Abstract
Objectives
Reconstituted HDL (rHDL), is of interest as a potential novel therapy for atherosclerosis due to its ability to promote free cholesterol (FC) mobilization after intravenous administration. We performed studies to identify the underlying molecular mechanisms by which rHDL promote FC mobilization from whole body in vivo and macrophages in vitro.
Methods and results
Wild type (WT), SR-BI-KO, ABCA1-KO and ABCG1-KO mice received either rHDL or PBS intravenously. Blood was drawn before and at several time points after injection for apoA-I, phosphatidylcholine and FC measurement. In WT mice, serum FC peaked at 20 min and rapidly returned towards baseline levels by 24 h. Unexpectedly, ABCA1-KO and ABCG1-KO mice did not differ from WT mice regard to the kinetics of FC mobilization. In contrast, in SR-BI-KO mice the increase in FC level at 20 min was only 10% of that in control mice (p<0.01). Bone marrow-derived macrophages from WT, SR-BI-KO, ABCA1-KO and ABCG1-KO mice were incubated in vitro with rHDL and cholesterol efflux determined. Efflux from SR-BI KO and ABCA1 KO macrophages was not different from WT macrophages. In contrast, efflux from ABCG1-KO macrophages was ∼ 50% lower as compared with WT macrophages (p<0.001).
Conclusions
The bulk mobilization of FC observed in circulation after rHDL administration is primarily mediated by SR-BI. However, cholesterol mobilization from macrophages to rHDL is primarily mediated by ABCG1.
Keywords: reconstituted HDL, cholesterol efflux, apolipoprotein A-I, SR-BI, ABCA1, ABCG1
Circulating high density lipoprotein (HDL) and its major protein apolipoprotein A-I (apoA-I) are generally considered to protect against atherosclerosis. The main mechanism by which HDL and apoA-I are thought to be protective is through the promotion of reverse cholesterol transport (RCT), the process by which excess cholesterol from the periphery is transported back to the liver to be further metabolized and excreted 1. Novel therapeutic approaches that promote RCT and hold the potential to be a major tool to reduce cardiovascular disease risks are currently being tested. One promising approach is the use of syntheticdiscoidal particles containing either human apoA-I or one of its variants, apoA-IMilano and phospholipids, and sometimes cholesterol2, frequently referred as reconstituted HDL (rHDL). The intravenous administration in humans of such particles has been associated with regression of coronary atherosclerosis3 and improvement in plaque characteristics4, 5, endothelial function 6 and anti-inflammatory markers 7. It is also associated with a transitory increase in plasma free cholesterol (FC) in both mouse models8, 9 and humans10, 11, as well as with increased cholesterol synthesis in peripheral tissues, consistent with cholesterol movement from tissues to plasma in mice 8, and increased fecal sterol excretion in humans11, 12, suggesting that rHDL is able to promote RCT. However, the molecular mechanisms by which rHDL promote cholesterol mobilization from tissues are not understood.
The first step of the RCT pathway is the efflux of FC from peripheral cells to extracellular acceptor particles. Four different mechanisms are known to contribute to this step: ABCA1-mediated efflux to apoA-I 13, SR-BI and ABCG1-mediated efflux to mature HDL14, 15, and aqueous diffusion to mature HDL 16. In vitro data suggest that rHDL can be an excellent cholesterol acceptor in the context of SR-BI- and ABCG1-mediated efflux pathways, but not in the context of an ABCA1- mediated efflux pathway17, 18. The goal of this study was to investigate the molecular mechanism(s) underlying the FC mobilization observed after intravenous administration of rHDL and specifically to test the hypothesis that administration of rHDL in vivo promotes cholesterol mobilization via SR-BI-and ABCG1- mediated, but not ABCA1-mediated, pathways. As we were interested in investigating the mechanisms at both the systemic and macrophage levels, we conducted both in vivo studies in mice models and in vitro studies in primary macrophages.
Methods
A detailed description of animals, reagents and methods used in the studies reported in this manuscript are described in the supplemental materials appendix. Following is a brief description of the methods used.
Preparation and Characterization of Discoidal Reconstituted Lipid/Protein
rHDL containing human apoA-I (∼3 mg) and egg phosphatidylcholine (PC; ∼7 mg) were prepared by the cholate dialysis method as previously described 19. The final rHDL particles had an average hydrodynamic diameter of approximately 10nm and a protein to phospholipid ratio of about 1:2 (w/w). The concentration of apoA-I in rHDL is similar to that used in other animal 8 and human 11, 12 studies.
Cholesterol Efflux Assays in Bone Marrow–Derived Macrophages
Bone marrow-derived macrophages (BMM) were isolated from femurs and tibias of SR-BI-, ABCA1- and ABCG1-KO mice and respective controls, and cultured in DMEM supplemented with 10% FBS and 30% L929 conditioned media. as described before 20. Cellular cholesterol efflux assays were performed as previously described 20.
Mice study protocol
Wild-type (WT) control mice were obtained from Jackson Labs. SR-BI- ABCA1- and ABCG1-deficient mice were bred in house. Mice were fed a standard chow diet ad libitum. rHDL were administered by intravenous bolus injection. Serum was obtained by retro-orbital bleeding while the mice were under isofluorane anesthesia and collected in heparinized tubes. All mice experiments were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Lipid and Lipoprotein Analysis
Total cholesterol, HDL cholesterol, FC and phospholipid levels were measured using Wako Pure Chemical Industries reagents. The enzymatic method used to measure phospholipids selectively measures choline-containing phospholipids, i.e. PC and sphingomyelin. In the context of this paper, where high doses of PC are administered into mice in the form of rHDL, we assume PC represent the majority of the phospholipids and report the results as such. Pooled plasma from each group was separated by fast protein liquid (FPLC) gel filtration and total cholesterol and PC in each fraction was measured.
