Abstract
The molecular definition of major histocompatibility complex (MHC) class I-presented CD8+ T-cell epitopes from clinically relevant Mycobacterium tuberculosis (Mtb) target proteins will aid in the rational design of T-cell-based diagnostics of tuberculosis (TB) and the measurement of TB vaccine-take. We used an epitope discovery system, based on recombinant MHC class I molecules that cover the most frequent Caucasian alleles [human leucocyte antigen (HLA)-A*0101, A*0201, A*0301, A*1101, A*2402, B*0702, B*0801 and B*1501], to identify MHC class I-binding peptides from overlapping 9-mer peptides representing the Mtb protein TB10.4. A total of 33 MHC class I-binding epitopes were identified, spread across the entire amino acid sequence, with some clustering at the N- and C-termini of the protein. Binding of individual peptides or closely related peptide species to different MHC class I alleles was frequently observed. For instance, the common motif of xIMYNYPAMx bound to six of eight alleles. Affinity (50% effective dose) and off-rate (half life) analysis of candidate Mtb peptides will help to define the conditions for CD8+ T-cell interaction with their nominal MHC class I-peptide ligands. Subsequent construction of tetramers allowed us to confirm the recognition of some of the epitopes by CD8+ T cells from patients with active pulmonary TB. HLA-B alleles served as the dominant MHC class I restricting molecules for anti-Mtb TB10.4-specific CD8+ T-cell responses measured in CD8+ T cells from patients with pulmonary TB.
Keywords: CD8+ T cells, epitopes, immune dominance, Mycobacterium tuberculosis, TB10.4
Introduction
Tuberculosis (TB) is a major health problem world-wide; increasing resistance and coinfection with the human immunodeficiency virus (HIV) lead to an increased disease burden in many countries. Although anti-mycobacterial drugs and a vaccine, Bacillus Calmette–Guérin (BCG), are available, neither has proved to be the solution in controlling the disease. The immune mechanisms controlling Mycobacterium tuberculosis (Mtb) are not fully understood, but it is known that both the innate and adaptive parts of the immune system are involved in Mtb control,1 and cell-mediated immunity, involving both CD4+ and CD8+ T cells, has been shown to be important for effective Mtb containment.1,2 Eliciting a strong T helper type 1 (Th1) response and a robust Mtb antigen-specific CD8+ T-cell response represent important goals for vaccine developers.3,4
The repertoire of the CD8+ T-cell response is shaped by the entry of antigen into the major histocompatibility complex (MHC) class I processing pathway, binding of peptides to MHC class I molecules and, ultimately, recognition of the trimolecular MHC–β2 microglobulin–peptide complex by CD8+ T cells. The interaction between the T-cell receptor (TCR) and the MHC–β2 microglobulin–peptide complex is a two-step process. In the first step, the TCR docks on the MHC molecule in a peptide-independent fashion. This is followed by contact between the TCR and the peptide, which stabilizes the MHC–TCR complex.5 Therefore, at least two variables determine the outcome of antigen presentation by MHC class I molecules: (i) the nature of the presenting MHC class I allele and (ii) the amino acid (aa) composition of the nominal target peptide. Different peptides bind to the MHC molecule with different affinity and off-rate (the time of peptide binding to the MHC class I molecule), thereby affecting the magnitude and outcome of the priming phase, i.e. the close interaction of antigen-presenting cells (APCs) and CD8+ T cells.6–8
A better understanding of the cellular immune response to Mtb will be of value in determining the nature of clinically relevant anti-Mtb immune responses, but also in gauging ‘vaccine-take’, for example for novel TB vaccines.9 Measuring cellular immune response induced by vaccination requires the identification of dominant and subdominant epitopes from individual Mtb proteins. The enumeration of antigen-specific T cells in TB infection is currently limited by inadequate knowledge of CD8+ epitopes. Some Mtb-specific CD8+ T-cell epitopes have been identified both in peripheral blood mononuclear cells (PBMCs) from Mtb-infected humans and in murine models.10,11 Yet, a broader peptide repertoire needs to be identified to appreciate the breadth of the CD8+ T-cell response.
We choose the Mtb protein TB10.4 (Rv0288), a component of several new TB vaccine candidates.12,13 TB10.4 is part of the 6 kDa early secretory antigenic target (esat-6) gene family, which encodes a number of secreted immunodominant molecules such as TB10.3 and TB12.9.14 Rv0288 is expressed both in virulent Mtb and BCG vaccine strains.14,15 A few CD8+ T-cell epitopes have previously been described for this protein,16,17 but a systematic approach covering the most frequent MHC class I alleles is lacking. In the current study, we used immobilized recombinant MHC class I molecules, covering a large part of the world’s population (approximately 95% of Caucasians, approximately 65% of Asians and approximately 40% of Africans),18 to define candidate epitopes from TB10.4 in a first screening step. This was followed by affinity and off-rate analysis and subsequent MHC class I tetramer construction, which were used to enumerate epitope-specific CD8+ T cells by flow cytometric analysis in PBMCs from patients with active pulmonary TB. This MHC-guided peptide mapping represented a fast and convenient way of identifying antigenic epitopes presented by multiple MHC alleles simultaneously.
