Abstract
The contribution of zinc-mediated neuronal death in the process of both acute and chronic neurodegeneration has been increasingly appreciated. Phosphatase and tensin homologue, deleted on chromosome 10 (PTEN), the major tumor suppressor and key regulator of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, plays a critical role in neuronal death in response to various insults. NEDD4-1-mediated PTEN ubiquitination and subsequent degradation via the ubiquitin proteosomal system have recently been demonstrated to be the important regulatory mechanism for PTEN in several cancer types. We now demonstrate that PTEN is also the key mediator of the PI3K/Akt pathway in the neuronal response to zinc insult. We used primary cortical neurons and neuroblastoma N2a cells to show that zinc treatment results in a reduction of the PTEN protein level in parallel with increased NEDD4-1 gene/protein expression. The reduced PTEN level is associated with an activated PI3K pathway as determined by elevated phosphorylation of both Akt and GSK-3 as well as by the attenuating effect of a specific PI3K inhibitor (wortmannin). The reduction of PTEN can be attributed to increased protein degradation via the ubiquitin proteosomal system, as we show NEDD4-1 to be the major E3 ligase responsible for PTEN ubiquitination in neurons. Moreover, PTEN and NEDD4-1 appear to be able to counter-regulate each other to mediate the neuronal response to zinc. This reciprocal regulation requires the PI3K signaling pathway, suggesting a feedback loop mechanism. This study demonstrates that NEDD4-1-mediated PTEN ubiquitination is crucial in the regulation of PI3K/Akt signaling by PTEN during the neuronal response to zinc, which may represent a common mechanism in neurodegeneration.
Keywords: Neurodegeneration, PI 3-Kinase, Protein Degradation, Synapses, Ubiquitination, NEDD4–1, PTEN
Introduction
Zinc is increasingly recognized as an important molecule that plays both physiological and pathological roles in a variety of biological processes. The role of zinc in the central nervous system has also been discovered over the past 2 decades, as a neurotransmitter in modulating synaptic plasticity (1, 2). Although zinc is selectively stored in the pre-synaptic vesicles of a specific type of neuron, sequestered or tightly bound to cellular compartments, it can be rapidly released in response to extracellular signals. The same neurons also secrete glutamate and are the so-called “gluzinergic” neurons found primarily in the mammalian cerebral cortex. Neurons store up to 300 μm free zinc in their termini (3) and release it through several types of cation channels (e.g. N-methyl-d-aspartic acid receptors) when they are depolarized, reaching high concentrations that are known to cause acute neuronal death. Together with glutamate, zinc mediates excitotoxicity, as manifested in acute brain injuries, ischemia, and seizures (4, 5). Zinc is also implicated in chronic neurodegenerative diseases such as Alzheimer disease via direct chelation with the amyloid precursor protein, thereby modulating its processing and generating oxidative stress (6).
In cortical cell cultures, 100 μm zinc, with 25 mm potassium for depolarization, caused neuronal death with signs of both necrosis and apoptosis (7). The mechanism of zinc-triggered apoptosis has been modestly studied and shows induced neurotrophic receptor p75NTR and its associated death executor, which further activates the caspase cascade. Several main signaling pathways involved in zinc-induced neuronal death have also been identified, including PI3K3/Akt/GSK-3β/p70S6K, Ras/mitogen-activated protein kinases (MAPK), NF-κB, and translation initiation factor, eIF-2 (8–12).
PTEN is one of the most frequently mutated tumor suppressors in human cancers (13, 14). The major and best characterized function of PTEN is its lipid phosphatase activity, which recognizes and dephosphorylates phosphatidylinositol 3,4,5-triphosphate to generate phosphatidylinositol 4,5-bisphosphate and thus antagonizes the PI3K in the subsequent activation of protein kinase B/Akt (15–17). Although PTEN has been extensively studied in the field of cancer research, recent studies have also revealed that PTEN is crucial in the central nervous system for modulating neuronal differentiation (18, 19) and synaptogenesis (20). In addition, PTEN deficiencies can cause serious defects in brain function such as seizures, ataxia, and brain enlargement (21, 22). Furthermore, PTEN is closely associated with neuronal cell death by direct physical interaction with the N-methyl-d-aspartic acid receptor and regulation of the major survival PI3K/Akt signaling pathway (23).
Because PTEN is the major regulator of the PI3K/Akt signaling pathway, we speculated that it is also a mediator of zinc-induced neuronal death. We therefore aimed to study molecular regulation of PTEN upon zinc treatment in neuronal models. Protein interactions between PTEN with other partners such as MAGI-2 (24), PICT-1 (25), and PCAF (26) through its C-terminal PDZ (postsynaptic density 95, PSD-85; discs large, Dlg; zonula occludens-1, ZO-1)-binding motif have been known to regulate both the protein stability and enzymatic activity of PTEN (27). Numerous studies have also shown that post-translational modifications, including phosphorylation, oxidation, acetylation, and ubiquitination, are critical for PTEN regulation (28). Among these modifications, phosphorylation has been most extensively studied in PTEN. Phosphorylation at five sites (Thr-366, Ser-370, Ser-380, Thr-382, and Ser-385) dramatically reduced PTEN stability and, inversely, increased its enzymatic activity (29). Recently, NEDD4-1 (neural precursor cell expressed, developmentally down-regulated 4-1) was identified as the first ubiquitin ligase (E3) for PTEN that regulates PTEN degradation in multiple types of cancer cells (human bladder, gastric, and colorectal carcinomas) (30, 31). It is intriguing to note that cellular PTEN is generally very stable, and gene knock-out of NEDD4-1 does not result in a further increase of PTEN stability in the several cell types tested (32, 33). Therefore, it is possible that the enzymatic activity of NEDD4-1 toward PTEN is under tight control (34). Consistent with this model, it has been shown most recently that the tyrosine kinase RAK can inhibit NEDD4-1-mediated PTEN degradation by phosphorylating PTEN (35). However, under which physiological or pathological conditions NEDD4-1 degrades PTEN is still not defined. Unlike NEDD4-2 (the closely related family member of NEDD4-1), which has been most frequently associated with a series of neuronal functions such as synaptic plasticity, commissural axon guidance, as well as neuronal survival (36–42), the central nervous system substrate for NEDD4-1 is unknown. Hence, it was intriguing to determine which NEDD4 member(s) (−1 and/or −2) is responsible for modulating PTEN activity in the central nervous system.
We now demonstrate that zinc treatment in primary cultured cortical neurons selectively induced protein expression of NEDD4-1 but not NEDD4-2, resulting in enhanced interaction between PTEN and NEDD4-1 and increased PTEN degradation by UPS, accordingly. Moreover, we show that these processes are dependent on the PI3K/Akt signaling pathway because a specific inhibitor (wortmannin) completely abolishes the changes to PTEN/NEDD4-1 upon zinc treatment. However, the ubiquitinated/degraded PTEN led to Akt activation, suggesting the existence of a feedback regulatory loop. Down-regulation of NEDD4-1 in N2a cells by its specific siRNA, or by partial immunodepletion of NEDD4-1 in primary cultured neurons, resulted in markedly reduced PTEN ubiquitination and degradation, indicating that NEDD4-1 is the major E3 ligase for PTEN in neuronal systems. We have further validated the notion of reciprocal protein levels of PTEN and NEDD4-1 in rodent brains after systemic administration of zinc to mice. This is the first report on NEDD4-1 as one major E3 ligase for PTEN in the central nervous system, and it shows that ubiquitination of PTEN is crucial for the regulation of PTEN for the PI3K/Akt signaling during the neuronal response to zinc.