Kinetic analysis
Transport and fractional catabolic rates (FCR) were calculated using the WinSAAM version 3.0.621. Human apoA-I, PC and FC masses were calculated by multiplying their plasma concentrations for each mouse by the estimated plasma pool size (approximately 3.5% of body weight).
Statistical Analysis
All values are shown as the mean±SD. A 2-tailed Student t test was used to test for statistical significance. A probability value of <0.05 was considered significant.
Results
rHDL effectively mobilizes cholesterol in wild-type mice in vivo
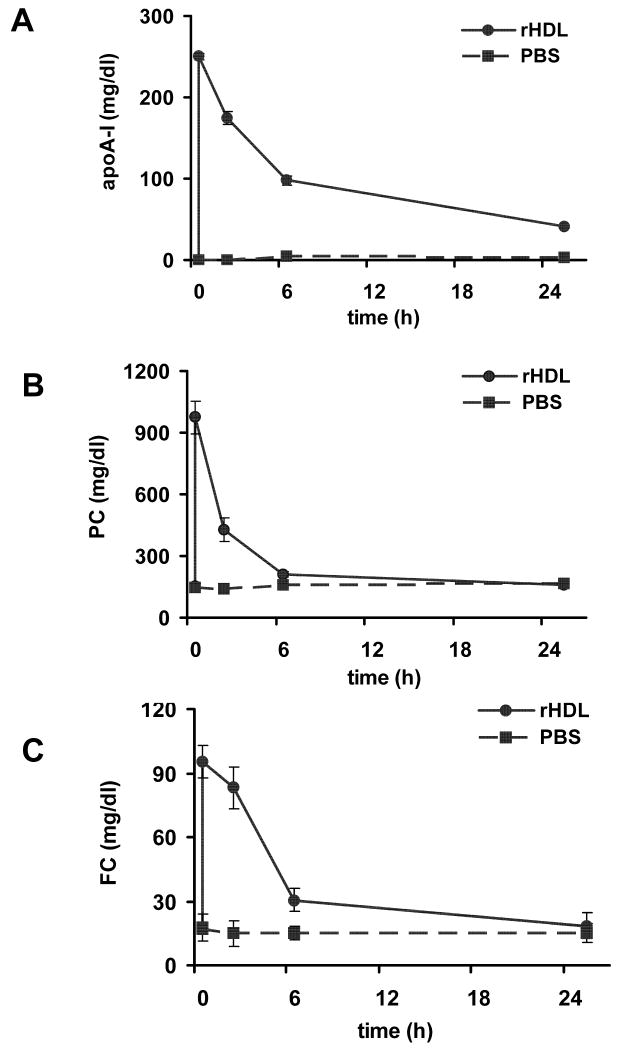
We did not observe any changes in serum levels of either PC or FC following intravenous administration of PBS in WT mice (Figure 1). In contrast, following the iv administration of rHDL we observed the rapid appearance of human apoA-I (Figure 1A), and a rapid and significant increase in PC (Fig. 1B) in serum. In addition we observed a significant increase in serum FC (Figure 1C). At 20 min post injection, serum PC was increased more than 6 fold, and FC more than 5-fold as compared with the serum levels at baseline. PC and FC levels returned to baseline levels by 24 h post injection (Figure 1). HDL particles observed in the FPLC profile of serum obtained from wild type mice 20 min after injection of rHDL were larger and enriched in PC and cholesterol as compared with HDL particles observed in the FPLC profile of serum obtained before injection (Supplemental Figure 1A, 1B). Moreover, more than half of the cholesterol present in these particles was FC (Supplemental Figure 1C).
Figure 1.
Human apolipoprotein A-I (apoA-I, panel A), phosphatidylcholine (PC, panel B), and free cholesterol (FC, panel C) levels in serum from C56BL/6 female mice before and 20 minutes, 2, 6 and 24 hours after i.v administration of either PBS (n=4, dashed line) or rHDL (n=8, solid line). See methods section for details.
