-dependent participants, 18 (35%) were abstainers and 33 (65%) were resumers. Ninety percent of resumers met Project MATCH criteria for an alcohol relapse. There were no significant baseline differences in indexes of psychosocial functioning (e.g., education, socioeconomic status, percentage gainfully employed) between abstainers and resumers. Approximately 75% of abstainers and 80% of resumers had at least one previous inpatient or outpatient treatment for an AUD. Abstainers and resumers were equivalent on age, predicted premorbid verbal intelligence, and body mass index (see Table 1). Abstainers and resumers were also equivalent on average number of drinks per month over 1 and 3 years before enrollment, number of months of heavy drinking, and years of regular drinking. Resumers tended to consume more drinks per month over 8 years before enrollment and over lifetime than abstainers (both ps = .09). The frequency of chronic smoking was equivalent between resumers (55%) and abstainers (50%), and they were not different on measures of cigarette consumption (Table 1). Table 2 provides alcohol-use characteristics for resumers over the interval between the baseline assessment and follow-up.
Comorbid psychiatric, medical, and substance-use disorders
Resumers and abstainers were equivalent on the frequency of comorbid psychiatric (primarily major depression or substance-induced mood disorder with depressive features), medical (primarily hypertension and hepatitis C) and substance-use disorders. Resumers and abstainers were also equivalent on the Beck Depression Inventory and State-Trait Anxiety Inventory, form Y-2 (see Table 1). Approximately 30% of participants diagnosed with a unipolar mood disorder took an antidepressant medication, and approximately 70% percent of participants with hypertension took antihypertensive medications; there were no differences between resumers and abstainers in frequency of use of antidepressant or antihypertensive medications.
Baseline metabolite levels in the brain reward system
Comparisons among abstainers, resumers, and light-drinking nonsmoking controls.
In each region of interest in the following, a test statistic was considered significant when the reported p value is equal to, or less than, the stated corrected p value (Table 3).
Table 3.
Baseline regional metabolite concentrations
| Effect size (Cohen's d) |
||||||
| Region | Metabolite | nsLD (n = 26) M (SD) | Abstainers (n = 18) M (SD) | Resumers (n = 33) M (SD) | Resumers vs. nsLD | Resumers vs. abstainers |
| DLPFC | NAA | 34.0 (3.5)a | 35.6 (4.3)b | 31.1 (3.8)a,b | 0.87 | 1.10 |
| Cr | 22.3 (2.3) | 35.6 (4.3)b | 21.6 (2.6)b | 0.90 | ||
| Cho | 5.3 (0.8) | 5.7 (0.8) | 5.1 (0.7) | |||
| mI | 17.6 (3.2) | 19.3 (4.5) | 18.6 (3.3) | |||
| ACC | NAA | 31.2 (4.1)a | 31.8 (3.5)b | 29.1 (4.1)a,b | 0.52 | 0.71 |
| Cr | 20.2 (3.1) | 21.7 (2.7) | 20.1 (3.2) | |||
| Cho | 6.5 (0.9) | 6.6 (0.9) | 6.0 (1.3) | |||
| mI | 18.2 (2.6) | 19.9 (3.7) | 18.4 (4.2) | |||
| Insula | NAA | 33.0 (3.2)a | 32.7 (3.4)b | 31.1 (2.8)a,b | 0.63 | 0.52 |
| Cr | 23.0 (2.6) | 23.3 (3.1) | 23.2 (2.9) | |||
| Cho | 6.5 (0.8) | 6.1 (1.1) | 6.4 (0.9) | |||
| mI | 19.1 (3.3) | 20.2 (4.8) | 20.4 (3.2) | |||
| Vermis | NAA | 33.5 (3.3)a | 35.6 (4.3)b | 31.4 (3.7)a,b | 0.60 | 1.10 |
| Cr | 32.7 (3.4) | 34.7 (5.6)b | 31.7 (4.6)b | 0.65 | ||
| Cho | 8.5 (1.1) | 8.2 (1.7) | 8.0 (1.4) | |||
| mI | 24.1 (3.9) | 26.2 (9.6) | 23.8 (5.1) | |||
| SCR | NAA | 31.8 (3.4)a | 31.5 (2.8)b | 29.4 (3.0)a,b | 0.75 | 0.72 |
| Cr | 18.6 (2.0) | 19.6 (2.0)b | 18.1 (2.2)a,b | 0.71 | ||
| Cho | 5.9 (0.8)a | 5.8 (0.7)b | 5.3 (0.7)a,b | 0.83 | 0.74 | |
| mI | 16.9 (2.3) | 18.1 (3.1) | 17.2 (2.8) | |||
Notes: nsLD = light-drinking nonsmoking controls; DLPFC = dorsolateral prefrontal cortex; NAA = N-acetylaspartate; Cr = creatine-containing metabolites; Cho = choline-containing metabolites; mI = myoinositol; ACC = anterior cingulate cortex;
resumer < nsLD, p < .05;
resumer < abstainer, p < .05.