EXPERIMENTAL PROCEDURES
Cell Culture, Treatment of Zn2+, and Transfection
Primary cultured cortical neurons were prepared as described previously (43, 44) using E17 pregnant rats. For the majority of experiments, zinc was added at 300 μm to 2-week-cultured neurons for 4 h before cell lysates were prepared. In this study, we used ZnSO4 instead of ZnCl2 because it has less cellular toxicity. Neuroblastoma N2a cells were grown to confluency in 6-well plates or 100-mm tissue culture dishes in Dulbecco's modified Eagle's medium/F-12 (HyClone)/Opti-MEM (Invitrogen) supplemented with 5% heat-inactivated fetal bovine serum (HyClone), 1% antibiotic/antimycotic mixture (Invitrogen), 4 mm glutamine (Invitrogen) and maintained in a humidified atmosphere of 5% CO2 at 37 °C (24). U87 (PTEN null glioma cell line) cells were grown to confluence on 6-well plates or 100-mm dishes in Dulbecco's modified Eagle's medium/F-12 (HyClone) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 1% antibiotic/antimycotic mixture (Invitrogen), and 200 mg/liter G418 (Invitrogen).
Transient transfections were performed with vector plasmids for pEF-PTEN-WT, pEF-PTEN-C124G, pSUPER-siNEDD4, pGIPZ-shPTEN, pCMV-Tag2B-AKT-WT, and Stealth negative siRNA (Invitrogen). All transfections were performed with LipofectamineTM 2000 (Invitrogen) on subconfluent N2a cells in 6-well culture plates according to the manufacturer's protocol. Cells were rinsed twice in ice-cold phosphate-buffered saline, pH 7.4, after the transfections, and 48 h later cell lysates were prepared for further analysis.
RNA Interference-mediated Silencing of PTEN and NEDD4-1
NEDD4-1-specific siRNA, which targets human and murine Nedd4-1 (NM_006154;TGGCGATTTGTAAACCGAAT), was constructed into the pSUPER-puro vector (OligoEngine, Seattle) according to the company's protocol. Briefly, primers (Nedd4-1 siRNA(+), 5′-GATCCCCTGGCGATTTGTAAACCGAATTCAAGAGATTCGGTTTACAAATCGCCATTTTTA-3′ and Nedd4-1 siRNA(−); 5′-AGCTTAAAAATGGCGATTTGTAAACCGAATCTCTTGAATTCGGTTTACAAATCGCCAGGG-3′) were annealed at 90 °C for 4 min and then at 70 °C for 10 min. Subsequently, these mixtures were step-cooled to 37 °C for 15–20 min and then to 10 °C. Annealed oligonucleotides were cloned into the BglII and HindIII sites of the vector and named pSUPER-siNEDD4-1. For silencing PTEN expression, we purchased the pGIPZ-shPTEN plasmid from Open Biosystems (Huntsville, AL). To validate off-target gene silencing, we used Stealth negative siRNA (Invitrogen) and pGIPZ-nonsilencing shRNA (Open Biosystem) as a negative control.
Quantitative Reverse Transcription-PCR
Total RNA was extracted from cells by using TRIzol reagent (Invitrogen). The SuperScript First Strand kit (Invitrogen) was used to synthesize the first strand cDNA from the samples with an equal amount of RNA, according to the manufacturer's instructions. Synthesized cDNAs were then amplified using IQTM SYBR Green Supermix and ICycler from Bio-Rad with 3 min of preincubation at 95 °C followed by 40 cycles of 30 s at 95 °C, 30 s at 55 °C, and 30 s at 72 °C. The data were analyzed by using Bio-Rad MyIQ 2.0. PCR products were verified by melting curve analysis and agarose gel electrophoresis. Primers used for NEDD4-1 and GAPDH amplification were as follows: NEDD4-1(+), 5′-CCAGGTTGGGAAGAAAAACA-3′, and NEDD4-1(−), 5′-ATTTCAGATGGCTGGGTCAC-3′; and GAPDH(+), 5′-CCCTTCATTGACCTCAACTA-3′, and GAPDH(−), 5′-CCTTCTCCATGGTGGTGAA-3′, respectively. The level of NEDD4-1 mRNA was normalized to that of GAPDH. Relative amounts of NEDD4-1 and GAPDH were determined based on the standard curves prepared by serial dilution of cDNA from mouse N2a cells.
Immunoprecipitation and Western Blot Analysis
Cells were lysed in ice-cold lysis buffer consisting of 1% Nonidet P-40, 150 mm NaCl, 50 mm Tris, pH 8.0, and a mixture of protease inhibitors (Roche Applied Science). The protein concentration of each sample was determined by Bio-Rad protein assay. Cell lysates (300 μg) were immunoprecipitated with specific antibody against PTEN (Santa Cruz Biotechnology, Santa Cruz, CA) using protein A-Sepharose (Amersham Biosciences). Following immunoprecipitation, immunoprecipitants or cell lysates were heated at 90 °C for 5 min in 2× TG sample loading buffer and separated on NuPAGETM 4–12% Tris glycine gel (Invitrogen) and transferred to a polyvinylidene difluoride membrane (Amersham Biosciences). Membranes were blocked with 5% skim milk in phosphate-buffered saline for 1 h at room temperature and probed at 4 °C overnight with the primary antibody in 5% skim milk/PBST. In this study, the following antibodies (Abs) were used: rabbit anti-PTEN Ab (Cell Signaling, Danvers, MA); goat anti-PTEN (Santa Cruz Biotechnology); mouse anti-α-tubulin Ab (Sigma); rabbit anti-p-Akt Ab (Cell Signaling); rabbit Akt 1/2/3 (Santa Cruz Biotechnology); mouse anti-mono-poly-Ub Ab (BioMol, Plymouth Meeting, PA); rabbit anti-NEDD4 Ab (Upstate, Charlottesville, VA); rabbit anti-NEDD4-2 Ab (Abcam, Cambridge, MA); mouse anti-FLAG Ab (Sigma); mouse anti-β-actin Ab (Sigma); and anti-mouse IgG and anti-rabbit IgG horseradish peroxidase-conjugated Abs (Chemicon, Temecula, CA). The membranes were washed three times for 10 min each with phosphate-buffered saline containing 0.05% Tween 20 (PBST, pH 7.4) and incubated with horseradish peroxidase-conjugated secondary antibodies in 5% skim milk for 30 min at room temperature. After washing three times with PBST, immunoreactive bands were visualized by using ECL Plus (Amersham Biosciences) chemiluminescence reagent.
Cycloheximide Chase Assay
Neuronal cells were incubated with 50 μg/ml cycloheximide (Sigma) for the indicated times in the presence or absence of 300 μm ZnSO4. Cycloheximide was simultaneously treated with ZnSO4. Cells were rinsed twice in ice-cold phosphate-buffered saline, pH 7.4, and cell lysates were then prepared for further analysis.
