Abstract
The decomposition of peroxidized lipids of low-density lipoprotein (LDL) has been suggested to be involved in atherosclerosis. In this study, an in vitro system with 13-hydroperoxylinoleic acid (13-HPODE) was used to determine the effects of antioxidants on its decomposition. Decomposition of 13-HPODE was not affected by α-tocopherol, several other antioxidants, or antioxidant enzymes. Moreover, the inclusion of α-tocopherol during the decomposition of 13-HPODE resulted in an accumulation of aldehydes. Further oxidation of aldehydes to carboxylic acids by a number of oxidases was prevented by α-tocopherol. Conversely, the formation of carboxylic acids may be conducive to plaque stabilization via immunomodulation, rapid degradation, and by calcium sequestration. Thus, the inhibition of formation of carboxylic acids could be a serious deleterious effect of antioxidant treatment. In contrast, α-keto acids, like pyruvic acid, promoted the conversion of 13-HPODE to 13-hydroxylinoleic acid (13-HODE) by readily undergoing decarboxylation into acetate. These observations suggest that agents that promote the reduction of lipid peroxides into lipid hydroxides could be far more effective in treating cardiovascular diseases as opposed α-tocopherol–like antioxidants that could affect additional steps in the oxidation cascade. Antioxid. Redox Signal. 11, 1237–1248.
Introduction
The oxidation of low-density lipoprotein (LDL) has been suggested to play a major role in the development of early atherosclerotic lesions (7, 8, 39, 41). That recent human clinical trials reported failure of antioxidants to affect human cardiovascular disease (4, 7, 9, 13, 16, 17, 19, 37, 51), despite reduction in specific biomarkers of oxidative stress, is of great concern.
Some of the strongest evidence for the oxidation hypothesis was derived from animal studies that tested the effects of various antioxidants on atherosclerotic development (27, 41, 45). These include WHHL rabbits, cholesterol-fed rabbits, hypercholesterolemic hamsters and monkeys, and various mouse models of atherosclerosis. Animal models were developed to study the formation of early atherosclerotic lesions in an isolated environment so that contribution of individual risk factors and associated biochemical events could be segregated and studied. Results from these studies unambiguously showed that antioxidants attenuate the early atherogenic process (27).
The human form of atherosclerotic disease differs considerably from the disease models in animals. The animal models were created to study the human disease in a short and meaningful period of time. In contrast, the macrophage foam cell rich-fatty-streak lesions start very early in life in humans and progress with age (20, 38). Infants and children younger than 15 years have been noted to have foam cell fatty-streak lesions (20, 22, 24, 25, 38). Maternal hypercholesterolemia seems to influence strongly the development of atherosclerotic lesions in the young (22, 24, 28, 32). Yet another major difference between human and animal atherosclerosis is the contribution of inflammatory cytokines, calcification, and matrix-digesting enzymes (MMPs) to the advancement of the vulnerable plaque (32, 33, 43). The human clinical trials using α-tocopherol as one of the major antioxidant ingredients were mostly negative and failed to show any evidence of protection against cardiovascular diseases (4, 7, 9, 13, 16, 17, 19, 51). More important, antioxidants seemed to have an adverse effect on clinical end points, including HDL levels (5). Numerous explanations were offered for this failure (27, 28, 29, 40, 48), including the inability of pharmacologic doses of α-tocopherol to affect lipid peroxidation and thromboxane biosynthesis in healthy subjects with a mild degree of oxidant stress. These findings were interpreted to suggest that the basal rate of lipid peroxidation is a major determinant of the response to α-tocopherol supplementation (29). The bottom line remains that the human atherosclerosis at the time when patients are seen in the clinic manifests in a different form that is not amenable to attenuation by antioxidants. Moreover, the burden is not only to explain the failure of antioxidants to retard the progression of the disease but also to explain why they may have an adverse effect.
A not-well-studied reaction in the lipid-peroxidation cascade is the oxidation of lipid peroxide–derived aldehydes into carboxylic acids. In this study, we propose that such reactions may not be amenable to inhibition by antioxidants. The oxidation hypothesis took into consideration that the aldehyde products covalently modify the ɛ-amino groups of lysine residues to generate the oxidatively modified LDL (12, 15). Considering that even the simplest PUFA, linoleic acid, could generate several aldehyde products (e.g., 2-hydroxy hexanal, 4-HNE, oxo-nonanoic acid) (15, 30, 34–36), one would end up with a glut of such products during the oxidation of LDL. In addition, the lysosomal proteolysis would release from modified apoproteins, aldehydes-bound lysine peptides, as well as free aldehydes, because of the instability of Schiff bases in such acidic environments. Aldehydes formed from peroxidized linoleic acid (18:2) have been extensively studied (34–36), and such products themselves have a plethora of potent proatherogenic effects.
Materials and Methods
Linoleic acid, soybean lipoxygenase (SLO) type V, human MPO, superoxide dismutase (SOD), XAO, catalase, ascorbic acid, α-tocopherol, tetramethylpentamine-2,4-dinitrophenyl hydrazine, bromocresol green, pyruvic acid, pelargonyl aldehyde (nonenal), AZA, 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole-4-amino-5-hydrazino-1,2,4-triazole-3-thiol (Purpald), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and silica gel G TLC plates were purchased from Sigma (St. Louis, MO); leucomethylene blue (LMB) and 4-nitrobenzylpyridine were bought from Alfa Aesar (Ward Hill, MA); and 13-S-hydroperoxy octadecadienoicacid and 4-HNE were obtained from Cayman chemicals (Ann Arbor, MI). Radioactive calcium chloride was obtained from Amersham Biosciences (Piscataway, NJ). Solvents were of HPLC grade and were free of aldehydes.
Preparation of linoleic acid hydroperoxide
Linoleic acid, 100 nmol/ml in phosphate-buffered saline (PBS, pH 7.4), was oxidized with the addition of 10 U soybean lipoxygenase. The oxidation reaction was allowed to complete at room temperature over a period of 1 h. Increased absorption at 234 nm was used to monitor the reaction by using an Uvikon XL (Biotech Instruments, El Cajon, CA) spectrophotometer. The amount of conjugated diene formed was determined from its molar extinction coefficient 23 mM/C at 234 nm. Lipid peroxide generated in the reaction system was analyzed with the leucomethylene blue assay (3). In general, the conversion of linoleic acid to HPODE was >90%, and the major (>82%) product was 13-HPODE. In almost all experiments, the isolated HPODE was used immediately. We have not detected any free aldehydes in fresh preparations.
From 50 to 60 nmols of HPODE was made up to 1 ml with PBS and incubated at 4°C and 37°C over a period of 72 h. Reaction mixture at various time intervals was collected, and conjugated diene and lipid peroxide levels were determined. To eliminate any residual effects of lipoxygenase, the studies were performed with extracted and purified HPODE, as well as with a pure commercial HPODE.