SR-BI is required for the rapid mobilization of free cholesterol observed in circulation after the administration of rHDL
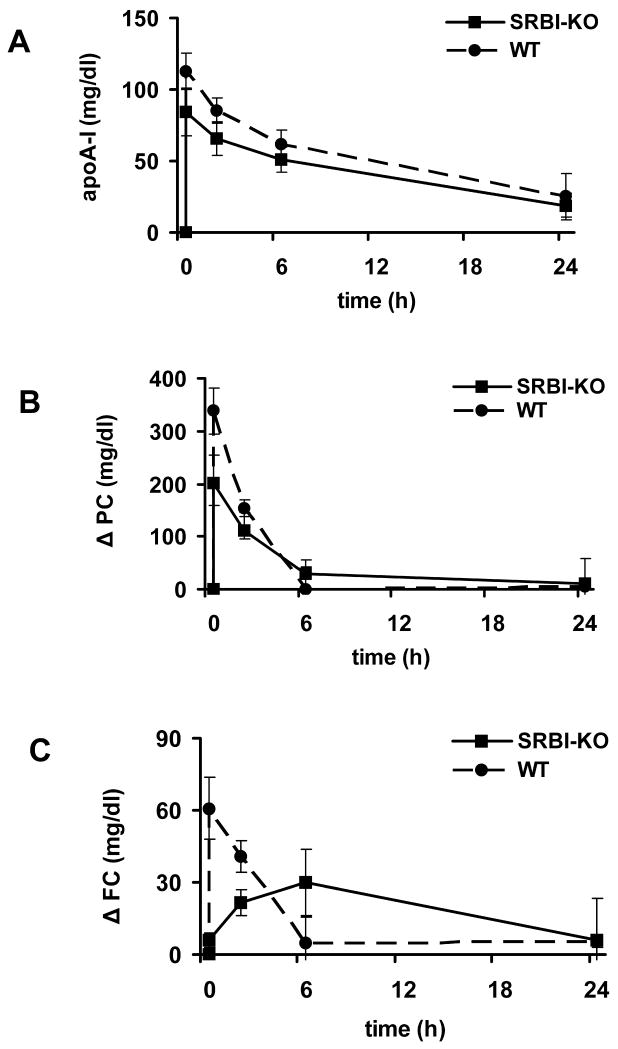
We evaluated the effects of administration of rHDL in mouse models that lacked SR-BI, ABCA1 or ABCG1 to assess their role in mediating cholesterol efflux to rHDL. Baseline lipid levels for the knock-out (KO) mouse models and their respective controls are shown in Supplemental Table 1. Following administration of rHDL into SR-BI deficient mice, the human apoA-I peak at 20 min was lower than that observed in the control mice (85±17 vs. 113±13 mg/dl, p=0.051); however, by 24 h the levels in the two groups of animals were similar (19±9 vs. 25±16 mg/dl, p=0.58). Similarly, when expressed as change from baseline, the increase in PC levels in SR-BI deficient mice (Figure 2B) were lower than that in control mice at the 20 min peak (201±53 vs. 338±43 mg/dl, p=0.01), but similar 24 h post-injection (10±10 vs. 4±54 mg/dl, p=0.93). The kinetic analysis of these data support the concept that the overall human apoA-I and PC clearance (FCR) and the PC transport is similar in SR-BI KO and control mice (Table 1). The most striking difference observed between SR-BI KO mice and their controls were the FC levels, as SR-BI deficient mice lacked the rapid increase from baseline seen in the control mice in response to the rHDL injection (Figure 2C). At 20 min post-injection, changes in FC levels from baseline in SR-BI KO mice were only 10% of the changes observed in control mice (6±3 vs. 61±13 mg/dl, p<0.01) and increased rather slowly up to 6 h post injection. The kinetic analysis suggests that the FCR of FC as well as the rapid input of FC into circulation were markedly reduced in the SR-BI deficient mice as compared with their controls (p< 0.01, Table 1).
Figure 2.
Human apolipoprotein A-I (apoA-I, panel A), phosphatidylcholine (PC, panel B), and free cholesterol (FC, panel C) levels in serum from SR-BI KO (n=3) and control (n=4) male mice before and 20 minutes, 2, 6 and 24 hours after i.v. administration of rHDL. PC and FC levels are expressed as change from baseline. See methods section for details. Solid line: KO mice; dashed line: control mice.
Table 1.
Kinetic parameters following administration of rHDL in mice
| apoA-I FCR pool/h |
PC FCR pool/h |
PC trans. mg/kg/h |
FC FCR pool/h |
FC trans. AUC (mg/kg)*h |
Rapid FC trans. AUC (mg/kg)*h |
|
|---|---|---|---|---|---|---|
| C57BL/6 (n=8) | 0.09 ± 0.01 | 0.67 ± 0.19 | 26.16 ± 5.89 | 0.95 ± 0.22 | 182.28 ± 50.86 | 23.11 ± 5.47 |
| SRBI-WT (n=4) | 0.04 ± 0.03 | 0.50 ± 0.07 | 30.39 ± 4.88 | 1.01 ± 0.06 | 241.00 ± 49.64 | 27.96 ± 9.99 |
| SRBI-KO (n=3) | 0.04 ± 0.01 | 0.37 ± 0.20 | 33.51 ± 19.60 | 0.18 ± 0.01 | 274.59 ± 15.25 | 1.01 ± 1.75 |
| p value | 0.88 | 0.27 | 0.76 | <0.001 | 0.33 | 0.006 |
| ABCA1-WT (n=4) | 0.07 ± 0.01 | 0.51 ± 0.14 | 25.96 ± 6.89 | 1.25 ± 0.14 | 267.35 ± 39.93 | 53.86 ± 4.19 |
| ABCA1-KO (n=4) | 0.18 ± 0.04 | 0.54 ± 0.08 | 14.94 ± 2.96 | 1.36 ± 0.16 | 230.36 ± 35.36 | 63.95 ± 12.72 |
| p value | 0.002 | 0.73 | 0.03 | 0.36 | 0.21 | 0.18 |
| ABCG1-WT (n=5) | 0.08 ± 0.01 | 0.40 ± 0.04 | 16.05 ± 5.31 | 1.09 ± 0.12 | 311.74 ± 28.43 | 17.73 ± 10.06 |
| ABCG1-KO (n=5) | 0.07 ± 0.01 | 0.48 ± 0.04 | 21.21 ± 2.42 | 1.16 ± 0.15 | 311.02 ± 36.73 | 21.83 ± 2.75 |
| p value | 0.13 | 0.01 | 0.08 | 0.45 | 0.97 | 0.41 |
ApoA-I, human apolipoprotein A-I; FCR, fractional catabolic rate; PC, phosphatidylcholine; trans, transport; FC, free cholesterol; AUC, area under the curve; WT, control wild type mice; KO, knockout mice.