Immunodepletion of NEDD4-1 in Primary Cultured Neurons
Zinc was added 300 μm to 2-week-cultured neurons for 4 h before cell lysates were prepared. Cell lysates were immunoprecipitated with a control mouse IgG and NEDD4-1-specific antibodies and labeled as Mock and NEDD4-1, respectively. The treated lysates (15 μg) were then used in an in vitro PTEN ubiquitination assay using recombinant hemagglutinin-tagged PTEN as substrate as described previously (30, 31, 34).
Immunocytochemistry for Neuronal Morphology/Dendritic Structure Study (MAP2) and Cell Death (Terminal dUTP Nick End Labeling)
Work was performed as described previously (44).
Administration of ZnSO4 into C57B6 Mice
Mouse handling was performed according to the standard procedures approved by the Animal Research Committee at Burnham Institute for Medical Research and National Institutes of Health Guidelines. A small volume (200 μl, 600 μm) of ZnSO4 or saline was administered to 12-month-old female C57B6 transgenic mice by intraperitoneal route at different time points (0, 6, 12, 18, and 24 h). To prepare brain lysates, mouse brains were removed, dissected, and minced by a Dounce homogenizer. Protein samples were prepared by lysing the smashed tissue with ice-cold lysis buffer consisting of 1% Nonidet P-40, 150 mm NaCl, 50 mm Tris, pH 8.0, and protease inhibitors (Roche Applied Science).
Statistics
All quantitative data were presented as means ± S.D. Comparison between groups were analyzed with unpaired analysis of variance using GraphPad PRIZM software (La Jolla, CA), and values of p < 0.05 were considered to be significant.
RESULTS
Treatment with Zn2+ Reduced PTEN Levels in Cultured Neuronal Models
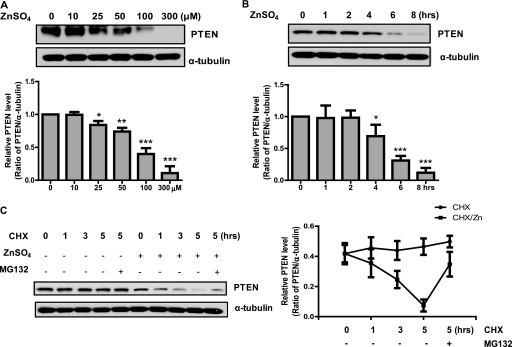
Zinc, as an essential catalytic and structural element of many proteins and a signaling messenger, is increasingly recognized as a key player in neuronal activities at many central excitatory synapses. Excessive synaptic release of zinc followed by its entry into vulnerable neurons contributes to severe neuronal cell death accompanied by Akt activation and its downstream signaling events. Because PTEN is known to be the major regulator of the phosphatidylinositol 3-kinase/Akt pathway via its phosphatase activity, which converts phosphatidylinositol 3,4,5-triphosphate to phosphatidylinositol 4,5-bisphosphate, we therefore speculate that zinc also modulates PTEN, making PTEN the major executer of neuronal response to zinc. Indeed, exposure to Zn2+ significantly reduced PTEN protein levels in primary cultured neurons in a concentration- and time-dependent manner, with a significant effect seen at 25 μm and a 4-h time point at 100 μm (Fig. 1, A and B). Maximum reduction of PTEN protein levels was observed with 300 μm Zn2+. To examine whether reduction of PTEN was induced nonspecifically by exposure to any heavy metal ions, we exposed neurons to various concentrations of two additional metal ions, MgSO4 and CuSO4. However, these ions did not show any effect on PTEN levels (supplemental Fig. 1), indicating that the PTEN level was specifically reduced by Zn2+.
FIGURE 1.
Reduced PTEN protein level/stability upon zinc treatment in primary cultured neurons. A and B, zinc reduces PTEN levels in a dose- and time-dependent manner in primary neurons. Primary neurons were exposed to different concentrations (0–300 μm) of Zn2+ for 4 h (A) and to 100 μm for various time points (0–8 h) (B). PTEN expression level was determined by Western blot analysis. α-Tubulin was used as a loading control. C, zinc treatment decreased the protein stability of PTEN due to proteasome-mediated degradation. Primary rat cortical neurons were exposed to 300 μm Zn2+ with or without pretreatment of 25 μm MG132 for 4 h. Then 50 μg/ml cycloheximide (CHX) was added to primary neurons for various time points (0–5 h). PTEN expression levels were determined by Western blot analysis. Densitometry quantification of PTEN level from the experiment presented was normalized by α-tubulin and was from a collection of four independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001, compared with untreated sample; n = 3.
To examine whether reduction of PTEN was due to a decrease of its protein stability in neurons, we performed a cycloheximide chase experiment (Fig. 1C). Although PTEN levels were stable in the untreated group, treatment with Zn2+-induced reduction in PTEN stability after 1 h, with a significant loss of PTEN after 5 h. These results indicated that the Zn2+-induced PTEN reduction in primary cortical neurons was due to its decreased protein stability. Furthermore, we found that pretreatment of cells with the specific proteasome inhibitor MG132 (25 μm for 30 min) before Zn2+ exposure completely rescued PTEN from degradation (Fig. 1C, last lane), indicating an involvement of the UPS.
Zn2+-induced PTEN Reduction Requires the Activated PI3K Signaling Cascade
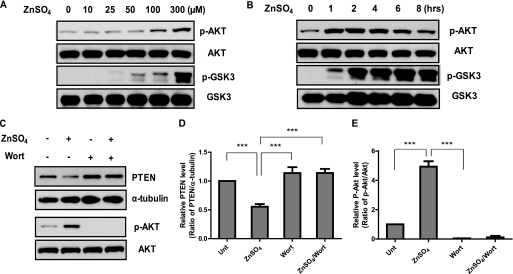
Zinc has been shown to induce neuronal death in immortalized embryonic hippocampal cells, accompanied by Akt activation (9). We report here a similar observation for primary cultured cortical neuronal cells. We assessed the effects of Zn2+ on p-Akt and p-GSK3α/β levels. Treatment with Zn2+ induced both p-Akt and p-GSK3α/β in neurons, in a dose- and time-dependent manner (Fig. 2, A and B). Moreover, we found that co-incubation or pretreatment with a PI3K-specific inhibitor (200 nm wortmannin) not only completely abolished Akt activation, as evidenced by knocking down p-Akt below the basal level, but also reversed the effect of zinc on reducing PTEN protein to its basal level (Fig. 2, C–E). These results indicated a negative feedback mechanism in PI3K signaling for regulating PTEN levels, although it is not clear which component(s) of the PI3K/Akt pathway mediates this effect.
FIGURE 2.
Zn2+-induced down-regulation of PTEN is mediated by the activated PI3K signaling pathway. A and B, zinc treatment induces Akt phosphorylation/activation and GSK3 phosphorylation/inactivation in a concentration- and time-dependent manner. C, PI3K/Akt-dependent effect. Zinc treatment at 300 μm for 4 h induced Akt activation in conjunction with reduced PTEN levels. This effect is completely abolished by the PI3K-specific inhibitor wortmannin (Wort) (200 nm). Densitometry quantification of PTEN and p-Akt level from the experiment presented in D and E, as normalized by α-tubulin and total Akt level, respectively; n = 5. ***, p < 0.001, compared with untreated (Unt) sample.
