Abstract
This paper provides an extensive overview of published studies on the development and applications of three-dimensional bone tissue engineering (TE) scaffolds with potential capability for the controlled delivery of therapeutic drugs. Typical drugs considered include gentamicin and other antibiotics generally used to combat osteomyelitis, as well as anti-inflammatory drugs and bisphosphonates, but delivery of growth factors is not covered in this review. In each case reviewed, special attention has been given to the technology used for controlling the release of the loaded drugs. The possibility of designing multifunctional three-dimensional bone TE scaffolds for the emerging field of bone TE therapeutics is discussed. A detailed summary of drugs included in three-dimensional scaffolds and the several approaches developed to combine bioceramics with various polymeric biomaterials in composites for drug-delivery systems is included. The main results presented in the literature are discussed and the remaining challenges in the field are summarized with suggestions for future research directions.
Keywords: bone tissue engineering, drug release, drug-delivery system, scaffolds, biodegradable materials, bioactive glass
1. Introduction
Even though osseous tissue has the unique internal repair capacity to heal and remodel without scarring, there are several conditions, both congenital and acquired, where bone replacement is needed (Buckwalter et al. 1996a,b). Furthermore, the clinical need to effectively treat bone defects is expected to increase as population ageing continues to grow. Autologous grafting is the therapeutically obvious gold standard in reconstructive surgery owing to high immunocompatibility (Reichert et al. 2009). However, this concept is bound by several constraints (e.g. requirement of secondary surgery, limited amount of tissue that can be harvested, increased risk of infection or recurrent pain) (Younger & Chapman 1989; Arosarena 2004). Allograft or xenograft graftings are optional treatments, but each process has its own disadvantages including the possibility of graft rejection by the immune system, risk of infections and transmission of donor pathogens (Kumar 2006). To overcome these problems, tissue engineering approaches are emerging as convenient alternatives to promote the regenerative ability of the host body (Goessler et al. 2007; Lee & Shin 2007; Bran et al. 2008; Kanczler & Oreffo 2008). One of the most important stages of bone tissue engineering (TE) is the design and processing of a porous, biodegradable three-dimensional structure called a ‘scaffold’, exhibiting high porosity, high pore interconnectivity and uniform pore distribution (Hutmacher 2000). These scaffolds should provide structural support for cells and the new tissue being formed, acting as a temporary extracellular matrix inducing the natural processes of tissue regeneration and development (Lee & Shin 2007). Thus, the improvement of the biological activity and performance of bone-substitute materials and scaffolds is one of the main concerns in bone regeneration (Le Bolay et al. 2009).
Further, bone tissue requires the action of growth factors that provide signals at local injury sites allowing progenitors and inflammatory cells to migrate and trigger the healing process (Furth et al. 2007; Lee & Shin 2007). Several attempts have been made to include growth factors and morphogens within bioactive scaffolds to stimulate cellular adhesion, proliferation and differentiation, in order to promote bone regeneration (Habraken et al. 2007; Lee & Shin 2007). In addition, enhancing further the functionality of these already complex matrices by loading drugs into them to treat bone disorders or to act on the surrounding tissues with an adequate therapeutic concentration level and for a desired time frame is recognized as being highly beneficial, hence the increasing interest in incorporating a drug-delivery function in TE applications (Gomes & Reis 2004; Duarte et al. 2009a). The increasing volume of work dealing with this approach is leading to the establishment of an emerging field which has been termed TE therapeutics (Baroli 2009).
Three-dimensional bioactive bone scaffolds can be fabricated from bioceramics, biodegradable polymers and their composites (Rezwan et al. 2006). Bioactive ceramic scaffolds alone used in bone TE can serve as a delivery vehicle of drugs but the drug release patterns are difficult to control (Habraken et al. 2007). On the other hand, biodegradable polymeric materials such as poly(lactic-co-glycolic acid) (PLGA) (Garvin & Feschuk 2005) and poly(propylene glycol-fumerate)/methylmethacrylate (Gerhart et al. 1993) can be used to control the local delivery of drugs; however, they show impaired osteoconduction and sometimes they provoke an adverse tissue response owing to inflammation as a consequence of acidic degradation (Böstman & Pihlajamäki 2000). Thus, the smart combination of bioceramics (including calcium phosphates (CaP), hydroxyapatite (HA) and silicate bioactive glasses (BGs)) and biodegradable polymers can not only improve the degradability of the inorganic material and alter its mechanical/physical properties (Rezwan et al. 2006), but also drug-release profiles can be controlled to a greater extent than on pure ceramics (Habraken et al. 2007). There is a wide range of different polymers that can be selected for this application, which show different degradation rates and mechanisms (Nair & Laurencin 2007), and a wide range of bioceramic/biopolymer composite scaffolds is available for bone TE (Rezwan et al. 2006; Guarino et al. 2007; Yunos et al. 2008).
There are different possible routes of administration for most drugs, which can be broadly divided into two categories: local and systemic (Somayaji et al. 1998). Drugs delivered systemically are absorbed into the blood stream and distributed throughout the body via the circulatory system, which can result in systemic toxicity with associated renal and liver complications, poor penetration into the targeted tissue and the need for hospitalized monitoring (Price et al. 1996; Somayaji et al. 1998; Ruszczak & Friess 2003). When a drug is delivered locally, the side effects and risk of overdose of systemic administration can be limited and there is a higher concentration of medication effectively reaching the targeted site (Somayaji et al. 1998). Moreover, controlled release systems might be helpful to improve drug bioavailability because they could be designed to deliver a drug molecule at a specific rate and for a specific period of time at the desired location (Roman & Madruga 1989). The rate and manner in which a particular drug is released are determined by the matrix into which the drug is loaded, the type of drug and its clearance rate (Wu & Grainger 2006; Zilberman & Elsner 2008; Baroli 2009). Different strategies followed to deliver specific growth factors (e.g. cytokines and hormones); morphogens and proteins using TE scaffolds to stimulate cellular adhesion, proliferation and differentiation, thus promoting bone regeneration, have been proposed and these have been reviewed in the literature (Saltzman & Olbricht 2002; Chung & Park 2007; Habraken et al. 2007; Lee & Shin 2007; Baroli 2009). However, there has been no previous review, to the authors' knowledge, focusing exclusively on TE approaches to deliver therapeutic (synthetic) drugs. The review, thus, focuses specifically on the rationale to load different therapeutic drugs relevant for treating pathologies associated with the bone repair process in three-dimensional scaffolds used in bone TE. Specific attention has been given to the most recent developments related to controlling the release rate of the relevant therapeutic drugs, which is the marker that defines the degree of success of a given strategy. Approaches followed to deliver growth factors and morphogens will not be covered in this review. Moreover, the review focuses on TE therapeutic strategies based only on solid, three-dimensional scaffolds, e.g. injectable systems are not included. The paper is organized in the following manner: §2 briefly discusses the key biomaterials used for bone tissue scaffolds, presenting their relative advantages and disadvantages. This section also covers general approaches used for the appropriate formulation of scaffolds with a focus on processing methods of relevance to scaffolds as drug-delivery carriers. Sections 3 and 4 present the specific strategies adopted to develop TE scaffolds with drug-delivery capability for treating relevant pathologies associated with bone, focusing on antimicrobial agents (§3) and on other relevant used drugs such as anti-inflammatory and antiresorptive pharmacotherapeutics (§4). Subsequently, §5 focuses on the key variables needed to be taken into account to investigate in vitro drug release from particular scaffolds used. Finally, remaining challenges in the field are summarized in §6, where directions for future research efforts are also highlighted.
2. Bioactive bone scaffolds as therapeutic drug carriers: the added value
2.1. Scaffold materials
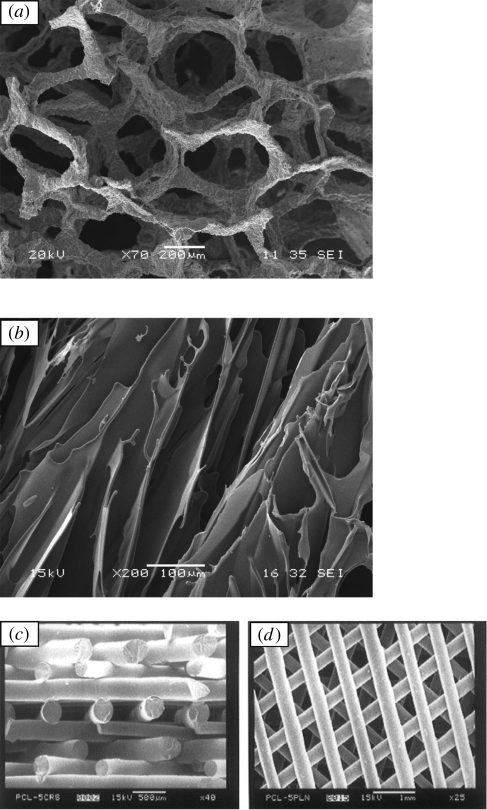
An optimal scaffold for bone TE should be osteoconductive and angiogenic and it should serve as a three-dimensional template to provide structural support to the newly formed bone through an interpenetrating network of pores (at least 100 µm wide) to allow cell migration, tissue in-growth and vascularization (Freyman et al. 2001; Hutmacher 2001; Guarino et al. 2007; Moroni et al. 2008). Analysis of the literature demonstrates that a wide range of three-dimensional bioactive scaffolds has been developed which can be potentially used as delivery systems for therapeutic drugs relevant for bone repair processes. Basically, three major scaffold materials are used: ceramics, polymers and composites and their main characteristics are described briefly below. Comprehensive descriptions of scaffold fabrication techniques are available in the specialized literature (Hutmacher 2000; Guarino et al. 2007; Chen et al. 2008; Moroni et al. 2008). Table 1 (Chen et al. 2008) summarizes the advantages and disadvantages of the scaffold materials used for bone TE, while the techniques already employed to fabricate bone scaffolds with potential therapeutic drug-release capabilities are listed in table 2. Figure 1 presents images showing the pore structure of selected ceramic and polymer scaffolds fabricated using different techniques, which are finding successful applications in bone TE developments (Hutmacher 2000; Hollister 2005; Chen, Q. Z. et al. 2006). Some of these techniques have already been considered for fabricating scaffolds with therapeutic drug-delivery function (summarized in table 2 and discussed in detail in sections below). These scaffolds can be developed with the aim of providing not only the physico-chemical environment and the structural integrity required for bone regeneration (which is the main scaffold function) but also with the added value of acting as a local regulator to control the dose and kinetics of therapeutic drug release without the need to use an additional drug carrier (Berger et al. 1997). Several comprehensive reports covering the state-of-the-art techniques developed to fabricate bone scaffolds are available and the field continues to expand (Hollister 2005; Guarino et al. 2007; Hutmacher et al. 2007; Lickorish et al. 2007; Moroni et al. 2008). However, while several of the proposed methods have been conveniently adapted for incorporating growth factors (e.g. Baroli 2009), only a limited number of techniques have been specifically used for fabricating scaffolds with therapeutic drug-delivery capability (table 2). In the following sections we summarize the progress in the field of bone TE scaffold development with potential application as therapeutic drug-delivery vehicles. The sections are organized by group of scaffold materials, e.g. inorganic, polymeric and composite scaffolds, with the discussion of processing techniques being limited to those methods already applied for therapeutic drug loading.
Table 1.
Advantages and disadvantages of scaffold materials used in three-dimensional bone TE: ceramics, polymers and composites (adapted from Chen et al. 2008). ++, excellent; +, good; HA, hydroxyapatite; β-TCP, β-tricalcium phosphate.
| material | advantages | disadvantages |
|---|---|---|
| inorganic | ||
| calcium phosphates (HA, β-TCP) |
|
|
| bioactive glasses and glass ceramics (bioglass, phosphate glasses) |
|
|
| organic | ||
| bulk biodegradable polymers (poly(lactic acid), poly(glycolic acid), poly(lactic-co-glycolic acid), poly(propylene fumarate)) |
|
|
| surface bioerodible polymers (poly(ortho esters), poly(anhydrides), poly(phosphazene)) |
|
|
| composites | ||
|
|
|
Table 2.
Techniques developed to elaborate porous three-dimensional scaffolds for bone TE already applied to fabricate scaffolds with therapeutic drug-delivery capability.
| technique | details of the process | references |
|---|---|---|
| melt moulding |
|
Di Nunzio & Verné (2005) |
| solvent-casting |
|
Thomson et al. (1998) |
| freeze-drying |
|
Whang et al. (1999); Cabañas et al. (2009) |
| liquid/liquid thermally induced separation technique |
|
Zhang & Zhang (2002) |
| foaming |
|
Mooney et al. (1996); Harris et al. (1998) |
| template technique |
|
Vitale-Brovarone et al. (2007); Chen, Q. Z. et al. (2006) |
| sol–gel |
|
Domingues et al. (2004) |
| powder compaction |
|
Kimakhe et al. (1999); Vallet-Regí et al. (2001); Castro et al. (2005); Miyai et al. (2008) |
Figure 1.
Scanning electron microscopy images showing typical pore structures of scaffolds successfully developed for bone TE: (a) bioactive glass–ceramic scaffolds fabricated by the foam replica method (Chen, Q. Z. et al. 2006); (b) PDLLA foam fabricated by thermally induced phase separation (Maquet & Jerome 1997); (c) polymer scaffold architectures developed by FDM (Hutmacher 2000). (Part (c) reproduced from Hutmacher (2000) with permission of Elsevier.)