For experiments using 100% serum, 13-HPODE was prepared in large quantities (30–50 μmols), extracted with ether, and dried. An aliquot was stored for the determination of peroxide and diene content. Dry 13-HPODE was suspended in fetal calf serum at 1 μmol/100 μl by vigorous vortexing for 2 min. An aliquot was immediately analyzed for peroxide content and diene levels. Within 5 min, 28 ± 3% of added peroxide was lost, as measured by the LMB reaction. This could be caused by either a variety of mechanisms, including the actions of specific enzymes such as paraoxonase, reaction with protein thiols or other amino acids, or by competition of the serum with the detection reagent.
At 5 min, several aliquots (hexaplicates) were stored in the freezer or incubated at 37°C for 72 h. The reactions were performed in 96-well plates. Three separate reactions of hexaplicates were performed. In some reactions, sodium pyruvate (final concentration of 5 μmol per 100 μl serum) was added at the beginning of the incubation. As 100 μl of serum contains a large amount of lipids, these concentrations were needed for the detection of peroxides, dienes, and aldehydes.
Effects of antioxidants on the decomposition reaction
HPODE (60–80 nmol peroxide equivalent) was incubated for various time intervals at 37°C with 10–100 μM α-tocopherol, vitamin C, butylated hydroxy toluene (BHT), or 20 μM ethylenediaminetetraacetic acid (EDTA). All antioxidants were added in 5 μl ethyl alcohol except Trolox, which was added as a sodium salt. The antioxidant enzymes such as SOD and catalase (10–100 units) were also used in this study, although they are of no relevance to the human clinical trials. The efficacy of these antioxidants to prevent the loss in conjugated dienes and lipid peroxides was evaluated over a period of 72 h.
Visualization of linoleic acid hydroperoxide decomposition products with thin-layer chromatography (TLC)
Lipid hydroperoxide was allowed to decompose completely (ascertained by complete loss of peroxides and conjugated dienes). Products obtained were extracted twice with chloroform/methanol (2:1, vol/vol). The organic phase was evaporated under nitrogen gas, and the resultant residue dissolved in known volume of acetone. The compounds in the residue were separated on silica gel G TLC plates by using hexane/diethylether/acetic acid (70:30:1.5, vol/vol) as the solvent system. Visualization of compounds was carried out with exposure to iodine vapor for unsaturated compounds, spraying the chromatogram with 2,4-dinitrophenyl hydrazine (DNP) reagent for aldehydes, chromosulfuric acid reagent for organic compounds, and 4-nitrobenzylpyrridine and tetraethylpentamine reagents for epoxides. Dipping of chromatogram in bromocresol green reagent (0.2% in alcohol) for organic acids was also performed.
IR spectral analysis
Oxidized linoleic acid (5 mg peroxide equivalent) was incubated at 60°C in a rotary evaporator for 24 h. The volatile compounds were collected on 2-ml cold acetone kept in the collecting flask. The collecting flask was kept cool on dry ice. The compound in acetone was then evaporated under nitrogen gas and spotted on a TLC plate. The separated compound was scraped out, and the IR spectrum was determined.
AZA formation from oxidized linoleic acid
13-HPODE (100 nmol/ml), prepared as described earlier, was incubated at 37°C for 1 week. After acidification to pH 4.5 with 1N HCl, the mixture was extracted with chloroform/methanol (1:1, vol/vol) and dried under nitrogen. The sample was then dissolved in methanol at 1 nM concentration and continuously injected in MS by using a Hamilton Syringe 4.6 mm in diameter and a flow rate of 10.00 μl/min. The negative ion electrospray ionization (ESI) mode was used, and a Q1MS Q1 scan was conducted to scan simultaneously for ions between 100 and 300 m/z. Heat-block temperatures for the analysis were set at 300°C, and ion-source gas flow was set at 20 l/min with the detector voltage at 5,500 eV AzA mass elute at a mass of 187. A 3,200 Q TRAP LC/MS/MS (Applied Biosystems/MDS Sciex, Foster City, CA) was used for the analysis.
Reaction of lipid hydroperoxides with pyruvic acid
The 500 nmols/ml of 13-HPODE was reacted with 1–7.5μmols/ml of PYR in 1 ml of PBS at pH 7.4 for 5 min at room temperature. After the incubation, a 50-μl aliquot of the sample (in duplicate) was taken for the LMB assay, as described in Methods. The remaining sample was acidified with 25 μl of 1N HCl and extracted with 2 ml of ether. The ether was then evaporated overnight, and the resultant extract was dissolved in methanol. The conjugated diene was measured by reading the spectrum at 234 nm with methanol as the blank, and the experiment was done in triplicate. The graph is an average of three experiments done with fresh 13-HPODE and PYR and expressed as the percentage of remaining diene and peroxide.
Generation of CO2 in lipid hydroperoxide and pyruvic acid reaction
Radioactive calcium hydroxide [45Ca](OH)2 was prepared by mixing supersaturated solutions of potassium hydroxide and [45Ca]calcium chloride. The reaction with PYR was carried out by incubating 50 nmols of 13-HPODE with similar concentrations of (1–7.5 μmols) PYR in a microtube at 37°C by using a dry-bath incubator. The carbon dioxide released during these reactions was trapped by using filter paper inserted in a lid containing 5 μl of radioactive calcium hydroxide. Approximately six to 10 filter papers were inserted per tube. After overnight incubation, the tube lid was opened carefully, and the filter papers were transferred gently to another set of tubes and washed with distilled water (500 μl × 3). The filter papers were then placed in scintillation vials, and the formation of radioactive calcium carbonate was counted on a scintillation counter (Beckman Coulter, Fullerton, CA).
NMR spectroscopy analysis for the reaction between pyruvate and 13-HPODE
Because of the lack of sensitivity of natural abundance NMR, higher concentrations of the reactants were used. HPODE, 12 μmoles, was allowed to react with 60 μmoles of PYR at room temperature. After overnight incubation, the samples were lyophilized and dissolved in 750 μl deuterium oxide and measured with 400-MHz 1H NMR.
Oxidation of nonylaldehyde or malondialdehyde (MDA) by XAO or MPO
The reaction mixture (1 ml) contained 5 mM nonyl aldehyde in 5 μl ethyl alcohol, Tris buffer (pH 7.4), cytochrome c (100 μg), and suitably diluted XAO (0.01 unit) was used. α-Tocopherol was added in ethyl alcohol in 1- to 4-μl volume at appropriate concentrations. The reduction of the cytochrome c reaction was monitored by monitoring changes in the spectrum between 500 and 600 nm. In some cases, the reaction was scaled down to 175 μl, keeping the concentrations of the reagents the same without cytochrome c. The reaction was carried out in microtiter plates with a total volume of 175 μl. At the end of the reaction, the remaining aldehyde was determined by adding the Purpald (2-1,2,4-triazoline-5-thione, 4-amino-3-hydrazino-1,2,4-triazolidin-3-one, 4-amino-5-thioxo-, 3-hydrazone) reagent (31). The optical density of the remaining aldehydes was determined by measuring the OD at 540 nm. Aliquots from the reaction were used for LC-MS detection of nonanoic acid.
