Abstract
While psoriasis is one of the most common skin disorders in humans, effective, safe and inexpensive treatments are still largely unavailable. Chinese herbal medicine (CHM) has been used for centuries for treating psoriasis and several reports claim that systemic administration of one such CHM, Tuhuai, mainly composed of flos sophorae, smilax glabra roxb and licorice, is effective in psoriasis. However, the mechanisms by which this CHM improves psoriasis are not yet clear. Two universal features of psoriasis are epidermal hyperplasia and inflammation. Moreover, drugs that specifically inhibit epidermal hyperplasia and/or inflammation are widely used to treat psoriasis. Here, we investigated whether topical applications of Tuhuai extract exhibit anti-proliferative and anti-inflammatory activities in two murine models of inflammatory dermatoses. To assess Tuhuai's potential anti-proliferative effect, we disrupted epidermal barrier function twice-daily for 4 days in normal hairless mice followed by topical applications of either 1% Tuhuai extract or Vehicle to both flanks immediately after each barrier perturbation. Changes in epidermal proliferation and apoptosis were evaluated by immunohistochemistry and TUNEL staining. To assess the anti-inflammatory effects of Tuhuai, both irritant (phorbol ester) and acute allergic contact dermatitis (oxazolone) models were used. Whereas topical Tuhuai extract did not alter epidermal proliferation or induce irritation in normal skin, it both reduced epidermal hyperplasia in the epidermal hyperproliferative model, and reduced inflammation in both irritant and allergic contact dermatitis models. As topical Tuhuai extract exhibits anti-proliferative and anti-inflammatory properties in a variety of human models of inflammatory dermatoses, Tuhuai could provide an effective, relatively safe and inexpensive therapeutic alternative for the treatment of inflammatory dermatoses, including psoriasis.
Keywords: Chinese herbal medicine, epidermal proliferation, inflammation, psoriasis
Introduction
Psoriasis is a common, chronic skin disorder characterized by epidermal hyperproliferation and inflammation. Although psoriasis affects up to 5% of the general population, a much smaller percentage of ethnic Chinese are affected (<0.3%). The tendency for psoriasis to frequently flare after cessation of therapy; to develop tachyphylaxis to most forms of therapy and its tenacious thick scale, represent a considerable therapeutic challenge. As a result, long-term systemic therapy or combinations of multiple agents are often used. These conventional approaches for the treatment of psoriasis include: systemic and topical anti-inflammatory, anti-proliferative, immunosuppressive and biological agents, as well as phototherapy. Yet, important side effects can occur with each of these systemic regimens, whether they are administrated systemically or topically. For example, cyclosporin A has been reported to be effective in the treatment of psoriasis (1,2), but a number of adverse effects, including kidney disease, hypertension or gingival hyperplasia, can occur (2–8). Glucocorticoids are also commonly used in the treatment of psoriasis but can produce severe side effects. Topical beta-methasone and clobetasol can induce skin atrophy, permeability barrier dysfunction, as well as increased skin infections (9–12). Liver damage can result after long-term systematic administration of methotrexate (13–15). In addition, decreased bone density has been observed with methotrexate therapy (16,17). 1,25 (OH) vitamin D3 and its analogues have proved to be effective in treating psoriasis (18,19), however, topical irritation and hypercalciuria can occur (18,20,21). Retinoids represent another group of biological agents, which show beneficial effects in treating psoriasis (22,23). Yet, alopecia, elevated blood triglycerides, and many other side effects limit acitretin use (24,25). TNFα inhibitors, such as etancercept and infliximab, although effective, also can cause serious adverse effects including infections, exacerbation of psoriasis and increased risk of lymphomas (26–31). Finally, the considerable medical costs incurred during treatment of psoriasis with currently available medications can interfere with quality of patients’ daily life (32). Thus, substantial risks coupled with modest efficacy, limit current usage of conventional medications for psoriasis, providing rationale for an effective, relatively safe and inexpensive alternative treatment for psoriasis.
Chinese herbal medicines (CHM), which have been used for centuries in the treatment of skin diseases, including psoriasis, are both inexpensive and widely available. Moreover, some controlled clinical studies suggest that these preparations are effective, safe and inexpensive treatment for inflammatory skin disorders in general, but psoriasis in particular (33–35). Although in vivo studies suggest that CHM inhibit cell proliferation (36), which could be relevant for how CHM improve psoriasis, controlled mechanistic studies have not yet been carried out. Whereas normal mouse vaginal and rectal epithelia are often used to assess the anti-proliferative activity of CHM (37), these models do not necessarily reflect how hyperproliferative epidermis would respond. Thus, an epidermal hyperproliferative model (ideally also accompanied by dermal inflammation or by other models of inflammation) would be preferred to assess the therapeutic effects and to study the mechanisms responsible for the efficacy of CHM. Therefore, in the present studies, we employed an epidermal hyperproliferative murine model to evaluate the ability of topical CHM to reduce epidermal hyperplasia, and both TPA-induced irritant dermatitis and oxazolone-induced, allergic contact dermatitis mouse models to assess the anti-inflammatory efficacy of topical CHM.