2.2. Inorganic scaffolds
2.2.1. Characteristics of bioactive ceramics and glasses
Synthetic bioactive ceramics such as some forms of CaP (HA, α-tricalcium phosphate (TCP)) and combinations of them have been widely studied as scaffold materials owing to their structural and chemical similarity with the inorganic component of bone (Yuan et al. 1999; Hutmacher 2000; Dong et al. 2001; Zhang et al. 2001; Ramay & Zhang 2003; Karageorgiou & Kaplan 2006). However, some of these ceramics degrade very slowly, with crystalline HA being a very stable CaP in the biological environment (table 1) (Marcacci et al. 2007; Chen et al. 2008). More recently, bioactive silicate glasses and glass–ceramics have been investigated in their application as scaffolds (Livingston et al. 2002; Di Nunzio et al. 2004; Vitale-Brovarone et al. 2004; Chen, Q. Z. et al. 2006; Fu et al. 2007; Vitale-Brovarone et al. 2008). This is a group of inorganic materials with several unique properties favourable for bone TE. Bioactive glass is a class A bioactive material exhibiting the capability to bond to both bone and soft tissue and to stimulate bone growth (Hench & Polak 2002). In vitro, BGs are able to form a surface layer of microcrystalline HA when in contact with simulated body fluid (SBF) (Kim et al. 1995; Hench 1998; Fujibayashi et al. 2003). More recently, it has been discovered that ionic dissolution products released from silica-based BGs upregulate seven families of genes found in osteoblasts (Xynos et al. 2001). Dissolution products of BGs have also been shown to promote angiogenesis (Day et al. 2004). Additionally, research efforts on mesoporous inorganic materials have led to the development of ceramic matrices with both drug delivery and bone tissue regeneration capabilities (Vallet-Regí et al. 2006), which has promoted these biomaterials to novel uses in the biomedical field (Izquierdo-Barba et al. 2008).
2.2.2. Processing techniques
Numerous techniques have been developed to fabricate porous ceramic and glass–ceramic scaffolds, including starch consolidation, incorporation of volatile organic particles, sol–gel, foaming, solid-free form fabrication methods and replication of polymeric sponges (figure 1a) (Livingston et al. 2002; Vitale-Brovarone et al. 2004, 2006, 2007; Chen, Q. Z. et al. 2006; Ahmad et al. 2008; Yun et al. 2008; Yunos et al. 2008). In order to maximize the mechanical strength of the scaffold, and depending on the fabrication technique used, different parameters such as solid loading of the ceramic slurry, type and amount of additives (binders, dispersants, etc.) should be optimized. However, the condition for preserving the activity of a drug is that the process of drug incorporation should not degrade it. Traditionally, fabrication of bioceramic scaffolds involves thermal treatment at high temperature (>800°C), hence this process cannot be used if a drug will be incorporated because most of it would be degraded (Vallet-Regí et al. 2008). Therefore, different methods have been proposed to tackle this problem. One of the simplest strategies to load drugs within ceramic scaffolds is conventional impregnation, which consists of immersing the scaffold in a drug containing phosphate-buffered saline (PBS) (Habraken et al. 2007). In this simple case, however, the drug release rate usually cannot be adequately controlled (Habraken et al. 2007). Sol–gel technology allows the introduction of drugs into glass-like materials at room temperature where pore formation is due to byproducts (water and alcohols) of the sol–gel synthesis (Nicoll et al. 1997; Santos et al. 1999). These byproducts are volatile and evaporate during the ageing and drying of the sol–gel materials, thus leaving pores (Nicoll et al. 1997; Santos et al. 1999). However, materials with optimal scaffold structure (exhibiting high porosity and large interconnected macropores) are in general unable to hold a large reservoir of drugs and they are not capable of retaining the drug at the site for a prolonged period of time (Malafaya et al. 2002; Ziegler et al. 2002). Further, solvents used in sol–gel methods have to be removed with the disadvantage of adding a drying step to the process. This drying step usually includes heating, which, even if at moderate temperatures, can degrade thermolabile substances. One of the first successful attempts to solve this difficulty was the use of uniaxial and isostatic pressure at room temperature to obtain gentamicin-loaded bioceramics (Vallet-Regí et al. 2001).
2.3. Polymer scaffolds
2.3.1. Characteristics of biodegradable polymers
An alternative approach to obtain multifunctional three-dimensional bioactive bone scaffolds includes their fabrication from biodegradable polymers (Maquet & Jerome 1997; Hutmacher 2000; Hollister 2005; Rezwan et al. 2006; Guarino et al. 2007). In this case, it is considered that biodegradable polymers can control the release of drugs since polymer degradation properties can be tailored for each specific application (Hutmacher 2000). Thus, a range of processes including cell growth, tissue regeneration and host response can be influenced by choosing suitable biopolymers (Lu et al. 1999; Hutmacher 2000). It is known that cells usually ‘prefer’ hydrophilic surfaces; however, hydrophobic materials have typically longer residence times in vivo (Streubel et al. 2000; Malafaya et al. 2002), meaning that the selection of the optimal biodegradable polymer for the drug-delivery function in TE scaffolds should be aimed at balancing both aspects. Synthetic biodegradable polymers that have been reported for various drug-eluting devices include PLGA copolymers (Garvin et al. 1994; Nie et al. 1995; Overbeck et al. 1995; Ambrose et al. 2003), polycaprolactone (Hendricks et al. 2001; Rutledge et al. 2003), polyanhydrides (Jacob et al. 1991; Nelson et al. 1997; Kanellakopoulou & Giamarellos-Bourboulis 2000; Li, L. C. et al. 2002; Li, W. 2002), polyhydroxybutyrate-co-hydroxyvalerate (PHBV) (Yagmurlu et al. 1999; Rossi et al. 2004) and other polyhydroxyalkanoates (Turesin et al. 2001). Natural polymers including proteins such as collagen (Lee et al. 2002; Park et al. 2004; Sripriya et al. 2004; Prabu et al. 2006; Shanmugasundaram et al. 2006) and polysaccharides such as alginate, hyaluronic acid and chitosan (Muzzarelli et al. 1990; Chung et al. 1994; Mi et al. 2002; Aoyagi et al. 2007; Rossi et al. 2007) are also attractive, since they exhibit superior biocompatibility and can facilitate cell growth. Many of these polymers are inexpensive and readily available. Moreover, ‘surface-eroding polymers’, which have the feature of being biodegraded only at their surfaces, are also candidates for this application. Poly(anhydrides), poly(ortho-esthers) and polyphosphazene show this property (‘surface eroding’) as opposed to ‘bulk degradation’ (Rezwan et al. 2006). Surface-eroding polymers offer advantages over bulk degradation polymers when used as multifunctional scaffold materials: (i) they are capable of releasing entrapped drugs by zero-order kinetics (when drug release can be limited to regions undergoing degradation) and (ii) the release rate is proportional to the surface area of the matrix (Heller 1985; Rezwan et al. 2006).
2.3.2. Processing techniques
Porous polymer scaffolds can be produced through thermally induced phase-separation (TIPS) (figure 1b), evaporation, freeze-drying, solid-free form fabrication (e.g. fused deposition modelling (FDM), three-dimensional printing, selective laser sintering) (figure 1c), solvent-casting and foam-coating among others techniques (Maquet & Jerome 1997; Thomson et al. 1998; Whang et al. 1999; Zhang & Zhang 2002; Boccaccini & Maquet 2003; Guan & Davies 2004; Cabañas et al. 2009). While a range of these methods have already been considered to incorporate therapeutic drugs, other suitable scaffold manufacturing methods, e.g. solid-free form fabrication methods (Hutmacher 2000; Hollister 2005) (figure 1c), have not yet been investigated with this purpose, thus remaining an area for future research.
Recently, impregnation of three-dimensional porous scaffolds using carbon dioxide (scCO2) has been used to develop alternative clean processes for the preparation of drug-loaded polymeric matrices when the drug is soluble in scCO2 and the polymer chosen can be swollen by the supercritical fluid (Mooney et al. 1996; Harris et al. 1998; Kikic & Sist 2000; Duarte et al. 2007; Heyde et al. 2007). Impregnation using supercritical fluid technologies has the advantage of using a supercritical fluid with high diffusivity in the polymer chosen in addition to its high solubility and plasticizing capability (Berens et al. 1992; Kazarian 2000; Duarte et al. 2007). Electrospinning is another technique with a potential to develop bioactive three-dimensional bone scaffolds with drug-release capabilities (Li, W. et al. 2002; Matthews et al. 2002; Ma et al. 2005; Gupta et al. 2007). However, this technique seems to have its limitations because some organic solvents used to prepare the polymer solutions may degrade the drugs (Han & Gouma 2006; Gupta et al. 2007; Ahmad et al. 2008; Baroli 2009). Finally, solid-free form fabrication technologies such as three-dimensional printing and FDM appear promising for further developments in this field, as mentioned above (Hollister 2005; Hutmacher & Cool 2007; Hutmacher et al. 2007; Pang et al. 2007; Wang et al. 2007).
2.4. Composite scaffolds
2.4.1. Characteristics of biodegradable and bioactive composites
In this approach, usually the matrix is prepared by using biodegradable polymers and inclusions in the form or particles of fibres of BG, CaP or HA are added to improve the mechanical strength and bioactivity (Roether et al. 2002; Rezwan et al. 2006; Guarino et al. 2007; Vallet-Regí et al. 2008). Polymers combined with ceramic particles, such as HA, can also be applied as coating on porous bioceramic scaffolds, fabricated using one of the techniques mentioned in §2.2.2, in order to tailor the controlled release of a drug (Kim et al. 2004a,b; Yunos et al. 2008). Composite scaffolds represent a convenient alternative as they combine the advantages of both biodegradable polymers and bioactive ceramics for bone engineering scaffolds. This combination leads to composites with improved mechanical properties because of the inherent higher stiffness and strength of the inorganic material. In addition, bioactive inorganic particles such as HA, bioglass or tricalcium phosphate induce the effective interaction of the scaffold with the surrounding bone tissue by forming a tenacious bond via the growth of a carbonate HA layer, as mentioned above (Rezwan et al. 2006). Moreover, addition of inorganic materials to bioresorbable polymers can change the polymer degradation behaviour by buffering the pH of the nearby solution, thus preventing the autocatalytic effect of the acidic end groups resulting from hydrolysis of polymer chains, e.g. in polylactic acid. It is well known that incorporation of bioactive inorganic phases in biodegradable polymers can enhance water ingress owing to the internal interfaces formed between the polymer and the more hydrophilic bioactive inclusions, hence enabling control of the degradation kinetics of scaffolds (Boccaccini & Maquet 2003).
2.4.2. Processing techniques
As mentioned above, one of the simplest strategies is the application of biodegradable polymer coatings loaded with the relevant drugs onto the three-dimensional structure of bioceramic scaffolds (Kim et al. 2004b). Other techniques reported are: solvent-casting, TIPS, evaporation, freeze-drying and foam-coating (table 2—Thomson et al. 1998; Whang et al. 1999; Zhang & Zhang 2002; Boccaccini & Maquet 2003; Cabañas et al. 2009). An interesting approach to drug delivery in TE is to combine drug-loaded microspheres within the scaffold macroporous structure or even to use the loaded microspheres to construct an open-pore three-dimensional scaffold structure (Malafaya et al. 2002; Vallet-Regí et al. 2008; Zhu & Kaskel 2009). The approach of combining microspheres and a matrix (scaffold) could be very useful in cases where the scaffold material is not suitable for the regulated release of specific drugs, such as low-molecular weight or water-soluble drugs (Malafaya et al. 2002). When microspheres are added to an inorganic scaffold, they can interact with the surface of the scaffold by electrostatic charges depending on the nature of the biodegradable polymer and the bioceramic used (Xu & Czernuszka 2008). Moreover, by using microspheres, drug-release profiles can be altered and tuned depending on the polymer selected, as mentioned above.
A summary of experimental research carried out on the development of three-dimensional scaffolds for bone TE with controlled-release capability is presented in table 3 including both in vitro and in vivo studies. From this table it appears that porous matrices based mainly on well-characterized biocompatible polymeric scaffolds or, in some cases, composites comprising polymeric matrices and added inorganic particles represent the best systems to date to incorporate therapeutic drug delivery in bone TE approaches. A schematic diagram summarizing the different strategies proposed so far is shown in figure 2. Although several novel techniques have been developed to introduce therapeutic drugs within scaffolds, in most cases the strategy followed has been the direct incorporation of the drug into the scaffold by immersion of the scaffold in a drug-containing buffer aqueous solution. Nevertheless, thermo-labile drugs can also be loaded within three-dimensional scaffolds in a one-step process using room temperature compaction of the powder mix, this being a solvent-free process, which avoids the use of toxic solvents (Kimakhe et al. 1999; Vallet-Regí et al. 2001; Castro et al. 2005). Further details of relevant studies are discussed in the following sections, highlighting the strengths and weaknesses of the different approaches.
Table 3.
Selected experimental trials carried out for three-dimensional bone scaffolds, both in vivo and in vitro, with a combination of controlled drug release. β-TCP, β-tricalcium phosphate; CP, calcium phosphate invert glasses; GS, gentamicin; HA, hydroxyapatite; HMS, mesoporous silica; PLA, poly(l-lactic acid); PLGA, poly(lactide-co-glycolide); PCL, poly-ε-caprolactone; PBS, phosphate-buffered saline; CPA, calcium phosphate-deficient apatite.
| drug | composition | end-product shape | methodology | experimental trial | references |
|---|---|---|---|---|---|
| gentamicin | β-TCP/CP/chitosan | porous matrix | thermally induced phase-separation technique + immersion in drug-containing PBS solution | in vitro | Zhang & Zhang (2002) |
| gentamicin | bioactive glass | bioactive glass pieces | uniaxial and isostatic compression at room temperature | in vivo | Vallet-Regí et al. (2001) |
| gentamicin | bioactive glass | mesoporous bioactive glass/bioactive glass | polyurethane sponge technique + immersion in drug-containing PBS solution | in vitro | Zhu & Kaskel (2009) |
| gentamicin | HMS–HA/PLGA microspheres | porous matrix | tensiactive template to obtain HMS–HA + double-emulsion evaporation technique to obtain GS-loaded PLGA microspheres + sinterization at 70°C | in vitro | Shi et al. (2009) |
| tetracycline | bioactive glass/β-cyclodextrin | bioactive glass pieces | sol–gel | in vivo | Domingues et al. (2004) |
| vancomycin | β-TCP/agarose | porous matrix | freeze-drying and heat desiccation at 37°C | in vitro | Cabañas et al. (2009) |
| polymyxin B | calcium phosphate | ceramic pieces | compaction | in vitro | Kimakhe et al. (1999) |
| gatifloxacin | β-TCP/PCL | porous matrix | compaction + sintering + immersion in drug-loaded PCL slurry | in vitro and in vivo | Miyai et al. (2008) |
| ciproflozacin | HA/β-TCP/PLA | porous matrix | compression | in vitro/in vivo | Castro et al. (2005) |
| silver | bioactive glass | bioctive glass pieces | melting and sintering at high temperature + introduction of drug by ion-exchange process | in vitro | Di Nunzio & Verné (2005) |
| dexamethasone | starch/PLA | porous matrix | supercritical phase-inversion technique | in vitro | Duarte et al. (2009a) |
| dexamethasone | chitosan | porous matrix | freeze-drying + drug impregnation by supercritical fluid technology | in vitro | Duarte et al. (2009b) |
| ibuprofen | bioactive glass/MCM-41 | porous matrix | MCM-41 spheres + melting and sintering at high temperature to obtain bioactive glass scaffold + drug impregnation | in vitro | Mortera et al. (2007) |
| alendronate | silica (SBA-15) | mesoporous silica matrix | triblock copolymers technique + functionalization + immersion in drug-containing buffer aqueous solution | in vitro | Nieto et al. (2008) |
| zoledronate | CPA | pellets | suspension of CDA in drug-containing water solution | in vitro | Fauchex et al. (2009) |
Figure 2.
