Abstract
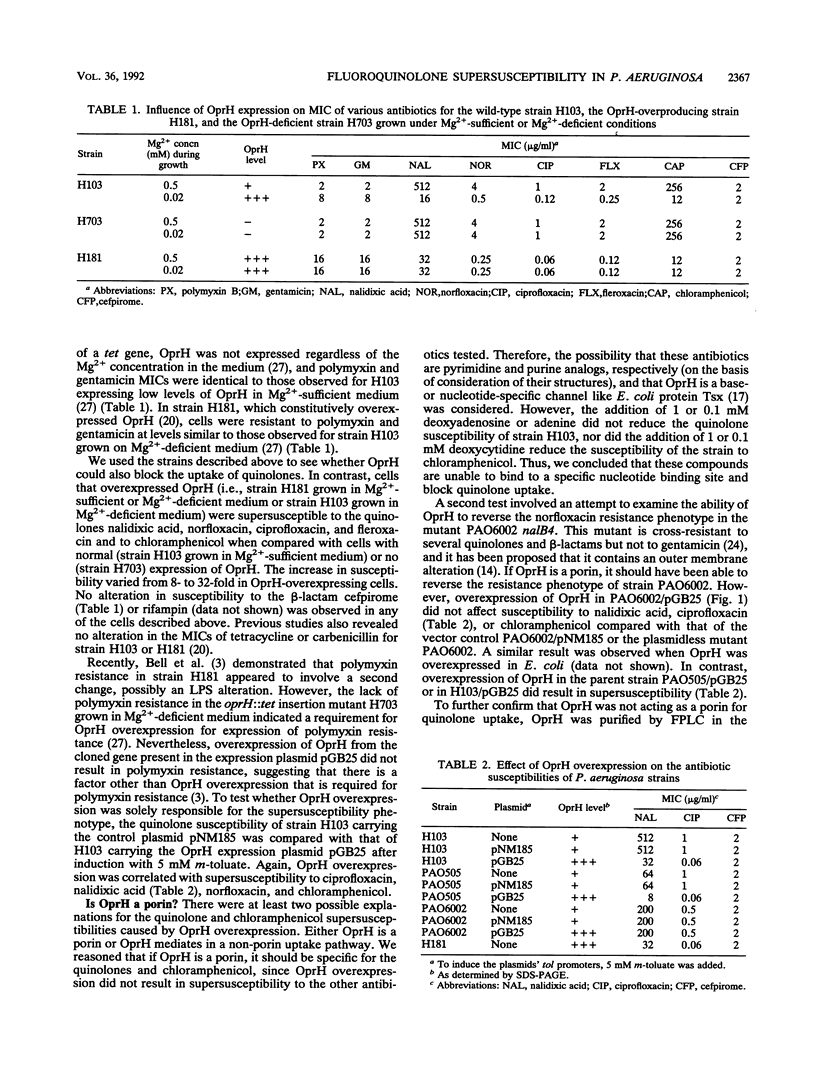
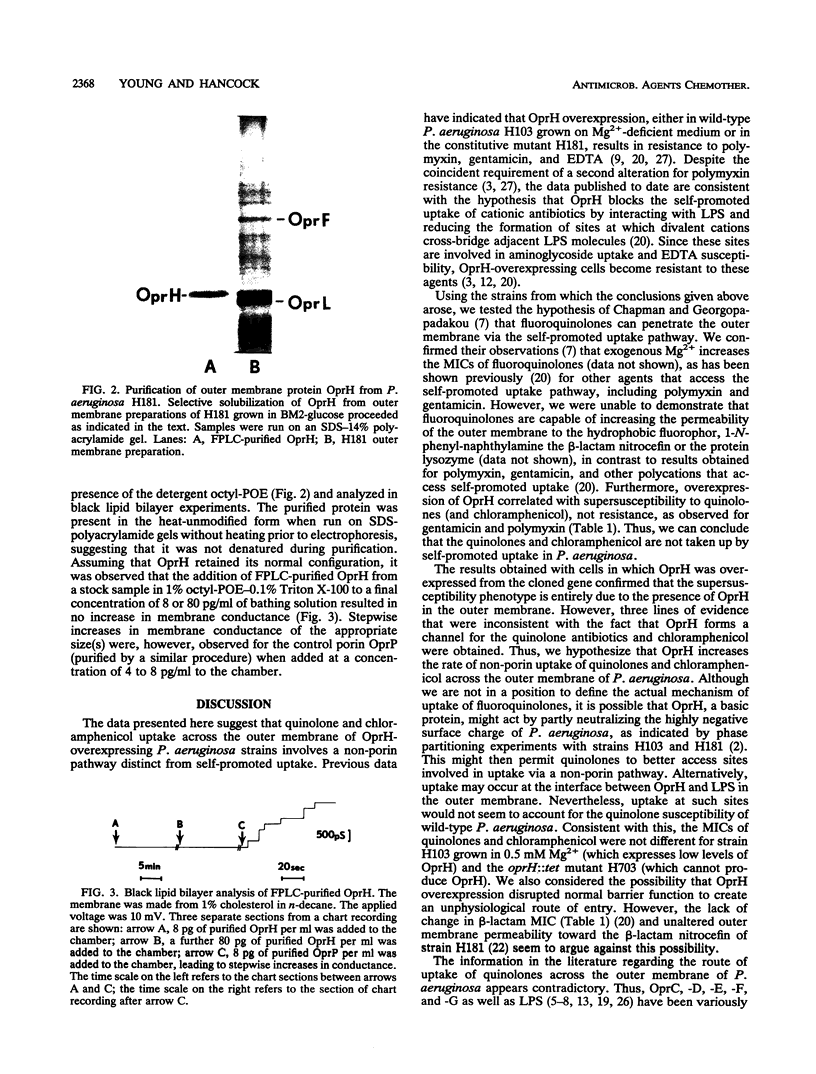
Overexpression of Pseudomonas aeruginosa outer membrane protein OprH led to an 8- to 32-fold increase in susceptibility to chloramphenicol and the quinolones nalidixic acid, norfloxacin, ciprofloxacin, and fleroxacin in comparison with the susceptibility of the wild-type strain H103 grown on Mg(2+)-sufficient medium. This was true regardless of whether OprH overexpression was induced by growth of strain H103 on Mg(2+)-deficient medium, the addition of 5 mM m-toluate to cells containing the cloned oprH gene behind the inducible tol promoter in plasmid pGB25, or mutation in the polymyxin-resistant derivative strain H181. In contrast, OprH overexpression failed to reverse the quinolone resistance phenotype of a nalB mutant. OprH was purified to homogeneity by selective detergent solubilization and fast protein liquid chromatography. The addition of OprH to the solution bathing a black lipid bilayer membrane failed to give rise to an increase in membrane conductance. This suggests that OprH is not a porin but, instead, may cause increased uptake of quinolones and chloramphenicol via a non-porin pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedard J., Chamberland S., Wong S., Schollaardt T., Bryan L. E. Contribution of permeability and sensitivity to inhibition of DNA synthesis in determining susceptibilities of Escherichia coli, Pseudomonas aeruginosa, and Alcaligenes faecalis to ciprofloxacin. Antimicrob Agents Chemother. 1989 Sep;33(9):1457–1464. doi: 10.1128/aac.33.9.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A., Bains M., Hancock R. E. Pseudomonas aeruginosa outer membrane protein OprH: expression from the cloned gene and function in EDTA and gentamicin resistance. J Bacteriol. 1991 Nov;173(21):6657–6664. doi: 10.1128/jb.173.21.6657-6664.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: purification of the protein and cloning and nucleotide sequence of the gene. J Bacteriol. 1989 Jun;171(6):3211–3217. doi: 10.1128/jb.171.6.3211-3217.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Bayer A. S., Schollaardt T., Wong S. A., Bryan L. E. Characterization of mechanisms of quinolone resistance in Pseudomonas aeruginosa strains isolated in vitro and in vivo during experimental endocarditis. Antimicrob Agents Chemother. 1989 May;33(5):624–634. doi: 10.1128/aac.33.5.