Abstract
Since W. D. Hamilton's seminal work on the evolution of sociality, a large body of research has accumulated on how kin selection might explain the evolution of cooperation in many group-living species. Our study examined the evolutionary basis of philopatry and cooperation; specifically, whether individuals benefit from the presence of close kin. We applied an individual fitness approach to a 16-year study of Columbian ground squirrels (Urocitellus columbianus) to investigate potential causal paths by which the presence of kin might act on individual fitness. Our results indicate that individual fitness benefits resulted from associations of philopatric female kin, and support the hypothesis that increased tolerance of proximity of kin is a proximate mechanism for these benefits. The major life-history influence of kin on individual fitness was through improved reproductive success, and this benefit may have been owing to philopatric settlement of kin that were recognized through familiarization in the natal burrow. Thus, we demonstrated an evolutionary basis necessary for ongoing kin-selected cooperation in Columbian ground squirrels, though the mechanism of familiarity may determine which kin individuals benefit from cooperative behaviours.
Keywords: individual fitness, philopatry, kin recognition, kin selection, path analysis, Urocitellus columbianus
1. Introduction
Since W. D. Hamilton's early discussion of the genetic evolution of social behaviour (Hamilton 1964), many advances have been made in our understanding of how kin selection might promote the evolution of cooperation and sociality (West-Eberhard 1975; Wilson 1975; Oli 2003; Bshary & Bergmüller 2008). Kin selection is likely to operate whenever the benefits of helping a relative reproduce overcome any fitness costs of the helping behaviour (Komdeur 1992; Komdeur et al. 1995; Oli 2003). For kin selection to favour cooperation, individuals with kin present should have enhanced fitness compared to individuals without kin. This expectation has seldom been tested with an appropriate fitness measure, though many studies have examined nepotism associated with just a few fitness traits (e.g. MacColl et al. 2000; Pope 2000; reviewed in primates, Silk 2007).
The individual fitness approach (McGraw & Caswell 1996; Oli 2003; Oli & Armitage 2008) should provide an alternative for testing whether kin presence is beneficial to fitness. Individual fitness can be estimated for animals that live together and have different degrees of kinship. An association of presence of kin and greater individual fitness would show that philopatry is adaptive, and that heritable behaviours associated with kinship might evolve through kin selection. While indirect, this expectation provides a test of whether kin selection could favour helping or cooperation within a population.
We investigated philopatric maternal kin in a local population of Columbian ground squirrels (Urocitellus columbianus). These ground squirrels are hibernating rodents with a short active season of three to four months (Dobson et al. 1992). They are relatively long lived (up to about 10 years old), and generation overlap (both spatial and temporal) allows for the presence of matrilineal kin and the occurrence of nepotistic behaviours (King & Murie 1985; King 1989a). Female philopatry produces kin associations that may result in both competition and cooperation among relatives (Dobson et al. 1997; Dobson 1998; for a review, Lawson Handley & Perrin 2007). Females exhibit territoriality (Murie & Harris 1978, 1988) and kin-differential behaviours, namely reduced aggressiveness and increased tolerance of close maternal kin (King 1989b). Such observations raise the question of whether fitness benefits are associated with cohabitation with kin.
We performed a detailed analysis of causal influences by which the presence of kin might increase individual fitness. First, we examined whether preference was given to territorial establishment around close kin (i.e. littermates and their mother) by recording and comparing distances between natal nest burrows for neighbouring females that were or were not closely related. We then examined variance in individual fitness of females, and compared this with the average number of kin present for each female over her lifespan. Further, we investigated whether fitness differences were observable for sisters (including full and half sisters) that were either littermates or non-littermates, as the recognition mechanism of close relatives might constrain kin-differential behaviour (King 1989a). If individual fitness were enhanced by kin selection but constrained by social learning in the natal nest, we would expect only the presence of kin from the same natal nest to positively affect direct fitness.