In similar incubations, MPO (0.1 unit) and H2O2 (1 mM) were used in a total volume of 175 μl, and freshly generated MDA (0.1–5 mM) was used as the aldehyde. The remaining aldehydes at the end of the incubation were determined with the TBA reaction (50). When nonenal was used, the formation of nonanoic acid was ascertained with LC-MS.
Results
13-HPODE undergoes temperature dependent decomposition generating products
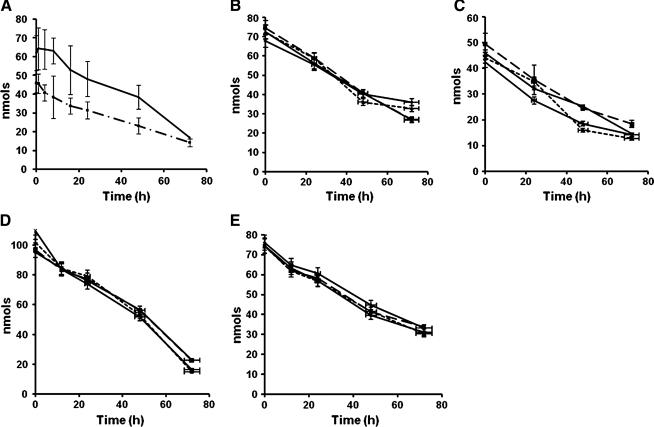
About 90% of linoleic acid was converted to its peroxides when subjected to oxidation with soybean lipoxygenase, as measured by conjugated diene formation and by LMB peroxide assay. Incubation of 13-HPODE at 4°C up to 72 h resulted in no changes in the peroxide content or in the conjugated diene content (data not shown). However, when incubated at 37°C, a time-dependent loss was found in conjugated diene and peroxides (Fig. 1A), suggesting decomposition of the peroxides into components that do not have the peroxide or the conjugated dienyl structures. A similar decrease in conjugated dienes and lipid peroxides also was observed when commercial purified 13- HPODE was incubated at 37°C (data not shown), indicating that the decomposition was not due to any residual lipoxygenase enzyme. Overall, these results suggest that once formed, peroxidized lipids could spontaneously undergo decomposition to generate products.
FIG. 1.
The decomposition of 13-HPODE with time. Linoleic acid was oxidized with soybean lipoxygenase, as described in Methods, and was incubated at 37°C for <72 h. At specified time periods, lipid peroxides and conjugated dienes were determined. (A) The determination of peroxides (▪) and conjugated diene (♦) during the decomposition of HPODE. The data are expressed as average ± SD from six different sets of incubations. (B, C) Determination of peroxides and conjugated dienes in the presence of α-tocopherol at 0 (♦), 5 (▪), 25 (▴), and 100 (×) μM concentrations. (D, E) Determination of peroxides and conjugated dienes in the presence of BHT at 0 (♦), 10 (▪), 50 (▴) and 100 (×) μM concentrations. The (B–E) values are too close, and the results are averages from four independent trials.
We have not performed similar studies with the other isomer of 13-HPODE because of the limited availability of the R-isomer. It would be interesting if the R-isomer had a different decomposition fate.
The oxidation of LDL is more likely to occur in the subendothelial space (42), perhaps in the extracellular milieu in which filtered serum components and albumin might be present. Although both phospholipid and cholesterol ester peroxides as well as various types of oxidized lipids and electronegative LDL subfractions have been detected in the plasma, because of extremely rapid clearance of oxidized LDL (half-life, t1/2 of <15 min), it is generally believed that these components are products of diffusion of oxidized lipid species from the intima.
The decomposition of HPODE in the presence of serum
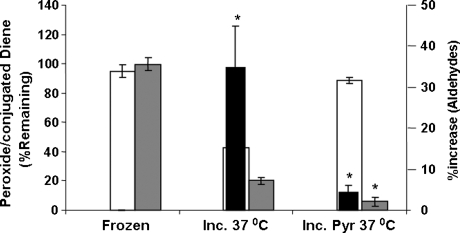
The presence of 1 and 10% heat-inactivated fetal calf serum (FCS) that contains ∼0.1–1 mg of albumin reduced the level of peroxide content immediately by ∼6.3 ± 0.8% and 23 ± 6%, respectively. Inclusion of 100% serum did not drastically increase the immediate decomposition of 13-HPODE (28 ± 3%). However, subsequent decomposition of the 13-HPODE even in the presence of 100% serum. The peroxide and conjugated diene content precipitately decreased to <50% of the level as compared with samples that were kept at −20°C (Fig. 2). As the presence of 100 or 10% serum imposed problems in subsequent extraction and conjugated diene determinations, we performed additional studies in the absence of serum.
FIG. 2.
Decomposition of HPODE in serum. HPODE was prepared (∼30 μmols); an aliquot was set aside for peroxide, conjugated diene, and aldehyde determinations. Dried HPODE was resuspended in fetal calf serum at a concentration of 1.0 μmol in 100 μl and aliquoted into 96-well microtiter plates. Aliquots were also stored at −20°C. When needed, pyruvate was added in 5-μl quantities to give a final concentration of 5.0 μmols per 100 μl. Samples were collected at 1, 2, and 3 days after incubation at 37°C for analytic determinations. Incubations were set in quintuplicates, and the values represent average ± SD. *p < 0.05 versus sample incubated at 37°C. Open bars, grey bars, and black solid bars represent conjugated dienes, peroxides, and aldehydes, respectively.
TLC, IR, GC-MS, and LC-MS determinations revealed that the decomposition of 13-HPODE was accompanied by the formation of a variety of products that included epoxides, aldehydes, and carboxylic acids, depending on the length and temperature of the incubations. Typically, at the end of the incubation, the formation of carboxylic acids, particularly the formation of AZA, was noted both by GC-MS and by LC-MS reactions.