Materials and methods
Materials
Six- to eight-week-old female hairless mice (hr/hr) and CD-1 male mice were purchased from Charles River Laboratories (Wilmington, MA, USA) and fed mouse diet (Ralston-Purina Co., St Louis, MO, USA) and water ad libitum. Phorbol 12-myristate 13-acetate (TPA), 4-ethoxymethylene-2-phenyl-2-oxazolin-5-one (oxazolone) and acetone were purchased from Sigma Chemical Co. (St Louis, MO, USA). Biotinylated anti-proliferating cell nuclear antigen (PCNA) antibody was from CalTag Laboratories (Burlingame, CA, USA). ABC-peroxidase kit was purchased from Vector Laboratories (Burlingame, CA, USA). The raw materials of Tuhuai mixture were purchased from Bozhou Tongrentang Pharmaceutical Company (Bozhou, China), where all herbals are collected under strict quality control conditions, according to standards set by the Chinese State Department of Drug Administration. The topical Tuhuai extract was prepared by soaking flos sophorae, smilax glabra roxb, Paeonia lactiflora, radix scutellariae, flos lonicerate and glycyrrhiza uralensis-containing mixture without further purification or analytical studies, in 100% ethanol (20%, w/v) for 3 weeks. The ethanol extract was used as the treatment agent.
Experimental protocols
All animal procedures were approved by the Animal Studies Subcommittee of the San Francisco Veterans Administration Medical Center and performed in accordance with their guidelines. An epidermal hyperproliferative model was established by repeated applications of cellophane tape on the flanks of hairless mice (hr/hr) twice daily for 4 days until transepidermal water loss reached 3–6 mg/cm2/h as measured with an electrolytic water analyzer (Meeco, Warrington, PA, USA). Either 60 μl of 1% Tuhuai extract or ethanol alone was applied to both flanks on the tape-stripped areas (4 × 4 cm) following each barrier disruption. Skin samples for immunohistochemistry were taken 12 h after the last treatment.
For anti-inflammatory studies, ear inflammation was induced by topical application of 10 μl of 0.03% TPA to both inner and outer surfaces of both ears of CD-1 mice (38). 10 μl of 1% Tuhuai in ethanol or ethanol alone was applied to both surfaces of the right or left ear at 45 min and 4 h following TPA treatment. Additionally, the oxazo-lone-induced ear inflammation model was also used to assess the anti-inflammatory effects of Tuhuai. CD-1 mice were sensitized by topical application of 3% oxazolone to the back once daily for 2 days. One week later, 10 μl of 0.5% oxazolone was applied to both inner and outer surfaces of both ears. 10 μl of 1% Tuhuai or ethanol alone was applied to both surfaces of right or left ear at 45 min and 4 h following last oxazolone treatment. Ear thickness was measured with a digital caliper (Mitutoyo, Tokyo, Japan) before and 20 h after the last oxazolone or TPA application, and samples were taken for H&E staining. The measurement of epidermal thickness of nucleated cell layers was taken at 100X on H&E sections at every 2 cm points along the epidermis.
Immunohistochemistry
For determination of epidermal proliferation, a previously described method was employed (39). Briefly, 5 μm paraffin sections were incubated with an antibody against PCNA (Ki67) (CalTag Laboratories, Burlingame, CA, USA) overnight at 4°C. Immunostaining was detected by ABC peroxidase method. Sections were visualized with a Zeiss Microscope (Jena, Germany), and digital images were taken with Axio Vision software 3.1 (Carl Zeiss Vision, Munich, Germany).
Keratinocyte apoptosis was determined with a Fluorescein-FragEL™ apoptosis detection kit (Oncogen Research Products, San Diego, CA, USA), according to manufacturer's protocol. Briefly, deparaffinized sections were incubated with proteinase K for 10 min at room temperature, followed by incubation with deoxynucleotidyl transferase for 60 min. The labelled cells were observed with a fluorescence microscope.
Epidermal thickness measurement and PCNA quantification
Thickness of the epidermal nucleated cell layers was measured on 100X micrographs taken every 2 cm along the epidermis from biopsies from normal untreated, Vehicle-treated, as well as, Tuhuai-treated skin. The data presented represent the mean of all measured points ± SEM. The number of PCNA positive cells was counted on the prints at every 1 cm segment along the epidermis, and presented as the mean of all PCNA positive cells/cm ± SEM. One-way anova test was used to determine significant differences.
Results
Topical Tuhuai does not alter epidermal proliferation in normal mice
The gross (macroscopic) appearance of mouse skin remained normal after 4 days of topical Tuhuai treatment. Notably, there were no signs of skin irritation either by visual inspection or histologically. Moreover, epidermal thickness and proliferation did not change, as assessed both by H&E and PCNA staining (Fig. S1). These results suggest that topical Tuhuai extract does not alter epidermal proliferation or produce inflammation in normal mouse skin, suggesting further that topical extract of Tuhuai is both non-toxic and non-atrophogenic.