Values for C57BL/6 mice are shown for comparison.
p values refer to comparison between the wild type control mice (WT) and the knockout (KO) mice.
ABCA1 and ABCG1 are not required for the rapid mobilization of free cholesterol observed in circulation after the administration of rHDL
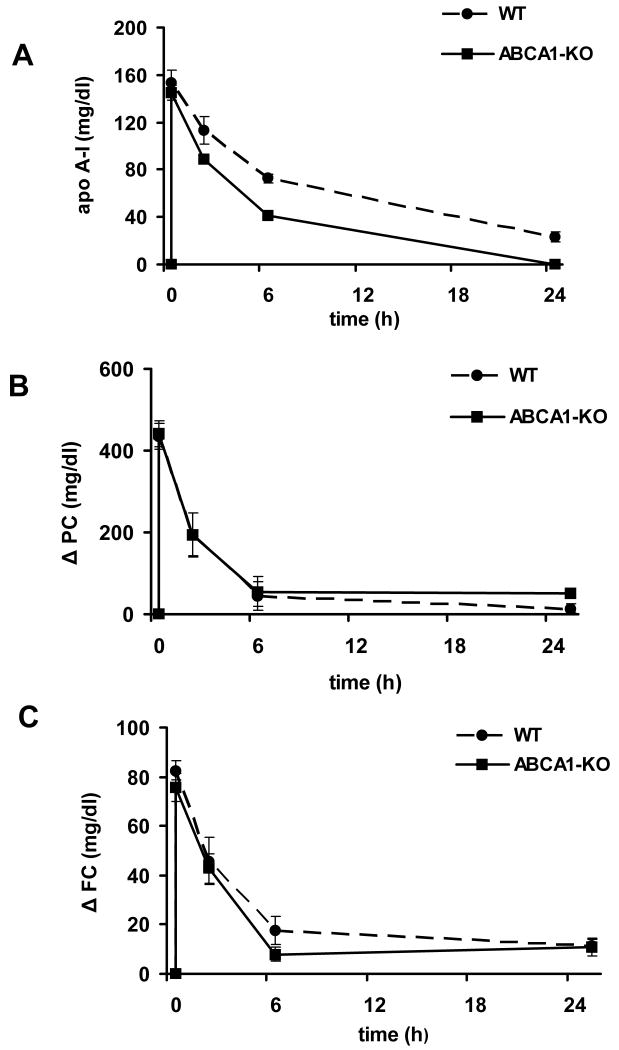
Following administration of rHDL, the serum concentration of human apoA-I in ABCA1 deficient mice was similar to that in control mice 20 min post injection, but declined more rapidly over time (Figure 3A), consistent with the known increase in apoA-I catabolism in ABCA1 deficiency. Kinetic analysis showed that the human apoA-I FCR was significantly faster in ABCA1-KO mice as compared with wild type mice (p=0.002; Table 1), explaining the more rapid decline in concentration over time. Changes in the serum concentrations of PC in ABCA1 KO mice after rHDL injection were similar to those observed in control mice (Figure 3B). Kinetic analysis showed that the PC FCR observed in ABCA1 deficient mice was similar to that in control mice while PC transport into the circulation was significantly lower (p=0.03; Table 1). The increase in FC levels was similar to that observed in control mice at the earlier time points; however, a modest but significant decrease in FC concentrations was observed 6 h post-injection (Figure 3C). Kinetic analysis showed that, following rHDL injection, FC FCR and transport into circulation in ABCA1 deficient mice were similar to those observed in control mice (Table 1).
Figure 3.
Human apolipoprotein A-I (apoA-I, panel A), phosphatidylcholine (PC, panel B), and free cholesterol (FC, panel C) levels in serum from ABCA1 KO (n=4) and control (n=4) female mice before and 20 minutes, 2, 6 and 24 hours after i.v. administration of rHDL. PC and FC levels are expressed as change from baseline. See methods section for details. Solid line: KO mice; dashed line: control mice.
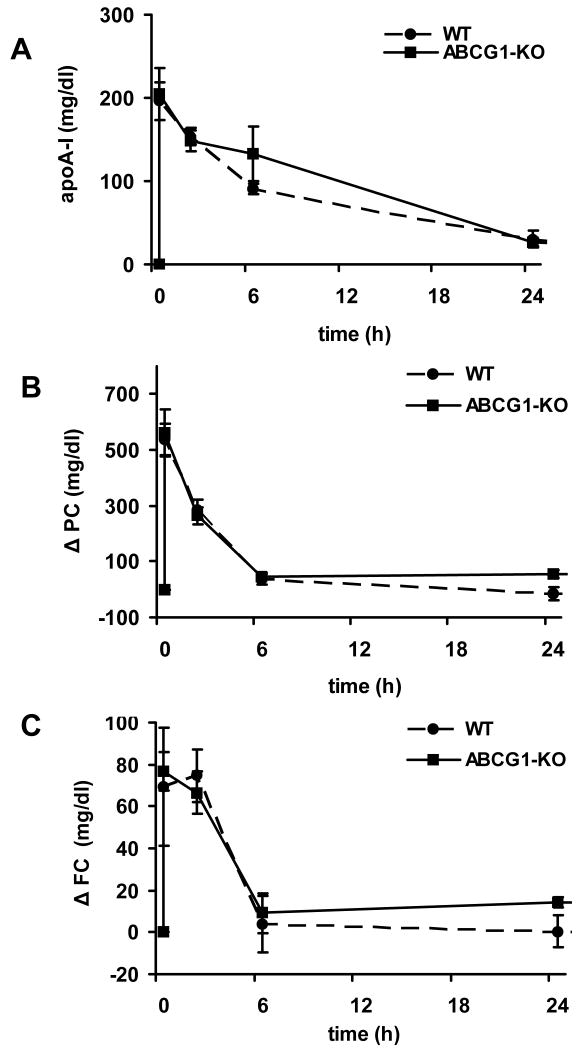
Following administration of rHDL into ABCG1 KO mice, the rapid appearance of human apoA-I and increase in PC and FC levels (Figure 4) was similar to those observed in control mice. Kinetic analysis showed that the human apoA-I and FC FCRs observed in ABCG1 deficient mice were similar, and that the slightly but significantly increased PC FCR (p=0.01) was balanced by a tendency of PC transfer to increase (p=0.08) as compared to control mice (Table 1).