Materials and methods
MHC class I–peptide binding assay
Binding assay
Nonamer peptides overlapping by 8 aa covering the entire TB10.4 sequence (total number of 88 peptides) were synthesized by JPT Peptide Technologies GmbH (Berlin, Germany). Peptide-binding, affinity and off-rate experiments were performed in duplicate in iTopia 96-well plates (Beckman Coulter, San Diego, CA) coated with eight different recombinant MHC class I molecules [human leucocyte antigen (HLA) A*0101, A*0201, A*0301, A*2402, A*1101, B*0702, B*0801 and B*1501, as described previously.19–21 Briefly, monomer-coated plates are stripped off the placeholder peptide leaving the heavy chain free to associate with a candidate peptide after addition of β2 microglobulin. Peptide binding to MHC class I molecules is detected after 18 hr of incubation at 21° with a fluorescent-labelled antibody [fluorescein isothiocyanate (FITC)-conjugated anti-HLA-A, -B and -C], which binds only to the trimeric MHC–β2 microglobulin–peptide complex.
Each candidate peptide was tested against an appropriate control peptide, specific for each MHC class I molecule, and results are reported as the percentage of binding compared with the control peptide. A more detailed analysis of the binding characteristics of each individual peptide was performed using affinity and off-rate assays. In silico prediction of peptide binding to individual MHC class I alleles was also performed using the SYFPEITHI database (http://www.syfpeithi.de).
Off-rate
MHC class I–peptide complex stability was analysed by incubating bound peptides at 37° for eight different times. The off-rate is expressed as a half-life (t1/2) value, which is defined as the time-point at which 50% of the initial peptide concentration has dissociated from the MHC class I–peptide molecule complex.
Affinity assay
MHC class I allele–peptide affinity for individual peptide species was measured using different peptide concentrations (10−4–10−9 m) and then the peptide quantity needed to achieve 50% binding saturation [the 50% effective dose (ED50)] was calculated.
Calculations
Values for peptide binding, affinity and off-rate were calculated using the iTopia™ System Software (Beckman Coulter). Sigmoidal dose–response curves were generated using prism® 4.0 (GraphPad, La Jolla, CA).
Cellular analysis
PBMCs from 14 Caucasian patients with pulmonary TB were obtained by separation on a Ficoll gradient. Patients were diagnosed with pulmonary TB based on acid-fast staining and bacterial culture, and gave their consent to participate in this study. Ethical approval was documented (on file with reference number 837.327.99-2272; 15 November 1999, University of Mainz, Mainz, Germany). The patients were MHC class I typed at the Blood Bank, University of Mainz. Sixteen tetramers were prepared for the MHC class I alleles HLA-A*0201, A*0301, A*1101, A*2402, B*0702, B*0801 and B*1501 (for epitopes, see Table 2) and labelled with strepavidin-phycoerytin (PE) except for HLA-A*0201, which was labelled with streptavidin-allophycocyanin (APC). Flow cytometric analysis was performed and positive events, i.e. antigen-specific T cells, were identified as a percentage of CD3+ CD8+ T cells. At least 50 000 events were obtained in the CD3+ CD8+ CD4− CD13− CD19− population. The following antibodies (Abs) obtained from Beckman Coulter were used: anti-CD3-phycoerythrin-Texas red (clone CHT1) and anti-CD8α-FITC (clone T8) for positive gating, and anti-CD4-PCy5 (clone 13B8.2), anti-CD13-Pcy5 (clone SJ1D1) and anti-CD19-Pcy5 (clone J4.119) for negative gating. Positive tetramer staining was compared with staining with the iTag negative control tetramer. This gating strategy has been found to reliably identify ‘low-frequency’ events, for example melanoma-specific and Melan-A/melanoma antigen recognized by T-cell-1 (MART-1)-1 reactive CD8+ T cells, if the negative control tetramer reagent (loaded with an irrelevant peptide) is used to set the negative gate.22 Flow cytometry analysis was performed using an FC500 flow cytometer from Beckman Coulter (Krefeld, Germany).
Table 2.