Schematic representation of the most common strategies to deliver drugs from three-dimensional scaffolds in bone TE. Drugs may be adsorbed onto the pore surface of the scaffolds in either their unprotected (a) or their protected (microsphere/matrix) (b) forms. Alternatively, drugs may be entrapped in the scaffold structure in either their unprotected (c) or their protected (microsphere/matrix) (d) forms.
3. Antimicrobial agents
3.1. Controlled release of antibiotics
Infection is defined as a homeostatic imbalance between host tissue and the presence of micro-organisms at a concentration that exceeds 105 organisms per gram of tissue or the presence of beta-haemolytic streptococci (Sussman & Bates-Jensen 2001; Zilberman & Elsner 2008). The principal aim of treating wound infections is to reduce the bacterial load in the wound to a level at which wound-healing processes can take place. If the drug is released too quickly, the entire drug amount could be released before the infection is stopped. On the other hand, if the release of the drug is delayed, infection may set in further, thus making it difficult to manage the healing of the wound (Gold & Moellering 1996; Gransden 1997; Zilberman & Elsner 2008). Bacterial infection remains a major problem affecting the service life of medical implants and scaffolds (Segreti 2000; Virk & Osmon 2001). Generally, sources for contamination include the air of the operating room and resident bacteria on the patient's skin and bacteria already present in the body (An & Friedman 1996; Zilberman & Elsner 2008). In addition to human pain and suffering, direct medical costs associated with such infections are often extremely high (Hetrick & Schoenfisch 2006). Similar complexities encountered with conventional orthopaedic implants can be expected in TE approaches based on implantation of engineered biomaterial scaffolds. Device-associated infections are the result of bacterial adhesion and subsequent bio-film formation at the implantation site. Inhibiting bacterial adhesion is often regarded as the most critical step in preventing infection. Upon implantation, there is a competition between the integration of the material into the surrounding tissue and adhesion of bacteria to the implant surface. Therefore, implantation will be successful only if tissue integration occurs prior to considerable bacterial adhesion, thus preventing colonization of the implant (Gristina 1987). Furthermore, certain bacterial species are able to attach to the implant surfaces and form a protective bio-film layer, which is extremely resistant to both the immune system and antibiotics. These bio-films are considered the primary cause of implant-associated infection (Hetrick & Schoenfisch 2006). Local antibiotic release profiles should exhibit a high initial release rate in order to respond to the elevated risk of infection from bacteria introduced during the initial shock, followed by a sustained release at an effective level for inhibiting the occurrence of latent infection (Zilberman & Elsner 2008). In the case of orthopaedic-related devices, including TE scaffolds, it is important to combat bacteria possibly introduced during implantation and also those introduced systemically afterwards. Therefore, sustained drug release is necessary (Zilberman & Elsner 2008).
The most common antibiotic carrier to treat osteomyelitis after debridement surgery to remove necrotic bone tissue is poly(methylmethacrylate) (PMMA) beads (Vallo et al. 2004; Habraken et al. 2007, 2008; Shi et al. 2009). However, PMMA is not a resorbable polymer and must be removed in a second surgical procedure (Vallet-Regí et al. 2007; Shi et al. 2009). In this context, it should be pointed out that most previous work on loading biomaterials with antibiotics for orthopaedic applications has been carried out on bone-filler materials and bone cements (Takechi et al. 1998; Armstrong et al. 2002; Diez-Peña et al. 2002; Gbureck et al. 2002; Joseph et al. 2003; Hanssen 2004; Joosten et al. 2004; Webb et al. 2005; Schnieders et al. 2006; Krasko et al. 2007; Zilberman & Elsner 2008). In one of the first attempts to develop multifunctional re-absorbable implants, Queiroz et al. (2001) prepared sodium ampicillin which was adsorbed onto HA and glass-reinforced HA composites as a potential pharmaceutical formulation for periodontitis. Ampicillin-loaded methylpyrrolidinone chitosan microparticles have been described for a similar purpose (Giunchedi et al. 1998). Moreover, calcium phosphate cement (CPC)–chitosan composites have been applied for drug release of the cephalosporin antibiotic flomoxef, which is active against methicillin-resistant Streptococcus aureus. For example, Takechi et al. (2002) added flomoxef sodium to the liquid phase of tetracalcium phosphate (TTCP) cement/chitosan composites and measured the drug release from preset discs for 3 days. Results showed a release pattern that was characterized by an initial burst, followed by a more sustained release. The total per cent of drug released in 24 h was 24–35% and it was found that the addition of chitosan in different amounts did not influence the total drug release after 72 h. Moreover, the release from these chitosan-enriched cements did not differ significantly from that of normal TTCP cement, though the maximum amount of chitosan used was 1.0 per cent w/v. Analysis of the literature reveals that new multifunctional composite materials, produced by different techniques, are being developed for specific application in bone TE and are detailed below, with a focus on specific antibiotics used in three-dimensional TE scaffolds.
3.2. Gentamicin
Gentamicin is a commonly employed antibiotic in trauma, widely used for the treatment of osteomyelitis because of its broad-spectrum characteristics (Li & Hu 2001). Most of the major bacteria causing chronic osteomyelitis are sensitive to gentamicin. This drug has been loaded in several scaffolds in order to evaluate their ability as controlled-release carriers (Li & Hu 2001). Zhang & Zhang (2002) prepared macroporous chitosan scaffolds reinforced by CaP particles such as β-TCP and CaP invert glasses using a thermally induced phase-separation technique. These porous composite materials were loaded with gentamicin sulphate (GS) by immersing them in drug-containing PBS solutions. In vitro tests showed that, in comparison with GS-loaded pure chitosan scaffolds, the initial high burst release of GS was decreased through incorporating CaP crystals and glass particles into the scaffolds, and a sustained release for more than three weeks was achieved (Zhang & Zhang 2002). The highest sustained release was observed from the particle-containing composite, which was suggested to occur owing to a higher extent of chitosan cross-linking. Scanning electron microscopy micrographs showed no apparent morphological differences for osteoblastic cells grown on the pure chitosan scaffolds and those grown on composite scaffolds. The cells attached and migrated on these scaffolds, suggesting a good cellular compatibility (Zhang & Zhang 2002; Habraken et al. 2007). Vallet-Regí and co-workers used uniaxial and isostatic pressure at room temperature to obtain GS-loaded bioceramic tablets (Vallet-Regí et al. 2001). These tablets were tested in vivo in New Zealand rabbit femurs for one, four, eight and 12 weeks to study their biological response. The bone response to the implant was of perfect osseo-integration. The local GS levels detected in bone tissue were above the minimal inhibitory concentration (MIC) and they were effective because they were toxic for the majority of the resident micro-organisms. In addition, there was progressive decrease of GS levels in bone tissue with time, but the levels were always above the MIC until the end of the assay. In related studies, Zhu & Kaskel (2009) compared in vitro the local drug-release behaviour of two bioactive silicate glass scaffolds: three-dimensional mesoporous (SiO2–CaO–P2O5) (MBG) and three-dimensional BG scaffolds. Both scaffolds were prepared by using the polyurethane sponge technique, which involves the burning-out of a sacrificial sponge to create the pore structure (figure 1a) (Chen, Q. Z. et al. 2006); but in case of MBG scaffold a surfactant was added. Afterwards, the scaffolds were immersed in a gentamicin solution for loading the drug. The results indicated that the mesoporous structure played an important role in the drug-loading capability and its release rate. The drug-uptake capacity of the MBG scaffold was over twofold higher than that of the BG scaffold. During the whole release period in SBF, gentamicin was released from the MBG scaffold at a much lower release rate than from the BG scaffold. A novel design of a TE scaffold with controlled drug-delivery capability has been developed by Shi et al. (2009). The scaffold is based on mesoporous silica–HA (HMS–HA) composite particles used as fillers in PLGA microspheres. HMS–HA particles were produced using dodecylamine as a template and GS-loaded PLGA microspheres were prepared using a double emulsion solvent evaporation technique (water/oil/water). PLGA/HMS–HA–GS composite microspheres were prepared using a single emulsion solvent evaporation method. Afterwards, PLGA or PLGA/HMS–HA–GS microsphere sintered scaffolds were fabricated by pouring PLGA or PLGA/HMS–HA–GS microspheres into cylindrical moulds, and subsequently sintering at 70°C for 2 h. The results showed that the presence of HA in PLGA/HMS–HA scaffolds could balance the decreased pH values caused by the acidic degradation product of PLGA. Moreover, HMS–HA improved the cytocompatibility and bioactivity of PLGA. It was also claimed that the compressive strength and elastic modulus of PLGA/HMS–HA scaffolds were higher than those of pure PLGA scaffolds, showing similar mechanical properties to human cancellous bone (Shi et al. 2009). In vitro drug-delivery testing in SBF of the PLGA/HMS–HA scaffolds showed that PLGA reduced the GS release from HMS–HA particles, and the release lasted for nearly one month (Shi et al. 2009).
3.3. Other antibiotics
A series of antibiotics has been further considered in combination with scaffolds for bone TE. Tetracycline was incorporated in a hybrid coating consisting of polycaprolactone (PCL) and HA powder, which was applied on the surface of a HA porous bone scaffold through dip-coating and solvent-casting method (Kim et al. 2004a). In vitro drug-delivery testing in PBS revealed that the release amount was controlled via the coating cycle and initial drug loading and a sustained release preceded by a burst release during the first 2 h was achieved (Kim et al. 2004a). Domingues et al. (2004) introduced tetracycline and an inclusion complex formed by tetracycline and β-cyclodextrin, at 1:1 molar ratio tetracyline/β-cyclodextrin, into a BG prepared by the sol–gel technique. The inclusion complex tetracycline/β-cyclodextrin was prepared by freeze-drying and either tetracycline hydrochloride or tetracycline in β-cyclodextrin inclusion compound was loaded into sol–gel solutions to prepare drug-loaded silicate BGs. An initial burst of 12 per cent was observed in vitro (in SBF), followed by a sustained release over 80 days achieving a total release of 22–25%. The in vivo test was carried out with three groups of female mice treated with BG without drugs, or associated with tetracycline (BT), or with tetracycline/β-cyclodextrin (BTC) by subcutaneous implantation. A considerable bacteriostatic activity was found with BT and BTC-loaded glasses, when compared with plain glass. The presence of cyclodextrin was important to slow down the release of tetracycline for a long period of time and it was verified that the presence of tetracycline or its inclusion complex, tetracycline/BTC, did not affect the bioactivity of the glass. Recently, Cabañas et al. (2009) loaded vancomycin (VAN), a drug susceptible to heat degrading, into β-TCP/agarose scaffolds by two different methods: freeze-drying and desiccation at 37°C. Poly(ethylene glycol) (PEG) was included in the formulation to tailor the release of VAN (Cabañas et al. 2009). The freeze-dried samples had a higher porosity structure than samples dried at 37°C. The pieces obtained showed a microstructure similar to that of human cancellous bone. In addition, the different pore architectures and the formation of an agarose–PEG–VAN complex yielded different drug-release patterns (Cabañas et al. 2009). Polymyxin B (PMB) is a polypeptidic antibiotic that undergoes thermodamage above 60°C. With this limitation to be taken into account, Kimakhe et al. (1999) made one of the first attempts to obtain a multifunctional inorganic matrix for a bioactive drug-delivery system (DDS) in which the effect of a released therapeutic agent is favoured by the biocompatibility, osteoconductivity and bioresorption of the ceramic material. PMB was loaded on CaP powders using a dynamic compaction method at high velocities (25 and 50 m s−1) without external heating. The compaction procedure did not cause any loss in PMB integrity and biological activity. PMB release in vitro began after 2–3 days of incubation for blocks compacted at 25 m s−1 velocity and on day 5 for those compacted at 50 m s−1 (Kimakhe et al. 1999).
Some important results have shown the potential effectiveness of quinolones in local DDS designed to treat bone infection using carriers such as HA–anionic collagen composite (Martins & Goissis 2000), biodegradable polymers (Overbeck et al. 1995; Nicolau et al. 1998; Nie et al. 1998; Ramchandani & Robinson 1998; Kanellakopoulou & Giamarellos-Bourboulis 2000; Désévaux et al. 2002) and mixtures of calcium phosphates and biodegradable polymers (Castro et al. 2003). A composite of gatifloxacine (GFLX)-loaded poly-ε-caprolactone combined with β-TCP porous ceramic was obtained by compression moulding, followed by sintering (Miyai et al. 2008). This process had the advantage of being a solvent-free process. GFLX mostly retained its bactericidal property after processing. In vitro testing in Hanks' balanced solution showed that the composite of GFLX-loaded PCL/βTCP ceramic released GFLX for four weeks and had sustained bactericidal activity against S. milleri and Bacteroides fragilis for at least one week (Miyai et al. 2008). In vivo tests in rabbits with osteomyelitis lesions induced by S. milleri and B. fragilis in the rabbit mandible showed that the composite of GFLX-loaded PCL/βTCP was effective in controlling infection at the bone defect formed by debridement. Moreover, after four weeks, new bone formation was observed on the surface of the composite. Further, after 50 weeks, ingrowing bone tissue with vascular vessels was observed along the PCL and β-TCP interface. Additionally, GFLX concentrations in the serum and soft tissues were very low suggesting a low risk of systemic toxicity (Miyai et al. 2008). Ciprofloxacin (CFX) is the most widely used fluoroquinolone for bacterial bone infection with a low MIC (0.25–2 µg ml−1). CFX has been considered in combination with bioceramics and polymers. For example CFX was loaded into a composite comprising HA, TCP and poly(dl-lactide) (PLA) to treat complicated multi-organism bone infections that required high antibiotic concentrations (40%) in bone for long periods (Castro et al. 2005). The composites were prepared by mixing the ingredients and by further uniaxial compression using a hydraulic press. In vitro results showed that the release rate decreased proportionally to the PLA/CaP ratio, drug-loading and compaction pressure as a more tightly packed matrix was obtained. In vivo tests in rabbits showed that CFX concentrations along the bond remained higher than the MIC against the most common pathogens causing osteomyelitis. A good in vivo/in vitro correlation was found; however, the release of the last 20 per cent CFX remaining within the matrix was faster in vivo than in vitro, as the release was enhanced in vivo by neovascular vessels formed inside the matrix (Castro et al. 2005).