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Malouin F., Rabin H. R., Schollaardt T., Parr T. R., Jr, Bryan L. E. Persistence of Pseudomonas aeruginosa during ciprofloxacin therapy of a cystic fibrosis patient: transient resistance to quinolones and protein F-deficiency. J Antimicrob Chemother. 1990 Jun;25(6):995–1010. doi: 10.1093/jac/25.6.995. [DOI] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Routes of quinolone permeation in Escherichia coli. Antimicrob Agents Chemother. 1988 Apr;32(4):438–442. doi: 10.1128/aac.32.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. Alterations in outer membrane proteins of Pseudomonas aeruginosa associated with selective resistance to quinolones. Antimicrob Agents Chemother. 1988 May;32(5):785–787. doi: 10.1128/aac.32.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H., Hosaka M., Hirai K., Iyobe S. New norfloxacin resistance gene in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1990 Sep;34(9):1757–1761. doi: 10.1128/aac.34.9.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Benz R. Demonstration and chemical modification of a specific phosphate binding site in the phosphate-starvation-inducible outer membrane porin protein P of Pseudomonas aeruginosa. Biochim Biophys Acta. 1986 Sep 11;860(3):699–707. doi: 10.1016/0005-2736(86)90569-9. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Carey A. M. Outer membrane of Pseudomonas aeruginosa: heat- 2-mercaptoethanol-modifiable proteins. J Bacteriol. 1979 Dec;140(3):902–910. doi: 10.1128/jb.140.3.902-910.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Raffle V. J., Nicas T. I. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1981 May;19(5):777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Mutations producing resistance to norfloxacin in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1987 Apr;31(4):582–586. doi: 10.1128/aac.31.4.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrebenda J., Heleszko H., Brzostek K., Bielecki J. Mutation affecting resistance of Escherichia coli K12 to nalidixic acid. J Gen Microbiol. 1985 Sep;131(9):2285–2292. doi: 10.1099/00221287-131-9-2285. [DOI] [PubMed] [Google Scholar]
- Legakis N. J., Tzouvelekis L. S., Makris A., Kotsifaki H. Outer membrane alterations in multiresistant mutants of Pseudomonas aeruginosa selected by ciprofloxacin. Antimicrob Agents Chemother. 1989 Jan;33(1):124–127. doi: 10.1128/aac.33.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier C., Bremer E., Schmid A., Benz R. Pore-forming activity of the Tsx protein from the outer membrane of Escherichia coli. Demonstration of a nucleoside-specific binding site. J Biol Chem. 1988 Feb 15;263(5):2493–2499. [PubMed] [Google Scholar]