Figure 4.
Human apolipoprotein A-I (apoA-I, panel A), phosphatidylcholine (PC, panel B), and free cholesterol (FC, panel C) levels in serum from ABCG1 KO (n=5) and control (n=5) male mice before and 20 minutes, 2, 6 and 24 hours after i.v. administration of rHDL. PC and FC levels are expressed as change from baseline. See methods section for details. Solid line: KO mice; dashed line: control mice.
ABCG1 contributes to the mobilization of free cholesterol from primary macrophages incubated in presence of rHDL
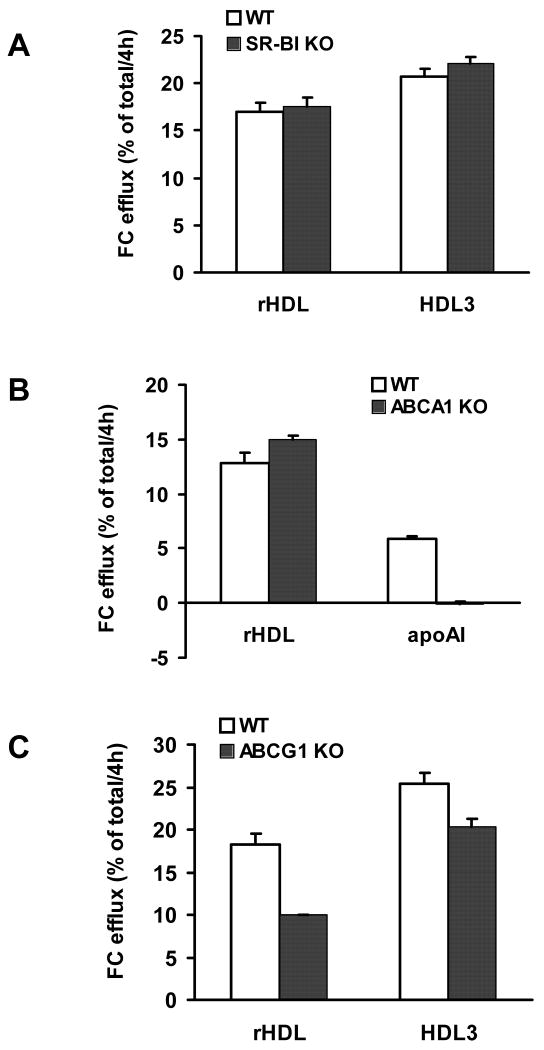
To directly assess the mechanism(s), if any, responsible for cholesterol mobilization from macrophages following the administration of rHDL we conducted in vitro studies using bone marrow-derived macrophages from SR-BI, ABCA1 and ABCG1 KO mice and appropriate controls. FC efflux from SR-BI KO-derived macrophages was not different than that from control cells when either HDL3 (20.70±0.80 vs 22.06±0.77 %FC/4h, p>0.1) or rHDL (16.92±0.99 vs 17.51±1.04 %FC/4h, p>0.5) were used as acceptors (Figure 5A). FC efflux from ABCA1 KO-derived macrophages to apoA-I, the acceptor for ABCA1, was as expected absent; while FC efflux to rHDL was present and slightly but significantly increased as compared to control cells (12.83±0.92 vs 14.97±0.39 %FC/4h, p<0.02, Figure 5B). Finally, FC efflux from ABCG1 KO-derived macrophages was significantly decreased as compared with controls when HDL3 was used as acceptor (25.37±1.23 vs 20.27±1.04 %FC/4h, p<0.005), and even more so when rHDL were used (18.18±1.37 vs 9.95±0.13 %FC/4h, p<0.001; Figure 5C).
Figure 5.
In vitro cholesterol efflux from bone marrow macrophages obtained from SR-BI (panel A), ABCA1 (panel B) and ABCG1 (panel C) deficient mice and respective controls in the presence of rHDL as acceptor. Cells were labeled with [3H]-cholesterol as described in the methods and incubated for 4 h with rHDL (25 μg/ml) and either free human apoA-I (10 μg/ml) or HDL3 (25 μg/ml) as controls. See methods section for details.
Discussion
Administration of rHDL is associated with increased FC in plasma both in humans10, 11 and mice8, and with increased fecal sterol excretion 11, 12, increased ex-vivo cholesterol efflux ability 7 and improvement of atherosclerosis burden4 in humans suggesting that administration of rHDL promotes RCT. The main objective of these studies was to evaluate the molecular mechanisms underlying the FC mobilization observed after the administration of rHDL at both systemic and macrophage levels.