3.4. Inorganic ions
Silver, in its oxidized form (Ag+), is an antibacterial agent and it has been proposed as an additive in bone TE scaffolds (Di Nunzio et al. 2004). Although the antibacterial activity of silver ions has been demonstrated in several works (Matsuura et al. 1997; Gatter et al. 1998; Kim et al. 1998; Adams et al. 1999; Kawashita et al. 2000; Bellantone et al. 2002; Blaker et al. 2004; Di Nunzio et al. 2004; Verné et al. 2005), the silver ion antimicrobial mechanism is not fully understood. It is well known that silver ions can interact with bacterial cells in different ways: they can bind to microbial DNA preventing bacterial replication or to sulphydryl groups of bacterial enzymes, inhibiting cell respiration and binding transport of important substances across the cell membrane and within the cells (Chen, W. et al. 2006). These different ways of interaction are the origin of low bacterial resistance to silver (Chen, W. et al. 2006; Hetrick & Schoenfisch 2006). The bactericidal activity of Ag+ is effective against a broad range of bacteria potentially found at the sites of scaffolds or implants including S. aureus (Hetrick & Schoenfisch 2006).
The incorporation of silver into bone TE scaffolds is a novel approach. In one of the earliest studies, Ag+ ions were introduced into three-dimensional bioactive glass–ceramic scaffold surfaces through a patented ion-exchange process (Di Nunzio & Verné 2005). The control of Ag+ content on the surface, as well as its diffusion profile throughout the ion-exchanged layer, was achieved by a careful choice of the ion-exchange parameters (temperature, time and silver concentration in the molten bath). According to the authors of the original investigation (Di Nunzio & Verné 2005), this technique allows, by tuning the process parameters, a controlled silver ion incorporation into the superficial layers of the scaffold, maintaining the scaffold structure and its characteristics unchanged (Di Nunzio et al. 2004; Vitale-Brovarone et al. 2008). Moreover, the possibility of using Ag+-doped BG as coating of polymeric fibres (sutures) for fabricating textile scaffolds was investigated by Blaker et al. (2004). The authors indicated that the Ag-loaded BG coating of textile structures can represent a convenient alternative to fabricate multifunctional biodegradable scaffolds (Blaker et al. 2004). In a related work, Pratten et al. (2004) investigated in vitro the ability of a silver-doped BG (AgBG) coating, which was elaborated using a slurry-dipping process, to prevent Staphylococcus epidermidis colonization on surgical sutures. The in vitro studies were carried out under both batch and flow conditions to quantify the number of viable cells adhered to the surface and to determine the attachment and detachment over time, respectively. The authors showed that AgBG coating had a significant effect on preventing S. epidermidis attachment when compared with coatings of standard 45S5 Bioglass.
4. Other used drugs
4.1. Anti-inflammatory drugs
Implantation of engineered biomaterials might cause local inflammation owing to the host immunoresponse (Corry & Moran 1998; Mendez et al. 2004; Gonzalez-Corchon et al. 2006; Chevalier et al. 2009), which therefore requires the use of anti-inflammatory agents, either steroids (glucocorticoids) or non-steroids. In particular, glucocorticoids have been shown to have strong inhibitory effects on cytokine-related inflammation by downregulating transcription of interleukin (IL)-1, tumour necrosis factor (TNF)-α, granulocyte macrophage–colony-stimulating factor (GM-CSF), IL-3, IL-4, IL-5, IL-6 and IL-8 (Norton et al. 2005; Novak et al. 2009). In particular, dexamethasone (DEX) is also used in TE for the preparation of osteogenic medium (Duarte et al. 2009a,b). In this context, Duarte et al. (2009b) impregnated DEX using supercritical fluid technology in chitosan porous scaffolds prepared by a freeze-drying process. The highest loading was obtained at low pressures and temperatures (8.0 MPa and 35°C), corresponding to a lower solubility of DEX in the supercritical fluid (carbon dioxide) and a low swelling of the polymeric matrix. Results from in vitro drug-release studies showed that the release of DEX from chitosan scaffolds exhibited a sustained profile, which is suitable for application in TE. Supercritical fluid technology can be adapted to prepare porous structures using a variety of polymeric systems or composites and other bioactive compounds in order to develop multifunctional scaffolds with improved mechanical properties and biocompatibility (Quirk et al. 2004). In this context, Duarte et al. (2009a) reported the feasibility of using supercritical fluid methods to process in one step a porous matrix loaded with a pharmaceutical agent for TE purposes. In order to investigate the scaffold's performance, the release of DEX loaded in a three-dimensional starch-based porous matrix was tested. A supercritical phase-inversion technique, using carbon dioxide as the supercritical fluid, was employed to prepare the composite scaffolds of DEX and a polymeric blend of starch and poly(l-lactic acid). In vitro drug-release studies were carried out in PBS and results showed that a sustained release of DEX was achieved over 21 days. The fitting of a power law to the experimental data demonstrated that drug release is governed by an anomalous transport, i.e. it was proposed that both the drug diffusion and the swelling of the matrix influence the release of DEX out of the scaffold (Duarte et al. 2009a).
Within non-steroids, ibuprofen (IBU) has been largely used orally, intravenously and even topically. Recently, Mortera et al. (2007) used silica-ordered mesophase (MCM-41) submicron spheres incorporated inside glass–ceramic (SiO2–CaO–K2O) bioactive scaffolds for the controlled delivery of IBU in TE. The scaffolds were fabricated by dipping the glass–ceramic scaffold into the MCM-41 synthesis solution and after that samples were dipped into an IBU solution for drug adsorption over 3 days. The in vitro drug-release study performed in SFB showed a fast release of IBU during the first 8 h followed by a slower release between 8 and 120 h (Mortera et al. 2007).
4.2. Bisphosphonates
Bisphosphonates (BPs) have been in widespread use since the 1970s for the treatment of a variety of bone diseases characterized by osteoclast-mediated bone resorption such as Paget's disease, tumour-induced hypercalcaemia, metastatic bone diseases and osteoporosis (Siris et al. 1996; Colucci et al. 1998; Major et al. 2000; Rodan & Martin 2000; Van Beek et al. 2003; Miller 2005; Nieto et al. 2008; Panzavolta et al. 2009). BPs act by inhibiting the osteoclastic resorption of bone tissue (Colucci et al. 1998; Van Beek et al. 2003), they bind strongly to HA crystals and are retained for a long time in bone, being excreted unmetabolized in urine (Papapoulos 2006; Panzavolta et al. 2009). Chemically, BPs are analogous to pyrophosphates, which are natural modulators of bone metabolism (Nancollas et al. 2006; Nieto et al. 2008). However, in BPs, the oxygen atom that binds the two phosphate groups of pyrophosphate (P–O–P) is substituted by a carbon atom (P–C–P) making the molecule less resistant to hydrolysis than pyrophosphates (Nancollas et al. 2006; Papapoulos 2006; Nieto et al. 2008). Recently, the positive effect of adjunct treatment with injectable antiresorptive zoledronic acid on the biological process induced by BG incorporation in bone defects has been proved (Välimäki et al. 2006). A member of the family of BPs, sodium alendronate (SA) was used by Nieto and collaborators (Nieto et al. 2008) as a drug model to test how the organic modification of the surface of silica-based ordered mesoporous scaffolds can control the drug dosage. The mesoporous silica structure (SBA-16) was synthesized using triblock copolymers followed by functionalization by acid catalysis. Finally, SA was adsorbed by immersing the specimens in a drug-containing buffered aqueous solution. The adsorption rates of SA, and consequently the SA release rates, were tuned by using a range of amine-functionalization degrees (Nieto et al. 2008). Recently, Fauchex et al. (2009) used calcium-deficient apatite as a carrier for delivery of zoledronate acid (ZA) in bone tissue. According to the in vitro tests carried out, the released ZA inhibited osteoclastic resorption without affecting osteoblasts. Future studies are planned to develop three-dimensional TE scaffolds loaded with BPs and the use of BPs in TE approaches is bound to increase as novel methodologies are developed for the efficient loading of BPs in porous three-dimensional matrices.
5. Evaluating the efficacy of bioactive bone scaffolds as drug-delivery systems
It is important to highlight that even though several in vitro tests have been performed in order to investigate the suitability of bone scaffolds as drug carriers, there is no in vitro standard technique available for this purpose yet. Indeed, several methods described in the literature try to simulate in vivo conditions and to avoid non-physiological turbulence. In most cases, loaded scaffolds are soaked in a beaker, a bottle or a sampling tube and/or in a culture chamber with or without controlled agitation (Guicheux et al. 1997; Paul & Sharma 1999; Krajewski et al. 2000; Perry et al. 2002; Hasegawa et al. 2004; Murugan & Ramakrishna 2004; Palazzo et al. 2005; Medvecky et al. 2007; Melville et al. 2008; Victor & Kumar 2008; Chevalier et al. 2009). Released tests are usually performed considering only low volumes (from 3 up to 150 ml) of various dissolution media (phosphate buffer, saline physiological solution, SBF, etc.). For bone TE, SBF is frequently the dissolution medium of choice as it allows the study of how the release of a drug can be affected by the formation of a surface layer of microcrystalline HA on the bioactive scaffold, which is the expected behaviour in vivo. There are several sampling methods reported for studying the release of drug substances from biomaterials (replacing the entire volume with fresh media after taking a sample, replacing the same volume withdrawn after taking a sample); sometimes ensuring sink conditions (ensuring that sufficient medium volume is available during the test to dissolve the total drug quantity from the loaded scaffold) and sometimes using continuous flow through chambers (flow rate from 0.02 to 2.5 ml min−1) (Perry et al. 2002; Hall et al. 2004; Chevalier et al. 2009). Moreover, it could be beneficial to mimic the blood/plasma flow speed observed at the site of the scaffold's implantation (Baroli 2009). Further, and surprisingly, there is very limited reported information regarding the stability of the drugs involved in the studies, in particular the thermal stability. If a drug is included within a scaffold in order to be released over a month or longer period, the normal stability tests usually carried out by suppliers will not be sufficient; further tests should be done in order to evaluate the activity and drug degradation rate at body temperature for a prolonged period of time. In addition, when in vivo tests were performed, little work reported on the drug clearance from the zone and how the new vascularization induced by the presence of the scaffold would affect the residence time of the drug, especially when the matrix is expected to sustain the release of the drug for a prolonged period of time (Castro et al. 2003). In this regard, also very little work has been carried out on the correlation of in vitro and in vivo results of drug release to evaluate the significance of establishing novel in vitro models, which would represent realistic studies, prior to in vivo tests on host response after scaffold implantation (Malafaya et al. 2002).
6. Conclusions and perspectives
In reviewing the published studies on bioactive bone TE scaffolds with additional drug-delivery capability, it becomes clear that there have been continued advances, particularly in recent years, towards the further development of the field. New materials and combinations of materials, as well as improved scaffold designs based on novel processing techniques, are being continuously proposed to advance the drug-delivery capability of bone tissue scaffolds. However, a large amount of biological information is still needed to fully understand the in vitro and in vivo performance of such scaffolds in specific applications, which once obtained will provide a rational scaffold design and optimization of their drug-delivery function. For example, analysis of the literature has revealed that there is a lack of biological data regarding the specific concentration in which a given drug is needed in relation to the particular local microenvironment and how this is affected by the interaction between the scaffold and new tissue, considering also the effect on vascularization. In addition, during the design and development of multifunctional scaffolds and in order to control the drug-release patterns, the effect of processing parameters on scaffold microstructure (i.e. porosity) and on the resulting drug-release profiles, stability, biodegradation behaviour, as well as the scaffold's mechanical and physical properties, must be further investigated. Despite significant efforts in this direction, several challenges have yet to be resolved. These include understanding the link between the key variables determining the scaffold-processing conditions and the physico-chemical properties of the novel three-dimensional delivery systems with special consideration given to the stability of the incorporated drug. These challenges also include achieving accurate control over time and space of specific quantities of drug for determined applications and engineering the release patterns, which are related to the interaction between the drug and the scaffold (matrix degradation and erosion and drug diffusion through the matrix), drug solubility and the amount of drug loaded available to be delivered. Further, only limited work has been reported on how processing parameters and sterilization can affect the homogeneous loading, the stability and the release kinetics of the drug incorporated. In addition, there is a lack of focus in several works in terms of the pathology they would like to address. It is clear that the combination of drug delivery and TE using advanced three-dimensional scaffold concepts is in its early years and it will take time to achieve clinical results of relevance. It is therefore expected that this field will keep growing within the next few years and related research outputs could lead to results with the potential to transform the clinical approach to bone-related pathologies. Specifically, in order to achieve these goals, it would be important to intensify the working collaboration of researchers and technologists from different relevant communities. The interdisciplinary character of TE, allowing the confluence of different scientific fields, backgrounds and knowledge, is mandatory for the advance of bone TE therapeutics.