Dorsolateral prefrontal cortex.
Significant group differences (corrected p ≤ .030) were observed for NAA, χ2(3) = 19.42, p < .001, and Cr concentrations, χ2(3) = 13.42, p = .004. No significant group differences were found for Cho or mI levels. Follow-up t tests (corrected p ≤ .033) indicated resumers demonstrated lower NAA than light-drinking nonsmoking controls and abstainers (both p ≤ .007), and resumers had a lower Cr level than abstainers (p = .001). No significant differences were observed between abstainers and light-drinking nonsmoking controls for NAA and Cr levels. Cr concentration among resumers and light-drinking nonsmoking controls was not significantly different.
Anterior cingulate cortex.
Groups were significantly different (corrected p ≤ .034) on NAA, χ2(3) = 10.56, p = .014, with a trend for Cho, χ2(3) = 5.34, p = .069. No group differences were apparent for Cr and ml levels. Follow-up t tests (corrected p ≤ .036) showed resumers demonstrated lower NAA than light-drinking nonsmoking controls (p = .031) and abstainers (p = .011). No significant differences were observed between light-drinking nonsmoking controls and abstainers for NAA concentration.
Insula.
Group differences (corrected p ≤ .029) were observed for NAA, χ2(3) = 8.34, p < .025. No group differences were found for Cho, Cr, or mI levels. Follow-up t tests (corrected p ≤ .032) indicated resumers had lower NAA than light-drinking nonsmoking controls (p = .017) and abstainers (p = .030). No significant differences were apparent between abstainers and light-drinking nonsmoking controls for NAA level.
Cerebellar vermis.
Significant group differences (corrected p ≤ .029) were observed for NAA, χ2(3) = 13.57, p < .004, and Cr, χ2(3) = 11.53 p = .009. No group differences were found for Cho or ml levels. Follow-up t tests (corrected p ≤ .033) showed resumers had lower NAA than light-drinking nonsmoking controls and abstainers (both p ≤ .015), and resumers had a lower Cr level than abstainers (p = .001). No significant differences were observed between abstainers and light-drinking nonsmoking controls in NAA and Cr or between resumers and light-drinking nonsmoking controls in level.
Superior corona radiata.
Groups were significantly different (corrected p ≤ .029) on concentrations of NAA, χ2(3) = 16.72, p = .001; Cr, χ2(3) = 16.22, p = .001; and Cho, χ2(3) = 17.24, p = .001. No group differences were observed for mI. Follow-up t tests (corrected p ≤ .033) indicated resumers had lower NAA, Cr, and Cho than light-drinking nonsmoking controls (all p ≤ .023) and abstainers (p ≤ .029). No significant differences were observed between abstainers and light-drinking nonsmoking controls in NAA, Cr, or Cho concentrations.
The lower Cr and/or NAA concentrations in resumers relative to abstainers remained significant for all regions of interest after covarying for the trends to greater alcohol consumption at 8 years before enrollment and over the lifetime in resumers. Additionally, the observed regional metabolite differences between resumers and abstainers were significant for all regions of interest after covarying for psychiatric, substance-use disorder, and medical comorbidities; these covariates were not significant predictors of any metabolite level in the regions of interest assessed. The above reported regional metabolite differences among light-drinking nonsmoking controls, abstainers, and resumers were also unchanged after covarying for education. Finally, the pattern of findings and reported effect sizes were not appreciably altered when analyses included only those resumers who met Project MATCH criteria for relapse.