Affinity and off-rate data for selected major histocompatibility complex (MHC) class I TB10.4 peptides
 |
Results
Identification of HLA-binding peptides from TB10.4
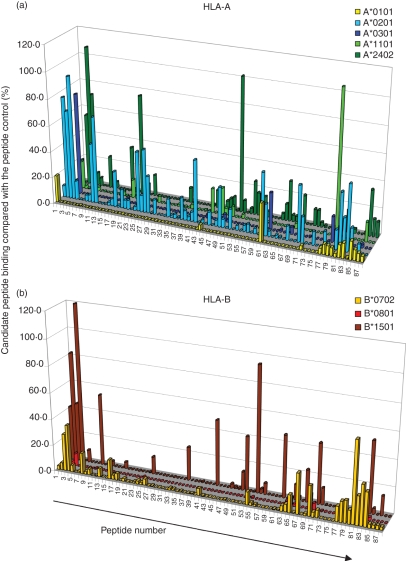
Eighty-eight overlapping peptides from TB10.4 were tested for binding to five HLA-A molecules (A*0101, A*0201, A*0301, A*1101 and A*2402) and three HLA-B molecules (B*0702, B*0801 and B*1501). Binding to each allele is reported as a percentage relative to a positive control peptide for the respective MHC class I allele. With a cut-off of 20% binding as compared with the positive control peptide, we identified the following numbers of positive binding epitopes: two of 88 for A*0101, 17 of 88 for A*0201, two of 88 for A*0301, three of 88 for A*1101, 10 of 88 for A*2402, seven of 88 for B*0702, zero of 88 for B*0801 and 12 of 88 for B*1501 (Fig. 1, Table 1). The alleles HLA-A*0201 and HLA-A*2402 were among the most frequent MHC class I–peptide binders; they bound 20% and 11% of the candidate peptides, respectively. Also, HLA- B*1501 was among the top MHC class I-binding alleles; it bound to 14% of the TB10.4 peptide library. The prediction program syfpeithi (http://www.syfpeithi.de) picked up most TB10.4 epitopes for HLA-A*0201, A*2402 and A*1101; 17 of 17, five of seven and two of three binding epitopes showed a syfpeithi score ≥ 10. For other MHC class I alleles, the program showed a lower success rate; for example, for B*0701 and B*1510, one of seven and five of 12 binding epitopes showed a syfpeithi score ≥ 10.
Figure 1.
Major histocompatibility complex (MHC) class I-binding peptides identified by binding to recombinant MHC class I molecules. Peptide binding to (a) human leucocyte antigen (HLA)-A molecules and (b) HLA-B molecules. The x-axis shows the peptide ID 1–88 (the aa sequence for the positive binders can be found in Table 1) and the z-axis shows the per cent binding for the respective peptides compared with an allele-specific positive control peptide. In (a), yellow colour indicates binding to HLA-A*0101, light blue binding to A*0201, dark blue binding to A*0301, light green binding to A*1101 and dark green binding to A*2402. In (b), yellow indicates binding to B*0702, red binding to B*0801 and brown binding to B*1501.
Table 1.
Screening of major histocompatibility complex (MHC) class I TB10.4 peptide binding
 |
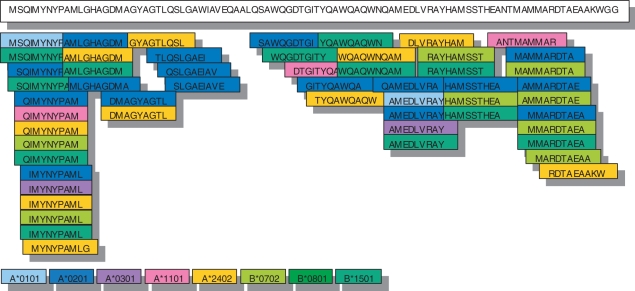
Thirty-three of 88 candidate peptides bound at least to one MHC class I allele; the epitopes could be found throughout the whole amino acid sequence but with some clustering at the N- and C-termini (Fig. 2).
Figure 2.
Alignment of the major histocompatibility complex (MHC) class I-binding peptides within the amino acid sequence of TB10.4. Peptides identified for HLA-A*0101 are shown in light blue, peptides for A*0201 are in dark blue, peptides for A*0301 are in purple, peptides for A*1101 are in pink, peptides for A*2402 are in yellow, peptides for B*0702 are in light green and peptides for B*1501 are in dark green. Clustering of peptides around the N- and C-termini and extensive ‘cross-binding’ of single peptides to different MHC class I alleles can be seen.
Screening of TB10.4 peptides for binding to the eight most frequent Caucasian alleles revealed extensive cross-binding of the identical or closely related peptides to different MHC class I molecules. Near the N-terminus of the amino acid sequence of TB10.4 reside the peptides QIMYNYPAM (TB10.43–11) and IMYNYPAML (TB10.44–12). These peptides share the common motif ‘IMYNYPAM’ and bound to six out of eight alleles (i.e. HLA-A*0201, A*0301, A*1101, A*2401, B*0702 and B*1501). At the C-terminus, we identified a different motif, MMARDTAE, shared by the peptides AMMARDTAE (TB10.482–90) and MMARDTAEA (TB10.483–91). This motif bound to three out of eight alleles (HLA-A*0201, B*0702 and B*1501; Table 1).