Our in vivo results are consistent with the hypothesis that SR-BI, but not ABCA1 or ABCG1, is responsible for most of the increase in FC levels observed in circulation following the administration of rHDL. Relative to WT mice, we observed an altered response to rHDL in SR-BI-KO mice, with a significantly lower peak in FC levels soon after rHDL administration (Figure 2C). The kinetic analysis also supports the concept that SR-BI plays a critical role in the rapid early efflux of FC into circulation in response to rHDL injection, as demonstrated by the reduction of the rapid FC transport observed in the SR-BI KO mice, as well as a role in FC uptake, as demonstrated by the reduction in FC FCR observed in the SR-BI KO mice. These results are consistent with the known role of liver SR-BI in HDL metabolism in mice22-24, the ability of SR-BI to mediate bi-directional cholesterol flux between cells and HDL16 and the ability to promote cholesterol efflux from a variety of cell types to mature HDL25 as well as to rHDL26, 27.
Although we measured cholesterol concentrations in blood cells over time in animals injected with rHDL and excluded the possibility that blood cells are a major source of the increased serum FC observed in our studies (data not shown), we did not assess which tissues are the primary sources of the FC mobilization in response to rHDL infusion. SR-BI is abundantly expressed in the liver28, and it is possible that a considerable proportion of the FC mobilized in response to rHDL is derived from this organ. Indeed, based on the data presented by Alam and colleagues8, the liver is one of the biggest contributors to the flux of FC into the circulation following the intravenous administration of rHDL. Peripheral tissues also contribute to the net flux of cholesterol into circulation and to the liver in the hours following administration of rHDL8.
The results of the in vivo studies suggest that an ABCA1- and ABCG1- mediated mechanism do not contribute substantially to the amount of FC mobilized following rHDL administration at least at the earlier time points. It is, however, interesting to notice that serum FC levels 6 h post-injection were significantly lower and human apoA-I was cleared from plasma more rapidly in ABCA1-deficient mice as compared with control mice. rHDL has been shown to undergo remodeling in circulation, including the generation of pre-beta particles 10, 11, 29. Thus the observed faster apoA-I clearance in ABCA1 deficient mice could be explained by impaired lipidation of lipid-poor apoA-I created by remodeling of rHDL in circulation, consistent with the decreased PC transport into circulation and the tendency to decreased FC transport in the ABCA1-deficient mice as compared with control mice shown by the kinetics analysis. Taken together, these data suggest that ABCA1 may be important in maintaining FC efflux at later time points following rHDL injection and may contribute together with ABCG1 in promoting RCT from macrophages after administration of rHDL and to the improvement in the atherosclerotic burden that may be associated with the administration of rHDL in humans3-5 and animals 9, 30.
As administration of rHDL is being considered for the treatment of atherosclerosis, we were interested in assessing the ability of rHDL in promoting cholesterol efflux specifically from macrophages and the underlying mechanisms. We conducted in vitro studies using bone marrow-derived macrophages isolated from SR-BI-, ABCA1- and ABCG1-KO mice and their respective controls, as described before20, and showed that rHDL promotes FC efflux from primary macrophages by an ABCG1-mediated pathway, while SR-BI seems not to play a significant role. Although SR-BI is expressed in macrophages 14, 31 and a protective role of macrophage SR-BI has been suggested from studies done using bone marrow transplantation 32, 33, these results are consistent with our previously published data, in which we have shown that macrophage SR-BI does not play a dominant role in either FC efflux from macrophages17, 34 or macrophage RCT20, contrary to the key role played by macrophage ABCG1.
Our data in primary macrophages support the hypothesis that rHDL promote efflux from macrophages via an ABCG1-mediated mechanism (Figure 5C). ABCG1 is highly expressed in macrophages35 and mediates macrophage cholesterol efflux to mature HDL as well as rHDL 18, 36. Although its role in atherosclerosis is still debated 37-39, its role in controlling cholesterol homeostasis in macrophages is well supported by published data 15, 20, 34-36, and our results suggest that an ABCG1-mediated mechanism is at least in part responsible for the improved atherosclerotic burden observed in previous studies following administration of rHDL.
Other pathways, in particular aqueous diffusion 34, must also mediated cholesterol efflux in presence of rHDL, as efflux is preserved, albeit reduced, in ABCG1-deficient macrophages. It is also reasonable to think that, following remodeling in vivo with generation of lipid-poor apoA-I, rHDL may also promote ABCA1-mediated macrophage cholesterol efflux. Contrary to the role that ABCG1 has in mediating cholesterol efflux in macrophages, ABCG1-mediated cholesterol mobilization does not appear to contribute significantly to the bulk of FC mobilized soon after rHDL mobilization. This is probably due to the fact that the mass of cholesterol mobilized by macrophages via ABCG1 is very a very small part of the overall cholesterol tissue pool.
One possible limitation of our in vivo studies is the large difference in HDL levels in the different mouse models. The difference in pool size could potentially affect how the different mouse models responded to rHDL administration. However, we do not expect an effect of pool size on FC mobilization, since we believe there are sufficient phospholipids in the rHDL to allow for ample FC uptake.
In conclusion, we demonstrate that in mice SR-BI, and not ABCA1 or ABCG1, is responsible for the bulk of the rapid FC mobilization to the circulation (primarily non-macrophage) that is observed after intravenous administration of rHDL. In contrast, ABCG1, and not SR-BI or ABCA1, is primarily responsible for FC mobilization to rHDL from macrophages. Because macrophage cholesterol efflux is arguably more relevant to atherosclerosis than efflux from non-macrophage tissues, these results call into question the concept of using serum FC as a surrogate for the anti-atherosclerotic efficacy of rHDL infusion.
