Abstract
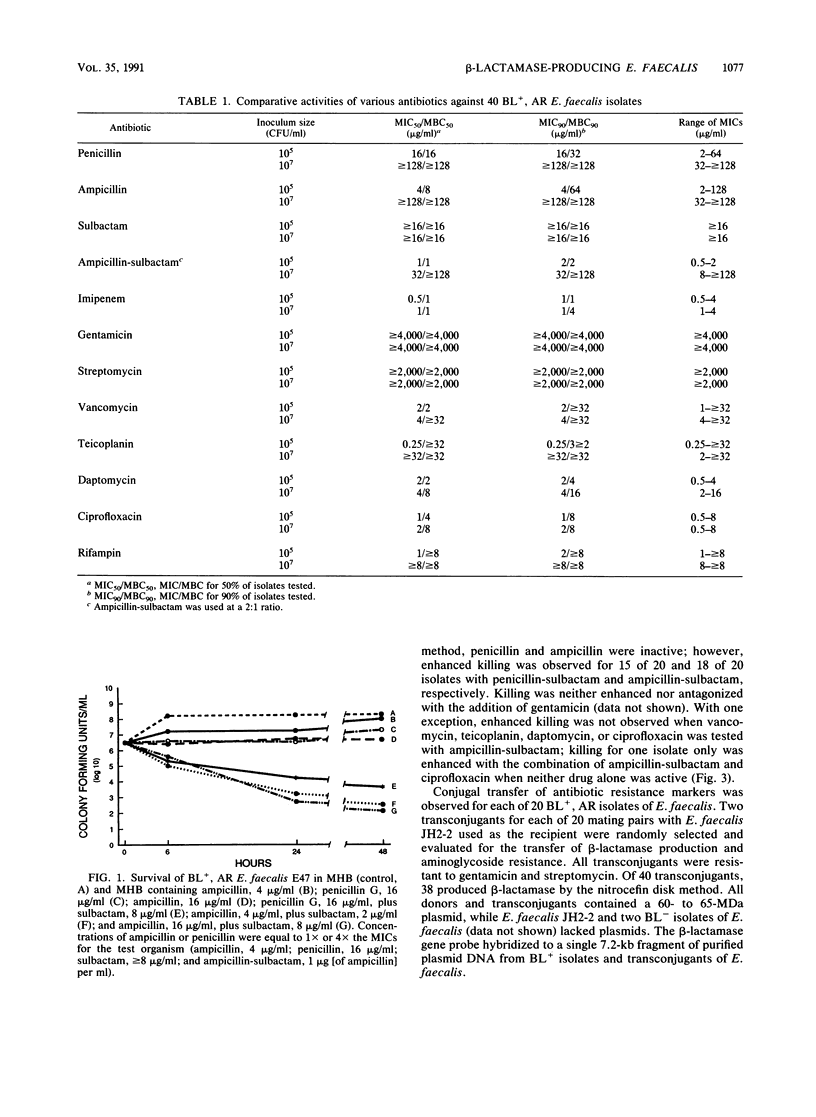
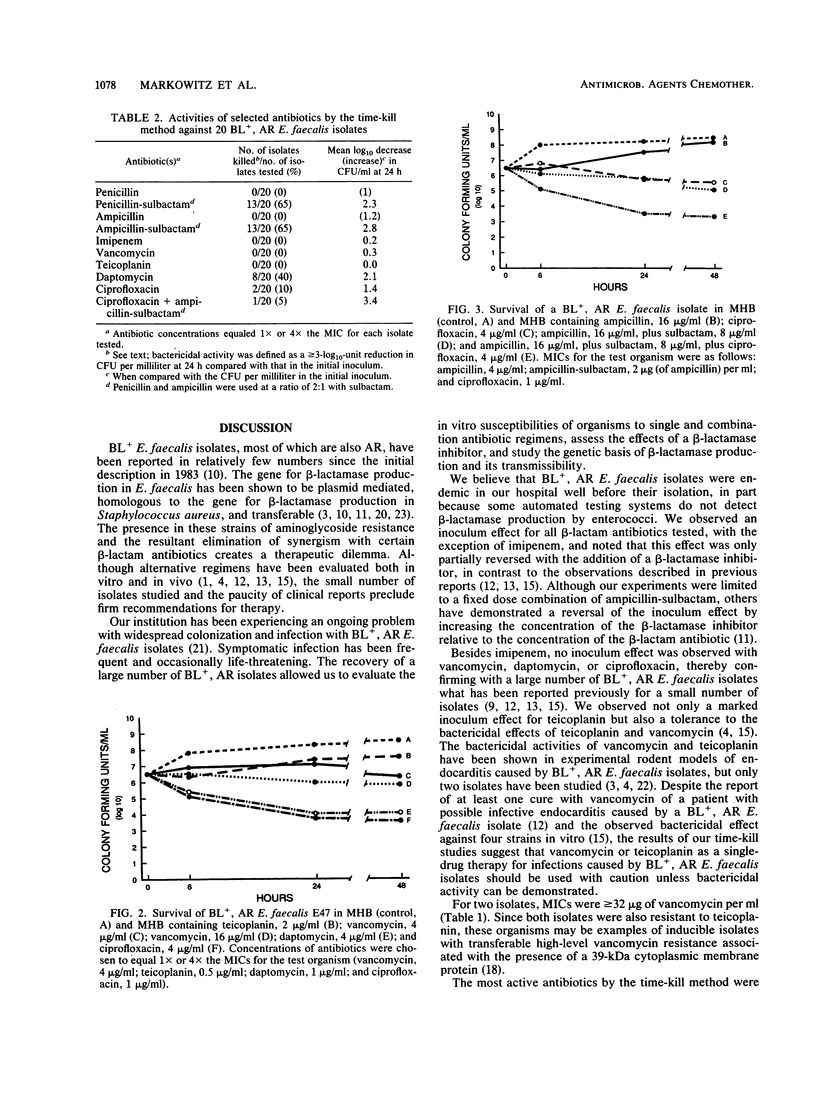
beta-Lactamase-producing (BL+), aminoglycoside-resistant (AR) Enterococcus faecalis is endemic in our hospital, having caused widespread colonization and infection. Suitable therapy for infections caused by these organisms has been problematic. We compared the antimicrobial and bactericidal activities, by broth macrodilution and time-kill methods, of several antibiotics, alone and in combination, against BL+, AR isolates of E. faecalis and determined the transmissibility of antibiotic resistance markers. Ampicillin-sulbactam, imipenem, daptomycin, and ciprofloxacin were the most active antibiotics with MICs for 90% of isolates tested of 2, 1, 2, and 1 microgram/ml, respectively, against inocula of 10(3) and 10(5) CFU/ml. Little inoculum effect was noted with imipenem, vancomycin, daptomycin, or ciprofloxacin, while the addition of sulbactam to ampicillin partially inhibited the effect of the increased inoculum. Penicillin-sulbactam and ampicillin-sulbactam combinations in a 2:1 ratio were most frequently bactericidal (greater than or equal to 3-log10-unit decrease in bacterial titers at 24 h for 13 of 20 isolates), followed by daptomycin (8 of 20 isolates) and ciprofloxacin (2 of 20 isolates). Bactericidal activity was not demonstrated for imipenem or teicoplanin. beta-Lactamase production and aminoglycoside resistance were associated with a 60- to 65-MDa plasmid which was easily transferred to a plasmid-free E. faecalis recipient. The 840-bp beta-lactamase gene probe hybridized to purified plasmid DNA from BL+ donor isolates of E. faecalis and transconjugants but not from BL- isolates. Ampicillin-sulbactam and daptomycin (an investigational antibiotic) seem to be reasonable choices for the empiric therapy of presumed enterococcal infections in hospitals in which BL+, AR E. faecalis strains are isolated. Their use should ideally be supported by tests for bactericidal activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush L. M., Boscia J. A., Kaye D. Daptomycin (LY146032) treatment of experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1988 Jun;32(6):877–881. doi: 10.1128/aac.32.