Zn2+-induced PTEN Degradation Is Mediated by Ubiquitination via the E3 Ligase, NEDD4-1
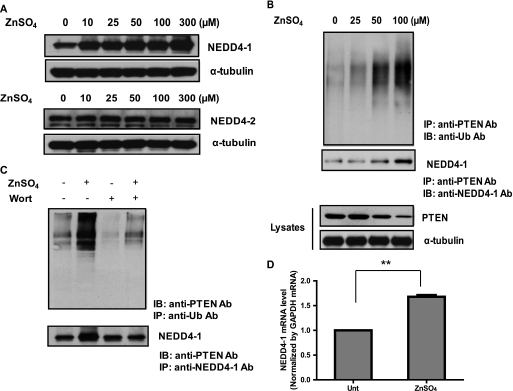
Because MG132 can fully prevent PTEN degradation via the UPS upon exposure to Zn2+, ubiquitination is likely necessary for the observed PTEN reduction. NEDD4-1, an E3 ubiquitin ligase from the HECT (homologous to E6-associated protein (E6AP) C terminus) family, has recently been identified as the enzyme responsible for PTEN ubiquitination in a number of cancer cell types (30, 31). We therefore examined the possibility that PTEN can be ubiquitinated by NEDD4-1 in neurons.
We first tested whether zinc could induce a change in the protein levels of the HECT family E3 ligases, NEDD4-1 and NEDD4-2, by Western blot analysis using specific antibodies to these two proteins. Interestingly, we found that only NEDD4-1 was selectively increased upon zinc treatment, although NEDD4-2 remained unchanged (Fig. 3A). We then studied PTEN ubiquitination by assessing the conjugation of ubiquitin to PTEN using co-immunoprecipitation. We found that exposure to Zn2+ induced ubiquitination of PTEN dose-dependently in primary neurons (Fig. 3B, top panel). Because the state of ubiquitination of a target molecule is closely correlated with its physical interaction with a specific E3 ligase, we therefore determined the level of interaction between PTEN and NEDD4-1 by co-immunoprecipitation (Fig. 3B, bottom panel). Accordingly, a gradual increase of protein-protein interaction between PTEN and NEDD4-1 was observed in a dose-dependent fashion upon treatment with Zn2+. Because wortmannin efficiently inhibited degradation of PTEN (Fig. 2C), we reasoned that the PI3K signaling pathway may be involved in regulating the ubiquitination of PTEN. To elucidate the specific mechanism, we treated neuronal cells with or without wortmannin before exposure to Zn2+ and determined the level of specific conjugation of ubiquitin to PTEN using co-immunoprecipitation. As predicted, the pretreatment of wortmannin (200 nm) potently suppressed the ubiquitination of PTEN upon exposure to Zn2+, indicating that PI3K signaling is involved in modulating the PTEN ubiquitination process (Fig. 3C, top panel). Furthermore, we found that wortmannin could interfere with the physical interaction between PTEN and NEDD4-1 (Fig. 3C, lower panel), suggesting that wortmannin-induced inhibition of PTEN ubiquitination was mediated by direct blockade on the physical interaction between PTEN and NEDD4-1. The increased NEDD4-1 protein level appeared to correlate with the increased PTEN ubiquitination upon zinc treatment (Fig. 3C, lane 2). Together with the enhanced physical interaction between these two molecules, we believed that NEDD4-1 is responsible for PTEN ubiquitination and the subsequent degradation events.
FIGURE 3.
Zn2+-induced degradation of PTEN is mediated by PI3K signaling as well as the proteosomal ubiquitin system involving E3 ligase NEDD4-1. A, zinc treatment selectively induces NEDD4-1 expression but not NEDD4-2 in neurons based on Western blot analysis. B, zinc treatment (6 h) induces protein-protein interaction between PTEN and ubiquitins in conjunction with PTEN ubiquitination events in a concentration-dependent manner. Protein-protein interaction was examined by co-immunoprecipitation with PTEN antibody followed by detection with NEDD4-1 antibody. PTEN ubiquitination was examined by immunoprecipitation (IP) with an anti-PTEN antibody, followed by Ub antibody. Maximum effect is seen with 100 μm zinc. 25 μm MG132 was pretreated for 30 min before zinc treatment. IB, immunoblot. C, zinc-mediated effects on induced PTEN-NEDD4-1 interaction and PTEN ubiquitination are mediated by a mechanism dependent on PI3K signaling. Neurons were pretreated with or without 200 nm wortmannin (Wort) for 1 h. These cells were then exposed to 300 μm Zn2+ for 4 h, and the resulting cell lysates were subjected to immunoprecipitation followed by Western blot analysis. D, zinc treatment induced NEDD4-1 gene transcription as measured by quantitative PCR. Neurons were treated with or without zinc for 4 h before cells were lysed with TRIzol to isolate RNA. **, p < 0.01, compared with untreated (Unt) sample; n = 3.
By quantitative RT-PCR, we demonstrated that exposure of neuronal cells to 100 μm Zn2+ increased NEDD4-1 mRNA levels (p < 0.0026) compared with untreated cells (Fig. 3D). Because the protein stability was not changed as measured by a cycloheximide chase experiment (supplemental Fig. 2), we believed that the regulation of NEDD4-1 herein may be mainly attributed to its transcriptional up-regulation. Although we are not certain whether Zn2+ can also modulate NEDD4-1 at the translational level as well as its enzymatic E3 ligase activity, these results at least suggest that Zn2+ can facilitate degradation of PTEN through the increase of NEDD4-1 transcription and subsequently the protein levels in neurons.
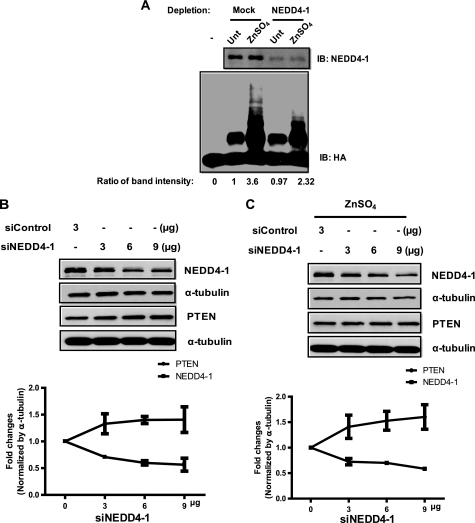
NEDD4-1 Is the Major E3 Ligase for PTEN upon Zinc Treatment in Primary Cultured Neurons
To provide further evidence for a causative role of the zinc-induced NEDD4-1 on the observed PTEN ubiquitination, we then immunodepleted NEDD4-1 with a specific antibody. Partial depletion of NEDD4-1 from primary neuronal cell lysates resulted in greatly reduced PTEN ubiquitination in an in vitro assay using hemagglutinin-tagged PTEN as a substrate (Fig. 4A). We also used a different approach to knock down NEDD4-1 using siRNA. Although transfection of NEDD4-1 siRNA at various concentrations (3, 6, and 9 μg) down-regulated NEDD4-1 levels in a dose-dependent manner, silencing of NEDD4-1 up-regulated PTEN to similar degrees with or without zinc treatment (Fig. 4, B and C). Taken together, these findings strongly suggest NEDD4-1 as the major E3 ligase for PTEN in neurons.