Supplementary Material
Acknowledgments
We thank Margaret Nickel, Vinh Nguyen, and Aisha Wilson for outstanding technical support.
Sources of Funding: This work was supported by a grant from NIH-NHLBI (HL22633). Dr Cuchel is supported by a K23 award from NIH-NHLBI (HL077146).
Footnotes
M. Cuchel: rHDL mobilizes cholesterol via different pathways
Disclosures: None.
Administration of reconstituted HDL (rHDL) is associated with increase in plasma free cholesterol, suggesting that rHDL promote cholesterol mobilization. We demonstrated that the bulk mobilization of free cholesterol observed in circulation after rHDL administration is primarily mediated by SR-BI. However, cholesterol mobilization from macrophages is primarily mediated by ABCG1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 2.Saito H, Lund-Katz S, Phillips MC. Contributions of domain structure and lipid interaction to the functionality of exchangeable human apolipoproteins. Prog Lipid Res. 2004;43:350–380. doi: 10.1016/j.plipres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. Jama. 2003;290:2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- 4.Tardif JC, Gregoire J, L'Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. Jama. 2007;297:1675–1682. doi: 10.1001/jama.297.15.jpc70004. [DOI] [PubMed] [Google Scholar]
- 5.Shaw JA, Bobik A, Murphy A, Kanellakis P, Blombery P, Mukhamedova N, Woollard K, Lyon S, Sviridov D, Dart AM. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ Res. 2008;103:1084–1091. doi: 10.1161/CIRCRESAHA.108.182063. [DOI] [PubMed] [Google Scholar]
- 6.Nieuwdorp M, Vergeer M, Bisoendial RJ, op 't Roodt J, Levels H, Birjmohun RS, Kuivenhoven JA, Basser R, Rabelink TJ, Kastelein JJ, Stroes ES. Reconstituted HDL infusion restores endothelial function in patients with type 2 diabetes mellitus. Diabetologia. 2008;51:1081–1084. doi: 10.1007/s00125-008-0975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin-Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA. Reconstituted high-density lipoprotein increases plasma high-density lipoprotein anti-inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53:962–971. doi: 10.1016/j.jacc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 8.Alam K, Meidell RS, Spady DK. Effect of up-regulating individual steps in the reverse cholesterol transport pathway on reverse cholesterol transport in normolipidemic mice. J Biol Chem. 2001;276:15641–15649. doi: 10.1074/jbc.M010230200. [DOI] [PubMed] [Google Scholar]
- 9.Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL, Drake S, Cercek B. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103:3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- 10.Nanjee MN, Doran JE, Lerch PG, Miller NE. Acute effects of intravenous infusion of ApoA1/phosphatidylcholine discs on plasma lipoproteins in humans. Arterioscler Thromb Vasc Biol. 1999;19:979–989. doi: 10.1161/01.atv.19.4.979. [DOI] [PubMed] [Google Scholar]
- 11.Nanjee MN, Cooke CJ, Garvin R, Semeria F, Lewis G, Olszewski WL, Miller NE. Intravenous apoA-I/lecithin discs increase pre-beta-HDL concentration in tissue fluid and stimulate reverse cholesterol transport in humans. J Lipid Res. 2001;42:1586–1593. [PubMed] [Google Scholar]
- 12.Eriksson M, Carlson LA, Miettinen TA, Angelin B. Stimulation of fecal steroid excretion after infusion of recombinant proapolipoprotein A-I: Potential reverse cholesterol transport in humans. Circulation. 1999;100:594–598. doi: 10.1161/01.cir.100.6.594. [DOI] [PubMed] [Google Scholar]
- 13.Wang N, Silver DL, Costet P, Tall AR. Specific Binding of ApoA-I, Enhanced Cholesterol Efflux, and Altered Plasma Membrane Morphology in Cells Expressing ABC1. J Biol Chem. 2000;275:33053–33058. doi: 10.1074/jbc.M005438200. [DOI] [PubMed] [Google Scholar]
- 14.Yancey PG, de la Llera-Moya M, Swarnakar S, Monzo P, Klein SM, Connelly MA, Johnson WJ, Williams DL, Rothblat GH. High density lipoprotein phospholipid composition is a major determinant of the bi-directional flux and net movement of cellular free cholesterol mediated by scavenger receptor BI. J Biol Chem. 2000;275:36596–36604. doi: 10.1074/jbc.M006924200. [DOI] [PubMed] [Google Scholar]
- 15.Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci U S A. 2004;101:9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yancey PG, Bortnick AE, Kellner-Weibel G, de la Llera-Moya M, Phillips MC, Rothblat GH. Importance of different pathways of cellular cholesterol efflux. Arterioscler Thromb Vasc Biol. 2003;23:712–719. doi: 10.1161/01.ATV.0000057572.97137.DD. [DOI] [PubMed] [Google Scholar]
- 17.Duong M, Collins HL, Jin W, Zanotti I, Favari E, Rothblat GH. Relative contributions of ABCA1 and SR-BI to cholesterol efflux to serum from fibroblasts and macrophages. Arterioscler Thromb Vasc Biol. 2006;26:541–547. doi: 10.1161/01.ATV.0000203515.25574.19. [DOI] [PubMed] [Google Scholar]