2. Material and methods
(a). Study population and data collection
Ground squirrels were followed from 1992 to 2007 at the Sheep River Wildlife Sanctuary, Alberta, Canada (110° W, 50° N and elevation 1500 m). Animals were live-trapped each year within a couple of days of emergence from hibernation using National Live Traps (Tomahawk Co., WI, USA; 13 × 13 × 40 cm3) baited with peanut butter. Each ground squirrel was marked with ear tags (Monel no. 1 National Band & Tag Co.) and was weighed to the nearest 5 g using a Pesola spring-slide balance. Zygomatic arch breadth (ZAB), an index of structural size, was measured to the nearest 0.1 mm by placing callipers over the animals' heads and clasping both sides of the zygomatic arch (described by Dobson et al. 1999). Complete litters were caught and marked when the young emerged from nest burrows near the time of weaning and mothers were thus associated with a single litter in each year.
(b). Inter-nest burrow distances
We considered as neighbours all reproductive females that established their natal nest burrow less than 30 m (roughly a home range diameter; Elliott & Flinders 1991) away from one another. Location of the nest sites were mapped using a Cartesian grid of flagged wires placed over the colony (cell size = 10 × 10 m), and nest burrows were determined either by live-trapping newly emerging juveniles at those specific sites, or by observing a lactating female there at morning emergence. We subsequently compared inter-burrow distances between focal individuals and close kin neighbours (littermate sisters and mothers), and between focal individuals and less-related individuals.
(c). Fitness estimates
Since a mother was associated with a single litter in each year, we were able to establish maternal and sibling (maternal component) relatedness for all females born since 1992. Reproductive data were complete and allowed us to estimate fitness indices for females. In order to integrate reproductive success and survival into a single measure of fitness, we established individual transition matrices (McGraw & Caswell 1996) for each of the 70 females of our study with complete life-time data. Transition matrices can be considered age-structured population projection matrices, applied to one individual. They are used to calculate the population growth rate of an individual (a genotype). An example of one such matrix follows:
 |
For each individual, the top row of the matrix represents reproductive success during each year of survival (reproductive success is 0.5 times the number of offspring weaned, since only half of the genome is passed on to future generations). Survival is reported in the matrix sub-diagonal and is binary (1, if the animal lives until the next year; 0, if not). The size of the matrix depends on the lifespan of the female (range = 2–10 years, average = 4.87 years). Thus, from age-specific fecundities and age-specific survival, the matrix allows the calculation of eigenvalues. The dominant eigenvalue is a population growth rate based on the individual, a measure of individual fitness. In the above case, this female (A958) lived for a total of 4 years, during which she weaned a total of (0 × 2) + (1.5 × 2) + (1.5 × 2) + (1.5 × 2) = 9 pups. Calculation yielded an eigenvalue (the individual fitness) of λ = 1.7025.
Eigenvalues thus represent an individual's propensity to survive and reproduce within a given community. However, individual fitness might be related to changes in population density, because some females have lifespans in increasing years, whereas others might have lifespans in decreasing years. For example, a mother with an individual fitness of 1 would be below mean fitness in years of population increase, but her fitness would be above average in years of population decline. We thus entered finite population growth rates into a matrix that yielded a relative index of population fitness that took into account annual population growth rates during different years of the female's lifetime. The regression of adult females' individual fitnesses on this relative population index was highly significant (r2 = 0.33, F = 33.387, d.f. = 1, p < 0.001, n = 70), and we used the residuals as individual fitness estimates adjusted for changes in population size. Analyses of fitness with and without this adjustment yielded very similar results and identical conclusions, but we present individual fitness adjusted for changes in population size because they should be better estimates.