FIGURE 4.
NEDD4-1 is the E3 ligase for PTEN in neurons. A, partial protein depletion of NEDD4-1 from neuronal lysates by specific immunoprecipitation results in greatly reduced PTEN ubiquitination in an in vitro assay. Relative band intensity was normalized by intensity of mock (untreated (Unt) sample). IB, immunoblot; HA, hemagglutinin. B, down-regulated NEDD4-1 expression results in increased PTEN levels. Specific NEDD4 siRNA transfected into N2a cells for 48 h leads to NEDD4-1 down-regulation in a dose-dependent manner. Densitometry quantification of PTEN and NEDD4-1 in A is normalized by α-tubulin. Error bars represent S.D.; n = 6. C, forced down-regulation of NEDD4-1 by siRNA antagonizes zinc-mediated down-regulation of PTEN, resulting in slightly increased PTEN levels. In these experiments, cells were exposed to 300 μm Zn2+ for 4 h, and cell lysates were prepared. As a negative control, Stealth negative siRNA (labeled as siControl, Invitrogen) were utilized. Densitometry quantification of PTEN and NEDD4-1 in panel is co-normalized by α-tubulin. Error bars represent S.D.; n = 6.
Zn2+-induced NEDD4-1 Expression Is Dependent on PTEN-regulated PI3K Signaling Pathway
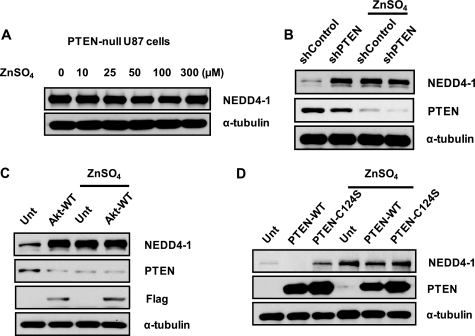
To further validate the functional importance of the PTEN-mediated PI3K/Akt pathway in NEDD4-1 regulation, we conducted additional experiments to examine the consequences of the down-regulated/inactivated PTEN as well as compensated Akt expression on NEDD4-1. Although exposure to Zn2+ up-regulated NEDD4-1 expression in neurons, NEDD4-1 expression levels remained unchanged by Zn2+ in glioma U87 cells that are PTEN-null (Fig. 5A). Moreover, shRNA of PTEN, which specifically knocks down PTEN expression, resulted in a significant increase of NEDD4-1 expression in the absence of zinc; zinc treatment did not induce a further increase in NEDD4-1 (Fig. 5B). In parallel, forced expression of Akt exerted a similar effect as down-regulated PTEN (Fig. 5C), suggesting the importance of the PI3K signaling cascade and possibly a direct role of activated Akt in NEDD4-1 regulation. To further validate this notion, we transfected wild type PTEN (PTEN-WT) and catalytic site dead (C124G) PTEN mutants (PTEN-C124G) into mouse neuroblastoma N2a cells. Both PTEN and NEDD4-1 levels of these cells were analyzed after treatment with or without Zn2+ (Fig. 5D). Overexpression of PTEN-WT considerably down-regulated NEDD4-1 levels in N2a cells. However, transient transfection of PTEN-C124G, which has no phosphatase activity, failed to diminish NEDD4-1 expression levels. These results are consistent with the previous data with wortmannin and confirm the involvement of PTEN-regulated PI3K signaling in NEDD4-1 regulation.
FIGURE 5.
Counter-regulation between PTEN and NEDD4-1. A, zinc-induced NEDD4-1 expression requires PTEN. Zinc fails to induce NEDD4-1 expression at concentrations as high as 300 μm in the PTEN-null glioma line U87. B, down-regulation of PTEN by shRNA induces NEDD4-1 protein expression in the absence of zinc treatment (1st and 2nd lanes), revealing an inverse relationship in N2a cells. Further down-regulation of PTEN by shRNA (5 μg) during the cellular response to zinc abolished zinc-mediated NEDD4-1 induction (3rd and 4th lanes). As a negative control, pGIPZ-nonsilencing shRNA (labeled as shControl, Open Biosystems, Huntsville, AL). C, forced expression of FLAG-tagged Akt-WT in N2a cells resulted in similar consequences to down-regulated PTEN, indicating the involvement of a PI3K-dependent mechanism. D, PTEN-mediated NEDD4-1 expression requires functional PTEN activity. Overexpression of wild type PTEN (PTEN-WT) results in suppressed NEDD4-1 expression in the absence of zinc treatment (1st and 2nd lanes), although a functionally inactive PTEN (PTEN-C124S mutant, 3rd lane) leads to slightly increased NEDD4-1 levels. Similarly, after zinc treatment, overexpression of PTEN-WT not only abolishes zinc-induced NEDD4-1 but leads to further reduced levels of NEDD4-1, which is reversed by the inactive PTEN-C124S. Unt, untreated.
Role of PTEN in Zinc-induced Neuronal Toxicity
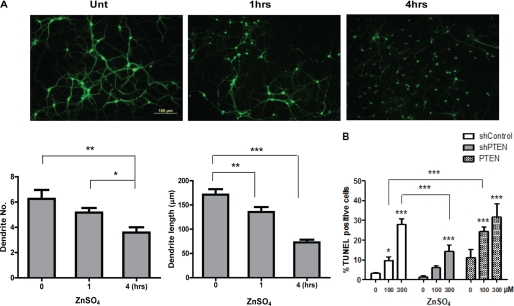
Before we studied the PTEN pathway in zinc-mediated cytotoxicity, we established an acute zinc neurotoxicity model in primary cultured neurons. Like the effect seen with brief zinc treatment (15 min at 100 μm and then washed away) on the induced NEDD4-1 protein (supplemental Fig. 4), zinc treatment induced dramatic morphological change in neurons as shown by immunostaining on the MAP2 (microtubule-associated protein 2). As shown in Fig. 6A, 4 h after zinc treatment caused nearly complete loss of dendritic structures of all the neurons, as quantified by both the number of dendrites per neuron and the length of the remaining dendrites. Furthermore, the zinc-treated neurons displayed condensed nuclei and cell shrinkage, which was confirmed to be apoptotic cells as measured by terminal dUTP nick end-labeling staining (Fig. 6B). We then used shRNA gene silencing and overexpression approaches to manipulate the PTEN levels in neurons. We found that overexpression and down-regulation of PTEN resulted in opposing effects on the number of apoptotic cells induced by zinc. Further down-regulation of PTEN by shRNA in the presence of zinc made the neurons more resistant to zinc-induced cytotoxicity, although overexpression of PTEN caused more cell death.
FIGURE 6.
Effect of Zn2+ on dendritic morphology and neuronal cell death. A, zinc-induced neuronal apoptosis. Primary rat cortical neurons were grown for 2 weeks and exposed to ZnSO4 (300 μm) for 0, 1, and 4 h, respectively. The neurons were then immunostained with a monoclonal antibody against MAP2 to investigate the effect of zinc on changes of dendrite number as well as dendritic length. B, PTEN pathway plays an important role in neuronal response to zinc. Mouse blastoma N2a cells transfected with PTEN plasmid, shPTEN, and control shRNA were treated with various doses of ZnSO4 (0, 100, and 300 μm) for 4 h. Cells were then fixed 48 h after transfection, and terminal dUTP nick end-labeling staining was performed to examine the effect of zinc to neuronal cell death. As a negative control, pGIPZ-nonsilencing shRNA (labeled as shControl, Open Biosystems). Values represent the mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001; n = 3.