Antioxidants, antioxidant enzymes and EDTA did not affect the spontaneous decomposition of 13-HPODE
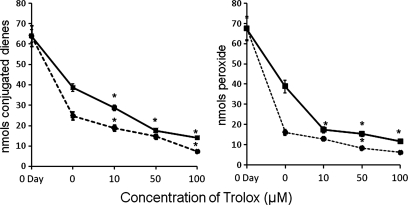
Radical-mediated decomposition of lipid peroxides has been described in literature (6, 14, 19). However, addition of α-tocopherol (5–100 μM) (Fig. 1B and C) and BHT (10–100 μM) (Fig. 1D and E) did not inhibit the decomposition reaction. No significant changes in the levels of peroxides (Fig. 1B and D) or conjugated dienes (Fig. 1C and E) in the absence or presence of these antioxidants. To eliminate the possibility that the observed effects were restricted to phenolic antioxidants, we tested the effects of N-acetyl cysteine and ascorbic acid and noted similar effects (or the lack of it) (Results are not included). It is unlikely that a radical-mediated decomposition process was involved as a major reaction in the decomposition of preexisting peroxides. In many experiments, an accelerated decomposition of 13-HPODE was noted in the presence of these antioxidants, suggesting that inhibition of radical side-reactions might actually help to promote decomposition. For example, Trolox, a water-soluble carboxylic acid analogue of tocopherol, not only failed to inhibit the decomposition of HPODE, but also seemed even to accelerate the decomposition (Fig. 3). The inability of three phenolic antioxidants, α-tocopherol, BHT, and the sodium salt of Trolox to prevent the decomposition of 13-HPODE might suggest that antioxidant partitioning in the lipid phase may play a limited role.
FIG. 3.
Effect of Trolox on the decomposition of HPODE. Linoleic acid was oxidized with soybean lipoxygenase, as described in Methods, and was incubated at 37°C for ≤72 h. Trolox was added as sodium salt at 10-, 50-, and 100-μM levels. At specified time periods, lipid peroxides and conjugated dienes were determined. The left and the right panels represent conjugated dienes and peroxides, respectively. Squares and circles, values at 1- and 2-day incubations. The data are expressed as average ± SD from six different sets of incubations. *Values significantly different from control (0 time) values.
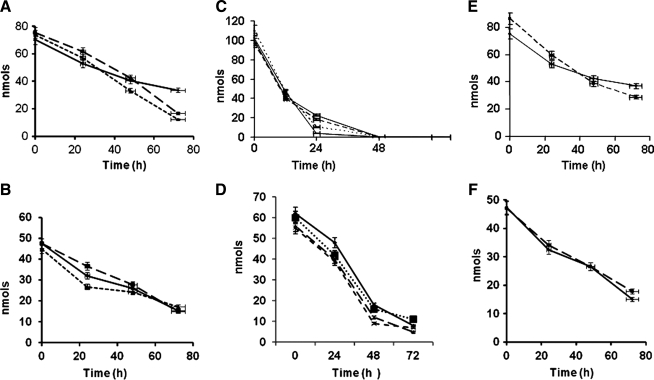
The human clinical trials were performed by using chemical antioxidants. Because of our findings that α-tocopherol and BHT did not prevent the decomposition of 13-HPODE, we tested to see whether antioxidant enzymes would have an effect, although the results would have no bearing on the clinical findings. In addition, superoxide release has been reported during amine-catalyzed decomposition of HPODE. Enzymes such as SOD (100 U/ml) (Fig. 4A and B) and catalase (10–100 U/ml) (Fig. 4C) had no effect on the loss of peroxides or conjugated dienes as compared with controls without antioxidant enzymes. Samples treated with catalase (10–100 U/ml) showed an increased and accelerated loss of lipid peroxide (Fig. 4C) and did not prevent the loss of conjugated dienes (Fig. 4D). Similar results were obtained when LOOH was incubated in the presence of EDTA (Fig. 4E and F), suggesting that the observed outcome was not due to chelation of any redox metal(s). The use of buffers prepared with “metal-free” water, along with the use of several other chelators/antioxidants, showed no differences. The use of TLC and identification of epoxides, aldehydes, and carboxylic acids showed that, in all cases, the parent compound 13-HPODE was degraded into simpler compounds during the long incubation. (Data not shown.)
FIG. 4.
Effects of SOD, catalase, and EDTA on the decomposition of HPODE. HPODE (60- to 80-nmol peroxide equivalent) was allowed to decompose at 37°C over a period of 72 h in presence of 0 (♦), 5 (▪), and 100 (▴) U/ml SOD (A, B); catalase 0 (♦), 10 (▪), 50 (▴), and 100 (×) U/ml (C, D); or EDTA 0 (♦) and 20 (▪) μM (E, F). Peroxides (top) and conjugated dienes (bottom) were measured. Figure 3B–E show representative experiments from four independent trials.
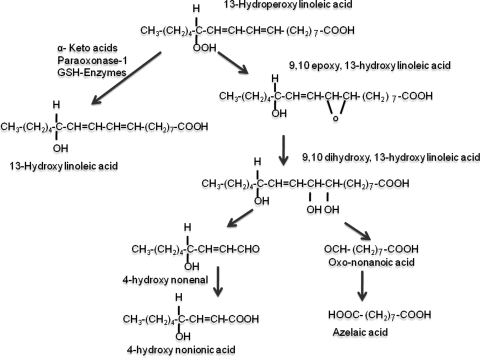
Based on one of our previous publications (11) that suggested that an intramolecular oxidation and epoxidation was feasible, we considered the following scheme of events (Fig. 5). According to Fig. 5, the 13-HPODE would oxidize the delta-9 double bond of the molecule, leading to the formation of epoxides. The hydration and further oxidation of the vicinal hydroxyl groups might lead to the formation of the aldehydes (4-hydroxynonenal and oxononanoic acid). Whereas it is tempting to speculate whether the vicinal diols would be cleaved by another molecule of LOOH or peracid, we do not have any data suggesting such a reaction. A corollary to this hypothesis would be that agents that would reduce hydroperoxides into hydroxides (HPODE to HODE), as shown in the left side of Fig. 5, would prevent the decomposition reactions.
FIG. 5.
Breakdown of 13-HPODE into azelaic and 4-hydroxynonenoic acids.
13-HPODE undergoes reductive reaction with α-keto acids
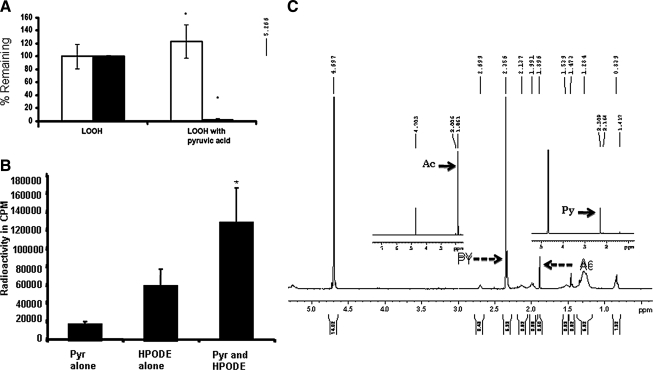
α-Keto acids readily undergo a decarboxylation reaction, and such a reaction is enhanced in the presence of hydrogen peroxide (H2O2). The decomposition of pyruvate (PYR) in the presence of H2O2 has been well described in the literature (2, 23, 46). We incubated 13-HPODE with PYR acid to determine whether such a decarboxylation reaction would convert 13-HPODE into 13-HODE. Incubation of LOOH by itself for up to 60 min had no effect on the peroxides or conjugated dienes (Fig. 6A, left panels). Conversely, incubation of LOOH with PYR for as little as 5 min at room temperature resulted in the complete loss of LMB reactivity (p < 0.05; Fig. 6A, right panel, solid bar) without any loss of conjugated dienes (Fig. 6A, right panel, open bar), suggesting a reduction of LOOH to LOH. LOH will retain the conjugated diene structure without the peroxides. Even in the presence of 100% serum, the addition of PYR decreased the peroxide content in a concentration-dependent manner (Fig. 2). Similar results were obtained with α-ketoglutarate (results not shown).