Affinity and off-rate studies
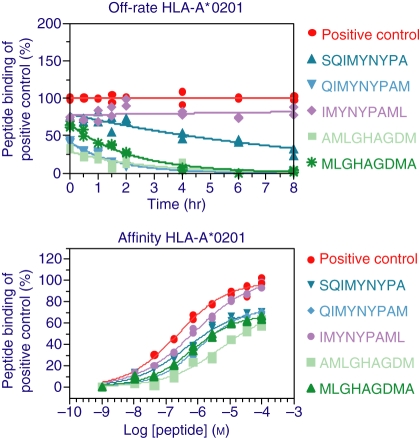
We chose TB10.4 peptides and performed affinity (ED50) and off-rate (t1/2) analysis for (i) peptides identified as binders (above 20% compared with the positive control peptide), and (ii) MHC class I-binding epitopes below the 20% cut-off if they represented the only peptides that bound to MHC class I alleles; for example, AMMARDTAE and MMARDTAEA for A*0101, and MMARDTAEA for B*0801. Affinity between candidate peptides and the respective MHC class I complex was found to be in the range of 60 nm to 800 μm, with the majority (75%) in the range of 1–80 μm. Different TB10.4 peptides bound with different affinity to the same MHC allele; for example, the peptide QIMYNYPAM (TB10.43–11) bound with an affinity of 800 μm to the allele HLA-B*0702, while the peptide AMMARDTAE (TB10.482–90) bound with an affinity of 80 nm to the same MHC class I allele. Also, the identical peptide could bind with different affinity to different MHC class I alleles. For example, the peptide IMYNYPAML (TB10.44–12) bound to HLA-A*0201 with an affinity of 800 nm, to A*0301 with an affinity of 700 nm, to A*2402 with an affinity of 100 nm, to B*0702 with an affinity of 30 μm and to B*1501 with an affinity of 20 μm. Overall, the TB10.4 peptides bound with higher affinity to HLA-A alleles than to HLA-B alleles (Fig. 3, Table 2).
Figure 3.
Affinity and off-rate curves for a set of TB10.4 candidate peptides and human leucocyte antigen (HLA) HLA-A*0201. Raw data for (a) affinity and (b) off-rate are shown for a representative major histocompatibility complex (MHC) class I allele (HLA-A*0201) with five individual candidate peptides. The affinity graph shows individual peptide binding for different peptide concentrations (in m) compared with a positive control. The 50% effective dose (ED50) value can be calculated using sigmoidal curve-fitting. The off-rate graph shows in a similar manner individual peptide off-rates (in hours) compared with an allele-specific positive control; the half life (t½) value was calculated using sigmoidal curve-fitting. Curves were fitted using Graphpad prism software.
The off-rate assay was used to evaluate the relative stability of each MHC class I complex. The dissociation rate of the peptides spanned a wide range of < 1 to 27 hr, with the majority of epitopes (27 of 52) in the range of 1–3 hr. Four peptides, for example HLA-B*0702 RAYHAMSST (TB10.467–75), exhibited a dissociation rate of < 1 hr, while nine of 52 peptides showed a t1/2 value of more than 5 hr, for example HLA-A*0201 AMMARDTAE (TB10.482–90). We could identify differences both (i) within a single MHC class I allele presenting different peptides, for example HLA-A*0201 which presents the peptide IMYNYPAML (TB10.44–12) with an off-rate of approximately 27 hr and GITYQAWQA (TB10.448–56) with an off-rate of 0·7 hr, and (ii) between different alleles presenting identical peptides, for example the peptide IMYNYPAML (TB10.44–12) which exhibited an off-rate of approximately 27 hr for HLA-A*0201, approximately 1 hr for A*0301, approximately 1·5 hr for A*2402/B*0702 and approximately 4 hr for B*1501. We could not find any correlation between affinity and off-rate; some peptides with high affinity had very long off-rates, while other peptides showed the opposite dissociation pattern (Fig. 3 and Table 2).
TB10.4 peptides and CD8+ T-cell recognition in patients with active pulmonary TB
In order to determine if the TB10.4 peptides act as targets for CD8+ T cells in PBMCs from patients with pulmonary TB, we performed tetramer-guided analysis of 13 peptides identified by peptide binding. Sixteen tetramers were constructed: four tetramers covering A*0201, three tetramers covering A*2402 and B*0702, and two tetramers covering B*1501 and A*1101; B*0801 and A*0301 were covered with a single tetramer (Table 2). No tetramers were constructed for HLA-A*0101 as the MHC class I–peptide complexes did not exhibit sufficient stability. PBMCs from 14 MHC class I typed patients were analysed for epitope-specific T cells using MHC allele-matched tetramers.