Correlations between baseline metabolite levels in regions of interest and pretreatment alcohol and cigarette consumption in the alcohol-dependent group
In the alcohol-dependent cohort (i.e., abstainers and resumers combined), higher insula mI level was positively related to the average number of drinks per month over 1 year (r = .37), 3 years (r = .40), and 8 years (r = .36) before enrollment (all ps ≤ .009), and months of heavy drinking (r = .32, p = .016). Lower insula NAA was correlated with a higher Fagerstrom total score (r = -.40, p = .02) and greater pack-years (r = -.37, p = .04).
Associations between baseline brain reward system metabolite levels and posttreatment alcohol consumption in resumers
There were several moderate-to-strong (.46-.75) correlations between posttreatment alcohol-consumption variables and regional baseline NAA levels in resumers. Generally, a greater number of drinking days were related to lower baseline insula and SCR NAA levels, a greater average number of drinks per day were associated with lower NAA concentrations in the ACC and SCR, and a greater number of total drinks were related to lower NAA levels in the DLPFC, insula, and SCR (see Table 4).
Table 4.
Correlations (Spearman's rho) between baseline regional NAA levels and posttreatment measures of alcohol consumption in resumers
| Variable | DLPFC | ACC | Insula | SCR | Vermis |
| Duration of abstinence, days | .09 | .57* | .31 | .27 | .35 |
| No. of drinkings days | −.11 | −.09 | −.49* | −.46* | −.34 |
| Average no. of drinks per day | −.40 | −.48* | .08 | −.48* | −.03 |
| Total no. of drinks | −.48* | −.31 | −.59* | −.75* | .17 |
Notes: NAA= N-acetylaspartate; duration of abstinence = number of consecutive abstinent days from baseline assessment to first drink; DLPFC = dorsolateral prefrontal cortex; ACC = anterior cingulate cortex; SCR = superior corona radiata.
p < .05.
Discussion
In this sample of predominately Caucasian, male, alcohol-dependent veterans in treatment, the primary findings were as follows: (a) Resumers demonstrated lower baseline NAA levels than light-drinking nonsmoking controls and abstainers in the DLPFC, ACC, insula, SCR, and cerebellar vermis; resumers also exhibited lower Cr than abstainers in the DLPFC, SCR, and cerebellar vermis. (b) Abstainers did not differ from light-drinking nonsmoking controls on any metabolite concentration in any region. (c) In the alcohol-dependent cohort, associations between baseline metabolite levels and prestudy measures of alcohol (for mI) and cigarette consumption (for NAA) were observed only for the insula. (d) Several moderately strong relationships were apparent between regional baseline NAA levels and posttreatment alcohol-consumption variables in resumers.
The pattern of significantly decreased NAA and Cr concentrations in resumers, compared with both abstainers and light-drinking nonsmoking controls, suggests resumers experienced compromised neuronal integrity and abnormalities in cellular bioenergetics in tissue comprising crucial nodes and white-matter interconnectivity of the brain reward pathway at entry into treatment. The DLPFC, ACC, and insula are fundamentally involved in decision making, problem solving, impulse control, regulation of mood and affect, craving, and evaluation and anticipation of stimulus salience and hedonics (Baler and Volkow, 2006; Paulus, 2007; Redish et al., 2008; Sinha and Li, 2007). The cerebellum is associated with aspects of learning and memory, working memory, executive skills, and reward processing (Anderson et al., 2006; Martin-Solch et al., 2001; Paul et al., 2009; Sullivan, 2003; Sullivan et al., 2003), and the corona radiata provides reciprocal connections linking frontal, parietal, and temporal neocortical regions and subcortical structures (Aralasmak et al., 2006; Makris et al., 1999; Schmahmann et al., 2007). Neurobiological abnormalities in these regions are linked to cognitive, emotional, and behavior disturbances that may confer risk for the relapse/remit cycle commonly observed in all substance-use disorders (Crews and Boettinger, 2009; Kalivas and O'Brien, 2008; Kalivas and Volkow, 2005).