Topical Tuhuai inhibits epidermal proliferation in an epidermal hyperproliferative model
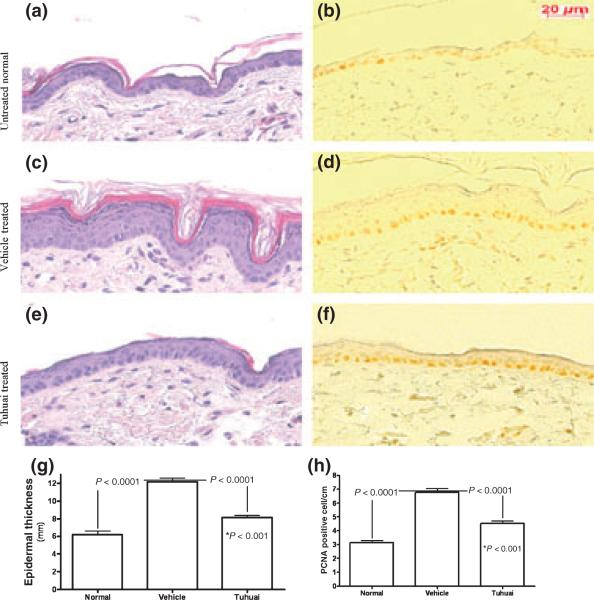
We next assessed whether topical Tuhuai inhibits epidermal proliferation in a hyperproliferative model, produced by twice-daily tape-stripping for 4 days. As shown in Fig. 1, repeated disruption of permeability barrier function induced epidermal hyperproliferation as measured by PCNA staining (Fig. 1b versus 1d), which was accompanied by increased epidermal thickness (Fig. 1a versus 1c). Barrier disruption followed by ethanol Vehicle applications did not reduce epidermal hyperplasia. In contrast, topical Tuhuai treatment inhibited the development of epidermal hyper-plasia (Fig. 1c,d versus Fig. 1e,f,g), although it did not completely normalize the epidermal thickness (Fig. 1g,h). Thus, in contrast to normal skin, topical Tuhuai exerts antiproliferative effects in an epidermal hyperpoliferative mouse model. As no changes in keratinocyte apoptosis occurred in hyperproliferative skin following Tuhuai treatment (data not shown), it is likely that the anti-hyperplastic mechanism of the Tuhuai extract is inhibiting epidermal DNA synthesis.
Figure 1.
Topical application of Tuhuai extract inhibits epidermal proliferation in an epidermal hyperproliferative model: an epidermal hyperproliferative model was established by repeated barrier disruption. Either Vehicle or 1% Tuhuai extract was applied topically following each barrier disruption. Figure 1a, c, e are H&E staining and b, d, f are anti-PCNA staining. Figure 1a, b are normal skin. Figure 1c, d are hyperproliferative skin treated with Vehicle. And figure 1e, f are hyperproliferative skin treated with Tuhuai extract. The magnifications for all are the same. Scale bar = 20 μm. Epidermal thickness of nucleated cell layer was measured on 100X H&E sections at every 2 cm points along the epidermis (Figure 1g, n = 10 for normal; n = 30 for Vehicle treated and n = 34 for Tuhuai treated). Number of PCNA positive cells was counted on every 1 cm segment along epidermis (Figure 1h, n = 60 for normal; n = 63 for Vehicle treated, and n = 67 for Tuhuai treated). The data are expressed as mean of all measured points. The significances are shown on the figure. And *P < 0.001 is for the difference between normal and Tuhuai treated.
Topical Tuhuai exhibits anti-inflammatory activities in both irritant and acute allergic contact dermatitis models
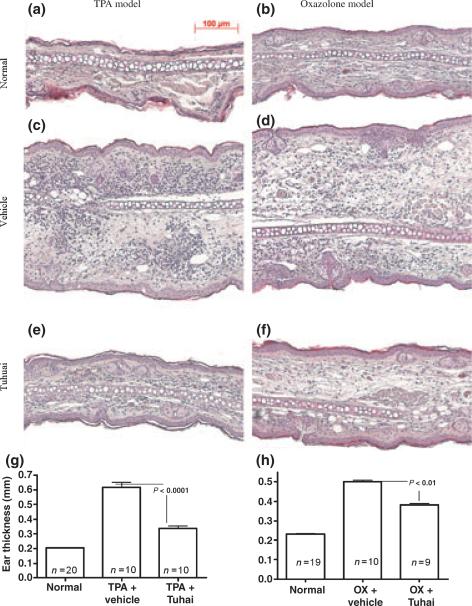
Reduction of dermal inflammation is a major goal in the treatment of inflammatory dermatoses, including psoriasis. Hence, we next assessed whether topical applications of Tuhuai decrease inflammation in the TPA model of irritant contact dermatitis, measuring the changes in ear thickness. As shown previously (40), topical TPA treatment caused a dramatic increase in ear thickness, which was not reduced by Vehicle treatment (Fig. 2c,g). In contract, topical Tuhuai application following TPA treatment significantly reduced the development of increased ear thickness (Fig. 2e,g).
Figure 2.
Topical application of Tuhuai extract reduces the inflammatory response in both TPA and oxazolone models: ear inflammation was induced by topical TPA or oxazolone treatment. Skin samples were obtained 20 h after treatment. Significant reductions in inflammatory cell infiltration and ear thickness were seen in Tuhuai-treated ear. a and b are normal ear treated with Vehicle. c (TPA) and d (oxazolone) are inflammatory ear treated with Vehicle. e and f are inflammatory ear treated with Tuhuai. Scale bar = 100 μm. The ear thickness was measured with a digital caliper as described in Methods. Fig 2g is TPA model (P < 0.001 between all groups) and Fig 2h is oxazolone model (P < 0.01 between all groups). Data are expressed as mean + SEM. Numbers for each group are indicated in the figure.