We identified three patterns: (i) some of the tetramers showed no T-cell binding compared with the negative control tetramer, for example A*2402 GYAGTLQSL (TB10.420–28); (ii) other tetramers showed T-cell binding in PBMCs from some patients but not in others, for example B*1501 WQAQWNQAM (TB10.454–62); (iii) and other tetramers identified peptide-specific T cells in all patients with matching MHC alleles, for example B*0702 MAMMARDTA (TB10.481–89). This epitope exhibited the most frequent T-cell population; up to 2% of all CD8+ T cells recognized this peptide in one patient (Table 3). In general, and as validated by the negative control tetramer-binding data, the frequencies of tetramer-binding T cells for HLA-A*0201 and A*2402 were relatively low, while the opposite was found to be true for HLA-B*0702 and B*0801.
Table 3.
Percentage of TB10.4 peptide-specific T cells in the CD3+ CD8+ CD4− CD13− CD19− population
 |
For the peptides IMYNYPAML (TB10.44–12) and MMARDTAEA (TB10.483–91) several different tetramers were constructed; for example, the peptide IMYNYPAML was used for HLA-A*0201, A*2402 and B*0702. This peptide was strongly recognized if presented by the HLA-B allele but not as strongly if presented by HLA-A alleles. The other ‘cross-presented’ peptides showed a similar recognition pattern.
Discussion
Identification of novel MHC class I-presented peptides is useful for the development of TB diagnostics and to gauge TB vaccine-take. TB10.4 is present in M. tuberculosis and environmental mycobacterial species, including the vaccine strain BCG. The value of testing TB10.4 CD8+ T-cell responses lies in the gauging of vaccines containing TB10.4 antigens. We confirmed the previous identification of some TB10.4 peptides, i.e. QIMYNYPAM (TB10.43–11) (H-2kd), IMYNYPAML (TB10.44–12) (HLA-A*0201) and GYAGTLQSL (TB10.420–28) (HLA-A*2402 and H-2kd),13,16,17,23 but the majority of TB10.4 peptides identified have not previously been reported or were previously identified as peptides binding to an ‘unknown allele’. Binding peptides were found for all the investigated alleles, and yet the frequency of peptide binding was different among the alleles. For instance, A*0201 showed a very high number of binding peptides (20%) while the opposite was true for A*0101 and B*0801. A previous study, using a similar approach, concluded that this is not a problem linked to the control peptides used in the assay: if the control peptides bind with different affinity, the 20% cut-off could identify more peptide-binders for alleles with low-affinity peptide control.20 It is more likely that some alleles exhibit a less restrictive peptide binding while other MHC class I alleles show a more restrictive peptide binding pattern.
Most MHC class I molecules have been studied in detail and peptide anchor positions have been identified. HLA-A*0201 preferentially recognizes peptides with the amino acids leucine or methionine at position 2 and valine or leucine at the C-terminus.24 Only two out of 17 epitopes showed ‘correct’ anchor residues; others had either one ‘correct’ anchor residue or residues with similar hydrophobic groups at these positions. Other peptides, such as SQIMYNYPA (TB10.42–10), shared almost none of the previously reported preferred residues, but they strongly stabilized the HLA-A*0201 monomer, sufficient for tetramer production.
For many of the TB10.4 peptides, we could identify extensive ‘cross-binding’ to different MHC class I molecules. MHC molecules have been divided into supertypes based on similar binding preferences for peptides.25 Some of the cross-binding could be a result of the fact that the alleles belong to the same supertype, or to supertypes with similar binding preferences. However, the majority of the peptides identified in the current study bound to alleles that exhibited very different binding preferences; for example, they bound both to HLA-A and HLA-B alleles. This observation has previously been postulated to represent an exception rather than a rule.26,27 Only recently, more systematic studies of HIV epitopes and human papillomavirus (HPV) epitopes showed that this phenomenon may be more common.28,29 In the context of nonviral pathogens, ‘cross-binding’ has previously been reported for the Mtb protein Ag85B19 and the tumour-associated protein 5T4.20 The extensive promiscuity of peptides in their binding to different MHC class I molecules may certainly be of clinical importance considering the vast number of alleles that exist. A peptide capable of binding to many different MHC class I molecules is more likely to be presented by a majority of people, a situation that could facilitate vaccine design, as fewer epitopes are needed to cover large population cohorts. While this might be positive from the perspective of vaccine design, it may also mean that a narrow focus on a few epitopes, presented by a high number of alleles to CD8+ T cells, could lead to ‘immune exhaustion’ or escape mutations. Escape mutations are common in viral epitopes but have not yet been reported for Mtb epitopes. This may be a result of the fact that the mutation rate for immunogenic Mtb proteins has been shown to be quite low;30 in addition, data comparing a comprehensive panel of Mtb isolates using genome-wide analysis are lacking at this time. In the case of TB10.4, similar epitopes from the closely related proteins TB10.3 and TB12.9 may also affect T-cell recognition and thereby the fine specificity and focus of the CD8+ T-cell response.