Despite long-term hazardous alcohol consumption and a high frequency of comorbid chronic smoking, unipolar mood disorders, and medical conditions (primarily hypertension and hepatitis C), abstainers were not different from light-drinking nonsmoking controls on any metabolite level in any region of interest of the reward circuit investigated. The potential “protective” or mitigating factors associated with the uncompromised regional metabolite levels in abstainers (relative to light-drinking controls) were not identified in this study. The differences in regional metabolite levels between resumers and abstainers showed medium to large effect sizes (see Table 3) and were not related to education; alcohol consumption; or psychiatric, medical, or substance-misuse comorbidities. For the vast majority of resumers, the magnitude and duration of their drinking episode(s) were more than a simple “slip,” with 90% meeting Project MATCH criteria for relapse. Additionally, abstainers also reported a mean of 917 (SD = 502) days of continuous sobriety following outpatient treatment at long-term follow-up. However, it is unknown if the abstainers and resumers demonstrated the same pattern of metabolite levels observed in this article at the times of their other previous treatment attempts.
Results from the present study are consistent with literature implicating plastic changes in multiple nodes of the brain reward network in the development, maintenance, and/ or resumption of substance use (Baler and Volkow, 2006; Kalivas and O'Brien, 2008; Kalivas et al., 2009; McFarland et al., 2003; Sher et al., 2005; Volkow and Fowler, 2000; Volkow et al., 2001, 2004). The overall pattern of results from the present study parallels the morphological findings by Wrase and colleagues (2008), who observed lower amygdala volume in alcohol-dependent individuals who relapsed after treatment, compared with those who remained abstinent and relative to controls (the amygdala is a crucial component of the brain reward system involved in the determination of the emotional valence of a stimulus; Kalivas and Volkow, 2005). The authors also found abstainers did not differ from controls on amygdala or hippocampal volumes, and pre-enrollment alcohol and cigarette consumption were not related to the volumes of the regions assessed. We observed that measures of pre-enrollment alcohol and cigarette consumption were related to only mI and NAA levels in the insula in the alcohol-dependent cohort. Results from Wrase and colleagues and the present study suggest that some degree of the neurobiological abnormalities demonstrated by the resumers in the brain reward system were potentially evident before the inception of alcohol dependence and possibly served as a premorbid condition that increased vulnerability to the development of an AUD. It is also possible that chronic and excessive alcohol consumption and/or cigarette smoking exacerbated premorbid neurobiological abnormalities in the resumers’ brain reward system, thereby increasing the risk for recurrent relapse.
The clinical relevance of the metabolite abnormalities in resumers is suggested by the associations of their baseline NAA concentrations with alcohol-consumption measures during posttreatment relapse. More specifically, higher NAA levels in all regions of interest except the vermis were related to lesser severity of relapse in resumers. These findings parallel our earlier work (Durazzo et al., 2008), in which lower frontal white-matter and temporal gray-matter NAA were related to a greater number of drinking days at follow-up in resumers. However, we consider the associations between baseline NAA levels and posttreatment alcohol-consumption in this study to be preliminary, because they were not corrected for multiple comparisons.
Limitations of this study include the reliance on self-report and/or medical records for the determination of drinking status at follow-up for some participants, the inability to examine for sex effects because of the small number of female participants, and the modest number of participants in the abstainer group. We did not examine the influence of coping skills, self-esteem/self-efficacy, social support, neurocognition and personality disorders, neurocognitive variables, or gene polymorphisms reported to predict relapse after treatment for an AUD (e.g., Bradizza et al., 2006; Krampe et al., 2006; Miller et al., 1996; Teichner et al., 2001; Walter et al., 2006; Wojnar et al., 2009). It is also likely that the magnitude and chronicity of alcohol consumption before and after treatment in our alcohol-dependent cohort were influenced by genetic or other premorbid and environmental factors not assessed in this research.