- Mermod N., Ramos J. L., Lehrbach P. R., Timmis K. N. Vector for regulated expression of cloned genes in a wide range of gram-negative bacteria. J Bacteriol. 1986 Aug;167(2):447–454. doi: 10.1128/jb.167.2.447-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michéa-Hamzehpour M., Auckenthaler R., Regamey P., Pechère J. C. Resistance occurring after fluoroquinolone therapy of experimental Pseudomonas aeruginosa peritonitis. Antimicrob Agents Chemother. 1987 Nov;31(11):1803–1808. doi: 10.1128/aac.31.11.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Alteration of susceptibility to EDTA, polymyxin B and gentamicin in Pseudomonas aeruginosa by divalent cation regulation of outer membrane protein H1. J Gen Microbiol. 1983 Feb;129(2):509–517. doi: 10.1099/00221287-129-2-509. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Outer membrane protein H1 of Pseudomonas aeruginosa: involvement in adaptive and mutational resistance to ethylenediaminetetraacetate, polymyxin B, and gentamicin. J Bacteriol. 1980 Aug;143(2):872–878. doi: 10.1128/jb.143.2.872-878.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. Mechanisms of resistance to quinolones and clinical perspectives. J Antimicrob Chemother. 1989 Apr;23(4):475–480. doi: 10.1093/jac/23.4.475. [DOI] [PubMed] [Google Scholar]
- Rella M., Haas D. Resistance of Pseudomonas aeruginosa PAO to nalidixic acid and low levels of beta-lactam antibiotics: mapping of chromosomal genes. Antimicrob Agents Chemother. 1982 Aug;22(2):242–249. doi: 10.1128/aac.22.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J., Scarpa A. L. Genetic and physiological characterization of ciprofloxacin resistance in Pseudomonas aeruginosa PAO. Antimicrob Agents Chemother. 1988 Apr;32(4):535–539. doi: 10.1128/aac.32.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano Y., Nishikawa T., Komatsu Y. Outer membrane proteins responsible for the penetration of beta-lactams and quinolones in Pseudomonas aeruginosa. J Antimicrob Chemother. 1990 Aug;26(2):175–184. doi: 10.1093/jac/26.2.175. [DOI] [PubMed] [Google Scholar]
- Young M. L., Bains M., Bell A., Hancock R. E. Role of Pseudomonas aeruginosa outer membrane protein OprH in polymyxin and gentamicin resistance: isolation of an OprH-deficient mutant by gene replacement techniques. Antimicrob Agents Chemother. 1992 Nov;36(11):2566–2568. doi: 10.1128/aac.36.11.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]