(d). Path analyses: the influence of kinship on female's direct component of fitness
We used standardized partial regression analysis (viz. path analysis; Li 1981) to evaluate variables that might explain variance in individual fitness for our 70 females. Because they are influential in the life-history matrix, we examined influences of female lifespan (i.e. longevity; mean = 4.87, s.d. = 3.11), age at maturity (mean = 2.3, s.d. = 0.77) and offspring production (i.e. mean litter size weaned; mean = 1.55, s.d. = 0.68) in order to partition their contribution to the individual fitness measure. This subsequently allowed us to estimate indirect effects in the path model. In turn, these life-history traits might be influenced by maternal structural size (i.e. ZAB; mean = 33.69, s.d. = 0.97), maternal body condition at spring emergence (estimated by the regression residuals of body mass on ZAB; Dobson 1992; Schulte-Hostedde et al. 2005; mean = 0.01, s.d. = 1) and the Julian date of spring emergence (i.e. the first time the animal was seen above ground; mean = 116.13, s.d. = 7.96; Dobson et al. 1999; Broussard et al. 2005).
For each of our 70 females, the number of kin present in the colony during the breeding season each year was averaged over her lifespan (mean = 2.28, s.d. = 1.51, range = 0–5.60) and was included as an independent variable in the model. This allowed us to investigate the indirect influences of kinship on individual fitness through offspring production, age at maturity and lifespan.
Data were tested for normality and transformed wherever necessary using rank transformation procedures from SPSS. Age at maturity was neither normally distributed nor greatly improved by transformation (skew = −0.02, kurtosis = 2.66), but we included this variable because of its known influence on λ (Oli & Dobson 2003). Analyses without this variable revealed very similar patterns to those reported below. Data were standardized, which is necessary when analysing variables measured on different scales (such as mass, number of individuals, frequency and fitness value). Independent variables were checked for collinearity using variance inflation factors (VIFs) and collinearity diagnostic procedures from SPSS.
Additionally, we considered the issue of kin discrimination. Kin-biased behaviour in Columbian ground squirrels might be based on social learning of individual identities (King 1989a; Hare & Murie 1996). We conducted a multiple regression allowing us to test our assumption that only natal kin should positively affect a female's direct fitness. For our 70 females, we examined the average number of individuals present in the colony over their lifespan in three categories: (i) natal kin (including a mixture of full and half sibs owing to multiple paternity; Murie 1995; mean = 1.22, s.d. = 0.69, range = 0–2.75); (ii) non-natal kin (i.e. non-littermate sisters, which may also contain full and half sibs; mean = 1.05, s.d. = 1.12, range = 0–3.67); (iii) distantly related and virtually non-kin individuals (mean = 48.80, s.d. = 19.45, range = 0–72.50). All analyses were performed using SPSS v.16.0 and R v.2.6.1 statistical software, and one-tailed tests based on a priori predictions carried out at α ≤ 0.05.
3. Results
(a). Inter-burrow distances
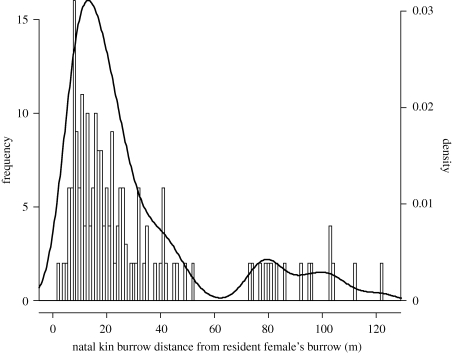
Inter-burrow distance was significantly less between lactating mothers that shared common natal environments as pups (viz. littermates and their mother) than between more distantly or unrelated individuals (Wilcoxon's signed-rank test; W = 10 131, p < 0.0001, n = 117). For focal adult females, female natal kin neighbours averaged 25 per cent closer than non-natal kin neighbours (means ± s.e. = 15.06 ± 0.05 m versus 20.09 ± 0.04 m). On average for a given female and a given year, 0.49 ± 0.002 natal kin versus 2.9 ± 0.008 non-natal kin or virtually unrelated individuals were located within 30 m of the nest burrow (means ± s.e.). When considering the spatial distribution of kin females over all the years and over the whole meadow, we found that, on average, and for each female having at least one kin present (n = 171), 72 per cent of their natal kin were established within their immediate surroundings (viz. within the 30 m home range of the focal females; figure 1).
Figure 1.
The distance between natal nest locations for adult females that were natal kin and reproduced concurrently on the study area. A histogram of the frequency distribution and a density function (viz. a kernel density plot) are shown.