The current investigation demonstrates the importance of the integrity of the brain reward circuit in the maintenance of abstinence in those with alcohol dependence. It is unclear if premorbid factors contributed to the regional metabolite levels observed in abstainers and resumers at the time of this study. Additionally, it is unknown if the regional metabolite concentrations demonstrated by abstainers and resumers in this study were apparent at the times of other treatment attempts. Longitudinal assessment over periods of sustained abstinence, combined with potential markers of genetic vulnerability (e.g., apolipoprotein E genotype, single nucleotide polymorphisms in brain-derived neurotrophic factor, dopamine receptor D2, catechol-O-methyl transferase) (see Wojnar et al., 2009), will assist in determining if the metabolite abnormalities observed in our cohort of resumers (or the uncompromised regional levels in abstainers) were influenced by premorbid factors, a consequence of chronic and excessive alcohol consumption, and/or an interaction between genetic vulnerability and alcohol/cigarette consumption. In general, more detailed investigations of the nature and magnitude of dysfunction in the brain reward system are required to inform the development of more efficacious pharmacological and behavioral interventions for AUDs.
Acknowledgments
We thank Mary Rebecca Young and Bill Clift of the Veterans Affairs Substance Abuse Day Hospital and Dr. David Pating, Karen Moise, and their colleagues at the Kaiser Permanente Chemical Dependency Recovery Program in San Francisco for their valuable assistance in recruiting participants. We also extend our gratitude to the study participants, who made this research possible.
Footnotes
This research was supported by National Institute on Alcohol Abuse and Alcoholism grant AA10788 awarded to Dieter J. Meyerhoff and National Institute on Drug Abuse grant DA24136 awarded to Timothy C. Durazzo, and with resources and the use of facilities at the San Francisco Veterans Administration Medical Center, San Francisco, CA.
References
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: Implications for relapse. Alcoholism: Clinical and Experimental Research. 2005;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 1994. [Google Scholar]
- Anderson CM, Maas LC, Frederick B, Bendor JT, Spencer TJ, Livni E, Kaufman MJ. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2006;31:1318–1326. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Aralasmak A, Ulmer JL, Kocak M, Salvan CV, Hillis AE, Yousem DM. Association, commissural, and projection pathways and their functional deficit reported in literature. Journal of Computer Assisted Tomography. 2006;30:695–715. doi: 10.1097/01.rct.0000226397.43235.8b. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Beck AT. Depression Inventory. Philadelphia, PA: Center for Cognitive Therapy; 1978. [Google Scholar]
- Bendszus M, Weijers HG, Wiesbeck G, Warmuth-Metz M, Bartsch AJ, Engels S, Solymosi L. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: Correlation with clinical and neuropsychological data. AJNR: American Journal of Neuroradiology. 2001;22:1926–1932. [PMC free article] [PubMed] [Google Scholar]
- Bottlender M, Soyka M. Efficacy of an intensive outpatient rehabilitation program in alcoholism: Predictors of outcome 6 months after treatment. European Addiction Research. 2005;11:132–137. doi: 10.1159/000085548. [DOI] [PubMed] [Google Scholar]
- Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2005;132:29–37. doi: 10.1002/ajmg.b.30080. [DOI] [PubMed] [Google Scholar]
- Bradizza CM, Stasiewicz PR, Paas ND. Relapse to alcohol and drug use among individuals diagnosed with co-occurring mental health and substance use disorders: A review. Clinical Psychology Review. 2006;26:162–178. doi: 10.1016/j.cpr.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental Neuroscience. 1993;15:289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Meyerhoff DJ, Song E, Weiner MW. Chronic active heavy drinking and family history of problem drinking modulate regional brain tissue volumes. Psychiatry Research. 2005;138:115–130. doi: 10.1016/j.pscychresns.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis of the behavioral sciences. New York: Academic Press; 1988. [Google Scholar]