FIG. 6.
(A) Reaction between lipid hydroperoxide and pyruvic acid. The 500 nmols/ml of 13-HPODE was reacted with 7.5 μmols/ml of PYR in 1 ml of PBS at pH 7.4 for 5 min at room temperature. After the incubation, a 50-μl aliquot of the sample (in duplicate) was taken for the LMB assay, as described in Methods. The remaining sample was acidified with 25 μl of 1N HCl and extracted with 2 ml of ether. Ether was then evaporated overnight, and the resultant residue was dissolved in methanol. The conjugated diene was measured by reading the spectrum at 234 nm, with methanol as blank. The experiment was done in triplicate. The graph is an average of three experiments done with fresh LOOH and PYR and expressed as a percentage of the remaining diene and peroxide. The Y axis represents the percentage of the remaining diene and peroxide in the reaction; this is statically significant (p < 0.05). (B) CO2 trapping in lipid hydroperoxide and pyruvic acid reaction: the 50 μM LOOH reacted with a similar concentration of PYR in a microtube. The mixture was incubated at 37°C in a dry-bath incubator. The carbon dioxide was trapped by using filter paper, as previously described in Methods. The Y axis represents the percentage of radioactive calcium carbonate present in the filter paper. Formation of calcium carbonate and rate of CO2 released was significant (p < 0.05). (C) NMR spectroscopy of the reaction between pyruvate and 13-HPODE. The 12 μmoles of HPODE was allowed to react with 60 μmoles of PYR at room temperature. After overnight incubation, the samples were lyophilized and dissolved in 750 μl deuterium oxide and measured in 400-MHz 1H-NMR. Inserts are spectra of acetate and pyruvate for comparison.
The reaction is expected to be accompanied by the decarboxylation of PYR to acetate and would lead to the release of increased levels of CO2 (Fig. 6B), as established by CO2 trapping. Carbon dioxide evolution was higher when PYR was treated with LOOH (taken as 100%) as compared with PYR alone (10%; p < 0.005) or LOOH alone (52%). (In many of these experiments, we noted an autodecomposition of the carboxylic acid moiety of LOOH, a reaction not understood at present, presumably the result of formation of peracids). The latter could be important in the oxidation of vicinal diols). In summary, α-keto acids appear to reduce LOOH to lipid hydroxide by undergoing oxidative decarboxylation.
A concomitant formation of acetate from PYR decarboxylation occurred, as determined by proton NMR (Fig. 6C). The 1H-NMR spectrum of sodium pyruvate in deuterated water exhibited a sharp singlet signal at δ 2.309 (PY indicated by solid arrow in the lower right insert), showing the presence of a methyl group, whereas the methyl group in sodium acetate resonated at δ 1.861 (Ac, indicated by solid arrow in the lower left insert). The presence of an electron-withdrawing keto group adjacent to the methyl group in sodium pyruvate deshielded the methyl protons and shifted the signal toward downfield. The 1H-NMR spectrum of the PYR and LOOH reaction mixture in deuterated water (D2O) gave two singlets, indicating the presence of both the reactant and the product (PY and Ac indicated by broken arrows), suggesting the formation of acetate during the reaction. The proton NMR spectrum of LOOH alone did not yield any of those signals, indicating the change in chemical shift value. This must have occurred because of the loss of a keto group in sodium pyruvate catalyzed by LOOH, yielding sodium acetate.
Enzymatic oxidation of aldehydes is prevented by α-tocopherol
Aldehydes have been identified as among the end products of lipid peroxidation. Numerous studies have measured the release of MDA during the preparation of oxidized LDL, and antibodies that recognize MDA-LDL, as well as other aldehyde-modified proteins (e.g., 4-HNE), have been well described (15). However, aldehydes are also chemically unstable and readily undergo further oxidation. In other words, if lipoprotein fatty acids undergo oxidation, no reason exists to suspect or expect that the reaction would terminate at the aldehyde level. Chemical oxidation of aldehydes is arrested by antioxidants. We, therefore, considered the possibility that antioxidants might also prevent the further “biologic” oxidation of aldehydes. A number of different oxidases, for example, XAO, aldehyde dehydrogenase, MPO (6, 44, 47, 49), and others, have been shown to act on aldehydes and oxidize them to carboxylic acids. For example, Winterbourn and Carr (49) described the loss of MDA after treatment with HOCl and MPO.
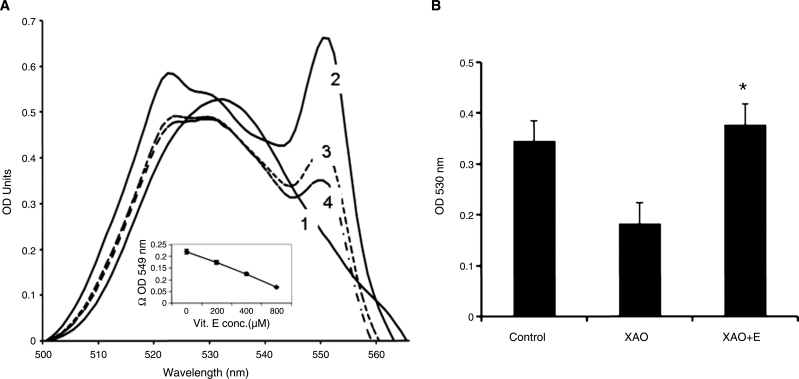
The release of superoxide also has been noted under these conditions, as typified by the acetaldehyde-XAO reaction. To determine whether α-tocopherol inhibited the XAO-mediated oxidation of aldehyde into the corresponding carboxylic acid, we incubated nonenal with XAO in the presence or absence of α-tocopherol and measured the free aldehyde at the end of the incubation. When we incubated nonenal in the presence of XAO, a substantial reduction of cytochrome c was found, which was effectively quenched by α-tocopherol (Fig. 7A). Increasing concentrations of α- tocopherol resulted in a decreased reduction of cytochrome c (curves 1 to 4 and also insert showing dose dependency). Of course, the possibility remained that α-tocopherol reacted only with the superoxide radical, thereby preventing the latter's interaction with the cytochrome c. In other words, although indicative, these reactions did not demonstrate that α-tocopherol prevented the conversion of an aldehyde into the carboxylic acid.
FIG. 7.