- 18.Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP, Phillips MC, Rothblat GH. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res. 2009;50:275–284. doi: 10.1194/jlr.M800362-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matz CE, Jonas A. Reaction of human lecithin cholesterol acyltransferase with synthetic micellar complexes of apolipoprotein A-I, phosphatidylcholine, and cholesterol. J Biol Chem. 1982;257:4541–4546. [PubMed] [Google Scholar]
- 20.Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stefanovski D, Moate PJ, Boston RC. WinSAAM: a windows-based compartmental modeling system. Metabolism. 2003;52:1153–1166. doi: 10.1016/s0026-0495(03)00144-6. [DOI] [PubMed] [Google Scholar]
- 22.Varban ML, Rinninger F, Wang N, Fairchild-Huntress V, Dunmore JH, Fang O, Gosselin ML, Dixon KL, Deeds JD, Acton SL, Tall AR, Huszar D. Targeted mutation reveals a central role for SR-BI in hepatic selective uptake of high density lipoprotein cholesterol. Proc Natl Acad Sci USA. 1998;95:4619–4624. doi: 10.1073/pnas.95.8.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. doi: 10.1172/JCI25327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zannis VI, Chroni A, Krieger M. Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med. 2006;84:276–294. doi: 10.1007/s00109-005-0030-4. [DOI] [PubMed] [Google Scholar]
- 25.de la Llera-Moya M, Rothblat GH, Connelly MA, Kellner-Weibel G, Sakr SW, Phillips MC, Williams DL. Scavenger receptor BI (SR-BI) mediates free cholesterol flux independently of HDL tethering to the cell surface. J Lipid Res. 1999;40:575–580. [PubMed] [Google Scholar]
- 26.Ji Y, Jian B, Wang N, Sun Y, de la Llera Moya M, Phillips MC, Rothblat GH, Swaney JB, Tall AR. Scavenger receptor BI promotes high density lipoprotein-medicated cellular cholersterol efflux. The Journal of Biological Chemistry. 1997;272:20982–20985. doi: 10.1074/jbc.272.34.20982. [DOI] [PubMed] [Google Scholar]
- 27.Jian B, de la Llera-Moya M, Ji Y, Wang N, Phillips MC, Swaney JB, Tall AR, Rothblat GH. Scavenger receptor class B type I as a mediator of cellular cholesterolefflux to lipoproteins and phospholipid acceptors. J Biol Chem. 1998;273:5599–5606. doi: 10.1074/jbc.273.10.5599. [DOI] [PubMed] [Google Scholar]
- 28.Rigotti A, Miettinen HE, Krieger M. The role of the high-density lipoprotein receptor SR-BI in the lipid metabolism of endocrine and other tissues. Endocr Rev. 2003;24:357–387. doi: 10.1210/er.2001-0037. [DOI] [PubMed] [Google Scholar]
- 29.Kujiraoka T, Nanjee MN, Oka T, Ito M, Nagano M, Cooke CJ, Takahashi S, Olszewski WL, Wong JS, Stepanova IP, Hamilton RL, Egashira T, Hattori H, Miller NE. Effects of intravenous apolipoprotein A-I/phosphatidylcholine discs on LCAT, PLTP, and CETP in plasma and peripheral lymph in humans. Arterioscler Thromb Vasc Biol. 2003;23:1653–1659. doi: 10.1161/01.ATV.0000089328.23279.3F. [DOI] [PubMed] [Google Scholar]
- 30.Ibanez B, Vilahur G, Cimmino G, Speidl WS, Pinero A, Choi BG, Zafar MU, Santos-Gallego CG, Krause B, Badimon L, Fuster V, Badimon JJ. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J Am Coll Cardiol. 2008;51:1104–1109. doi: 10.1016/j.jacc.2007.09.071. [DOI] [PubMed] [Google Scholar]
- 31.Hirano K, Yamashita S, Nakagawa Y, Ohya T, Matsuura F, Tsukamoto K, Okamoto Y, Matsuyama A, Matsumoto K, Miyagawa J, Matsuzawa Y. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ Res. 1999;85:108–116. doi: 10.1161/01.res.85.1.108. [DOI] [PubMed] [Google Scholar]
- 32.Covey SD, Krieger M, Wang W, Penman M, Trigatti BL. Scavenger receptor class B type I-mediated protection against atherosclerosis in LDL receptor-negative mice involves its expression in bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2003;23:1589–1594. doi: 10.1161/01.ATV.0000083343.19940.A0. [DOI] [PubMed] [Google Scholar]
- 33.Zhang W, Yancey PG, Su YR, Babaev VR, Zhang Y, Fazio S, Linton MF. Inactivation of macrophage scavenger receptor class B type I promotes atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation. 2003;108:2258–2263. doi: 10.1161/01.CIR.0000093189.97429.9D. [DOI] [PubMed] [Google Scholar]
- 34.Adorni MP, Zimetti F, Billheimer JT, Wang N, Rader DJ, Phillips MC, Rothblat GH. The roles of different pathways in the release of cholesterol from macrophages. J Lipid Res. 2007;48:2453–2462. doi: 10.1194/jlr.M700274-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC, Frank J, Francone OL, Edwards PA. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M, Cartland S, Packianathan M, Kritharides L, Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 37.Curtiss LK. Is Two out of Three Enough for ABCG1? Arterioscler Thromb Vasc Biol. 2006;26:2175–2177. doi: 10.1161/01.ATV.0000243741.89303.27. [DOI] [PubMed] [Google Scholar]
- 38.Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117:3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Van Eck M. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28:258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.