(b). Potential causal influences of kinship on female fitness
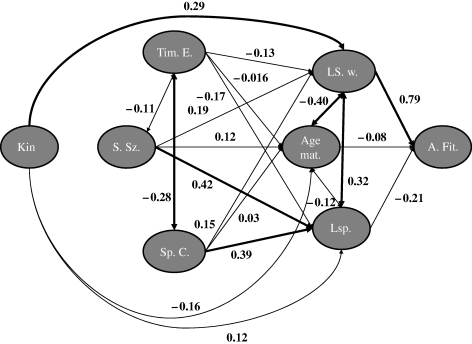
A path model was used to identify variables that contributed to variation among females in individual fitness (figure 2; r2 = 0.61, F = 34.74, d.f. = 3, p < 0.0001, n = 70). The standardized partial regression coefficient (viz. path coefficient) for the influence of offspring production (i.e. litter size weaned) was high and significant (β = 0.79, t = 8.97, p < 0.0001), but age at maturity and mother's lifespan did not have significant influences on individual fitness (respectively, β = −0.08, t = −0.98, p = 0.33; β = −0.21, t = −2.65, p > 0.95). Note that the path coefficient for mother's lifespan was negative, contrary to prediction, supporting the null hypothesis of no positive influence. Although offspring numbers were significantly correlated with maternal lifespan and age at maturity, condition indices and VIFs did not indicate a significant bias owing to collinearity. VIFs were between 1.12 and 1.31 (suggested cut-off about 10; Myers 1990), and condition indices were between 1.25 and 1.71 (suggested cut-off about 30; Belsley et al. 1980).
Figure 2.
Influence of kinship on female fitness: overall path diagram with path coefficients. Variables: Kin, mean number of kin present over lifetime; Sp. C., mother's spring body condition; S. Sz., mother's structural size; Tim. E., timing of emergence; LS. w., litter size at weaning; Lsp., mother's lifespan; Age mat., age at maturity; A. Fit., adjusted fitness. Significant paths (p < 0.05) appear in bold. Single-headed arrows indicate causality, whereas double-headed arrows indicate correlations (n = 70).
Multiple regressions of offspring production or age at maturity on maternal spring body condition, structural size and timing of spring emergence were not significant and did not explain substantial amounts of the variation (figure 2; r2 = 0.08, F = 2.06, d.f. = 3, p = 0.11, n = 70 and r2 = 0.016, F = 0.37, d.f. = 3, p = 0.77, n = 70, respectively; 1.01 < VIFs < 1.10). However, the multiple regression explained significant variation in mother's lifespan (r2 = 0.38, F = 13.55, d.f. = 3, p < 0.0001, n = 70). Mother's body condition and structural size had a noticeable effect on female lifespan (respectively, β = 0.39, t = 3.86, p < 0.0001; β = 0.42, t = 4.31, p < 0.0001), but the effect of timing of spring emergence was not significant (β = −0.17, t = −1.69, p = 0.09).
Mean number of kin was significantly associated with mean litter size at weaning (r = 0.29, p = 0.01, n = 70). An indirect influence of kin on individual fitness through the influence of offspring production on fitness was significant (figure 2; pathindirect = 0.23; indirect paths are generally considered significant when both direct paths are significant; Cohen & Cohen 1983).
(c). Natal kin, non-natal kin and individual fitness
Multiple regression of individual fitness on numbers of individuals present per year (averaged over lifetime) for three categories of individuals (natal kin, non-natal kin and distant or non-kin) was significant (r2 = 0.15, F = 3.84, d.f. = 3, p < 0.05, n = 70, 1.04 < VIFs < 1.18). Consistent with our prediction, natal kin had a positive effect on female fitness (β = 0.37, t = 3, p < 0.01, n = 70), whereas the two other classes of individuals explained little of the variation observed in individual female fitness (β = 0.011, t = 0.093, p = 0.92, n = 70 for non-natal kin; β = −0.18, t = −1.56, p = 0.12, n = 70 for non-kin). Although the effect is not significant, the negative path coefficient (−0.18) for non-kin suggests a negative effect of this class on individual fitness.