Effect of Zn2 on PTEN Stability and NEDD4-1 Expression in Vivo
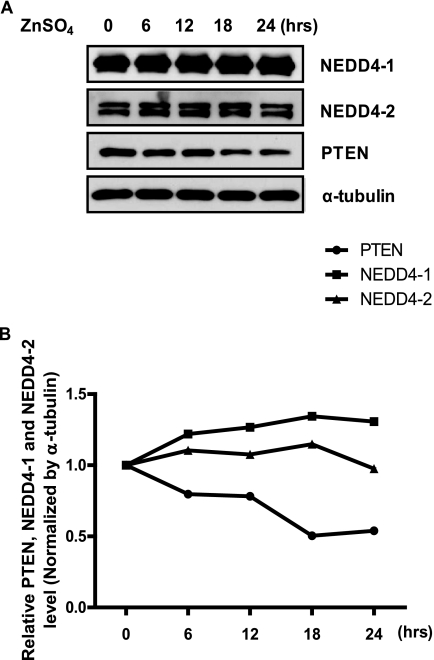
We have demonstrated that Zn2+ ions reduce the protein stability of PTEN and conversely increase NEDD4-1 expression in vitro. Therefore, to elucidate these effects in vivo, we systemically administered 200 μl of 600 μm Zn2+ to 1-year-old female C57B6 mice and analyzed PTEN, NEDD4-1, and NEDD4-2 levels in brain lysates prepared on a time course (0, 6, 12, 18, and 24 h). Because many studies have shown that Zn2+ can easily penetrate the blood brain barrier by intraperitoneal or oral administration, we predicted that infiltration of Zn2+ would show significant effects on the PTEN level in a relatively early time frame. Internal exposure to Zn2+ resulted in notably reduced PTEN levels in the brain 18 h after administering Zn2+ intraperitoneally (Fig. 7A). As expected, an inverse correlation was observed in NEDD4-1 and PTEN levels (Fig. 7B). However, NEDD4-2 was not changed by exposure to Zn2+. Thus, these results suggest that Zn2+-induced PTEN degradation is mediated by up-regulation of NEDD4-1 levels in vivo as well as in vitro.
FIGURE 7.
Administration of Zn2+in vivo induces NEDD4-1 and reduces PTEN. A, inverse protein levels of PTEN and NEDD4-1 in treated mouse brains. Systemic administration of zinc (200 μl of 600 μm ZnSO4 or saline via intraperitoneal injection in adult C57B6 mice) with protein levels determined by Western blot analysis on brain lysates prepared at different time points after zinc treatment. B, densitometry quantification of PTEN, NEDD4-1, and NEDD4-2 in A normalized by α-tubulin.
DISCUSSION
Zinc is an important signaling mediator that can modulate various neuronal activities. Upon extracellular cues, zinc is released into the synapse and subsequently activates various downstream signaling cascades such as Akt/PI3K, NF-κB, and c-Jun N-terminal kinase (JNK) in the brain (45). In this study, we examined the potential role of Zn2+ on PTEN stability and the PI3K signaling pathway in neuronal models. Exposure to Zn2+ leads to down-regulated PTEN protein levels due to increased protein degradation by the UPS, as evidenced by induced PTEN ubiquitination. We also demonstrate that NEDD4-1 is the main E3 ligase for PTEN in neurons. Furthermore, we found that PTEN and NEDD4-1 are inversely regulated through the PI3K signaling pathway via a negative feedback loop mechanism.
Our observation of the Zn2+-induced PTEN protein degradation in neuronal cells is consistent with the previous reports in various cell systems (46, 47). Furthermore, the use of PI3K-specific inhibitor wortmannin in primary cultured cortical neurons completely suppressed the Zn2+-induced PTEN reduction at the protein level, an effect equivalent to the proteasome inhibitor MG132 (Fig. 3). These findings indicate that the activated PI3K signaling pathway, as a consequence of the reduced PTEN activity, also exerts a feedback mechanism on modulating PTEN ubiquitination and its degradation. This observation is also consistent with the previously published data in lung airway BEAS-2B cells (47). The conserved mechanism indicates that this feedback loop between PTEN and PI3K signaling may be a common regulatory mode in the stress response to zinc in various cell types.
In this study, we investigated the potential role of the ubiquitin ligase NEDD4-1 in PTEN ubiquitination in neuronal systems. We observed that exposure to Zn2+ progressively enhanced physical interactions between PTEN and NEDD4-1, which induced degradation of PTEN likely via activated UPS (Fig. 3). Moreover, siRNA-down-regulated NEDD4-1 in neuronal cells caused the cells to fail to respond to zinc with reduced PTEN protein levels. We therefore conclude that NEDD4-1 is the major E3 ligase for PTEN in neuronal systems. Although X-linked inhibitor of apoptosis protein is also reported to be the E3 ligase for PTEN in cancer cells and primary mouse embryonic fibroblast cells (48), our preliminary data showed no change in X-linked inhibitor of apoptosis protein level upon zinc treatment.4 Our results also exclude the involvement of NEDD4-2 in regulating neuronal PTEN in response to zinc, despite the fact that this member of the family has been frequently reported to be associated with a number of neuronal activities, synaptic plasticity, commissural axon guidance, as well as neuronal survival; a number of central nervous system substrates have been identified for NEDD4-2, including a number of membrane ion channels and transporters, TrkA, and dopamine transporter (36–42). We should stress that, to our knowledge, these findings are the first to identify PTEN as one of the central nervous system substrates for NEDD4-1.
Interestingly, as in previous studies with various cancer cells (30, 49), we observed an inverse correlation between PTEN and NEDD4-1 protein levels upon zinc treatment in mouse brains (Fig. 7). These findings strongly suggest that PTEN and NEDD4-1 levels are counter-regulated by each other through the PI3K pathway via a negative feedback loop mechanism, similar to the reported relationship of p53 and Mdm2 (50, 51). However, it is not clear which component of the PI3K pathway plays the key role. Although there are reports indicating that Zn2+ metal ions can contribute to cellular physiology (e.g. neuropeptide production and insulin signaling pathway) through regulation of protein stability (46) instead of protein activity (52), we found that Zn2+ modulates NEDD4-1 mRNA transcripts instead of protein stability. It may not be surprising that certain downstream molecules of the PI3K/Akt pathway such as FOXO can regulate the components of basic transcriptional machinery (53). Further characterization of the NEDD4-1 promoter may shed some light on which signaling pathways and/or transcription factors can regulate NEDD4-1 transcription in neuronal cells, which is the key to elucidating the feed-back loop mechanism.