To further evaluate the anti-inflammatory effects of topical Tuhuai, another inflammatory model was also employed. As seen in Fig. 2d,f,h, topical Tuhuai also inhibited inflammation in the oxazolone model of acute allergic contact dermatitis (38,41). The anti-inflammatory effects of topical Tuhuai were further demonstrated in H&E-stained sections, which demonstrated reductions in both ear thickness and inflammatory cell infiltration in the ears of both the TPA and oxazolone-treated mice (Fig. 2, Fig. S2). Together, these results show that topical Tuhuai exhibits anti-inflammatory effects in both irritant and allergic contact dermatitis mouse models.
Discussion
Although widely used for millennia in China, until recently CHM has attracted minimal attention outside China as potential therapy for skin diseases. CHM are given systemically to patients with psoriasis, based upon the type and extent of the patient's condition. According to traditional Chinese medical theory, psoriasis is generally classified into four types, i.e. blood heat, blood dryness, blood stasis and blood toxicity. Tuhuai is a mixture of CHM, which has shown evidence of clinical effectiveness when used systemically in the treatment of psoriasis (42,43). Most Chinese herbals used in the treatment of psoriasis must be heated for more than 1 h to extract the active ingredients prior to use, which is inconvenient for most patients, and could also inactivate some ingredients. Moreover, these herbals often leave an unpleasant taste after oral use. Furthermore, ready-to-eat herbal preparations are convenient, but usually large amounts of sugar are added to make the preparation more edible, which is not acceptable for many patients with diabetes. Thus, further development of topical CHM would be a highly desirable alternative for the treatment of psoriasis.
The pharmacological mechanisms by which CHM influence inflammatory dermatoses, including psoriasis, are not clear. It has been known that these medicinal herbs contain many ingredients, some of which are known to be active (see below). In addition, chemical reactions may occur to produce still-unknown, new chemical entities during preparation or in complex mixtures. Nevertheless, Tse et al. studied the effects of 60 CHM, previously shown to display variable effectiveness in the treatment of psoriasis, on cell proliferation in HaCaT keratinocyte cell culture (44). Significant inhibition of cell proliferation has been observed with some of ingredients in Tuhuai mixture, such as glycyrrhizin (45,46). In agreement with these in vitro finding, we demonstrated here that topical Tuhuai also inhibits epidermal proliferation in an in vivo model of hyperproliferative epidermis. Moreover, scutellarin, flavonoids and paeonol, which are chemical compounds found in the Tuihuai mixture, inhibit cell proliferation in cancer cell lines (47–50). Yet the molecular mechanism by which Tuhuai inhibits keratinocyte proliferation is not known. Furthermore, the decreased epidermal thickness induced by topical Tuhuai extract is likely due to the inhibition of keratinocyte proliferation, as keratinocyte apoptosis did not change in either normal or hyperproliferative epidermis following topical Tuhuai treatment even though some anti-psoriatic agents induce keratinocyte apoptosis (51,52). As these active chemicals are ethanol extractable, it can be assumed that they were in the Tuhuai extract used in the present study.
Cutaneous inflammation is another prominent pathological feature of certain dermatoses, including psoriasis. Accordingly, immunomodulators and steroids often are used in the treatment of psoriasis. Previous clinical observations demonstrated that systematic administration of CHM decreased cutaneous inflammation in psoriasis and eczema (53,54). Topical applications of either glycyrrhiza containing gel or Atopiclair®, a US FDA approved glycyrrhetinic acid-containing cream for atopic dermatitis treatment, improve atopic dermatitis (55–57). Moreover, CHM inhibit the production of cytokines from stimulated human monocytes (58). Smilax glabra roxb and astilbin (a chemical from smilax glabra roxb) inhibit inflammation by decreasing TNF alpha expression, lymphocyte migration, as well as matrix metalloproteinase (MMP-2, MMP-9) activities (59–64). Both blood IgE and IL-4 levels decreased in atopic dermatitis patients treated with glycyrrhizin (65). Here, we demonstrated that topical applications of Tuhuai, containing all of the above ingredients, inhibits cutaneous inflammation, as indicated by a reduction of inflammation in both irritant and acute allergic contact dermatitis models. Yet, again the molecular mechanism(s) whereby Tuhuai inhibits inflammation remain unknown. Nevertheless, while topical Tuhuai does not completely eliminate skin inflammation, it could be useful for human inflammatory dermatoses, including psoriasis.
The epidermal hyperproliferative model is easy to establish and provides a large skin area for experimental study. Previously, we have utlilized this model to successfully evaluate the anti-proliferative effects of topical PPAR and LXR activators (66). Although this model is not an exact analogue of psoriasis, the epidermal changes, i.e. hyperplasia and abnormal barrier function mimic similar changes in psoriasis, making it a useful tool for assessing potential therapies for psoriasis. Likewise, the inflammatory models employed here are also easy to establish, and highly reproducible, and therefore useful models to assess anti-inflammatory therapies (38,41). Coupling the epidermal hyperproliferative model with the inflammatory models provides a useful matrix for the testing and development of topical anti-inflammatory and proliferative medications.
In conclusion, a combination of the epidermal hyperproliferative model and the ear inflammation models provide a reliable and useful approach to assess the efficacy of anti-proliferative and inflammatory agents. In addition to its anti-inflammatory effect in both TPA and oxazolone models, topical Tuhuai extract also inhibits epidermal proliferation in the epidermal hyperproliferative model, but not in normal skin. These results explain, at least partially, the mechanism by which psoriasis reportedly is effectively treated with Tuhuai, and suggest that topical Tuhuai may be clinically useful in the treatment other inflammatory dermatoses as well. In addition to delineating the molecular mechanisms accounting for Tuhuai's activity, further clinical studies will also be required to evaluate the efficacy of topical Tuhuai on psoriasis and other inflammatory skin disorders.