4. Discussion
The Hamiltonian theory of kin selection has received considerable support over the past 30 years in taxa as diverse as insects (West-Eberhard 2005), spiders (Anthony 2003), birds (Komdeur 1992; Komdeur et al. 1995; MacColl et al. 2000) and mammals (Holekamp & Smale 1991; Armitage & Schwartz 2000). However, to our knowledge, few studies have investigated the effect that kin have on the fitness of close relatives (see Armitage & Schwartz 2000; MacColl et al. 2000). We report evidence of direct fitness benefits associated with matriline establishment via kin selection for female Columbian ground squirrels.
Estimating individual fitness can be achieved by constructing individual transition matrices that reflect reproduction and survival (McGraw & Caswell 1996). However, when considering life-history data and comparing fitness estimates, special attention should be given to individual fitness relative to other individuals in the population. Comparisons could be biased, for instance, if some individuals reproduce primarily in years with increasing population densities, when average individual fitness is high, whereas others reproduce mainly in years with decreasing population densities. We thus adjusted individual fitness by taking changes in population size into account. In Columbian ground squirrels, population density is likely to vary over the years, as environmental conditions, such as food availability, vary (Dobson & Kjelgaard 1985; Dobson 1995; Dobson & Oli 2001).
Offspring production had the strongest effect in explaining differences observed in individual fitness and was by far the most important demographic factor contributing to individual fitness among the elements of the matrix population model (figure 2). In addition, mothers' traits such as body size (structural) and body condition had strong influences only on longevity among the demographic variables, and they appeared to have little or no indirect influence on individual fitness. Our analyses provide support for individual fitness benefits from the presence of natal kin in Columbian ground squirrels. Females with more close kin around them exhibit higher direct fitness values than females with fewer kin present. The presence of kin acts positively on fitness through a direct effect on mean litter size at weaning (β = 0.29). Kinship did not have a strong influence on age at maturity or lifespan through direct or indirect effects.
Our analysis of natal inter-burrow distances of natal kin versus more distant kin and unrelated individuals supported a role for shared space and decreased aggression between natal kin. Davis (1984) reported that female Richardson's ground squirrels (Spermophilus richardsonii) were less likely to be chased away from a resident female's core area if they were closely related to the resident. In Columbian ground squirrels, most natal kin individuals established themselves close to one another (figure 1), in spite of the fact that non-natal kin and unrelated individuals outnumber natal kin as neighbours by six to one. This finding supports previous results of kin spatial overlap and inheritance of territories in Columbian ground squirrels (Harris & Murie 1984; King 1989b), and ensuing benefits might be seen in the form of increased energy for reproduction (Davis 1984; King 1989a). Moreover, protection of young against infanticide might be an additional benefit of close cohabitation of natal kin. We found that although natal kin showed strong preference for clustering, resident females still had substantial amounts of non-natal kin and virtually unrelated individuals around their nest burrows. Perpetrators of infanticide are usually other lactating females near the time of the first emergence of juveniles above ground from natal burrows (Dobson 1990). Over a 2-year study, conspecifics were observed to kill 16 out of 209 (8%) newly emerged juveniles from 9 out of 72 (13%) litters; only two of the victims in the study were closely related to the perpetrator (i.e. relatedness coefficient r ≥ 0.25; Stevens 1998).
Comprehensive reviews on rodent sociality (Solomon 2003; Hare & Murie 2007) emphasize the role of philopatry in social evolution. Benefits for females of remaining philopatric include inheritance of territories, monitoring of nearby territories, mating with unrelated group members and increased direct fitness benefits (Davis 1984; Armitage & Schwartz 2000). Life-history advantages such as increased developmental rate or survival might also favour philopatric establishment. After philopatry, which brings kin into association, kin selection provides a basis for the evolution of cooperation and sociality. Whereas some studies have demonstrated indirect fitness benefits (Sherman 1977; McLean 1982) or greater tolerance (Michener 1973; Davis 1984; King 1989b; Hare 2004) resulting from kin-selected nepotism, others have called into question the extent to which kin selection might explain the evolution of ground squirrel sociality (Armitage 1987). Our study reports direct fitness benefits occurring from matriline establishment in Columbian ground squirrels, supporting kin selection as promoting the evolution of sociality. In our case, rather than measure kin selection directly, we demonstrated the fitness differences necessary for evolution via kin selection.