In summary, our present work identifies NEDD4-1 as the E3 ligase for neuronal PTEN and suggests that PTEN ubiquitination is a critical regulatory step in modulating PTEN level/activity and thus the subsequent PI3K/Akt pathway. Given the importance of the role of the PTEN-regulated PI3K/Akt pathway in neuronal responses to various insults, including zinc, a full understanding of the key mechanisms underlying PTEN regulation during the responses is crucial and is instrumental to the future design of therapeutics in neuroprotection. Although our data showed that down-regulation of PTEN conferred neurons more resistant to neurotoxin-induced apoptosis in our acute neuronal death model, presumably by activating the Akt survival signaling, the PTEN level is also found to be reduced in conjunction with elevated Akt phosphorylation in Alzheimer disease brains (54, 55). The latter observation suggests that loss of PTEN may contribute to neurodegeneration despite the fact of elevated Akt phosphorylation. It is not clear whether the Akt signaling pathway is impaired in Alzheimer disease brains because chronic Akt activation may be detrimental to neurons as exemplified in other studies (56). Alternatively, the observed increase in phospho-Akt in the Alzheimer disease brains does not indicate increased Akt activation and signaling as suggested by a recent report showing that increased phospho-Akt is associated with impaired Akt signaling (57). Nevertheless, it remains to be determined whether NEDD4-1-mediated ubiquitination is one of the major mechanisms that lead to PTEN reduction in chronic neurodegenerative processes.
Supplementary Material
Acknowledgments
We thank Dr. Wu Hong (UCLA Medical School, Los Angeles) and Dr. Masao Kaneki (Harvard Medical School, Boston) for providing pEF-PTEN/pEF-PTEN C124G and pCMV-Tag2B-AKT-WT plasmids, respectively. Robert Thompson and Yaomin Chen provided excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 NS054880 and AG031893 (to F.-F. L.). This work was also supported by American Cancer Society grant (to X. J.), Geoffrey Beene Cancer Research Fund (to X. J.), and the Alzheimer Association Investigator Initiated Research Award IIRG-06-26070 (to F.-F. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
Y.-D. Kwak, unpublished data.
- PI3K
- phosphatidylinositol 3-kinase
- PTEN
- phosphatase and tensin homologue, deleted on chromosome 10
- UPS
- ubiquitin/proteosomal system
- shRNA
- small hairpin RNA
- siRNA
- small interference RNA
- PDZ
- postsynaptic density 95, PSD-85
- Dlg
- discs large
- ZO-1
- zonula occludens-1
- qRT
- quantitative reverse transcription
- Ab
- antibody
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
REFERENCES
- 1.Izumi Y., Auberson Y. P., Zorumski C. F. (2006) J. Neurosci. 26, 7181–7188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qian J., Noebels J. L. (2006) J. Neurosci. 26, 6089–6095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frederickson C. J., Klitenick M. A., Manton W. I., Kirkpatrick J. B. (1983) Brain Res. 273, 335–339 [DOI] [PubMed] [Google Scholar]
- 4.Frederickson C. J., Koh J. Y., Bush A. I. (2005) Nat. Rev. Neurosci. 6, 449–462 [DOI] [PubMed] [Google Scholar]
- 5.Koh J. Y. (2001) Mol. Neurobiol. 24, 99–106 [DOI] [PubMed] [Google Scholar]
- 6.Huang X., Atwood C. S., Moir R. D., Hartshorn M. A., Vonsattel J. P., Tanzi R. E., Bush A. I. (1997) J. Biol. Chem. 272, 26464–26470 [DOI] [PubMed] [Google Scholar]
- 7.Weiss J. H., Hartley D. M., Koh J. Y., Choi D. W. (1993) Neuron 10, 43–49 [DOI] [PubMed] [Google Scholar]
- 8.Kim S., Jung Y., Kim D., Koh H., Chung J. (2000) J. Biol. Chem. 275, 25979–25984 [DOI] [PubMed] [Google Scholar]
- 9.Min Y. K., Lee J. E., Chung K. C. (2007) Exp. Cell Res. 313, 312–321 [DOI] [PubMed] [Google Scholar]
- 10.Samet J. M., Graves L. M., Quay J., Dailey L. A., Devlin R. B., Ghio A. J., Wu W., Bromberg P. A., Reed W. (1998) Am. J. Physiol. Lung Cell. Mol. Physiol. 275, L551–L558 [DOI] [PubMed] [Google Scholar]
- 11.Chung K. C., Park J. H., Kim C. H., Lee H. W., Sato N., Uchiyama Y., Ahn Y. S. (2000) J. Neurosci. Res. 59, 117–125 [PubMed] [Google Scholar]
- 12.Alirezaei M., Nairn A. C., Glowinski J., Prémont J., Marin P. (1999) J. Biol. Chem. 274, 32433–32438 [DOI] [PubMed] [Google Scholar]
- 13.Li J., Yen C., Liaw D., Podsypanina K., Bose S., Wang S. I., Puc J., Miliaresis C., Rodgers L., McCombie R., Bigner S. H., Giovanella B. C., Ittmann M., Tycko B., Hibshoosh H., Wigler M. H., Parsons R. (1997) Science 275, 1943–1947 [DOI] [PubMed] [Google Scholar]
- 14.Teng D. H., Hu R., Lin H., Davis T., Iliev D., Frye C., Swedlund B., Hansen K. L., Vinson V. L., Gumpper K. L., Ellis L., El-Naggar A., Frazier M., Jasser S., Langford L. A., Lee J., Mills G. B., Pershouse M. A., Pollack R. E., Tornos C., Troncoso P., Yung W. K., Fujii G., Berson A., Steck P. A. (1997) Cancer Res. 57, 5221–5225 [PubMed] [Google Scholar]
- 15.Gimm O., Perren A., Weng L. P., Marsh D. J., Yeh J. J., Ziebold U., Gil E., Hinze R., Delbridge L., Lees J. A., Mutter G. L., Robinson B. G., Komminoth P., Dralle H., Eng C. (2000) Am. J. Pathol. 156, 1693–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachibana M., Shibakita M., Ohno S., Kinugasa S., Yoshimura H., Ueda S., Fujii T., Rahman M. A., Dhar D. K., Nagasue N. (2002) Cancer 94, 1955–1960 [DOI] [PubMed] [Google Scholar]
- 17.Whiteman D. C., Zhou X. P., Cummings M. C., Pavey S., Hayward N. K., Eng C. (2002) Int. J. Cancer 99, 63–67 [DOI] [PubMed] [Google Scholar]
- 18.Lachyankar M. B., Sultana N., Schonhoff C. M., Mitra P., Poluha W., Lambert S., Quesenberry P. J., Litofsky N. S., Recht L. D., Nabi R., Miller S. J., Ohta S., Neel B. G., Ross A. H. (2000) J. Neurosci. 20, 1404–1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue Q., Groszer M., Gil J. S., Berk A. J., Messing A., Wu H., Liu X. (2005) Development 132, 3281–3291 [DOI] [PubMed] [Google Scholar]
- 20.van Diepen M. T., Eickholt B. J. (2008) Dev. Neurosci. 30, 59–64 [DOI] [PubMed] [Google Scholar]
- 21.Kwon C. H., Zhu X., Zhang J., Knoop L. L., Tharp R., Smeyne R. J., Eberhart C. G., Burger P. C., Baker S. J. (2001) Nat. Genet. 29, 404–411 [DOI] [PubMed] [Google Scholar]