- Crews FT, Boettinger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacology Biochemistry and Behavior. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Anatomic and behavioral aspects of frontal-subcortical circuits. Annals of the New York Academy of Sciences. 1995;769:1–13. doi: 10.1111/j.1749-6632.1995.tb38127.x. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Journal of Psychosomatic Research. 1998;44:627–628. doi: 10.1016/s0022-3999(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Grant BF. Rates and correlates of relapse among individuals in remission from DSM-IV alcohol dependence: A 3-year follow-up. Alcoholism: Clinical and Experimental Research. 2007;31:2036–2045. doi: 10.1111/j.1530-0277.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- Donovan DM. Assessment issues and domains in the prediction of relapse. Addiction. 1996;91(12 Suppl):S29–S36. [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Banys P, Meyerhoff DJ. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcoholism: Clinical and Experimental Research. 2004;28:1849–1860. doi: 10.1097/01.alc.0000148112.92525.ac. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Yeh PH, Meyerhoff DJ. Combined neuroimaging, neurocognitive and psychiatric factors to predict alcohol consumption following treatment for alcohol dependence. Alcohol and Alcoholism. 2008;43:683–691. doi: 10.1093/alcalc/agn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ. Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Frontiers in Bioscience. 2007;12:4079–4100. doi: 10.2741/2373. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO, Heatherton TF, Kozlowski LT. Nicotine addiction and its assessment. Ear Nose & Throat Journal. 1991;69:763–765. [PubMed] [Google Scholar]
- Ferguson KJ, MacLullich AM, Marshall I, Deary IJ, Starr JM, Seckl JR, Wardlaw JM. Magnetic resonance spectroscopy and cognitive function in healthy elderly men. Brain. 2002;125:2743–2749. doi: 10.1093/brain/awf278. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders—Patient Edition (SCID-I/P, version 2.0, 8/98 revision) New York: Biometrics Research Department, New York State Psychiatric Institute; 1998. [Google Scholar]
- Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Annals of Neurology. 2008;63:652–657. doi: 10.1002/ana.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Parsons OA. Prediction of resumption of drinking in posttreatment alcoholics. International Journal of the Addictions. 1991;26:237–254. doi: 10.3109/10826089109053186. [DOI] [PubMed] [Google Scholar]
- Goodman A. Neurobiology of addiction: An integrative review. Biochemical Pharmacology. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Heinz A, Schafer M, Higley JD, Krystal JH, Goldman D. Neurobiological correlates of the disposition and maintenance of alcoholism. Pharmacopsychiatry. 2003;36(Suppl. No. 3):S255–258. doi: 10.1055/s-2003-45139. [DOI] [PubMed] [Google Scholar]
- Jin H, Rourke SB, Patterson TL, Taylor MJ, Grant I. Predictors of relapse in long-term abstinent alcoholics. Journal of Studies on Alcohol. 1998;59:640–646. doi: 10.15288/jsa.1998.59.640. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56(Suppl. No. 1):169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: Allostasis and beyond. Alcoholism: Clinical and Experimental Research. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Krampe H, Wagner T, Stawicki S, Bartels C, Aust C, Kroener-Herwig B, Ehrenreich H. Personality disorder and chronicity of addiction as independent outcome predictors in alcoholism treatment. Psychiatric Services. 2006;57:708–712. doi: 10.1176/ps.2006.57.5.708. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yucel M, Pantelis C. Addiction, a condition of compulsive behaviour? Neuroimaging and neuropsychological evidence of inhibitory dysregulation. Addiction. 2004;99:1491–1502. doi: 10.1111/j.1360-0443.2004.00808.x. [DOI] [PubMed] [Google Scholar]
- McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. Journal of Neuroscience. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ. Relapse in the addictive behaviors: Integration and future directions. Clinical Psychology Review. 2006;26:229–231. doi: 10.1016/j.cpr.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Makris N, Meyer JW, Bates JF, Yeterian EH, Kennedy DN, Caviness VS. MRI-based topographic parcellation of human cerebral white matter and nuclei: II. Rationale and applications with systematics of cerebral connectivity. Neuroimage. 1999;9:18–45. doi: 10.1006/nimg.1998.0384. [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, Harris GJ. Decreased volume of the brain reward system in alcoholism. Biological Psychiatry. 2008;64:192–202. doi: 10.1016/j.biopsych.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin PR, Gibbs SJ, Nimmerrichter AA, Riddle WR, Welch LW, Willcott MR. Brain proton magnetic resonance spec-troscopy studies in recently abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:1078–1082. doi: 10.1111/j.1530-0277.1995.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Martin-Solch C, Magyar S, Kunig G, Missimer J, Schultz W, Leen-ders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers: A positron emission tomography study. Experimental Brain Research. 2001;139:278–286. doi: 10.1007/s002210100751. [DOI] [PubMed] [Google Scholar]