Our study supported King's (1989a) suggestion that recognition mechanisms constrain the degree of kinship over which direct fitness benefits occur because of the presence of kin. Cooperation among kin and the evolution of sociality in Columbian ground squirrels seem to favour only natal littermate kin because of this important constraint.
Acknowledgements
The Biogeoscience Institute (especially E. Johnson, Director and J. Mappin-Buchannan, Manager), University of Calgary, provided field camp and laboratory facilities. A host of field assistants over the years made our data collection possible. We are grateful to S. Servanty for help with matrix analyses. The manuscript benefited from the helpful comments and critical thinking of D. T. Blumstein and C. Saraux. This research was funded by Natural Sciences and Engineering Research Council of Canada grant to J.O.M. and by a National Science Foundation grant (DEB-0089473) to F.S.D.
References
- Anthony C. D.2003Kinship influences cannibalism in the Wolf spider, Pardosa milvina. J. Insect Behav. 16, 23–36 (doi:10.1023/A:1022893127216) [Google Scholar]
- Armitage K. B.1987Social dynamics of mammals: reproductive success, kinship and individual fitness. Trends Ecol. Evol. 2, 279–284 (doi:10.1016/0169-5347(87)90037-1) [DOI] [PubMed] [Google Scholar]
- Armitage K. B., Schwartz O. A.2000Social enhancement of fitness in yellow-bellied marmots. Proc. Natl Acad. Sci. USA 97, 12 149–12 152 (doi:10.1073/pnas.200196097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsley D. A., Kuh E., Welsch R. E.1980Regression diagnostics New York, NY: John Wiley & Sons, Inc [Google Scholar]
- Broussard D. B., Dobson F. S., Murie J. O.2005The effects of capital on an income breeder: evidence from female Columbian ground squirrels. Can. J. Zool. 83, 546–552 (doi:10.1139/z05-044) [Google Scholar]
- Bshary R., Bergmüller R.2008Distinguishing four fundamental approaches to the evolution of helping. J. Evol. Biol. 21, 405–420 (doi:10.1111/j.1420-9101.2007.01482.x) [DOI] [PubMed] [Google Scholar]
- Cohen J., Cohen P.1983Applied multiple regression/correlation for the behavioral sciences, 2nd edn.Hillsdale, NJ: Erlbaum Press [Google Scholar]
- Davis L. S.1984Kin selection and adult female Richardson's ground squirrels: a test. Can. J. Zool. 62, 2344–2348 (doi:10.1139/z84-343) [Google Scholar]
- Dobson F. S.1990Environmental influences on infanticide in Columbian ground squirrels. Ethology 84, 3–14 [Google Scholar]
- Dobson F. S.1992Body mass, structural size, and life-history patterns of the Columbian ground squirrel. Am. Nat. 140, 109–125 (doi:10.1086/285405) [DOI] [PubMed] [Google Scholar]
- Dobson F. S.1995Regulation of population size: evidence from Columbian ground squirrels. Oecologia 102, 44–51 [DOI] [PubMed] [Google Scholar]
- Dobson F. S.1998Social structure and gene dynamics in mammals. J. Mammal. 79, 667–670 [Google Scholar]
- Dobson F. S., Kjelgaard J. D.1985The influences of food resources on life history in Columbian ground squirrels. Can. J. Zool. 63, 2105–2109 (doi:10.1139/z85-309) [Google Scholar]
- Dobson F. S., Oli M. K.2001The demographic basis of population regulation in Columbian ground squirrels. Am. Nat. 158, 236–247 (doi:10.1086/321322) [DOI] [PubMed] [Google Scholar]