References
- Adams A. P., Santschi E. M., Mellencamp M. A. 1999. Antibacterial properties of a silver chloride-coated nylon wound dressing. Vet. Surg. 28, 219–225. [DOI] [PubMed] [Google Scholar]
- Ahmad Z., Zhang H. B., Farook U., Edirisinghe M., Stride E., Colombo P. 2008. Generation of multilayered structures for biomedical applications using a novel tri-needle coaxial device and electrohydrodynamic flow. J. R. Soc. Interface 5, 1255–1261. ( 10.1098/rsif.2008.0247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C. G., Gogola G. R., Clyburn T. A., Raymond A. K., Peng A. S., Mikos A. G. 2003. Antibiotic microspheres: preliminary testing for potential treatment of osteomyelitis. Clin. Orthop. Relat. Res. 415, 279–285. ( 10.1097/01.blo.0000093920.26658.ae) [DOI] [PubMed] [Google Scholar]
- An Y. H., Friedman R. J. 1996. Prevention of sepsis in total joint arthroplasty. J. Hosp. Infect. 33, 93–108. ( 10.1016/S0195-6701(96)90094-8) [DOI] [PubMed] [Google Scholar]
- Aoyagi S., Onishi H., Machida Y. 2007. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int. J. Pharm. 330, 138–145. ( 10.1016/j.ijpharm.2006.09.016) [DOI] [PubMed] [Google Scholar]
- Armstrong M. S., Spencer R. F., Cunningham J. L., Gheduzzi S., Miles A. W., Learmonth I. D. 2002. Mechanical characteristics of antibiotic-laden bone cement. Acta Orthop. Scand. 73, 688–690. ( 10.1080/000164702321039697) [DOI] [PubMed] [Google Scholar]
- Arosarena O. 2004. Tissue engineering. Curr. Opin. Otolaryngol. Head Neck Surg. 13, 233–241. [DOI] [PubMed] [Google Scholar]
- Baroli B. 2009. From natural bone graft to tissue engineering therapeutics: brainstorming on pharmaceutical formulative requirements and challenges. J. Pharm. Sci. 98, 1317–1375. ( 10.1002/jps.21528) [DOI] [PubMed] [Google Scholar]
- Bellantone M., Williams H. D., Hench L. L. 2002. Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrob. Agents Chemother. 46, 1940–1945. ( 10.1128/AAC.46.6.1940-1945.2002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens A. R., Huvard G. S., Korsmeyer R. W., Kunig F. W. 1992. Application of compressed carbon dioxide in the incorporation of additives into polymers. J. Appl. Polym. Sci. 46, 231–242. ( 10.1002/app.1992.070460204) [DOI] [Google Scholar]
- Berger R. A., Jacobs J. J., Quigley L. R., Rosenberg A. G., Galante J. O. 1997. Primary cementless acetabular reconstruction in patients younger than 50 years old. 7- to 11-year results. Clin. Orthop. Relat. Res. 344, 216–226. [PubMed] [Google Scholar]
- Blaker J. J., Nazhat S. N., Boccaccini A. R. 2004. Development and characterisation of silver doped bioactive glass-coated sutures for tissue engineering and wound healing applications. Biomaterials 25, 1319–1329. ( 10.1016/j.biomaterials.2003.08.007) [DOI] [PubMed] [Google Scholar]
- Boccaccini A. R., Maquet V. 2003. Bioresorbable and bioactive polymer/bioglassR composites with tailored pore structure for tissue engineering applications. Comp. Sci. Technol. 63, 2417–2429. ( 10.1016/S0266-3538(03)00275-6) [DOI] [Google Scholar]
- Böstman O., Pihlajamäki H. 2000. Clinical biocompatibility of biodegradable orthopaedic implants for internal fixation: a review. Biomaterials 21, 2615–2621. ( 10.1016/S0142-9612(00)00129-0) [DOI] [PubMed] [Google Scholar]
- Bran G. M., Stern-Straeter J., Hörmann K., Riedel F., Goessler U. R. 2008. Apoptosis in bone for tissue engineering. Arch. Med. Res. 39, 467–482. ( 10.1016/j.arcmed.2008.02.007) [DOI] [PubMed] [Google Scholar]
- Buckwalter J. A., Glimcher M. J., Cooper R. R., Recker R. 1996a. Bone biology. I. Structure, blood supply, cells, matrix, and mineralization. Instr. Course Lect. 45, 371–386. [PubMed] [Google Scholar]
- Buckwalter J. A., Glimcher M. J., Cooper R. R., Recker R. 1996b. Bone biology. II. Formation, form, modeling, remodeling, and regulation of cell function. Instr. Course Lect. 45, 387–399. [PubMed] [Google Scholar]
- Cabañas M. V., Peña J., Román J., Vallet-Regí M. 2009. Tailoring vancomycin release from β-TCP/agarose scaffolds. Eur. J. Pharm. Sci. 37, 249–256. ( 10.1016/j.ejps.2009.02.011) [DOI] [PubMed] [Google Scholar]
- Castro C., Sánchez E., Delgado A., Soriano I., Núñez P., Baro M., Perera A., Évora C. 2003. Ciprofloxacin implants for bone infection. In vitro–in vivo characterization. J. Control. Release 93, 341–354. ( 10.1016/j.jconrel.2003.09.004) [DOI] [PubMed] [Google Scholar]
- Castro C., Évora C., Baro M., Soriano I., Sánchez E. 2005. Two-month ciprofloxacin implants for multibacterial bone infections. Eur. J. Pharm. Sci. 60, 401–406. [DOI] [PubMed] [Google Scholar]
- Chen Q. Z., Thompson I. D., Boccaccini A. R. 2006. 45S5 Bioglass-derived glass ceramic scaffolds for bone tissue engineering. Biomaterials 27, 2414–2425. ( 10.1016/j.biomaterials.2005.11.025) [DOI] [PubMed] [Google Scholar]
- Chen W., Liu Y., Courtney H. S., Bettenga M., Agrawal C. M., Bumgardner J. D., Ong J. L. 2006. In vitro anti-bacterial and biological properties of magnetron co-sputtered silver-containing hydroxyapatite coating. Biomaterials 27, 5512–5517. ( 10.1016/j.biomaterials.2006.07.003) [DOI] [PubMed] [Google Scholar]
- Chen Q. Z., Bretcanu O., Boccaccini A. R. 2008. Inorganic and composite bioactive scaffolds for bone tissue engineering. In Biomaterials fabrication and processing handbook (eds Chu P. K., Liu X.). Boca Raton, FL: CRC Press. [Google Scholar]
- Chevalier E., Viana M., Artaud A., Haddouchi S., Chulia D. 2009. A novel application of the T-cell for flow-through dissolution: the case of bioceramics used as ibuprofen carrier. Talanta 77, 1545–1548. ( 10.1016/j.talanta.2008.09.046) [DOI] [PubMed] [Google Scholar]
- Chung H. J., Park T. G. 2007. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 59, 249–262. ( 10.1016/j.addr.2007.03.015) [DOI] [PubMed] [Google Scholar]
- Chung L. Y., Schmidt R. J., Hamlyn P. F., Sagar B. F., Andrews A. M., Turner T. D. 1994. Biocompatibility of potential wound management products: fungal mycelia as a source of chitin/chitosan and their effect on the proliferation of human F1000 fibroblasts in culture. J. Biomed. Mater. Res. 24, 463–469. [DOI] [PubMed] [Google Scholar]
- Colucci S., Minielli V., Zambonin G., Cirulli N., Mori G., Serra M., Patella V., Zambonin Zallone A., Grano M. 1998. Alendronate reduces adhesion of human osteoclast-like cells to bone and bone protein-coated. Surf. Calcif. Tissue Int. 63, 230 ( 10.1007/s002239900519) [DOI] [PubMed] [Google Scholar]
- Corry D., Moran J. 1998. Assessment of acrylic bone cement as a local delivery vehicle for the application of non-steroidal anti-inflammatory drugs. Biomaterials 19, 1295 ( 10.1016/S0142-9612(98)00012-X) [DOI] [PubMed] [Google Scholar]
- Day R. M., Boccaccini A. R., Shurey S., Roether J. A., Forbes A., Hench L. L., Gabe S. M. 2004. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 25, 5857–5866. ( 10.1016/j.biomaterials.2004.01.043) [DOI] [PubMed] [Google Scholar]
- Désévaux C., Lenaerts V., Girard C., Dubreuil P. 2002. Characterization of crosslinked high amylose starch matrix implants. 2. In vivo release of ciprofloxacin. J. Control. Release 82, 95–103. ( 10.1016/S0168-3659(02)00132-3) [DOI] [PubMed] [Google Scholar]
- Diez-Peña E., Frutos G., Barrales-Rienda J. 2002. Gentamicin sulphate release from a modified commercial acrylic surgical radiopaque bone cement. I. Influence of the gentamicin concentration on the release process mechanism. Chem. Pharm. Bull. 50, 1201–1208. ( 10.1248/cpb.50.1201) [DOI] [PubMed] [Google Scholar]
- Di Nunzio S., Verné E. 2005. Process for the production of silver-containing prosthetic devices. Italian Patent no. PCT/EP2005/056391. [Google Scholar]
- Di Nunzio S., Vitale-Brovarone C., Spriano S., Milanese D., Verné E., Bergo V., Maina G., Spinelli P. 2004. Silver containing bioactive glasses prepared by molten salt ion-exchange. J. Eur. Ceram. Soc. 24, 2935–2942. [Google Scholar]
- Domingues Z. R., Cortés M. E., Gomes T. A., Diniz H. F., Freitas C. S., Gomes J. B., Fariac A. M., Sinisterra R. D. 2004. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials 25, 327–333. ( 10.1016/S0142-9612(03)00524-6) [DOI] [PubMed] [Google Scholar]
- Dong J., Kojima H., Uemura T., Kikuchi M., Tateishi T., Tanaka J. 2001. In vivo evaluation of a novel porous hydroxyapatite to sustain osteogenesis of transplanted bone marrow-derived osteoblastic cells. J. Biomed. Mater. Res. 57, 208–216. ( 10.1002/1097-4636(200111)57:2%3C208::AID-JBM1160%3E3.0.CO;2-N) [DOI] [PubMed] [Google Scholar]
- Duarte A. R., Simplicio A. L., Vega-González A., Paternault P. S., Coimbra P., Gil M. H., de Sousa H. C., Duarte C. M. 2007. Supercritical fluid impregnation of a biocompatible polymer for ophthalmic drug delivery. J. Supercritical Fluids 42, 373–377. ( 10.1016/j.supflu.2007.01.007) [DOI] [Google Scholar]
- Duarte A. R., Mano J. F., Reis R. L. 2009a. Dexamethasone-loaded scaffolds prepared by supercritical-assisted phase inversion. Acta Biomater. 5, 2054–2062. ( 10.1016/j.actbio.2009.01.047) [DOI] [PubMed] [Google Scholar]
- Duarte A. R., Mano J. F., Reis R. L. 2009b. Preparation of chitosan scaffolds loaded with dexamethasone for tissue engineering applications using supercritical fluid technology. Eur. Polym. J. 45, 141–148. ( 10.1016/j.eurpolymj.2008.10.004) [DOI] [Google Scholar]
- Fauchex C., et al. 2009. Controlled release of bisphosphonate from a calcium phosphate biomaterial inhibits osteoclastic resorption in vitro. J. Biomed. Mater. Res. Part A 86, 46–56. [DOI] [PubMed] [Google Scholar]
- Freyman M., Yannas Y. V., Gibson L. 2001. Cellular materials as porous scaffolds for tissue engineering. J. Prog. Mater. Sci. 46, 273–282. ( 10.1016/S0079-6425(00)00018-9) [DOI] [Google Scholar]
- Fu Q., Rahaman M. N., Bal B. S., Huang W., Day D. E. 2007. Preparation and bioactive characteristics of a porous 13–93 glass, and fabrication into the articulating surface of a proximal tibia. J. Biomed. Mater. Res. A 82, 222–229. [DOI] [PubMed] [Google Scholar]
- Fujibayashi S., Neo M., Kim H. M., Kokubo T., Nakamura T. 2003. A comparative in vivo bone ingrowth and in vitro apatite formation on Na2O–CaO–SiO2 glasses. Biomaterials 24, 1349–1356. ( 10.1016/S0142-9612(02)00511-2) [DOI] [PubMed] [Google Scholar]
- Furth M. E., Atala A., Van Dyke M. E. 2007. Smart biomaterials design for tissue engineering and regenerative medicine. Biomaterials 28, 5068–5073. ( 10.1016/j.biomaterials.2007.07.042) [DOI] [PubMed] [Google Scholar]
- Garvin K., Feschuk C. 2005. Polylactide-polyglycolide antibiotic implants. Clin. Orthop. Relat. Res. 437, 105–110. [DOI] [PubMed] [Google Scholar]
- Garvin K. L., Miyano J. A., Robinson D., Giger D., Novak J., Radio S. 1994. Polylactide/polyglycolide antibiotic implants in the treatment of osteomyelitis. A canine model. J. Bone Joint Surg. Am. 76, 1500–1506. [DOI] [PubMed] [Google Scholar]
- Gatter N., Kohnen W., Jansen B. 1998. In vitro efficacy of hydrophilic central venous catheter loaded with silver to prevent microbial colonization. Zentbl. Bakteriol. 287, 157–169. [DOI] [PubMed] [Google Scholar]
- Gbureck U., Probst J., Thull R. 2002. Surface properties of calcium phosphate particles for self setting bone cements. Biomol. Eng. 19, 51–55. ( 10.1016/S1389-0344(02)00010-2) [DOI] [PubMed] [Google Scholar]
- Gerhart T. N., Roux R. D., Hanff P. A., Horowitz G. L., Renshaw A. A., Hayes W. C. 1993. Antibiotic-loaded biodegradable bone-cement for prophylaxis and treatment of experimental osteomyelitis in rats. J. Orthop. Res. 11, 250–255. ( 10.1002/jor.1100110212) [DOI] [PubMed] [Google Scholar]
- Giunchedi P., Genta I., Conti B., Muzzarelli R. A., Conte U. 1998. Preparation and characterization of ampicillin loaded methylpyrrolidinone chitosan and microspheres. Biomaterials 19, 157–161. ( 10.1016/S0142-9612(97)00181-6) [DOI] [PubMed] [Google Scholar]