- Mega MS, Cummings JL. Frontal-subcortical circuits and neuropsychiatric disorders. Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6:358–370. doi: 10.1176/jnp.6.4.358. [DOI] [PubMed] [Google Scholar]
- Mertens JR, Weisner C, Ray GT, Fireman B, Walsh K. Hazardous drinkers and drug users in HMO primary care: Prevalence, medical conditions, and costs. Alcoholism: Clinical and Experimental Research. 2005;29:989–998. doi: 10.1097/01.alc.0000167958.68586.3d. [DOI] [PubMed] [Google Scholar]
- Meyerhoff D, Blumenfeld R, Truran D, Lindgren J, Flenniken D, Cardenas V, Weiner H. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcoholism: Clinical and Experimental Research. 2004;28:650–661. doi: 10.1097/01.ALC.0000121805.12350.CA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR, Walters ST, Bennett ME. How effective is alcoholism treatment in the United States? Journal of Studies on Alcohol. 2001;62:211–220. doi: 10.15288/jsa.2001.62.211. [DOI] [PubMed] [Google Scholar]
- Miller WR, Westerberg VS, Harris RJ, Tonigan JS. What predicts relapse? Prospective testing of antecedent models. Addiction. 1996;91(12 Suppl.):S155–S172. [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-acetylaspartate in the CNS: From neurodiagnostics to neurobiology. Progress in Neurobiology. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan SC, Finney JW. Explaining abstinence rates following treatment for alcohol abuse: A quantitative synthesis of patient, research design and treatment effects. Addiction. 1996;91:787–805. doi: 10.1046/j.1360-0443.1996.9167876.x. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Rates and predictors of relapse after natural and treated remission from alcohol use disorders. Addiction. 2006;101:212–222. doi: 10.1111/j.1360-0443.2006.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbrich HM, Valerius G, Paris C, Hagenbuch F, Ebert D, Juengling FD. Brain activation during craving for alcohol measured by positron emission tomography. Australian and New Zealand Journal of Psychiatry. 2006;40:171–178. doi: 10.1080/j.1440-1614.2006.01765.x. [DOI] [PubMed] [Google Scholar]
- Parekh RS, Klag MJ. Alcohol: Role in the development of hypertension and end-stage renal disease. Current Opinion in Nephrology and Hypertension. 2001;10:385–390. doi: 10.1097/00041552-200105000-00014. [DOI] [PubMed] [Google Scholar]
- Parks MH, Dawant BM, Riddle WR, Hartmann SL, Dietrich MS, Nickel MK, Martin PR. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcoholism: Clinical and Experimental Research. 2002;26:1368–1380. doi: 10.1097/01.ALC.0000029598.07833.2D. [DOI] [PubMed] [Google Scholar]
- Parsons OA, Schaeffer KW, Glenn SW. Does neuropsychological test performance predict resumption of drinking in posttreatment alcoholics? Addictive Behaviors. 1990;15:297–307. doi: 10.1016/0306-4603(90)90073-7. [DOI] [PubMed] [Google Scholar]
- Paul R, Grieve SM, Chaudary B, Gordon N, Lawrence J, Cooper N, Gordon E. Relative contributions of the cerebellar vermis and prefrontal lobe volumes on cognitive function across the adult lifespan. Neurobiology of Aging. 2009;30:457–465. doi: 10.1016/j.neurobiolaging.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Paulus MP. Neural basis of reward and craving—a homeostatic point of view. Dialogues in Clinical Neuroscience. 2007;9:379–387. doi: 10.31887/DCNS.2007.9.4/mpaulus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Disruption of brain white matter microstructure by excessive intracellular and extracellular fluid in alcoholism: Evidence from diffusion tensor imaging. Neuropsychopharmacology. 2005;30:423–432. doi: 10.1038/sj.npp.1300623. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neuroscience & Biobehavioral Reviews. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Redish AD, Jensen S, Johnson A. A unified framework for addiction: Vulnerabilities in the decision process. Behavioral and Brain Sciences. 2008;31:415–437. doi: 10.1017/S0140525X0800472X. discussion 437–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Bluml S. Magnetic resonance spectroscopy of the human brain. Anatomical Record. 2001;265:54–84. doi: 10.1002/ar.1058. [DOI] [PubMed] [Google Scholar]