- Dobson F. S., Badry M. J., Geddes C.1992Seasonal activity and body mass of Columbian ground squirrels. Can. J. Zool. 70, 1364–1368 (doi:10.1139/z92-192) [Google Scholar]
- Dobson F. S., Chesser R. K., Hoogland J. L., Sugg D. W., Foltz D. W.1997Do black-tailed prairie dogs minimize inbreeding? Evolution 51, 970–978 (doi:10.2307/2411170) [DOI] [PubMed] [Google Scholar]
- Dobson F. S., Risch T. S., Murie J. O.1999Increasing returns in the life history of Columbian ground squirrels. J. Anim. Ecol. 68, 73–86 (doi:10.1046/j.1365-2656.1999.00268.x) [Google Scholar]
- Elliott C. L., Flinders J. T.1991Spermophilus columbianus Mamm. Species 372, 1–9 [Google Scholar]
- Hamilton W. D.1964The genetical evolution of social behaviour I. and II. J. Theor. Biol. 7, 1–52 (doi:10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- Hare J. F.2004Kin discrimination by asocial Franklin's ground squirrels (Spermophilus franklinii): is there a relationship between kin discrimination and ground squirrel sociality? Ethol. Ecol. Evol. 16, 157–169 [Google Scholar]
- Hare J. F., Murie J. O.1996Ground squirrel sociality and the quest for the ‘holy grail’: does kinship influence behavioral discrimination by juvenile Columbian ground squirrels? Behav. Ecol. 7, 76–81 (doi:10.1093/beheco/7.1.76) [Google Scholar]
- Hare J. F., Murie J. O.2007Ecology, kinship, and ground-squirrel sociality: insights from comparative analyses. In Rodent societies: an ecological and evolutionary perspective (eds Wolff J. O., Sherman P. W.), ch. 29, pp. 345–355 Oxford, UK: Oxford University Press [Google Scholar]
- Harris M. A., Murie J. O.1984Inheritance of nest sites in female Columbian ground squirrels. Behav. Ecol. Sociobiol. 15, 354–356 [Google Scholar]
- Holekamp K. E., Smale L.1991Dominance acquisition during mammalian social development: the ‘inheritance’ of maternal rank. Am. Zool. 31, 306–317 [Google Scholar]
- King W. J.1989aKin-differential behavior of adult female Columbian ground-squirrels. Anim. Behav. 38, 354–356 (doi:10.1016/S0003-3472(89)80097-1) [Google Scholar]
- King W. J.1989bSpacing of female kin in Columbian ground squirrels (Spermophilus columbianus). Can. J. Zool. 67, 91–95 (doi:10.1139/z89-014) [Google Scholar]
- King W. J., Murie J. O.1985Temporal overlap of female kin in Columbian ground-squirrels (Spermophilus columbianus). Behav. Ecol. Sociobiol. 16, 337–341 (doi:10.1007/BF00295546) [Google Scholar]
- Komdeur J.1992Importance of habitat saturation and territory quality for evolution of cooperative breeding in the Seychelles warbler. Nature 358, 493–965 (doi:10.1038/358493a0) [Google Scholar]
- Komdeur J., Huffstadt A., Prast W., Castle G., Mileto R., Wattel J.1995Transfer experiments of Seychelles warblers to new islands: changes in dispersal and helping behaviour. Anim. Behav. 49, 695–708 [Google Scholar]
- Lawson Handley L. J., Perrin N.2007Advances in our understanding of mammalian sex-biased dispersal. Mol. Ecol. 16, 1559–1578 (doi:10.1111/j.1365-294X.2006.03152.x) [DOI] [PubMed] [Google Scholar]
- Li C. C.1981Path analysis—a primer Pacific Grove, CA: Boxwood Press [Google Scholar]
- MacColl A. D. C., Piertney S. B., Moss R., Lambin X.2000Spatial arrangement of kin affects recruitment success in young male red grouse. Oikos 90, 261–270 (doi:10.1034/j.1600-0706.2000.900206.x) [Google Scholar]