(A) α-Tocopherol inhibits the reduction of Cyt C during the XAO-catalyzed oxidation of nonenal. Nonenal (5 mM) was incubated with the XAO assay system in the presence of 200–800 μM α-tocopherol for up to 15 min. Wavelength scan was performed between 500 and 600 nm. Lines 1 to 5 represent control without enzyme (1), with XAO alone (2), and with 200, 400, and 800 μM α-tocopherol (3–5) at the end of 15 min. The figure is a representation from six separate trials. Insert represents optical-density difference at 549 nm in the absence and presence of α-tocopherol. (B) α-Tocopherol inhibits the conversion of nonenal to nonanoic acid. Experiments were set as described for Fig. 6A, but in the absence of cytochrome c. Reactions were scaled down and performed in 96-well microtiter plates with a final volume of 175 μl. At the end of incubations at 37°C for 30 min, 25 μl of Purpald solution (20 mg in 0.5 ml of 1N NaOH) was added, and the color was read at 540 nm. Appropriate controls (without enzyme or aldehyde) were performed. The figure represents average ± SD of three separate trials done in triplicate. *Statistically significant values (p < 0.05).
We therefore measured the aldehydes remaining at the end of the incubation with xanthine oxidase. Results, as shown in Fig. 7B, indicated that a significant loss of aldehydes occurred at the end of the incubation. Inclusion of α-tocopherol in the incubations prevented the loss of the aldehyde. Similarly, we incubated MDA or 4-HNE (data not shown) with MPO, an enzyme that has been suggested to be involved in the development of atherosclerosis. α-Tocopherol was able to prevent the loss of the aldehydes.
We extracted the products after similar incubations with 4-HNE and subjected them to mass spectrometry. As expected, the control reaction, in the presence of XAO, showed the formation of a negative ion at 171 (4-hydroxy nonenoic acid). The formation of this ion was considerably reduced in the presence of α-tocopherol, with an increase in mass at 155 (4-HNE). Similar results were obtained when we used 9-oxo-nonenoic acid with the formation of AZA at mass of 187 (data not given).
Discussion
Secondary decomposition of fatty acid hydroperoxide has gained wide importance, as they are mainly responsible for causing rancidity in foods and also damage biologic materials. Large bodies of evidence suggest that metal ions, radicals, hemoglobin, hematin, and vitamin C induce decomposition of HPODE.
In the current study, we showed that, when incubated at physiologic temperature, 13-HPODE lost almost 80–90% of its diene structure and lipid peroxide levels during a period of 72 h. Thin-layer chromatography analysis showed the presence of various epoxides and aldehydes during the early period of incubation, as well as at later hours. Some of the epoxides generated during the early hours were found to increase over time, whereas other epoxides and aldehydes were not found during the later time (data not shown), indicating that epoxide may be an intermediate compound in the decomposition reaction. The decomposition was not affected by α-tocopherol or by EDTA, suggesting that the decomposition pathway is not radical mediated. Considering that α-tocopherol is well documented to prevent the initial oxidation of LDL, it is likely that α-tocopherol may have profound effects on the formation of new atherosclerotic lesions. It may also have effects if any further cellular oxidative events are involved in the pathobiologic actions of the decomposition products themselves.
The results will have far-reaching importance in the pathobiology of atherosclerosis. As mentioned earlier, a discrepancy exists between animal and human antioxidant trials. How do we reconcile the effects of α-tocopherol in the two systems? Convincing evidence from human studies is the prevalence of oxidized LDL in subjects with various forms of coronary artery diseases (40, 48), as determined by detection with antibodies that recognize oxidatively tailored lipids. Thus, it is likely that the decomposition events (as described in the current study) are the hallmarks of progression and the development of vulnerable lesions and not the initial formation of peroxidized lipids or early atherosclerotic lesions, as in animals. If this be the case, infants or children, at very early stages of lesion development, might benefit from antioxidants, and the treatment of adult disease might require novel strategies that would limit and detoxify the formation of secondary oxidation products. Glutathione-dependent enzymes or α-keto acids, such as pyruvate, might serve to accomplish the purpose of “detoxifying” lipid peroxides and generate alcohols that might undergo natural fatty acid oxidation mechanisms.
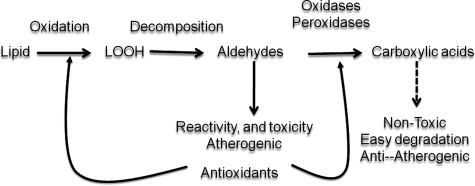
Conversely, dicarboxylic acids (further oxidation product of malondialdehyde or that of oxo-nonanoic acid), such as AZA, would have important antiatherogenic properties: as do other dicarboxylic acids, they would have great affinity for calcium and precipitate calcium in lipid-rich domains. This might account for the unusually high calcification associated with lipid-core domains. It is too premature to speculate about the effects of increased aortic calcification, as the literature is divided on the topic. Although calcium is definitely correlated with plaque burden, calcification also protects against plaque vulnerability. In addition, AZA has been shown to have antiinflammatory properties and has been noted to decrease cytokine production by macrophages and inflammatory cells (10). It also has been noted to reduce oxidant production by leukocytes (1). Thus, it has been added as an active ingredient in topical skin medications, particularly as an antiacne agent. In conclusion, the role of antioxidants in controlling the atherosclerotic process should be studied in the context of steps that are involved beyond initial oxidation, as outlined in Fig. 8. In animal atherosclerosis, which is studied in the short term, the emphasis is on establishing the lesions. Thus, antioxidants, such as α-tocopherol, might affect predominantly the initial formation and progression of the lesion. In humans, particularly in those who already have clinically significant events, the early steps might have already occurred. In such cases, α-tocopherol and similar antioxidants could affect the conversion of aldehydes into carboxylic acids. The latter, are presumed to be nonatherogenic and are easily degraded via fatty-acid degradation pathways.
FIG. 8.
Antioxidants might prevent the oxidation of aldehydes into carboxylic acids.
Acknowledgments
This work was supported by NIH grants HL69038 and DK056353 (S.P.). The authors thank Dr. Desikan Rajagopal and Dr. Tanya Young for assistance with NMR experiments.
Abbreviations
AZA, azelaic acid; BHT, butylated hydroxy toluene; DNP, 2,4-dinitrobenzene; D2O, deuterated water; EDTA, ethylenediaminetetraacetic acid; FCS, fetal calf serum; GC-MS, gas chromatography–mass spectrometry; 4-HNE, 4-hydroxynonenal; 13-HODE, 13-hydroxylinoleic acid; 13-HPODE, 13-hydroperoxylinoleic acid; IR, infrared; LC-MS, liquid chromatography–mass spectrometry; LDL, low-density lipoprotein; LMB, leucomethylene blue; LOH, lipid hydroxide; LOOH, lipid peroxide; MDA, malondialdehyde; MMPs, matrix-digesting enzymes; MPO, myeloperoxidase; NMR, nuclear magnetic resonance; PBS, phosphate-buffered saline; PYR, pyruvate; SLO, soybean lipoxygenase; SOD, superoxide dismutase; TLC, thin-layer chromatography; XAO, xanthine oxidase; Trolox, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid; WHHL, watanabe heritable hyperlipidemic rabbit.