- Saint-Cyr JA. Frontal-striatal circuit functions: Context, sequence, and consequence. Journal of the International Neuropsychological Society. 2003;9:103–127. doi: 10.1017/s1355617703910125. [DOI] [PubMed] [Google Scholar]
- Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Statistics in Medicine. 1997;16:2529–2542. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN, Wang R, Dai G, D'Arceuil HE, De Crespigny AJ, Wedeen VJ. Association fibre pathways of the brain: parallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Videen JS, Alhassoon OM, Patterson TL, Grant I. Elevated myo-inositol in gray matter of recently detoxified but not long-term alcoholics: A preliminary MR spectroscopy study. Alcoholism: Clinical and Experimental Research. 2000;24:699–770. [PubMed] [Google Scholar]
- Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annual Review of Clinical Psychology. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug and Alcohol Review. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten RZ, Allen JP, editors. Measuring alcohol consumption: Psychosocial and biochemical methods. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Journal of Studies on Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Soher BJ, Young K, Govindaraju V, Maudsley AA. Automated spectral analysis III: Application to in vivo proton MR spectroscopy and spectroscopic imaging. Magnetic Resonance in Medicine. 1998;40:822–831. doi: 10.1002/mrm.1910400607. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. State-Trait Anxiety Inventory, form Y-2, (STAI): Self-Evaluation Questionnaire. Palo Alto, CA: Consulting Psychologist Press; 1977. [Google Scholar]
- Stinson FS, Grant BF, Dawson DA, Ruan WJ, Huang B, Saha T. Comorbidity between DSM-IV alcohol and specific drug use disorders in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Drug and Alcohol Dependence. 2005;80:105–116. doi: 10.1016/j.drugalcdep.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Sullivan EV. Human brain vulnerability to alcoholism: Evidence from neuroimaging studies. In: Noronha A, Eckardt M, Warren K, editors. Review of NIAAA's neuroscience and behavioral research portfolio (NIAAA Research Monograph No. 34, NIH Publication No. 00-4520. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2000. pp. 473–508. [Google Scholar]
- Sullivan E. Compromised pontocerebellar and cerebellothalamocortical systems: Speculations on their contributions to cognitive and motor impairment in nonamnesic. Alcoholism: Clinical and Experimental Research. 2003;27:1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney RJ, Dlugos CA, Martin PR, Parks MH, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: A substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Teichner G, Horner MD, Harvey RT. Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. International Journal of Neuroscience. 2001;106:253–263. doi: 10.3109/00207450109149753. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. Journal of Neuroscience. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain viewed in the light of imaging studies: Brain circuits and treatment strategies. Neuropharmacology. 2004;47(Suppl. No. 1):3–13. doi: 10.1016/j.neuropharm.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Telang F. Overlapping neuronal circuits in addiction and obesity: Evidence of systems pathology. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2008;363:3191–3200. doi: 10.1098/rstb.2008.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Gerhard U, Duersteler-MacFarland KM, Weijers HG, Boening J, Wiesbeck GA. Social factors but not stress-coping styles predict relapse in detoxified alcoholics. Neuropsychobiology. 2006;54:100–106. doi: 10.1159/000096991. [DOI] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: Recent advances and challenges. Journal of Neuroscience. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Strobbe S, Ilgen M, Matsumoto H, Nowosad I, Burmeister M. Association between Val66Met brain derived neurotrophic factor (BDNF) gene polymorphism and post-treatment relapse in alcohol dependence. Alcoholism: Clinical and Experimental Research. 2009;33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. American Journal of Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Stout RL, Trefry WB, Glasser I, Connors GJ, Maisto SA, Westerberg VS. Alcohol relapse repetition, gender, and predictive validity. Journal of Substance Abuse Treatment. 2006;30:349–353. doi: 10.1016/j.jsat.2006.03.004. [DOI] [PubMed] [Google Scholar]