The ability to determine the affinities and off-rates of peptides binding to MHC class I molecules will help to elucidate the time frame for which an individual epitope is available for T-cell priming. Previous studies have shown a correlation between high-affinity peptides and immunogenicity,31 while other studies failed to identify such a link.32 HLA-A alleles showed a wide range of both peptide affinity and off-rate; generally the peptide affinity was lower and the off-rate faster for the HLA-B alleles reported here. This is consistent with a previous study, in which the affinity of peptide epitopes generally tended to be lower for HLA-B alleles than for HLA-A alleles.33 We also observed that the ‘promiscuous peptides’ bound with different affinities and off-rates to MHC class I molecules; this behaviour may determine which MHC class I–peptide complexes are ‘immunodominant’ or ‘subdominant’ in CD8+ T-cell responses.
Using tetramer technology, we confirmed the presence of TB10.4 epitope-specific CD8+ T cells for most of the candidate peptides in patients with TB. The fact that some of the identified epitopes do not seem to be recognized by any CD8+ T cells may have several explanations. One explanation could be that certain peptides may not be generated in vivo because of proteasomal processing, or because of differential affinity for the transporter protein (TAP) and trimming by aminopeptidases.34 Other reasons may be that no TCRs are able to bind to the MHC class I–peptide complex, or antigen-specific T cells may not have been expanded by APC contact.35 In addition, we analysed PBMCs from patients with active pulmonary TB. It could very well be that local pulmonary immune responses36 show a different profile or that the focus of the CD8+ T-cell response changes over time after reduction of bacterial load as a result of anti-TB treatment.37 The fact that most TB10.4 antigen-specific T cells were identified using HLA-B tetramers supports the notion that the CD8+ T-cell response to Mtb antigens appears to be mainly HLA-B restricted. This is consistent with previous studies on TB,19,38 but also with those on viral diseases, i.e. infcetions with HIV,39 Epstein–Barr virus (EBV32) and cytomegalovirus (CMV40). The cause of this ‘immunodominance’ is not that HLA-B alleles have a broader peptide-binding repertoire than HLA-A alleles;33 in fact, our current study confirms that HLA-A alleles exhibit a more diverse peptide-binding repertoire. HLA-B immunodominance may be linked to either differences at the MHC expression level on APCs and/or differences in the TCR repertoire that is available to recognize the respective MHC class I–peptide complex. One may speculate that the lower affinity and shorter off-rate between the candidate peptides and HLA-B alleles may prevent the ‘immune exhaustion’ that may occur in MHC class I high-affinity binding epitopes in chronic infections, including TB. Other, not mutually exclusive, reasons for HLA-B dominance may be the selective interference of pathogens with HLA-A processing or up-regulation of HLA-B alleles.38 It will be of interest to study differential cytokine production in CD8+ T cells associated with differential TB10.4 peptide recognition, i.e. if an identical peptide presented by different MHC class I alleles elicits similar cytokine patterns. This could not be tested in the current study, as PBMCs were obtained from individuals with untreated, newly diagnosed pulmonary TB. This is usually associated with low TCR zeta chain expression41 and defective cytokine production,19 a situation described as ‘anergy’42 which would also lead to negative purified protein derivative (PPD) skin tests. Finally, as this study and most of the other reports focused on ‘Caucasian’ MHC class I alleles, we cannot exclude the possibility that other, less common MHC class I alleles might show a different pattern of immunodominance.
In summary, in the current study we identified 33 MHC class I peptides from the Mtb protein TB10.4. The peptides showed a high degree of promiscuity in binding to MHC class I alleles. These reagents can be included in studies monitoring TB10.4 vaccine-take and they will also be useful in elucidating the dynamics of anti-Mtb restricted T-cell responses in patients with active and latent TB.
Acknowledgments
The study was supported in part by grants from the AERAS Foundation, SIDA-SAREC, Vetenskapsrådet and the Söderberg Foundation to MM and from the Karolinska Institutet (KID) to RAR.
Glossary
Abbreviations:
- Mtb
Mycobacterium tuberculosis
- TB
tuberculosis
Disclosures
The authors have no conflict of interest.