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froggatt J. W., Johnston J. L., Galetto D. W., Archer G. L. Antimicrobial resistance in nosocomial isolates of Staphylococcus haemolyticus. Antimicrob Agents Chemother. 1989 Apr;33(4):460–466. doi: 10.1128/aac.33.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindes R. G., Willey S. H., Eliopoulos G. M., Rice L. B., Eliopoulos C. T., Murray B. E., Moellering R. C., Jr Treatment of experimental endocarditis caused by a beta-lactamase-producing strain of Enterococcus faecalis with high-level resistance to gentamicin. Antimicrob Agents Chemother. 1989 Jul;33(7):1019–1022. doi: 10.1128/aac.33.7.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman M., Pitsakis P. G., Rosenberg A., Hessen M. T., Abrutyn E., Murray B. E., Levison M. E. beta-Lactamase production in experimental endocarditis due to aminoglycoside-resistant Streptococcus faecalis. J Infect Dis. 1987 Jun;155(6):1226–1232. doi: 10.1093/infdis/155.6.1226. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Reider J. L., Virgili S. S., Kopecko D. J. Survey of the extrachromosomal gene pool of Streptococcus mutans. Infect Immun. 1977 Jul;17(1):215–226. doi: 10.1128/iai.17.1.215-226.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Church D. A., Wanger A., Zscheck K., Levison M. E., Ingerman M. J., Abrutyn E., Mederski-Samoraj B. Comparison of two beta-lactamase-producing strains of Streptococcus faecalis. Antimicrob Agents Chemother. 1986 Dec;30(6):861–864. doi: 10.1128/aac.30.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samoraj B., Foster S. K., Brunton J. L., Harford P. In vitro studies of plasmid-mediated penicillinase from Streptococcus faecalis suggest a staphylococcal origin. J Clin Invest. 1986 Jan;77(1):289–293. doi: 10.1172/JCI112289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Colodny S. M., Zervos M. J. Serious infection due to beta-lactamase-producing Streptococcus faecalis with high-level resistance to gentamicin. J Infect Dis. 1988 Nov;158(5):1144–1145. doi: 10.1093/infdis/158.5.1144. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Masecar B. L., Zervos M. J. Characterization and comparison of two penicillinase-producing strains of Streptococcus (Enterococcus) faecalis. Antimicrob Agents Chemother. 1988 Jan;32(1):122–124. doi: 10.1128/aac.32.