Supplementary Material
Supplementary material
The following supplementary material is available for this article online:
Figure S1. Topical application of Tuhuai extract does not effect epidermal proliferation significantly in normal skin: Either Vehicle or 1% Tuhuai extract was applied topically to normal intact skin twice daily for 4 days. Skin samples were taken 12 h after last treatment. a and c are H&E staining, and b and d are anti-PCNA staining. a and b are Vehicle-treated skin. c and d are Tuhuai-treated skin. The magnifications for all are the same. Scale bar = 20 μm.
Figure S2. Topical application of Tuhuai extract reduces the inflammatory cell infiltration in both TPA and oxazo-lone models: Ear inflammation was induced by topical TPA or oxazolone treatment. Skin samples were obtained 20 h after treatment. Significant reductions in inflamma-tory cell infiltration and ear thickness were seen in Tuhuai-treated ear. a is normal ear. b (oxazolone) and c (TPA) are inflammatory ear treated with Vehicle. d (oxazolone) and e (TPA) are inflammatory ear treated with Tuhuai. Scale bar = 100 μm.
This material is available as part of the online article from http://www.blackwell-synergy.com.
Please note: Blackwell publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Acknowledgements
This work was supported by National Institutes of Health grants AR 050629, AR 19098 and PO 39448 and the Medical Research Service, Department of Veterans Affairs Medical Center.
Footnotes
Please cite this paper as: Chinese herbal medicine (Tuhuai extract) exhibits topical anti-proliferative and anti-inflammatory activity in murine disease models. Experimental Dermatology 2008; 17: 681-687.
References
- 1.Mueller W, Hermann B. Cyclosporin A for psoriasis. N Engl J Med. 1979;301:355. doi: 10.1056/NEJM197909063011016. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths CE, Powles AV, Leonard JN, Fry L, Baker BS, Valdimarsson H. Clearance of psoriasis with low dose cyclosporin. Br Med J. 1986;293:731–732. doi: 10.1136/bmj.293.6549.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korstanje MJ, Bilo HJG, Stoof TJ. Sustained renal function loss in psoriasis patients after withdrawal of low dose cyclosporin therapy. Br J Dermatol. 1992;127:501–504. doi: 10.1111/j.1365-2133.1992.tb14848.x. [DOI] [PubMed] [Google Scholar]
- 4.Powles AV, Hardman CM, Porter WM, Cook T, Hulme B, Fry L. Renal function after 10 years treatment with cyclosporin for psoriasis. Br J Dermatol. 1998;138:443–449. doi: 10.1046/j.1365-2133.1998.02122.x. [DOI] [PubMed] [Google Scholar]
- 5.Oettinger-Barak O, Machtei EE, Peled M, Barak S, L-Naaj IA, Laufer D. Cyclosporine A-induced gingival hyperplasia pemphigus vulgaris: literature review and report of a case. J Periodontol. 2000;71:650–656. doi: 10.1902/jop.2000.71.4.650. [DOI] [PubMed] [Google Scholar]
- 6.Kirby B, Rogers S. Gingival hyperplasia in psoriasis patients treated with cyclosporin. Clin Exp Dermatol. 2000;25:97–98. doi: 10.1046/j.1365-2230.2000.00582.x. [DOI] [PubMed] [Google Scholar]
- 7.Markham T, Watson A, Rogers S. Adverse effects with long-term cyclosporin for severe psoriasis. Clin Exp Dermatol. 2002;27:111–114. doi: 10.1046/j.1365-2230.2002.00998.x. [DOI] [PubMed] [Google Scholar]
- 8.V'lckova-Laskoska MT. Cyclosporin A-induced gingival hyperplasia in psoriasis: review of literature and case reports. Acta Dermatovenerol Croat. 2005;13:108–113. [PubMed] [Google Scholar]
- 9.Kolbe L, Kligman AM, Schreiner V, Stoudemayer T. Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin. Skin Res Technol. 2001;7:73–77. doi: 10.1034/j.1600-0846.2001.70203.x. [DOI] [PubMed] [Google Scholar]
- 10.Kumaran MS, Kaur I, Kumar B. Effect of topical calcipotriol, betamethasone dipropionate and their combination in the treatment of localized vitiligo. J Eur Acad Dermatol Venereol. 2006;20:269–273. doi: 10.1111/j.1468-3083.2006.01420.x. [DOI] [PubMed] [Google Scholar]
- 11.Kragballe K, Austad J, Barnes L, et al. A 52-week randomized safety study of a calcipotriol/betamethasone dipropionate two-compound product (Dovobet/Daivobet/Taclonex) in the treatment of psoriasis vulgaris. Br J Dermatol. 2006;154:1155–1160. doi: 10.1111/j.1365-2133.2006.07236.x. [DOI] [PubMed] [Google Scholar]
- 12.Ahn SK, Bak HN, Park BD, et al. Effects of a multilamellar emulsion on glucocorticoid-induced epidermal atropgy and barrier impairment. J Dermatol. 2006;33:80–90. doi: 10.1111/j.1346-8138.2006.00018.x. [DOI] [PubMed] [Google Scholar]