- Goessler U. R., Stern-Straeter J., Riedel K., Bran G., Hörmann K., Riedel F. 2007. Tissue engineering in head and neck reconstructive surgery: what type of tissue do we need? Eur. Arch. Otorhinolaryngol. 264, 1343–1356. ( 10.1007/s00405-007-0369-y) [DOI] [PubMed] [Google Scholar]
- Gold H. S., Moellering R. S., Jr 1996. Antimicrobial-drug resistance. N. Engl. J. Med. 335, 1445–1453. ( 10.1056/NEJM199611073351907) [DOI] [PubMed] [Google Scholar]
- Gomes M. E., Reis R. L. 2004. Biodegradable polymers and composites in biomedical applications from catgut to tissue engineering. Part II. Systems for temporary replacement and advanced tissue regeneration. Int. Mater. Rev. 49, 274–285. ( 10.1179/095066004225021927) [DOI] [Google Scholar]
- Gonzalez-Corchon M. A., Salvado M., De la Torre B. J., Collia F., De Pedro J. A., Vazquez B., San Roman J. 2006. Injectable and self-curing composites of acrylic/bioactive glass and drug systems. A histomorphometric analysis of the behaviour in rabbits. Biomaterials 27, 1778. [DOI] [PubMed] [Google Scholar]
- Gransden W. R. 1997. Antibiotic resistance. Nosocomial gram-negative infection. J. Med. Microbiol. 46, 436–439. [PubMed] [Google Scholar]
- Gristina A. G. 1987. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 237, 1588–1595. ( 10.1126/science.3629258) [DOI] [PubMed] [Google Scholar]
- Guan L. M., Davies J. E. 2004. Preparation and characterization of a highly macroporous biodegradable composite tissue engineering scaffold. J. Biomed. Mater. Res. Part A 71A, 480–487. ( 10.1002/jbm.a.30173) [DOI] [PubMed] [Google Scholar]
- Guarino V., Causa F., Ambrosio L. 2007. Bioactive scaffolds for bone and ligament tissue. Exp. Rev. Med. Devices 4, 405–418. ( 10.1586/17434440.4.3.405) [DOI] [PubMed] [Google Scholar]
- Guicheux J., Grimandi G., Trécant M., Faivre A., Takahashi S., Daculsi G. 1997. Apatite as carrier for growth hormone: in vitro characterization of loading and release. J. Biomed. Mater. Res. 34, 165–170. ( 10.1002/(SICI)1097-4636(199702)34:2%3C165::AID-JBM4%3E3.0.CO;2-O) [DOI] [PubMed] [Google Scholar]
- Gupta A., Seifalian A. M., Edirisinghe M. J., Winslet M. C. 2007. Novel electrohydrodynamic printing of nanocomposite biopolymer scaffolds. J. Bioactive Compatible Polym. 22, 265–280. ( 10.1177/0883911507078268) [DOI] [Google Scholar]
- Habraken W. J., Wolke J. G., Jansen J. A. 2007. Ceramic composites as matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 59, 234–248. ( 10.1016/j.addr.2007.03.011) [DOI] [PubMed] [Google Scholar]
- Habraken W. J., Wolke J. G., Mikos A. G., Jansen J. A. 2008. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J. Biomat. Sci. Polym. E17, 1057–1074. [DOI] [PubMed] [Google Scholar]
- Hall E. W., Rouse M. S., Jacofsky D. J., Osmon D. R., Hanssen A. D., Steckelberg J. M., Patel R. 2004. Release of daptomycin from polymethylmethacrylate beads in a continuous flow chamber. Diagn. Microbiol. Infect. Dis. 50, 261 ( 10.1016/j.diagmicrobio.2004.03.004) [DOI] [PubMed] [Google Scholar]
- Han D., Gouma P.-I. 2006. Electrospun bioscaffolds that mimic the topology of extracellular matrix. Nanomed. Nanotechnol. Biol. Med. 2, 37–41. ( 10.1016/j.nano.2006.01.002) [DOI] [PubMed] [Google Scholar]
- Hanssen A. D. 2004. Prophylactic use of antibiotic bone cement: an emerging standard-in opposition. J. Arthroplast. 19, 73–77. ( 10.1016/j.arth.2004.04.006) [DOI] [PubMed] [Google Scholar]
- Harris L. D., Kim B., Mooney D. J. 1998. Open pore biodegradable matrices formed with gas foaming. J. Biomed. Mater. Res. 42, 396–402. ( 10.1002/(SICI)1097-4636(19981205)42:3%3C396::AID-JBM7%3E3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Sudo A., Komlev V. S., Barinov S. M., Uchida A. 2004. High release of antibiotic from a novel hydroxyapatite with bimodal pore size distribution. J. Biomed. Mater. Res. Part B: Appl. Biomater. 70, 332–339. [DOI] [PubMed] [Google Scholar]
- Heller J. 1985. Controlled drug release from poly(ortho esters)—a surface eroding polymer. J. Control. Release 2, 167–177. ( 10.1016/0168-3659(85)90042-2) [DOI] [Google Scholar]
- Hench L. L. 1998. Bioceramics. J. Am. Ceram. Soc. 81, 1705–1728. [Google Scholar]
- Hench L. L., Polak J. M. 2002. Third-generation biomedical materials. Science 295, 1014–1017. ( 10.1126/science.1067404) [DOI] [PubMed] [Google Scholar]
- Hendricks K. J., Lane D., Burd T. A., Lowry K. J., Day D., Phaup J. G., Anglen J. O. 2001. Elution characteristics of tobramycin from polycaprolactone in a rabbit model. Clin. Orthop. Relat. Res. 392, 418–426. ( 10.1097/00003086-200111000-00055) [DOI] [PubMed] [Google Scholar]
- Hetrick E. M., Schoenfisch M. H. 2006. Reducing implant-related infections: active release strategies. Chem. Soc. Rev. 35, 780–789. ( 10.1039/b515219b) [DOI] [PubMed] [Google Scholar]
- Heyde M., Partridge K. A., Howdle S. M., Oreffo R. O., Garnett M. C., Shakesheff K. M. 2007. Development of a controlled non-viral DNA release system from PDLLA scaffolds fabricated using a supercritical CO2 technique. Biotechnol. Bioeng. 98, 679–693. ( 10.1002/bit.21446) [DOI] [PubMed] [Google Scholar]
- Hollister S. 2005. Porous scaffold design for tissue engineering. Nat. Mater. 4, 518–524. ( 10.1038/nmat1421) [DOI] [PubMed] [Google Scholar]
- Hutmacher D. W. 2000. Polymeric scaffolds in tissue engineering bone and cartilage. Biomaterials 21, 2529–2543. ( 10.1016/S0142-9612(00)00121-6) [DOI] [PubMed] [Google Scholar]
- Hutmacher D. W. 2001. Scaffold design and fabrication technologies for engineering tissues: state of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 12, 107–124. ( 10.1163/156856201744489) [DOI] [PubMed] [Google Scholar]
- Hutmacher D. W., Cool S. 2007. Concepts of scaffold-based tissue engineering: the rationale to use solid free-form fabrication techniques. J. Cell. Mol. Med. 11, 654–669. ( 10.1111/j.1582-4934.2007.00078.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutmacher D. W., Schantz J. T., Lam C. X. F., Tan K. C., Lim T. C. 2007. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regen. Med. 1, 245–260. ( 10.1002/term.24) [DOI] [PubMed] [Google Scholar]
- Izquierdo-Barba I., Monserrat C., Vallet-Regí M. 2008. Nanostructured mesoporous silicas for bone tissue regeneration. J. Nanomater. 3, 1–14. [Google Scholar]
- Jacob E., Setterstrom J. A., Bach D. E., Heath J. R., III, McNiesh L. M., Cierny G., III 1991. Evaluation of biodegradable ampicillin anhydrate microcapsules for local treatment of experimental staphylococcal osteomyelitis. Clin. Orthop. Relat. Res. 267, 237–244. [PubMed] [Google Scholar]
- Joosten U., Joist A., Frebel T., Brandt B., Diederichs S., von Eiff C. 2004. Evaluation of an in situ setting injectable calcium phosphate as a new carrier material for gentamicin in the treatment of chronic osteomyelitis: studies in vitro and in vivo. Biomaterials 25, 4287–4295. ( 10.1016/j.biomaterials.2003.10.083) [DOI] [PubMed] [Google Scholar]
- Joseph T. N., Chen A. L., Di Cesare P. E. 2003. Use of antibiotic-impregnated cement in total joint arthroplasty. J. Am. Acad. Orthop. Surg. 11, 38–47. [DOI] [PubMed] [Google Scholar]
- Kanczler J. M., Oreffo R. O. 2008. Osteogenesis and angiogenesis: the potential for engineering bone. Eur. Cell Mater. 15, 100–114. [DOI] [PubMed] [Google Scholar]
- Kanellakopoulou K., Giamarellos-Bourboulis E. J. 2000. Carrier systems for the local delivery of antibiotics in bone infections. Drugs 59, 1223–1232. ( 10.2165/00003495-200059060-00003) [DOI] [PubMed] [Google Scholar]
- Karageorgiou V., Kaplan D. 2006. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26, 5474–5491. ( 10.1016/j.biomaterials.2005.02.002) [DOI] [PubMed] [Google Scholar]
- Kawashita M., Tsuneyama S., Miyaji F., Kokubo T., Yamamoto K. 2000. Antibacterial silver-containing silica glass prepared by the sol–gel method. Biomaterials 21, 393–398. ( 10.1016/S0142-9612(99)00201-X) [DOI] [PubMed] [Google Scholar]
- Kazarian S. G. 2000. Polymer processing with supercritical fluids. Polym. Sci. Ser. C 42, 78–101. [Google Scholar]
- Kikic I., Sist P. 2000. Applications of supercritical fluids to pharmaceuticals: controlled drug delivery systems, supercritical fluids: fundamentals and applications in. In Proc. Second NATO ASI on Supercritical Fluids, NATO Science Series, pp. 291–306. Dordrecht, The Netherlands: Kluwer Academic Publishers. [Google Scholar]
- Kim H. M., Miyaji F., Kokubo T., Ohtsuki C., Nakamura T. 1995. Bioactivity of Na2O–CaO–SiO2 glasses. J. Am. Ceram. Soc. 78, 2405–2411. ( 10.1111/j.1151-2916.1995.tb08677.x) [DOI] [Google Scholar]
- Kim T. N., Feng Q. L., Kim J. O., Wu J., Wang H., Chen G. C. 1998. Antimicrobial effects of metal ions (Ag+, Cu2+, Zn2+) in hydroxyapatite. J. Mater. Sci. Mater. Med. 9, 129–134. ( 10.1023/A:1008811501734) [DOI] [PubMed] [Google Scholar]
- Kim H. W., Knowles J. C., Kim H. E. 2004a. Development of hydroxyapatite bone scaffold for controlled drug release via poly(ε-caprolactone) and hydroxyapatite hybrid coatings. J. Biomed. Mater. Res. Part B: Appl. Biomater. 70, 240–249. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Knowles J. C., Kim E. 2004b. Hydroxyapatite and gelatine composite foams processed via novel freeze-drying and crosslinking for use as temporary hard tissue scaffolds. J. Biomed. Mater. Res. A 72, 136–145. [DOI] [PubMed] [Google Scholar]
- Kimakhe S., Bohic S., Larosse C., Reynaud A., Pilet P., Giumelli B., Heymann D., Daculsi G. 1999. Biological activities of sustained polymixin B release from calcium phosphate biomaterial prepared by dynamic compaction: an in vitro study. J. Biomed. Mater. Res. 47, 18–27. ( 10.1002/(SICI)1097-4636(199910)47:1%3C18::AID-JBM3%3E3.0.CO;2-T) [DOI] [PubMed] [Google Scholar]
- Krajewski A., Ravaglioli A., Roncari E., Pinasco P. 2000. Porous ceramic bodies for drug delivery. J. Mater. Sci. Mater. Med. 12, 763. [DOI] [PubMed] [Google Scholar]
- Krasko M., Golenser J., Nyska A., Nyska M., Brin Y. B., Domb A. J. 2007. Gentamicin extended release from an injectable polymeric implant. J. Control. Release 117, 90–96. ( 10.1016/j.jconrel.2006.10.010) [DOI] [PubMed] [Google Scholar]
- Kumar C. 2006. Tissue, cell and organ engineering, pp. 216 Weinheim, Germany: Wiley-VCH. [Google Scholar]
- Le Bolay N., Santran V., Dechambre G., Combes C., Drouet C., Lamure A., Rey C. 2009. Production, by co-grinding in a media mill, of porous biodegradable polylactic acid–apatite composite materials for bone tissue engineering. Powder Technol. 190, 89–94. ( 10.1016/j.powtec.2008.04.043) [DOI] [Google Scholar]
- Lee S. H., Shin H. 2007. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv. Drug Deliv. Rev. 59, 339–359. ( 10.1016/j.addr.2007.03.016) [DOI] [PubMed] [Google Scholar]
- Lee J. E., Park J. C., Kwang H. L., Sang H. O., Kim J. G., Hwal S. 2002. An infection-preventing bilayered collagen membrane containing antibiotic-loaded hyaluronan microparticles: physical and biological properties. Artif. Organs 26, 636–646. ( 10.1046/j.1525-1594.2002.06847.x) [DOI] [PubMed] [Google Scholar]
- Li X., Hu Y. 2001. The treatment of osteomyelitis with gentamicin—reconstituted bone xenograft-composite. J. Bone Joint Surg. Br. 83, 1063–1068. ( 10.1302/0301-620X.83B7.11271) [DOI] [PubMed] [Google Scholar]
- Li L. C., Deng J., Stephens D. 2002. Polyanhydride implant for antibiotic delivery: from the bench to the clinic. Adv. Drug Deliv. Rev. 54, 963–986. [DOI] [PubMed] [Google Scholar]
- Li W., Laurencin C. T., Caterson E. J., Tuan R. S., Ko F. K. 2002. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. J. Biomed. Mater. Res. 60, 613–621. ( 10.1002/jbm.10167) [DOI] [PubMed] [Google Scholar]
- Lickorish D., Guan L., Davies J. E. 2007. A three-phase, fully resorbable, polyester/calcium phosphate scaffold for bone tissue engineering: evolution of scaffold design. Biomaterials 28, 1495–1502. [DOI] [PubMed] [Google Scholar]