References
- 1.Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. doi: 10.1146/annurev.immunol.19.1.93. [DOI] [PubMed] [Google Scholar]
- 2.Lazarevic V, Flynn J. CD8+ T cells in tuberculosis. Am J Respir Crit Care Med. 2002;166:1116–21. doi: 10.1164/rccm.2204027. [DOI] [PubMed] [Google Scholar]
- 3.Behar SM, Woodworth JS, Wu Y. Next generation: tuberculosis vaccines that elicit protective CD8+ T cells. Expert Rev Vaccines. 2007;6:441–56. doi: 10.1586/14760584.6.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietrich J, Weldingh K, Andersen P. Prospects for a novel vaccine against tuberculosis. Vet Microbiol. 2006;112:163–9. doi: 10.1016/j.vetmic.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 5.Wu LC, Tuot DS, Lyons DS, Garcia KC, Davis MM. Two-step binding mechanism for T-cell receptor recognition of peptide MHC. Nature. 2002;418:552–6. doi: 10.1038/nature00920. [DOI] [PubMed] [Google Scholar]
- 6.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–43. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–22. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26:660–7. doi: 10.1016/j.it.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Lalvani A, Brookes R, Wilkinson RJ, et al. Human cytolytic and interferon gamma-secreting CD8+ T lymphocytes specific for Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1998;95:270–5. doi: 10.1073/pnas.95.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geluk A, van Meijgaarden KE, Franken KL, Drijfhout JW, D’Souza S, Necker A, Huygen K, Ottenhoff TH. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8+ T cells in HLA-transgenic mice and humans. J Immunol. 2000;165:6463–71. doi: 10.4049/jimmunol.165.11.6463. [DOI] [PubMed] [Google Scholar]
- 12.Dietrich J, Aagaard C, Leah R, Olsen AW, Stryhn A, Doherty TM, Andersen P. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005;174:6332–9. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 13.Radosevic K, Wieland CW, Rodriguez A, et al. Protective immune responses to a recombinant adenovirus type 35 tuberculosis vaccine in two mouse strains: CD4 and CD8 T-cell epitope mapping and role of gamma interferon. Infect Immun. 2007;75:4105–15. doi: 10.1128/IAI.00004-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skjot RL, Brock I, Arend SM, Munk ME, Theisen M, Ottenhoff TH, Andersen P. Epitope mapping of the immunodominant antigen TB10.4 and the two homologous proteins TB10.3 and TB12.9, which constitute a subfamily of the esat-6 gene family. Infect Immun. 2002;70:5446–53. doi: 10.1128/IAI.70.10.5446-5453.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skjot RL, Oettinger T, Rosenkrands I, Ravn P, Brock I, Jacobsen S, Andersen P. Comparative evaluation of low-molecular-mass proteins from Mycobacterium tuberculosis identifies members of the ESAT-6 family as immunodominant T-cell antigens. Infect Immun. 2000;68:214–20. doi: 10.1128/iai.68.1.214-220.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majlessi L, Rojas MJ, Brodin P, Leclerc C. CD8+-T-cell responses of Mycobacterium-infected mice to a newly identified major histocompatibility complex class I-restricted epitope shared by proteins of the ESAT-6 family. Infect Immun. 2003;71:7173–7. doi: 10.1128/IAI.71.12.7173-7177.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billeskov R, Vingsbo-Lundberg C, Andersen P, Dietrich J. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol. 2007;179:3973–81. doi: 10.4049/jimmunol.179.6.3973. [DOI] [PubMed] [Google Scholar]
- 18.Middleton D, Menchaca L, Rood H, Komerofsky R. New allele frequency database: http://www.allelefrequencies.net. Tissue Antigens. 2003;61:403–7. doi: 10.1034/j.1399-0039.2003.00062.x. [DOI] [PubMed] [Google Scholar]
- 19.Weichold FF, Mueller S, Kortsik C, Hitzler WE, Wulf MJ, Hone DM, Sadoff JC, Maeurer MJ. Impact of MHC class I alleles on the M. tuberculosis antigen-specific CD8+ T-cell response in patients with pulmonary tuberculosis. Genes Immun. 2007;8:334–43. doi: 10.1038/sj.gene.6364392. [DOI] [PubMed] [Google Scholar]
- 20.Shingler WH, Chikoti P, Kingsman SM, Harrop R. Identification and functional validation of MHC class I epitopes in the tumor-associated antigen 5T4. Int Immunol. 2008;20:1057–66. doi: 10.1093/intimm/dxn063. [DOI] [PubMed] [Google Scholar]
- 21.Bachinsky MM, Guillen DE, Patel SR, Singleton J, Chen C, Soltis DA, Tussey LG. Mapping and binding analysis of peptides derived from the tumor-associated antigen survivin for eight HLA alleles. Cancer Immun. 2005;5:6. [PubMed] [Google Scholar]