- 22.Kwon C. H., Zhu X., Zhang J., Baker S. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 12923–12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ning K., Pei L., Liao M., Liu B., Zhang Y., Jiang W., Mielke J. G., Li L., Chen Y., El-Hayek Y. H., Fehlings M. G., Zhang X., Liu F., Eubanks J., Wan Q. (2004) J. Neurosci. 24, 4052–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X., Hepner K., Castelino-Prabhu S., Do D., Kaye M. B., Yuan X. J., Wood J., Ross C., Sawyers C. L., Whang Y. E. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 4233–4238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okahara F., Ikawa H., Kanaho Y., Maehama T. (2004) J. Biol. Chem. 279, 45300–45303 [DOI] [PubMed] [Google Scholar]
- 26.Okumura K., Mendoza M., Bachoo R. M., DePinho R. A., Cavenee W. K., Furnari F. B. (2006) J. Biol. Chem. 281, 26562–26568 [DOI] [PubMed] [Google Scholar]
- 27.Georgescu M. M., Kirsch K. H., Akagi T., Shishido T., Hanafusa H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 10182–10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salmena L., Carracedo A., Pandolfi P. P. (2008) Cell 133, 403–414 [DOI] [PubMed] [Google Scholar]
- 29.Vazquez F., Ramaswamy S., Nakamura N., Sellers W. R. (2000) Mol. Cell. Biol. 20, 5010–5018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X., Trotman L. C., Koppie T., Alimonti A., Chen Z., Gao Z., Wang J., Erdjument-Bromage H., Tempst P., Cordon-Cardo C., Pandolfi P. P., Jiang X. (2007) Cell 128, 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trotman L. C., Wang X., Alimonti A., Chen Z., Teruya-Feldstein J., Yang H., Pavletich N. P., Carver B. S., Cordon-Cardo C., Erdjument-Bromage H., Tempst P., Chi S. G., Kim H. J., Misteli T., Jiang X., Pandolfi P. P. (2007) Cell 128, 141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fouladkou F., Landry T., Kawabe H., Neeb A., Lu C., Brose N., Stambolic V., Rotin D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 8585–8590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao X. R., Lill N. L., Boase N., Shi P. P., Croucher D. R., Shan H., Qu J., Sweezer E. M., Place T., Kirby P. A., Daly R. J., Kumar S., Yang B. (2008) Sci. Signal. 1, ra5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X., Shi Y., Wang J., Huang G., Jiang X. (2008) Biochem. J. 414, 221–229 [DOI] [PubMed] [Google Scholar]
- 35.Yim E. K., Peng G., Dai H., Hu R., Li K., Lu Y., Mills G. B., Meric-Bernstam F., Hennessy B. T., Craven R. J., Lin S. Y. (2009) Cancer Cell 15, 304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekberg J., Schuetz F., Boase N. A., Conroy S. J., Manning J., Kumar S., Poronnik P., Adams D. J. (2007) J. Biol. Chem. 282, 12135–12142 [DOI] [PubMed] [Google Scholar]
- 37.Sorkina T., Miranda M., Dionne K. R., Hoover B. R., Zahniser N. R., Sorkin A. (2006) J. Neurosci. 26, 8195–8205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arévalo J. C., Waite J., Rajagopal R., Beyna M., Chen Z. Y., Lee F. S., Chao M. V. (2006) Neuron 50, 549–559 [DOI] [PubMed] [Google Scholar]
- 39.Keleman K., Ribeiro C., Dickson B. J. (2005) Nat. Neurosci. 8, 156–163 [DOI] [PubMed] [Google Scholar]
- 40.Fotia A. B., Ekberg J., Adams D. J., Cook D. I., Poronnik P., Kumar S. (2004) J. Biol. Chem. 279, 28930–28935 [DOI] [PubMed] [Google Scholar]
- 41.Anan T., Nagata Y., Koga H., Honda Y., Yabuki N., Miyamoto C., Kuwano A., Matsuda I., Endo F., Saya H., Nakao M. (1998) Genes Cells 3, 751–763 [DOI] [PubMed] [Google Scholar]
- 42.Sang Q., Kim M. H., Kumar S., Bye N., Morganti-Kossman M. C., Gunnersen J., Fuller S., Howitt J., Hyde L., Beissbarth T., Scott H. S., Silke J., Tan S. S. (2006) J. Neurosci. 26, 7234–7244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han P., Dou F., Li F., Zhang X., Zhang Y. W., Zheng H., Lipton S. A., Xu H., Liao F. F. (2005) J. Neurosci. 25, 11542–11552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma T., Zhao Y., Kwak Y. D., Yang Z., Thompson R., Luo Z., Xu H., Liao F. F. (2009) J. Neurosci. 29, 11226–11236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Min Y. K., Park J. H., Chong S. A., Kim Y. S., Ahn Y. S., Seo J. T., Bae Y. S., Chung K. C. (2003) J. Neurosci. Res. 71, 689–700 [DOI] [PubMed] [Google Scholar]
- 46.Hwang S. R., Hook V. (2008) FEBS Lett. 582, 2527–2531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu W., Wang X., Zhang W., Reed W., Samet J. M., Whang Y. E., Ghio A. J. (2003) J. Biol. Chem. 278, 28258–28263 [DOI] [PubMed] [Google Scholar]
- 48.Van Themsche C., Leblanc V., Parent S., Asselin E. (2009) J. Biol. Chem. 284, 20462–20466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S. S., Yoo N. J., Jeong E. G., Kim M. S., Lee S. H. (2008) APMIS 116, 779–784 [DOI] [PubMed] [Google Scholar]
- 50.Freeman D. J., Li A. G., Wei G., Li H. H., Kertesz N., Lesche R., Whale A. D., Martinez-Diaz H., Rozengurt N., Cardiff R. D., Liu X., Wu H. (2003) Cancer Cell 3, 117–130 [DOI] [PubMed] [Google Scholar]
- 51.Zhou M., Gu L., Findley H. W., Jiang R., Woods W. G. (2003) Cancer Res. 63, 6357–6362 [PubMed] [Google Scholar]
- 52.Kim J. H., Cho H., Ryu S. E., Choi M. U. (2000) Arch. Biochem. Biophys. 382, 72–80 [DOI] [PubMed] [Google Scholar]
- 53.Salih D. A., Brunet A. (2008) Curr. Opin. Cell Biol. 20, 126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grundke-Iqbal I., Iqbal K., Tung Y. C., Quinlan M., Wisniewski H. M., Binder L. I. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 4913–4917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griffin R. J., Moloney A., Kelliher M., Johnston J. A., Ravid R., Dockery P., O'Connor R., O'Neill C. (2005) J. Neurochem. 93, 105–117 [DOI] [PubMed] [Google Scholar]
- 56.Nagoshi T., Matsui T., Aoyama T., Leri A., Anversa P., Li L., Ogawa W., del Monte F., Gwathmey J. K., Grazette L., Hemmings B. A., Hemmings B., Kass D. A., Champion H. C., Rosenzweig A. (2005) J. Clin. Invest. 115, 2128–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu M., Katta A., Gadde M. K., Liu H., Kakarla S. K., Fannin J., Paturi S., Arvapalli R. K., Rice K. M., Wang Y., Blough E. R. (2009) PLoS One 4, e6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.