- Livingston T., Ducheyne P., Garino J. 2002. In vivo evaluation of a bioactive scaffold for bone tissue engineering. J. Biomed. Mater. Res. 62, 1–13. ( 10.1002/jbm.10157) [DOI] [PubMed] [Google Scholar]
- Lu L., Garcia C. A., Mikos A. G. 1999. In vitro degradation of thin poly(dl-lactic-co-glycolic acid) films. J. Biomed. Mater. Res. 46, 236–244. ( 10.1002/(SICI)1097-4636(199908)46:2%3C236::AID-JBM13%3E3.0.CO;2-F) [DOI] [PubMed] [Google Scholar]
- Ma Z., Kotaki M., Inai R., Ramakrishna S. 2005. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 11, 101–109. ( 10.1089/ten.2005.11.101) [DOI] [PubMed] [Google Scholar]
- Major P., Lipton A., Berenson J., Hortobagyi G. 2000. Oral bisphosphonates. A review of clinical use in patients with bone metastases. Cancer 88, 6–14. ( 10.1002/(SICI)1097-0142(20000101)88:1%3C6::AID-CNCR3%3E3.0.CO;2-D) [DOI] [PubMed] [Google Scholar]
- Malafaya P. B., Silva G. A., Baran E. T., Reis R. L. 2002. Drug delivery therapies II: strategies for delivering bone regenerating factors. Curr. Opin. Solid State Mater. Sci. 6, 297–312. ( 10.1016/S1359-0286(02)00077-3) [DOI] [Google Scholar]
- Maquet V., Jerome R. 1997. Design of macroporous biodegradable polymer scaffolds for cell transplantation. Mat. Sci. Forum 250, 15–42. ( 10.4028/www.scientific.net/MSF.250.15) [DOI] [Google Scholar]
- Marcacci M., Kon E., Moukhachev V., Lavroukov A., Kutepov S., Quarto R., Mastrogiacomo M., Cancedda R. 2007. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 13, 947–955. ( 10.1089/ten.2006.0271) [DOI] [PubMed] [Google Scholar]
- Martins V. C., Goissis G. 2000. Nonstoichiometric hydroxyapatite–anionic collagen composite, a support for the double sustained release of gentamicin and norfloxacin/ciprofloxacin. Artif. Organs 24, 224–230. [DOI] [PubMed] [Google Scholar]
- Matsuura T., Abe Y., Sato Y., Okamoto K., Ueshige M., Akagawa Y. 1997. Prolonged antimicrobial effect of tissue conditioners containing silver-zeolite. J. Dent. 25, 373–377. ( 10.1016/S0300-5712(96)00050-4) [DOI] [PubMed] [Google Scholar]
- Matthews J. A., Wnek G. E., Simpson D. G., Bowlin G. L. 2002. Electrospinning of collagen nanofibers. Biomacromolecules 3, 232–238. ( 10.1021/bm015533u) [DOI] [PubMed] [Google Scholar]
- Medvecky L., Stulajterova R., Briancin J. 2007. Study of controlled tetracycline release from porous calcium phosphate/polyhydroxybutyrate composites. Chem. Pap. 61, 477–484. ( 10.2478/s11696-007-0065-9) [DOI] [Google Scholar]
- Melville A. J., Rodriguez-Lorenzo L. M., Forsythe J. S. 2008. Effects of calcination temperature on the drug delivery behaviour of Ibuprofen from hydroxyapatite powders. J. Mater. Sci. Mater. Med. 19, 1187–1195. ( 10.1007/s10856-007-3185-4) [DOI] [PubMed] [Google Scholar]
- Mendez J. A., et al. 2004. Injectable self-curing bioactive acrylic-glass composites charged with specific anti-inflammatory/analgesic agent. Biomaterials 25, 2381–2392. ( 10.1016/j.biomaterials.2003.09.004) [DOI] [PubMed] [Google Scholar]
- Mi F.-L., Wu Y.-B., Shyu S.-S., Schoung J.-Y., Huang Y.-B., Tsai Y.-H., Hao J.-Y. 2002. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 59, 438–449. ( 10.1002/jbm.1260) [DOI] [PubMed] [Google Scholar]
- Miller P. 2005. Optimizing the management of postmenopausal osteoporosis with bisphosphonates: the emerging role of intermittent therapy. Clin. Ther. 27, 361 ( 10.1016/j.clinthera.2005.04.005) [DOI] [PubMed] [Google Scholar]
- Miyai T., Ito A., Tamazawa G., Matsuno T., Sogo Y., Nakamura C., Yamazaki A., Satoh T. 2008. Antibiotic-loaded poly-ϵ-caprolactone and porous β-tricalcium phosphate composite for treating osteomyelitis. Biomaterials 29, 350–358. ( 10.1016/j.biomaterials.2007.09.040) [DOI] [PubMed] [Google Scholar]
- Mooney D. J., Baldwin D. F., Suh N. P., Vacanti J. P., Langer R. 1996. Novel approach to fabricate porous sponges of poly(d,l-lactic-co-glycolic acid) without the use of organic solvents. Biomaterials 17, 1417–1422. ( 10.1016/0142-9612(96)87284-X) [DOI] [PubMed] [Google Scholar]
- Moroni L., De Wijn J. R., Van Blitterswik C. A. 2008. The integrating novel technologies to fabricate smart scaffolds. J. Biomater. Sci. Polym. Ed. 19, 543–572. ( 10.1163/156856208784089571) [DOI] [PubMed] [Google Scholar]
- Mortera R., Onida B., Fiorilli S., Cauda V., Vitale-Brovarone C., Baino F., Vernè E., Garrone E. 2007. Synthesis and characterization of MCM-41 spheres inside bioactive glass–ceramic scaffold. Chem. Eng. J. 137, 54–61. ( 10.1016/j.cej.2007.07.094) [DOI] [Google Scholar]
- Murugan R., Ramakrishna S. 2004. Coupling of therapeutic molecules onto surface modified coralline hydroxyapatite. Biomaterials 25, 3073–3080. ( 10.1016/j.biomaterials.2003.09.089) [DOI] [PubMed] [Google Scholar]
- Muzzarelli R., Tarsi R., Filippini O., Giovanetti E., Biagini G., Varaldo P. E. 1990. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 34, 2019–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair L., Laurencin C. 2007. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32, 762–798. ( 10.1016/j.progpolymsci.2007.05.017) [DOI] [Google Scholar]
- Nancollas G. H., Tang R., Phipps R. J., Henneman Z., Gulde S., Wu W., Mangood A., Russell R. G. G., Ebetino F. H. 2006. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38, 617 ( 10.1016/j.bone.2005.05.003) [DOI] [PubMed] [Google Scholar]
- Nelson C. L., Hickmon S. G., Skinner R. A. 1997. Treatment of experimental osteomyelitis by surgical debridement and the implantation of bioerodable, polyanhydridegentamicin beads. J. Orthop. Res. 15, 249–255. ( 10.1002/jor.1100150214) [DOI] [PubMed] [Google Scholar]
- Nicolau D. P., Nie L., Tessier P. R., Kourea H. P., Nightingale C. H. 1998. Prophylaxis of acute osteomyelitis with absorbable ofloxacin-impregnated beads. Antimicrob. Agents Chemother. 42, 840–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll S. B., Radin S., Santos E. M., Tuan R. S., Ducheyne P. 1997. In vitro release kinetics of biologically active transforming growth factor-β1 from a novel porous glass carrier. Biomaterials 18, 853–859. ( 10.1016/S0142-9612(97)00008-2) [DOI] [PubMed] [Google Scholar]
- Nie L., Nicolau D. P., Nightingale C. H., Browner B. D., Quintilliani R. 1995. In vitro elution of ofloxacin from a bioabsorbable polymer. Acta Orthop. Scand. 66, 365–368. [DOI] [PubMed] [Google Scholar]
- Nie L., Nicolau D. P., Tessier P. R., Kourea H. P., Browner B. D. 1998. Use of bioabsorbable polymer for the delivery of ofloxacin during experimental osteomyelitis treatment. J. Orthop. Res. 16, 76–79. ( 10.1002/jor.1100160113) [DOI] [PubMed] [Google Scholar]
- Nieto A., Balas F., Colilla M., Manzano M., Vallet-Regí M. 2008. Functionalization degree of SBA-15 as key factor to modulate sodium alendronate dosage. Microporous Mesoporous Mater. 116, 4–13. ( 10.1016/j.micromeso.2008.03.025) [DOI] [Google Scholar]
- Norton L. W., Tegnell E., Toporek S. S., Reichert W. M. 2005. In vitro characterization of vascular endothelial growth factor and dexamethasone releasing hydrogels for implantable probe coatings. Biomaterials 26, 3285–3297. ( 10.1016/j.biomaterials.2004.07.069) [DOI] [PubMed] [Google Scholar]
- Novak M. T., Bryers J. D., Reichert W. M. 2009. Biomimetic strategies based on viruses and bacteria for the development of immune evasive biomaterials. Biomaterials 30, 1989–2005. ( 10.1016/j.biomaterials.2008.11.025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeck J. P., Winckler S. T., Meffert R., Törmälä P., Spiege H. U., Brug E. 1995. Penetration of ciprofloxacin into bone: a new bioabsorbable implant. J. Invest. Surg. 8, 155–162. ( 10.3109/08941939509023138) [DOI] [PubMed] [Google Scholar]
- Palazzo B., Sidoti M. C., Tampieri A., Sandri M., Bertolazzi L., Galbusera F., Dubini G., Vena P., Contro R. 2005. Controlled drug delivery from porous hydroxyapatite grafts: an experimental and theoretical approach. Mater. Sci. Eng. C 25, 207–213. ( 10.1016/j.msec.2005.01.011) [DOI] [Google Scholar]
- Pang L., Hu Y., Yan Y., Liu L., Xiong Z., Wei Y., Bai J. 2007. Surface modification of PLGA/s-TCP scaffold for bone tissue engineering: hybridization with collagen and apatite. Surf. Coat. Technol. 201, 9549–9557. ( 10.1016/j.surfcoat.2007.04.035) [DOI] [Google Scholar]
- Panzavolta S., Torricelli P., Bracci B., Fini M., Bigi A. 2009. Alendronate and Pamidronate calcium phosphate bone cements: setting properties and in vitro response of osteoblast and osteoclast cells. J. Inorg. Biochem. 103, 101–106. ( 10.1016/j.jinorgbio.2008.09.012) [DOI] [PubMed] [Google Scholar]
- Papapoulos S. 2006. Bisphosphonate actions: physical chemistry revisited. Bone 38, 613–616. ( 10.1016/j.bone.2006.01.141) [DOI] [PubMed] [Google Scholar]
- Park S. N., Kim J. K., Suh H. 2004. Evaluation of antibiotic-loaded collagen-hyaluronic acid matrix as a skin substitute. Biomaterials 25, 3689–3698. ( 10.1016/j.biomaterials.2003.10.072) [DOI] [PubMed] [Google Scholar]
- Paul W., Sharma C. P. 1999. Development of porous spherical hydroxyapatite granules: application towards protein delivery. J. Mater. Sci. Mater. Med. 10, 383–388. ( 10.1023/A:1008918412198) [DOI] [PubMed] [Google Scholar]
- Perry A. C., Rouse M. C., Khaliq Y., Piper K. E., Hanssen A. D., Osmon D. R., Steckelberg J. M., Patel R. 2002. Antimicrobial release kinetics from polymethylmethacrylate in a novel continuous flow chamber. Clin. Orthop. 403, 49–53. [DOI] [PubMed] [Google Scholar]
- Prabu P., Dharmaraj N., Aryal S., Lee B. M., Ramesh V., Kim H. Y. 2006. Preparation and drug release activity of scaffolds containing collagen and poly(caprolactone). J. Biomed. Mater. Res. A 79, 153–158. [DOI] [PubMed] [Google Scholar]
- Pratten J., Nazhat S. N., Blaker J. J., Boccaccini A. R. 2004. In vitro attachment of Staphylococcus epidermidis to surgical sutures with and without Ag-containing bioactive glass coating. J. Biomater. Appl. 19, 47–57. ( 10.1177/0885328204043200) [DOI] [PubMed] [Google Scholar]
- Price J. S., Tencer A. F., Arm D. M., Bohach G. A. 1996. Controlled release of antibiotics from coated orthopedic implants. J. Biomed. Mater. Res. 30, 281–286. ( 10.1002/(SICI)1097-4636(199603)30:3%3C281::AID-JBM2%3E3.0.CO;2-M) [DOI] [PubMed] [Google Scholar]
- Queiroz A. C., Santos J. D., Monterio F. J., Gibson I. R., Knowles J. C. 2001. Adsorbtion and release studies of sodium ampicillin from hydroxyapatite and glass-reinforced hydroxyapatite composites. Biomaterials 22, 1393–1400. ( 10.1016/S0142-9612(00)00296-9) [DOI] [PubMed] [Google Scholar]
- Quirk R. A., France R. M., Shakesheff K. M., Howdle S. M. 2004. Supercritical fluid technologies and tissue engineering scaffolds. Curr. Opin. Solid State Mater. Sci. 8, 313–321. ( 10.1016/j.cossms.2003.12.004) [DOI] [Google Scholar]
- Ramay H., Zhang M. 2003. Preparation of porous hydroxyapatite scaffolds by combination of the gel-casting and polymer sponge methods. Biomaterials 24, 3293–3302. ( 10.1016/S0142-9612(03)00171-6) [DOI] [PubMed] [Google Scholar]
- Ramchandani M., Robinson D. 1998. In vitro–in vivo release of ciprofloxacin from PLGA 50:50 implants. J. Control. Release 54, 167–175. ( 10.1016/S0168-3659(97)00113-2) [DOI] [PubMed] [Google Scholar]
- Reichert J. C., et al. 2009. The challenge of establishing preclinical models for segmental bone defect research. Biomaterials 30, 2149–2163. ( 10.1016/j.biomaterials.2008.12.050) [DOI] [PubMed] [Google Scholar]
- Rezwan K., Chen Q. Z., Blaker J. J., Boccaccini A. R. 2006. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 27, 3413–3431. ( 10.1016/j.biomaterials.2006.01.039) [DOI] [PubMed] [Google Scholar]
- Rodan G. A., Martin T. J. 2000. Therapeutic approaches to bone diseases. Science 289, 1508–1514. ( 10.1126/science.289.5484.1508) [DOI] [PubMed] [Google Scholar]