- McGraw J. B., Caswell H.1996Estimation of individual fitness from life-history data. Am. Nat. 147, 47–64 (doi:10.1086/285839) [Google Scholar]
- McLean I. G.1982The association of female kin in the arctic ground squirrel (Spermophilus parryii). Behav. Ecol. Sociobiol. 10, 91–99 (doi:10.1007/BF00300168) [Google Scholar]
- Michener G. R.1973Field observations on the social relationships between adult female and juvenile Richardson's ground squirrels. Can. J. Zool. 51, 33–38 (doi:10.1139/z73-006) [Google Scholar]
- Murie J. O.1995Mating behavior of Columbian ground squirrels. I. Multiple mating by females and multiple paternity. Can. J. Zool. 73, 1819–1826 (doi:10.1139/z95-214) [Google Scholar]
- Murie J. O., Harris M. A.1978Territoriality and dominance in male Columbian ground squirrels (Spermophilus columbianus). Can. J. Zool. 56, 2402–2412 (doi:10.1139/z78-325) [Google Scholar]
- Murie J. O., Harris M. A.1988Social interactions and dominance relationships between female and male Columbian ground squirrels. Can. J. Zool. 66, 1414–1420 (doi:10.1139/z88-207) [Google Scholar]
- Myers R. H.1990Classical and modern regression with applications, 2nd edn Boston, MA: P.W.S. Kent [Google Scholar]
- Oli M. K.2003Hamilton goes empirical: estimation of inclusive fitness from life-history data. Proc. R. Soc. Lond. B 270, 307–311 (doi:10.1098/rspb.2002.2227) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oli M. K., Armitage K. B.2008Indirect fitness benefits do not compensate for the loss of direct fitness in yellow-bellied marmots. J. Mammal. 89, 874–881 (doi:10.1644/07-MAMM-A-146.1) [Google Scholar]
- Oli M. K., Dobson F. S.2003The relative importance of life-history variables to population growth rate in mammals: Cole's prediction revisited. Am. Nat. 161, 422–440 (doi:10.1086/367591) [DOI] [PubMed] [Google Scholar]
- Pope T. R.2000Reproductive success increases with degree of kinship in cooperative coalitions of female red howler monkeys (Alouatta seniculus). Behav. Ecol. Sociobiol. 48, 253–267 (doi:10.1007/s002650000236) [Google Scholar]
- Schulte-Hostedde A. I., Zinner B., Millar J. S., Hickling G. J.2005Restitution of mass-size residuals: validating body condition indices. Ecology 86, 155–163 (doi:10.1890/04-0232) [Google Scholar]
- Sherman P. W.1977Nepotism and the evolution of alarm calls. Science 197, 1246–1253 (doi:10.1126/science.197.4310.1246) [DOI] [PubMed] [Google Scholar]
- Silk J. B.2007Social components of fitness in primate groups. Science 317, 1347–1351 (doi:10.1126/science.1140734) [DOI] [PubMed] [Google Scholar]
- Solomon N. G.2003A reexamination of factors influencing phylopatry in rodents. J. Mammal. 84, 1182–1197 (doi:10.1644/BLe-013) [Google Scholar]
- Stevens S. D.1998High incidence of infanticide by lactating females in a population of Columbian ground squirrels (Spermophilus columbianus). Can. J. Zool. 76, 1183–1187 (doi:10.1139/cjz-76-6-1183) [Google Scholar]
- West-Eberhard M. J.1975The evolution of social behaviour by kin selection. Quart. Rev. Biol. 50, 1–33 [Google Scholar]
- West-Eberhard M. J.2005Behavior of the primitively social wasp Montezumia cortesioides Willink (Vespidae Eumeninae) and the origins of vespid sociality. Ethol. Ecol. Evol. 17, 201–215 [Google Scholar]
- Wilson E. O.1975Sociobiology: the new synthesis Cambridge, MA: Harvard University Press [Google Scholar]