- 13.Whiting-O'Keefe QE, Fye KH, Sack KD. Methotrexate and histologic hepatic abnormalities: a meta-analysis. Am J Med. 1991;90:711–716. [PubMed] [Google Scholar]
- 14.Malatjialian DA, Ross JB, Williams CN, Colwell SJ, Eastwood BJ. Methotrexate hepatoxicity in psoriatics: report of 104 patients from Nova Scotia, with analysis of risks from obesity, diabetes and alcohol consumption during long term follow-up. Can J Gastroenterol. 1996;10:369–375. doi: 10.1155/1996/213596. [DOI] [PubMed] [Google Scholar]
- 15.Tilling L, Townsend S, David J. Methotrexate and hepatic toxicity in rheumatoid arthrithis and psoriatic arthritis. Clin Drug Invest. 2006;26:55–62. doi: 10.2165/00044011-200626020-00001. [DOI] [PubMed] [Google Scholar]
- 16.Cranney AB, McKendry RJ, Wells GA, et al. The effect of low dose methotrexate on bone density. J Rheumatol. 2001;28:2395–2399. [PubMed] [Google Scholar]
- 17.Minaur NJ, Kounali D, Vedi S, Compston JE, Beresford JN, Bhalla AK. Methotrexate in the treatment of rheumatoid arthritis. II. In vivo effects on bone mineral density. Rheumatology (Oxford) 2002;41:741–749. doi: 10.1093/rheumatology/41.7.741. [DOI] [PubMed] [Google Scholar]
- 18.Baker JN, Ashton RE, Marks R, Harris RI, Berth-Jones J. Topical maxacalcitol for the treatment of psoriasis vulgaris: a placebo-controlled, double-blind, dose-finding study with active comparator. Br J Dermatol. 1999;141:274–278. doi: 10.1046/j.1365-2133.1999.02975.x. [DOI] [PubMed] [Google Scholar]
- 19.Van de Kerkhof PCM, Berth-Jones J, Griffiths CEM, et al. Long-term efficacy and safety of tacalcitl ointment in patients with thronic plaque psoriasis. Br J Dermatol. 2002;146:414–422. doi: 10.1046/j.1365-2133.2002.04567.x. [DOI] [PubMed] [Google Scholar]
- 20.Georgiou S, Tsambaos D. Hypercalcaemia and hypercalciuria after topical treatment of psoriasis with excessive amounts of calcipotriol. Acta Derm Venereol. 1999;79:86. doi: 10.1080/000155599750011822. [DOI] [PubMed] [Google Scholar]
- 21.Kawahara C, Okada Y, Tanikawa T, Fukusima A, Misawa H, Tanaka Y. Severs hypercalcaemia and hypercalciuria associated with calcipotriol for treatment of psoriasis. J bone Miner Metab. 2004;22:159–162. doi: 10.1007/s00774-003-0465-z. [DOI] [PubMed] [Google Scholar]
- 22.Goldfarb MT, Ellis CN, Gupta AK, Tincoff T, Hamilton TA, Voorhees JJ. Acitretin improves psoriasis in a dose-dependent fashion. J Am Acad Dermatol. 1988;18:655–662. doi: 10.1016/s0190-9622(88)70086-9. [DOI] [PubMed] [Google Scholar]
- 23.Kragballe K, jansen CT, Geiger JM, et al. A double-blind comparison of acitretin and etretinate in the treatment of severe psoriasis. Results of a Nordic multicenter study. Acta Derm Venereol. 1989;69:35–40. [PubMed] [Google Scholar]
- 24.Gupta AK, Goldfarb MT, Ellis CN, Voorhees JJ. Aide-effect profile of acitretin therapy in psoriasis. J Am Acad Dermatol. 1989;20:1088–1093. doi: 10.1016/s0190-9622(89)70138-9. [DOI] [PubMed] [Google Scholar]
- 25.Pearce DJ, Higgins KB, Stealey KH, et al. Adverse events from systemic therapies for psoriasis are common in clinical practice. J Dermatolog Treat. 2006;17:288–293. doi: 10.1080/09546630600920041. [DOI] [PubMed] [Google Scholar]
- 26.Kary S, Worm M, Audring H, et al. New onset or exacerbation of psoriatic skin lesions in patients with definite rheumatoid arthritis receiving tumor mecrosis factor alpha antagonists. Ann Rheum Dis. 2006;65:405–407. doi: 10.1136/ard.2005.037424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux CH, Brocq O, Leccia N, et al. New-onset psoriatic palmoplantaris pustulosis following infliximab therapy: a class effect?. J Rheumatol. 2007;34:434–437. [PubMed] [Google Scholar]
- 28.Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as mono-therapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi: 10.1056/NEJMoa030409. [DOI] [PubMed] [Google Scholar]
- 29.Papp KA, Tyring S, Lahfa M, et al. A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol. 2005;152:1304–1312. doi: 10.1111/j.1365-2133.2005.06688.x. [DOI] [PubMed] [Google Scholar]
- 30.Bissonnette R. Etanercept for the treatment of psoriasis. Skin Therapy Lett. 2006;11:1–4. [PubMed] [Google Scholar]
- 31.Tobin AM, Kirby B. TNF alpha inhibitors in the treatment of psoriasis and psoriatic arthritis. BioDrugs. 2005;19:47–57. doi: 10.2165/00063030-200519010-00006. [DOI] [PubMed] [Google Scholar]
- 32.Feldman SR, Evans C, Russell MW. Systemic treatment for moderate to severe psoriasis: estimates of failure rates and direct medical costs in a north-eastern US managed care plan. J Dermatolog Treat. 2005;16:37–42. doi: 10.1080/09546630510025941. [DOI] [PubMed] [Google Scholar]