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Wanger A., Zscheck K. K., Zervos M. J., Murray B. E. Molecular epidemiology of beta-lactamase-producing enterococci. Antimicrob Agents Chemother. 1990 Feb;34(2):302–305. doi: 10.1128/aac.34.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Zervos M. J. Susceptibility and bactericidal activity studies of four beta-lactamase-producing enterococci. Antimicrob Agents Chemother. 1989 Feb;33(2):251–253. doi: 10.1128/aac.33.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinehart E., Smith N. E., Wennersten C., Gorss E., Freeman J., Eliopoulos G. M., Moellering R. C., Jr, Goldmann D. A. Rapid dissemination of beta-lactamase-producing, aminoglycoside-resistant Enterococcus faecalis among patients and staff on an infant-toddler surgical ward. N Engl J Med. 1990 Dec 27;323(26):1814–1818. doi: 10.1056/NEJM199012273232606. [DOI] [PubMed] [Google Scholar]
- Rice L. B., Eliopoulos G. M., Wennersten C., Goldmann D., Jacoby G. A., Moellering R. C., Jr Chromosomally mediated beta-lactamase production and gentamicin resistance in Enterococcus faecalis. Antimicrob Agents Chemother. 1991 Feb;35(2):272–276. doi: 10.1128/aac.35.2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlaes D. M., Bouvet A., Devine C., Shlaes J. H., al-Obeid S., Williamson R. Inducible, transferable resistance to vancomycin in Enterococcus faecalis A256. Antimicrob Agents Chemother. 1989 Feb;33(2):198–203. doi: 10.1128/aac.33.2.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E. Comparison of enterococcal and staphylococcal beta-lactamase plasmids. J Infect Dis. 1990 Jan;161(1):54–58. doi: 10.1093/infdis/161.1.54. [DOI] [PubMed] [Google Scholar]
- Yao J. D., Thauvin-Eliopoulos C., Eliopoulos G. M., Moellering R. C., Jr Efficacy of teicoplanin in two dosage regimens for experimental endocarditis caused by a beta-lactamase-producing strain of Enterococcus faecalis with high-level resistance to gentamicin. Antimicrob Agents Chemother. 1990 May;34(5):827–830. doi: 10.1128/aac.34.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zscheck K. K., Hull R., Murray B. E. Restriction mapping and hybridization studies of a beta-lactamase-encoding fragment from Streptococcus (Enterococcus) faecalis. Antimicrob Agents Chemother. 1988 May;32(5):768–769. doi: 10.1128/aac.32.5.768. [DOI] [PMC free article] [PubMed] [Google Scholar]