Disclosure Statement
No competing financial interests exist.
References
- 1.Akamatsu H. Komura J. Asada Y. Miyachi Y. Niwa Y. Inhibitory effect of azelaic acid on neutrophil functions: a possible cause for its efficacy in treating pathogenetically unrelated diseases. Arch Dermatol Res. 1991;283:162–166. doi: 10.1007/BF00372056. [DOI] [PubMed] [Google Scholar]
- 2.Andrae U. Singh J. Ziegler-Skylakakis K. Pyruvate and related α-ketoacids protect mammalian cells in culture against hydrogen peroxide-induced cytotoxicity. Toxicol Lett. 1985;28:93–98. doi: 10.1016/0378-4274(85)90015-3. [DOI] [PubMed] [Google Scholar]
- 3.Auerbach BJ. Kiely JS. Cornicelli JA. A spectrophotometric microtiter-based assay for the detection of hydroperoxy derivatives of linoleic acid. Anal Biochem. 1992;201:375–380. doi: 10.1016/0003-2697(92)90354-a. [DOI] [PubMed] [Google Scholar]
- 4.Brown BG. Cheung MC. Lee AC. Zhao XQ. Chait A. Antioxidant vitamins and lipid therapy: end of a long romance? Arterioscler Thromb Vasc Biol. 2002;22:1535–1546. doi: 10.1161/01.atv.0000034706.24149.95. [DOI] [PubMed] [Google Scholar]
- 5.Brown BG. Zhao XQ. Chait A. Fisher LD. Cheung MC. Morse JS. Dowdy AA. Marino EK. Bolson EL. Alaupovic P. Frohlich J. Albers JJ. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 6.Chen HJ. Chung FL. Epoxidation of trans-4-hydroxy-2-nonenal by fatty acid hydroperoxides and hydrogen peroxide. Chem Res Toxicol. 1996;9:306–312. doi: 10.1021/tx9501389. [DOI] [PubMed] [Google Scholar]
- 7.Cheung MC. Zhao XQ. Chait A. Albers JJ. Brown BG. Antioxidant supplements block the response of HDL to simvastatin-niacin therapy in patients with coronary artery disease and low HDL. Arterioscler Thromb Vasc Biol. 2001;21:1320–1326. doi: 10.1161/hq0801.095151. [DOI] [PubMed] [Google Scholar]
- 8.Chisolm GM. Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 9.Cook NR. Albert CM. Gaziano JM. Zaharris E. MacFadyen J. Danielson E. Buring JE. Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167:1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elewski B. Thiboutot D. A clinical overview of azelaic acid. Cutis. 2006;77:12–16. [PubMed] [Google Scholar]
- 11.Fruebis J. Parthasarathy S. Steinberg D. Evidence for a concerted reaction between lipid hydroperoxides and polypeptides. Proc Natl Acad Sci U S A. 1992;89:10588–10592. doi: 10.1073/pnas.89.22.10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haberland ME. Olch CL. Fogelman AM. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984;259:11305–11311. [PubMed] [Google Scholar]
- 13.Hodis HN. Mack WJ. LaBree L. Mahrer PR. Sevanian A. Liu CR. Liu CH. Hwang J. Selzer RH. Azen SP VEAPS Research Group. α-Tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: the Alpha Tocopherol Atherosclerosis Prevention Study (VEAPS) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 14.Iwahashi H. Some polyphenols inhibit the formation of pentyl radical and octanoic acradical in the reaction mixture of linoleic acid hydroperoxide with ferrous ions. Biochem J. 2000;346:265–273. [PMC free article] [PubMed] [Google Scholar]
- 15.Jurgens G. Lang J. Esterbauer H. Modification of human low density lipoproteins by the lipid peroxidation product 4-hydroxynonenal. Biochim Biophys Acta. 1986;875:103–114. doi: 10.1016/0005-2760(86)90016-0. [DOI] [PubMed] [Google Scholar]
- 16.Keith ME. Jeejeebhoy KN. Langer A. Kurian R. Barr A. O'Kelly B. Sole MJ. A controlled clinical trial of alpha tocopherol supplementation in patients with congestive heart failure. Am J Clin Nutr. 2001;73:219–224. doi: 10.1093/ajcn/73.2.219. [DOI] [PubMed] [Google Scholar]
- 17.Lee IM. Cook NR. Gaziano JM. Gordon D. Ridker PM. Manson JE. Hennekens CH. Buring JE. Alpha tocopherol in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 18.Lee SH. Oe T. Blair IA. Vitamin C induced decomposition of lipid hydroperoxides to endogenous genotoxins. Science. 2001;292:2083–2086. doi: 10.1126/science.1059501. [DOI] [PubMed] [Google Scholar]
- 19.Lonn E. Bosch J. Yusuf S. Sheridan P. Pogue J. Arnold JM. Ross C. Arnold A. Sleight P. Probstfield J. Dagenais GR HOPE and HOPE-TOO Trial Investigators. Effects of long-term alpha tocopherol supplementation on cardiovascular events and cancer: a randomized controlled trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 20.McGill HC., Jr McMahan CA. Zieske AW. Sloop GD. Walcott JV. Troxclair DA. Malcom GT. Tracy RE. Oalmann MC. Strong JP. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth: The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 2000;20:1998–2004. doi: 10.1161/01.atv.20.8.1998. [DOI] [PubMed] [Google Scholar]
- 21.Micheletta F. Natoli S. Misuraca M. Sbarigia E. Diczfalusy U. Iuliano L. Alpha tocopherol supplementation in patients with carotid atherosclerosis: reversal of altered oxidative stress status in plasma but not in plaque. Arterioscler Thromb Vasc Biol. 2004;24:136–140. doi: 10.1161/01.ATV.0000104028.07929.72. [DOI] [PubMed] [Google Scholar]
- 22.Napoli C. Glass CK. Witzum JL. Deutsch RD. Armiento FP. Palisky W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in child hood: Fate of Early Lesion In Children (FELIC) study. Lancet. 1999;354:1234–1241. doi: 10.1016/S0140-6736(99)02131-5. [DOI] [PubMed] [Google Scholar]
- 23.Nath KA. Ngo EO. Hebbel RP. Croatt AJ. Zhou B. Nutter LM. α-Ketoacids scavenge H2O2 in vitro and in vivo and reduce menadione induced DNA injury and cytotoxicity. Am J Physiol. 1995;268:C227–C236. doi: 10.1152/ajpcell.1995.268.1.C227. [DOI] [PubMed] [Google Scholar]
- 24.Palinski W. Napoli C. Pathophysiological events during pregnancy influence the development of atherosclerosis. Trends Cardiovasc Med. 1999;9:205–214. doi: 10.1016/s1050-1738(00)00022-0. [DOI] [PubMed] [Google Scholar]
- 25.Palinski W. Yamashita T. Freigang S. Napoli C. Developmental programming: maternal hypercholesterolemia and immunity influence susceptibility to atherosclerosis. Nutr Rev. 2007;65:S182–S187. doi: 10.1111/j.1753-4887.2007.tb00360.x. [DOI] [PubMed] [Google Scholar]