- 33.Liu HT, Zhang JM, Wang F, et al. Clinical study on Quyinheji for treatment of psoriasis. J Binzhou Med College. 2002;25:341–342. in Chinese. [Google Scholar]
- 34.Li HW, Zhang JA. Clinical observation of psoralen in the treatment of psoriasis. Chin J Dermatolvenereol. 2006;20:640–641. in Chinese. [Google Scholar]
- 35.Ye XY, Li XQ, Zhao JJ. Efficacy of SNMC in treatment of psoriasis vulgaris. Chin J Dermatovenereol. 2006;20:128–128. [Google Scholar]
- 36.Zhang JM, Zhang YJ, Wang F, Han ZD, Zhou SH, Song M. Study on the effects of Xiaoyin mixture on epidermal cell proliferation, immunomodulation in mouse and bacteriostasis. Chin J Leprosy Skin Dis. 2005;21:698–700. in Chinese. [Google Scholar]
- 37.Bonder RH, Van Scott EJ. Use of mouse vaginal and rectal epithelium to screen antimitotic effects of systemically administered drugs. Cancer Res. 1971;31:851–853. [PubMed] [Google Scholar]
- 38.Sheu MY, Fowler AJ, Kao J, et al. Topical peroxisome proliferator activated receptor-α activators reduce inflammation in irritant and allergic contact dermatitis models. J Invest Dermatol. 2002;118:94–101. doi: 10.1046/j.0022-202x.2001.01626.x. [DOI] [PubMed] [Google Scholar]
- 39.Komuves LG, Hanley K, Man MQ, Elias PM, Williams ML, Feingold KR. Keratinocyte differentiation in hyperproliferative epidermis: topical application of PPAR alpha activators restores tissue homoestasis. J Invest Dermatol. 2000;115:361–367. doi: 10.1046/j.1523-1747.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 40.Mao-Qiang M, Fowler AJ, Schmuth M, et al. Peroxisome-proliferator-activated receptor (PPAR)-γ activation stimulation keratinocyte differentiation. J Invest Dermatol. 2004;123:305–312. doi: 10.1111/j.0022-202X.2004.23235.x. [DOI] [PubMed] [Google Scholar]
- 41.Fowler AJ, Sheu MY, Schmuth M, et al. LXR avtivators display anti-inflammatory activity in irritant and allergic contact dermatitis models. J Invest Dermatol. 2003;120:246–255. doi: 10.1046/j.1523-1747.2003.12033.x. [DOI] [PubMed] [Google Scholar]
- 42.Hou PJ, Zhang JM. Treated of psoriasis with Tuhuai drink. Shandong J Traditional Chin Med. 2002;21:311. in Chinese. [Google Scholar]
- 43.Gong SM, Shi YJ. Clinical observation of psoriasis treated with the combination of Tuhuai and UVA+UVA. J Dermatol Venereol. 2003;25:28–29. in Chinese. [Google Scholar]
- 44.Tse WP, Che CT, Liu K, Lin ZX. Evaluation of the anti-proliferative properties of selected psoriasis-treating Chinese medicines on cultured HaCaT cells. J Ethnopharm. 2006;108:133–141. doi: 10.1016/j.jep.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Bai CC, Gao WT, Xia YK. Experimental study on the treatment of psoriasis with Tuhuai. Chin J Leprosy Skin Dis. 2006;22:297–298. in Chinese. [Google Scholar]
- 46.Zhang RZ, Zhu WY, Ma J, Jin HL, Han HF. The effect of complex glycyrrhizin on the keratinocytes cultured in vitro. Chin J Dermatovenereol. 2006;20:267–269. in Chinese. [Google Scholar]
- 47.Goh D, Lee YH, Ong ES. Inhibitory effects of a chemically standardized extract from scutellaria barbata in human colon cancer cell lines, LoVo. J Agric Food Chem. 2005;53:8197–8204. doi: 10.1021/jf051506+. [DOI] [PubMed] [Google Scholar]
- 48.Xu SP, Sun GP, Shen YX, Wei W, Peng WR, Wang H. Antiproliferation and apoptosis induction of paeonol in HepG2 cells. World J Gastroenterol. 2007;13:250–256. doi: 10.3748/wjg.v13.i2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan S, Ye J, Qian C, Ji C, Liu C, Wang J. Paeonol inhibits the proliferation of human colorectal carcinoma cells and synergic with chemotherapeutic agents. Saudi J. 2007;28:642–643. [PubMed] [Google Scholar]
- 50.Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenber MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143:58–65. doi: 10.1016/j.jss.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 51.El-Domyati M, Barakat M, Abdel-Razek R, El-Din Anbar T. Apoptosis, P53 and Bcl-2 expression in response to topical calcipotriol therapy for psoriasis. Int J Dermatol. 2007;46:468–474. doi: 10.1111/j.1365-4632.2007.03099.x. [DOI] [PubMed] [Google Scholar]
- 52.Kruger-Krasagakis S, Galanopoulos Vk, Giannikaki L, Stefanidou M, Tosca AD. Programmed cell death of keratinocytes in infliximab-treated plaque-type psoriasis. Br J Dermatol. 2006;154:460–466. doi: 10.1111/j.1365-2133.2005.07078.x. [DOI] [PubMed] [Google Scholar]