- Roether A., Boccaccini A. R., Hench L., Maquet V., Gautier S., Jérôme R. 2002. Development and in vitro characterisation of novel bioresorbable and bioactive composite materials based on polylactide foams and Bioglass® for tissue engineering applications. Biomaterials 23, 3871–3878. ( 10.1016/S0142-9612(02)00131-X) [DOI] [PubMed] [Google Scholar]
- Roman J. S., Madruga E. L. 1989. Polymers with pharmacological activity: synthesis and free radical polymerization of p-methacryloyloxyacetanilide. Polymer 30, 949–954. ( 10.1016/0032-3861(89)90198-5) [DOI] [Google Scholar]
- Rossi S., Azghani A. O., Omri A. 2004. Antimicrobial efficacy of a new antibiotic-loaded poly(hydroxybutyric-co-hydroxyvaleric acid) controlled release system. J. Antimicrob. Chemother. 54, 1013–1018. ( 10.1093/jac/dkh477) [DOI] [PubMed] [Google Scholar]
- Rossi S., Marciello M., Sandri G., Ferrari F., Bonferoni M. C., Papetti A., Caramella C., Dacarro C., Grisoli P. 2007. Wound dressings based on chitosans and hyaluronic acid for the release of chlorhexidine diacetate in skin ulcer therapy. Pharm. Dev. Technol. 12, 415–422. ( 10.1080/10837450701366903) [DOI] [PubMed] [Google Scholar]
- Ruszczak Z., Friess W. 2003. Collagen as a carrier for on-site delivery of antibacterial drugs. Adv. Drug Deliv. Rev. 55, 1679–1698. ( 10.1016/j.addr.2003.08.007) [DOI] [PubMed] [Google Scholar]
- Rutledge B., Huyette D., Day D., Anglen J. 2003. Treatment of osteomyelitis with local antibiotics delivered via bioabsorbable polymer. Clin. Orthop. Relat. Res. 411, 280–287. ( 10.1097/01.blo.0000065836.93465.ed) [DOI] [PubMed] [Google Scholar]
- Saltzman W. M., Olbricht W. L. 2002. Building drug delivery into tissue engineering. Nat. Rev. Drug Discov. 1, 177–186. ( 10.1038/nrd744) [DOI] [PubMed] [Google Scholar]
- Santos E. M., Radin S., Ducheyne P. 1999. Sol–gel derived carrier for the controlled release of proteins. Biomaterials 20, 1695–1700. ( 10.1016/S0142-9612(99)00066-6) [DOI] [PubMed] [Google Scholar]
- Schnieders J., Gbureck U., Thull R., Kissel T. 2006. Controlled release of gentamycin from calcium phosphate-poly(lactic acid-co-glycolic acid) composite bone cement. Biomaterials 27, 4239–4249. ( 10.1016/j.biomaterials.2006.03.032) [DOI] [PubMed] [Google Scholar]
- Segreti J. 2000. Prosthetic joint infections. Curr. Treat. Options Infect. Dis. 2, 200–207. [Google Scholar]
- Shanmugasundaram N., Sundaraseelan J., Uma S., Selvaraj D., Babu M. 2006. Design and delivery of silver sulfadiazine from alginate microspheres-impregnated collagen scaffold. J. Biomed. Mater. Res. B, Appl. Biomater. 77, 378–388. [DOI] [PubMed] [Google Scholar]
- Shi X., Wang Y., Rena L., Zhao N., Gong Y., Wang D. 2009. Novel mesoporous silica-based antibiotic releasing scaffold for bone repair. Acta Biomater. 5, 1697–1707. ( 10.1016/j.actbio.2009.01.010) [DOI] [PubMed] [Google Scholar]
- Siris E., et al. 1996. Comparative study of alendronate versus etidronate for the treatment of Paget's disease of bone. J. Clin. Endocrinol. Metab. 81, 961–967. ( 10.1210/jc.81.3.961) [DOI] [PubMed] [Google Scholar]
- Somayaji B. V., Jariwala U., Jayachandran P., Vidyalakshmi K., Dudhani R. V. 1998. Evaluation of antimicrobial efficacy and release pattern of tetracycline and metronidazole using a local delivery system. J. Periodontol. 69, 409–413. [DOI] [PubMed] [Google Scholar]
- Sripriya R., Kumar M. S., Sehgal P. K. 2004. Improved collagen bilayer dressing for the controlled release of drugs. J. Biomed. Mater. Res. B Appl. Biomater. 70, 389–396. [DOI] [PubMed] [Google Scholar]
- Streubel J., Siepmann N. A., Peppas, Bodmeier R. 2000. Bimodal drug release achieved with multi-layer matrix tablets: transport mechanisms and device design. J. Control. Release 69, 455–468. ( 10.1016/S0168-3659(00)00334-5) [DOI] [PubMed] [Google Scholar]
- Sussman C., Bates-Jensen B. M. 2001. Wound care: a collaborative practice manual for physical therapists and nurses, 2nd edn. Gaithersburg, MD: Aspen Publishers. [Google Scholar]
- Takechi M., Miyamoto Y., Ishikawa K., Nagayama M., Kon M., Asaoka K., Suzuki K. 1998. Effects of added antibiotics on the basic properties of antiwashout-type fast-setting calcium phosphate cement. J. Biomed. Mater. Res. 39, 308–316. ( 10.1002/(SICI)1097-4636(199802)39:2%3C308::AID-JBM19%3E3.0.CO;2-8) [DOI] [PubMed] [Google Scholar]
- Takechi M., Miyamoto Y., Momota Y., Yuasa T., Tatehara S., Nagayama M., Ishikawa K., Suzuki K. 2002. The in vitro antibiotic release from anti-washout apatite cement using chitosan. J. Mater. Sci. Mater. Med. 13, 973–978. ( 10.1023/A:1019816830793) [DOI] [PubMed] [Google Scholar]
- Thomson R. C., Yaszemski M. J., Powers J. M., Mikos A. G. 1998. Hydroxyapatite fiber reinforced poly(a-hydroxylester) foams for bone regeneration. Biomaterials 18, 1935–1943. [DOI] [PubMed] [Google Scholar]
- Turesin F., Gursel I., Hasirci V. 2001. Biodegradable polyhydroxyalkanoate implants for osteomyelitis therapy: in vitro antibiotic release. J. Biomater. Sci. Polym. Ed. 12, 195–207. ( 10.1163/156856201750180924) [DOI] [PubMed] [Google Scholar]
- Välimäki V., Moritz N., Yrjans J., Vuorio E., Aro H. 2006. Effect of zoledronic acid on incorporation of a bioceramic bone graft substitute. Bone 38, 432–443. ( 10.1016/j.bone.2005.09.016) [DOI] [PubMed] [Google Scholar]
- Vallet-Regí M., Ragel C. V., Arcos D., Clavel-Sainz M., Meseguer-Olmo L. 2001. Métodos para la obtención de implantes bioactivos útiles como sistemas de liberación controlada. Spanish Patent no. P22001–01386.2181593. [Google Scholar]
- Vallet-Regí M., Ruiz-González L., Izquierdo-Barba I., González-Calbet J. 2006. Revisiting silica based ordered mesoporous materials: medical applications. J. Mater. Chem. 16, 26–31. ( 10.1039/b509744d) [DOI] [Google Scholar]
- Vallet-Regí M., Balas F., Arcos D. 2007. Mesoporous materials for drug delivery. Angew Chem. Int. Ed. 46, 7548–7558. [DOI] [PubMed] [Google Scholar]
- Vallet-Regí M., Balas F., Colilla M., Manzano M. 2008. Bone-regenerative bioceramic implants with drug and protein controlled delivery capability. Prog. Solid State Chem. 36, 163–191. [Google Scholar]
- Vallo I., Abraham G. A., Cuadrado T. R., San Román J. 2004. Influence of cross-linked PMMA beads on the mechanical behavior of self-curing acrylic cements. J. Biomed. Mater. Res. B 70, 407–416. [DOI] [PubMed] [Google Scholar]
- Van Beek E. R., Cohen L. H., Leroy I. M., Ebetino F. H., Löwik C. W., Papapoulos S. E. 2003. Differentiating the mechanisms of antiresorptive action of nitrogen containing bisphosphonates. Bone 33, 805–811. [DOI] [PubMed] [Google Scholar]
- Verné E., Di Nunzio S., Bosetti M., Appendino P., Vitale-Brovarone C., Maina G., Cannas M. 2005. Surface characterization of silver-doped bioactive glass. Biomaterials 26, 5111–5119. ( 10.1016/j.biomaterials.2005.01.038) [DOI] [PubMed] [Google Scholar]
- Victor S. P., Kumar T. S. S. 2008. BCP ceramic microspheres as drug delivery carriers: synthesis, characterisation and doxycycline release. J. Mater. Sci. Mater. Med. 19, 283–290. ( 10.1007/s10856-006-0044-7) [DOI] [PubMed] [Google Scholar]
- Virk A., Osmon D. 2001. Prosthetic joint infections. Curr. Treat. Options Infect. Dis. 3, 287–300. [Google Scholar]
- Vitale-Brovarone C., Di Nunzio S., Bretcanu O., Verné E. 2004. Macroporous glass-ceramic materials with bioactive properties. J. Mater. Sci.: Mater. Med. 15, 209–217. ( 10.1023/B:JMSM.0000015480.49061.e1) [DOI] [PubMed] [Google Scholar]
- Vitale-Brovarone C., Verné E., Appendino P. J. 2006. Macroporous bioactive glass-ceramic scaffolds for tissue engineering. J. Mater. Sci.: Mater. Med. 17, 1069–1078. ( 10.1007/s10856-006-0533-8) [DOI] [PubMed] [Google Scholar]
- Vitale-Brovarone C., Verné E., Robiglio L., Appendino P., Bassi F., Martinasso G., Muzio G., Canuto R. 2007. Development of glass–ceramic scaffolds for bone tissue engineering: characterisation, proliferation of human osteoblasts and nodule formation. Acta Biomater. 2, 199–208. [DOI] [PubMed] [Google Scholar]
- Vitale-Brovarone C., Miola M., Balagna C., Verné E. 2008. 3D-glass–ceramic scaffolds with antibacterial properties for bone grafting. Chem. Eng. J. 137, 129–136. ( 10.1016/j.cej.2007.07.083) [DOI] [Google Scholar]
- Wang H., Li Y., Zuo Y., Li J., Ma S., Cheng L. 2007. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials 28, 3338–3348. ( 10.1016/j.biomaterials.2007.04.014) [DOI] [PubMed] [Google Scholar]
- Webb J. C. J., Spencer R. F., Lovering A. M., Learmonth I. D. 2005. Very late release of gentamicin from bone cement in total hip arthroplasty (THA). J. Bone Joint Surg. Br. 87-B(Suppl. 1), 52-b-. [Google Scholar]
- Whang K., et al. 1999. Engineering bone regeneration with bioabsorbable scaffolds with novel microarchitecture. Tissue Eng. 5, 35–51. [DOI] [PubMed] [Google Scholar]
- Wu P., Grainger D. W. 2006. Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 27, 2450–2467. ( 10.1016/j.biomaterials.2005.11.031) [DOI] [PubMed] [Google Scholar]
- Xu Q., Czernuszka J. T. 2008. Controlled release of amoxicillin from hydroxyapatite coated poly(lactic-co-glycolic acid) microspheres. J. Control. Release 127, 146–153. [DOI] [PubMed] [Google Scholar]
- Xynos I. D., Edgar A. J., Buttery L. D., Hench L. L. 2001. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 55, 151–157. ( 10.1002/1097-4636(200105)55:2%3C151::AID-JBM1001%3E3.0.CO;2-D) [DOI] [PubMed] [Google Scholar]
- Yagmurlu M. F., Korkusuz F., Gürsel I., Korkusuz P., Ors U., Hasirci V. 1999. Sulbactam-cefoperazone polyhydroxybutyrate-co-hydroxyvalerate (PHBV) local antibiotic delivery system: in vivo effectiveness and biocompatibility in the treatment of implant-related experimental osteomyelitis. J. Biomed. Mater. Res. 46, 494–503. ( 10.1002/(SICI)1097-4636(19990915)46:4%3C494::AID-JBM7%3E3.0.CO;2-E) [DOI] [PubMed] [Google Scholar]
- Younger E. M., Chapman M. W. 1989. Morbidity at bone graft donor sites. J. Orthop. Trauma 3, 192–195. [DOI] [PubMed] [Google Scholar]
- Yuan H., Kurashina K., Beuijn J. D., Li Y., De Groot K., Zhang X. 1999. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials 20, 1799–1806. ( 10.1016/S0142-9612(99)00075-7) [DOI] [PubMed] [Google Scholar]
- Yun H. S., Kim S. E., Hyun Y. T., Heo S. J., Shin J. W. 2008. Hierarchically mesoporous-macroporous bioactive glasses scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. 87B, 374–380. ( 10.1002/jbm.b.31114) [DOI] [PubMed] [Google Scholar]
- Yunos D. M., Bretcanu O., Boccaccini A. R. 2008. Polymer-bioceramic composites for tissue engineering scaffolds. J. Mater. Sci. 43, 4433–4442. [Google Scholar]
- Zhang Y., Zhang M. 2002. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. 62, 378–386. ( 10.1002/jbm.10312) [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang J., Feng H., Lu B., Song Z., Zhang X. 2001. Replacement of segmental bone defects using porous bioceramic cylinders: a biomechanical and X-ray diffraction study. J. Biomed. Mater. Res. 54, 407–411. ( 10.1002/1097-4636(20010305)54:3%3C407::AID-JBM140%3E3.0.CO;2-C) [DOI] [PubMed] [Google Scholar]
- Zhu Y., Kaskel S. 2009. Comparison of the in vitro bioactivity and drug release property of mesoporous bioactive glasses (MBGs) and bioactive glasses (BGs) scaffolds. Microporous Mesoporous Mater. 118, 176–182. ( 10.1016/j.micromeso.2008.08.046) [DOI] [Google Scholar]
- Ziegler J., Mayr-Wohlfart U., Kessler S., Breitig D., Gunther P. 2002. Adsorption and release properties of growth factors from biodegradable implants. J. Biomed. Mater. Res. 59, 422–428. ( 10.1002/jbm.1258) [DOI] [PubMed] [Google Scholar]
- Zilberman M., Elsner J. J. 2008. Antibiotic-eluting medical devices for various applications. J. Control. Release 130, 202–215. ( 10.1016/j.jconrel.2008.05.020) [DOI] [PubMed] [Google Scholar]