- 26.Parthasarathy S. Khan-Merchant N. Penumetcha M. Khan BV. Santanam N. Did the antioxidant trials fail to validate the oxidation hypothesis? Curr Atheroscler Rep. 2001;3:392–398. doi: 10.1007/s11883-001-0077-9. [DOI] [PubMed] [Google Scholar]
- 27.Parthasarathy S. Litvinov D. Selvarajan K. Garelnabi M. Lipid peroxidation and decomposition: conflicting roles in plaque vulnerability and stability. Biochim Biophys Acta. 2008;1781:221–231. doi: 10.1016/j.bbalip.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parthasarathy S. Santanam N. Ramachandran S. Meilhac O. Oxidants and antioxidants in atherogenesis: an appraisal. J Lipid Res. 1999;40:2143–2157. [PubMed] [Google Scholar]
- 29.Patrignani P. Panara MR. Tacconelli S. Seta F. Bucciarelli T. Ciabattoni G. Alessandrini P. Mezzetti A. Santini G. Sciulli MG. Cipollone F. Davi G. Galliana P. Bon GB. Patrono C. Effects of vitamin E supplementation on F2-isoprostane and thromboxane biosynthesis in healthy cigarette smokers. Circulation. 2000;102:539–545. doi: 10.1161/01.cir.102.5.539. [DOI] [PubMed] [Google Scholar]
- 30.Pryor WA. Porter NA. Suggested mechanisms for the production of 4-hydroxy-2-nonenal from the autooxidation of polyunsaturated fatty acids. Free Radic Biol Med. 1990;8:541–543. doi: 10.1016/0891-5849(90)90153-a. [DOI] [PubMed] [Google Scholar]
- 31.Rahn CH. Schlenk H. Detection of aldehydes with 4-amino-5-hydrazino-1, 2, 4-triazole-3-thiol as spray reagent. Lipids. 1973;8:612–616. doi: 10.1007/BF02533143. [DOI] [PubMed] [Google Scholar]
- 32.Rajavashisth TB. Liao JK. Galis ZS. Tripathi S. Laufs U. Tripathi J. Chai NN. Xu XP. Jovinge S. Shah PK. Libby P. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J Biol Chem. 1999;274:11924–11929. doi: 10.1074/jbc.274.17.11924. [DOI] [PubMed] [Google Scholar]
- 33.Rajavashisth TB. Xu XP. Jovinge S. Meisel S. Xu XO. Chai NN. Fishbein MC. Kaul S. Cercek B. Sharifi B. Shah PK. Membrane type 1 matrix metalloproteinase expression in human atherosclerotic plaques: evidence for activation by proinflammatory mediators. Circulation. 1999;99:3103–3109. doi: 10.1161/01.cir.99.24.3103. [DOI] [PubMed] [Google Scholar]
- 34.Schneider C. Boeglin WE. Yin H. Ste DF. Hachey DL. Porter NA. Brash AR. Synthesis of dihydroperoxides of linoleic and linolenic acids and studies on their transformation to 4-hydroperoxynonenal. Lipids. 2005;40:1155–1162. doi: 10.1007/s11745-005-1480-3. [DOI] [PubMed] [Google Scholar]
- 35.Schneider C. Porter NA. Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J Biol Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schneider C. Tallman KA. Porter NA. Brash AR. Two distinct pathway of formation of 4-hydroxynonenal: mechanisms of non enzymatic transformation of the 9- and 13- hydroperoxides of linoleic acids to 4-hydroxyalkenals. J Biol Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 37.Sesso HD. Buring JE. Christen WG. Kurth T. Belanger C. MacFadyen J. Bubes V. Manson JE. Glynn RJ. Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stary HC. Lipid and macrophage accumulations in arteries of children and the development of atherosclerosis. Am J Clin Nutr. 2000;72:1297S–1306S. doi: 10.1093/ajcn/72.5.1297s. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg D. Lewis A. Conner Memorial Lecture: oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 40.Steinberg D. Witztum JL. Is the oxidative modification hypothesis relevant to human atherosclerosis? Do the antioxidant trials conducted to date refute the hypothesis? Circulation. 2002;105:2107–2111. doi: 10.1161/01.cir.0000014762.06201.06. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg D. Parthasarathy S. Carew TE. Khoo JC. Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 42.Stocker R. Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 43.Sujiyama S. Okada Y. Sulkhova GK. Virmani R. Heinecke JW. Libby P. Macrophage myloperoxidase regulation by granulocyte macrophage colony stimulating factor in human atherosclerosis and implications in acute coronary syndromes. Am J Pathol. 2001;158:879–891. doi: 10.1016/S0002-9440(10)64036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tian Q. Shi D. Sha Y. CuO, and Ag2O/CuO catalyzed oxidation of aldehydes to the corresponding carboxylic acids by molecular oxygen. Molecules. 2008;13:948–957. doi: 10.3390/molecules13040948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verlangieri AJ. Bush MJ. Effects of D-α-tocopherol supplementation on experimentally induced primate atherosclerosis. J Am Coll Nutr. 1992;11:131–138. [PubMed] [Google Scholar]
- 46.Wang X. Perez E. Liu R. Yan LJ. Mallet RT. Yang SH. Pyruvate protect mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132:1–9. doi: 10.1016/j.brainres.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wildsmith KR. Albert CJ. Anbukumar DS. Ford DA. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J Biol Chem. 2006;281:16849–16860. doi: 10.1074/jbc.M602505200. [DOI] [PubMed] [Google Scholar]
- 48.Williams KJ. Fisher EA. Oxidation, lipoproteins, atherosclerosis: which is wrong, the antioxidant or theory? Curr Opin Clin Nutr Metab Care. 2005;8:139–146. doi: 10.1097/00075197-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 49.Winterbourn CC. Carr AC. Myeloperoxidase-dependent loss of malondialdehyde: a limitation for detecting neutrophil-mediated lipid peroxidation. Arch Biochem Biophys. 1993;302:461–467. doi: 10.1006/abbi.1993.1240. [DOI] [PubMed] [Google Scholar]
- 50.Yagi K. Nishigaki I. Ohama H. Measurement of serum TBA value. Vitamins. 1968;37:105–112. [Google Scholar]
- 51.Yusuf S. Dagenais G. Pogue J. Bosch J. Sleight P. Alpha tocopherol supplementation and cardiovascular events in high-risk patients: the Heart Outcomes Prevention Evaluation Study investigators. N Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]