- 53.Zhang JM, Wang XM, Liu HT. Clinical observation of psoriasis vulgaris treated with Xiaoyin mixture. Chin J Leprosy Skin Dis. 2004;20:249. in Chinese. [Google Scholar]
- 54.Long LP, Bai YP, Liu YS, et al. Observation on the effect of glycyrrhizin complex on subacute and chronic eczema. China Pharmacy. 2003;14:37–38. [Google Scholar]
- 55.Saeedi M, Morteza-Semnani K, Ghoreishi MR. The treatment of atopic dermatitis with licorice gel. J Dermatolog Treat. 2003;14:153–157. doi: 10.1080/09546630310014369. [DOI] [PubMed] [Google Scholar]
- 56.Abramovits W, Boguniewicz M. A multicenter, randomized, vehicle-controlled clinical study to examine the efficacy and safety of MAS063DP (Atopiclair) in the management of mild to moderate atopic dermatitis in adults. J Drugs Dermatol. 2006;5:236–244. [PubMed] [Google Scholar]
- 57.Belloni G, Pinelli S, Veraldi S. A randomised, double-blind, vehicle-controlled study to evaluate the efficacy and safety of MAS063DP (Atopiclair) in the treatment of mild to moderate atopic dermatitis. Eur J Dermatol. 2005;15:31–36. [PubMed] [Google Scholar]
- 58.Kawashima S, Hayashi M, Takii T, et al. Serontonin derivative N-(pcoumaroyl)- serotonin inhibits the production of TNF-α, IL-1α, IL-1β and IL-6 by endotoxin-stimulated human blood monocytes. J Interferon Cyt Res. 1998;18:423–428. doi: 10.1089/jir.1998.18.423. [DOI] [PubMed] [Google Scholar]
- 59.Xu Q, Yuan K, Lu J. A new strategy for regulating the immunological liver injury-effectiveness of DTH-inhibiting agents on DTH-induced liver injury to picryl chloride. Pharmacol Res. 1997;36:401–409. doi: 10.1006/phrs.1997.0249. [DOI] [PubMed] [Google Scholar]
- 60.Jiang J, Wu F, Lu J, Lu Z, Xu Q. Anti-inflammatory activity of the aqueous extract from Rhizoma smilacis glabrae. Pharmacol Res. 1997;36:309–314. doi: 10.1006/phrs.1997.0234. [DOI] [PubMed] [Google Scholar]
- 61.Jiang J, Xu Q. Immunomodulatory activity of the aqueous extract from rhizome of smilax glabra in the later phase of adjuvant-induced arthritis in rats. J Ethnopharmacol. 2003;85:53–59. doi: 10.1016/s0378-8741(02)00340-9. [DOI] [PubMed] [Google Scholar]
- 62.Fei M, Wu X, Xu Q. Astilbin inhibits contact hypersensitivity through negative cytokine regulation distinct cyclosporin A. J Allergy Clin Immunol. 2005;116:1350–1356. doi: 10.1016/j.jaci.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 63.Cai Y, Chen T, Xu Q. Astilbin suppresses delayed-type hypersensitivity by inhibiting lymphocyte migration. J Pharm Pharmacol. 2003;55:691–696. doi: 10.1211/002235703765344612. [DOI] [PubMed] [Google Scholar]
- 64.Cai Y, Chen T, Xu Q. Astilbin suppresses collagen-induced arthritis via the dysfunction of lymphocytes. Inflamm Res. 2003;52:334–340. doi: 10.1007/s00011-003-1179-3. [DOI] [PubMed] [Google Scholar]
- 65.Tu CX, Zhang XJ, Zhang YY, et al. Clinical and experimental study on treatment of atopic dermatitis with glycyrrhizin. Chin J Leprosy Skin Dis. 2007;23:393–395. in Chinese. [Google Scholar]
- 66.Demerijian M, Man MQ, Choi EH, et al. Topical treatment with thiazolidinediones, activators of peroxisome proliferator-activated receptor-gamma, normalizes epidermal homeostasis in a murine hyperproliferative disease model. Exp Dermatol. 2006;15:154–160. doi: 10.1111/j.1600-0625.2006.00402.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
The following supplementary material is available for this article online:
Figure S1. Topical application of Tuhuai extract does not effect epidermal proliferation significantly in normal skin: Either Vehicle or 1% Tuhuai extract was applied topically to normal intact skin twice daily for 4 days. Skin samples were taken 12 h after last treatment. a and c are H&E staining, and b and d are anti-PCNA staining. a and b are Vehicle-treated skin. c and d are Tuhuai-treated skin. The magnifications for all are the same. Scale bar = 20 μm.
Figure S2. Topical application of Tuhuai extract reduces the inflammatory cell infiltration in both TPA and oxazo-lone models: Ear inflammation was induced by topical TPA or oxazolone treatment. Skin samples were obtained 20 h after treatment. Significant reductions in inflamma-tory cell infiltration and ear thickness were seen in Tuhuai-treated ear. a is normal ear. b (oxazolone) and c (TPA) are inflammatory ear treated with Vehicle. d (oxazolone) and e (TPA) are inflammatory ear treated with Tuhuai. Scale bar = 100 μm.
This material is available as part of the online article from http://www.blackwell-synergy.com.
Please note: Blackwell publishing are not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.