- 22.Britten CM, Janetzki S, Ben-Porat L, et al. Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Cancer Immunol Immunother. 2009;58:1701–13. doi: 10.1007/s00262-009-0681-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamath A, Woodworth JS, Behar SM. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J Immunol. 2006;177:6361–9. doi: 10.4049/jimmunol.177.9.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Falk K, Rotzschke O, Stevanovic S, Jung G, Rammensee HG. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. 1991. J Immunol. 2006;177:2741–7. [PubMed] [Google Scholar]
- 25.Sette A, Sidney J. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics. 1999;50:201–12. doi: 10.1007/s002510050594. [DOI] [PubMed] [Google Scholar]
- 26.Sabbaj S, Bansal A, Ritter GD, et al. Cross-reactive CD8+ T cell epitopes identified in US adolescent minorities. J Acquir Immune Defic Syndr. 2003;33:426–38. doi: 10.1097/00126334-200308010-00003. [DOI] [PubMed] [Google Scholar]
- 27.Masemola AM, Mashishi TN, Khoury G, et al. Novel and promiscuous CTL epitopes in conserved regions of Gag targeted by individuals with early subtype C HIV type 1 infection from southern Africa. J Immunol. 2004;173:4607–17. doi: 10.4049/jimmunol.173.7.4607. [DOI] [PubMed] [Google Scholar]
- 28.Frahm N, Yusim K, Suscovich TJ, et al. Extensive HLA class I allele promiscuity among viral CTL epitopes. Eur J Immunol. 2007;37:2419–33. doi: 10.1002/eji.200737365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakagawa M, Kim KH, Gillam TM, Moscicki AB. HLA class I binding promiscuity of the CD8 T-cell epitopes of human papillomavirus type 16 E6 protein. J Virol. 2007;81:1412–23. doi: 10.1128/JVI.01768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musser JM, Amin A, Ramaswamy S. Negligible genetic diversity of mycobacterium tuberculosis host immune system protein targets: evidence of limited selective pressure. Genetics. 2000;155:7–16. doi: 10.1093/genetics/155.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sette A, Vitiello A, Reherman B, et al. The relationship between class I binding affinity and immunogenicity of potential cytotoxic T cell epitopes. J Immunol. 1994;153:5586–92. [PubMed] [Google Scholar]
- 32.Bihl F, Frahm N, Di Giammarino L, et al. Impact of HLA-B alleles, epitope binding affinity, functional avidity, and viral coinfection on the immunodominance of virus-specific CTL responses. J Immunol. 2006;176:4094–101. doi: 10.4049/jimmunol.176.7.4094. [DOI] [PubMed] [Google Scholar]
- 33.Rao X, Costa AI, van Baarle D, Kesmir C. A comparative study of HLA binding affinity and ligand diversity: implications for generating immunodominant CD8+ T cell responses. J Immunol. 2009;182:1526–32. doi: 10.4049/jimmunol.182.3.1526. [DOI] [PubMed] [Google Scholar]
- 34.Tenzer S, Wee E, Burgevin A, et al. Antigen processing influences HIV-specific cytotoxic T lymphocyte immunodominance. Nat Immunol. 2009;10:636–46. doi: 10.1038/ni.1728. [DOI] [PubMed] [Google Scholar]
- 35.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 36.Tully G, Kortsik C, Hohn H, et al. Highly focused T cell responses in latent human pulmonary Mycobacterium tuberculosis infection. J Immunol. 2005;174:2174–84. doi: 10.4049/jimmunol.174.4.2174. [DOI] [PubMed] [Google Scholar]
- 37.Hohn H, Kortsik C, Tully G, et al. Longitudinal analysis of Mycobacterium tuberculosis 19-kDa antigen-specific T cells in patients with pulmonary tuberculosis: association with disease activity and cross-reactivity to a peptide from HIVenv gp120. Eur J Immunol. 2003;33:1613–23. doi: 10.1002/eji.200323480. [DOI] [PubMed] [Google Scholar]
- 38.Lewinsohn DA, Winata E, Swarbrick GM, et al. Immunodominant tuberculosis CD8 antigens preferentially restricted by HLA-B. PLoS Pathog. 2007;3:1240–9. doi: 10.1371/journal.ppat.0030127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiepiela P, Leslie AJ, Honeyborne I, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature. 2004;432:769–75. doi: 10.1038/nature03113. [DOI] [PubMed] [Google Scholar]
- 40.Lacey SF, Villacres MC, La Rosa C, et al. Relative dominance of HLA-B*07 restricted CD8+ T-lymphocyte immune responses to human cytomegalovirus pp65 in persons sharing HLA-A*02 and HLA-B*07 alleles. Hum Immunol. 2003;64:440–52. doi: 10.1016/s0198-8859(03)00028-4. [DOI] [PubMed] [Google Scholar]
- 41.Seitzer U, Kayser K, Hohn H, et al. Reduced T-cell receptor CD3zeta-chain protein and sustained CD3epsilon expression at the site of mycobacterial infection. Immunology. 2001;104:269–77. doi: 10.1046/j.1365-2567.2001.01323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daley CL, Hahn JA, Moss AR, Hopewell PC, Schecter GF. Incidence of tuberculosis in injection drug users in San Francisco: impact of anergy. Am J Respir Crit Care Med. 1998;157:19–22. doi: 10.1164/ajrccm.157.1.9701111. [DOI] [PubMed] [Google Scholar]