Abstract
The epidermal growth factor receptor (EGFR) is overexpressed in ovarian carcinomas and promotes cellular responses that contribute to ovarian cancer pathobiology. In addition to modulation of mitogenic and motogenic behavior, emerging data identify EGFR activation as a novel mechanism for rapid modification of the cell surface proteome. The transmembrane collagenase membrane type 1 matrix metalloproteinase (MT1-MMP, MMP-14) is a major contributor to pericelluar proteolysis in the ovarian carcinoma microenvironment and is subjected to extensive post-translational regulation. In the present study, the contribution of EGFR activation to control of MT1-MMP cell surface dynamics was investigated. Unstimulated ovarian cancer cells display caveolar co-localization of EGFR and MT1-MMP whereas EGFR activation prompts internalization via distinct endocytic pathways. EGF treatment results in phosphorylation of the MT1-MMP cytoplasmic tail and cells expressing a tyrosine mutated form of MT1-MMP (MT1-MMP-Y573F) exhibit defective MT1-MMP internalization. As a result of sustained cell surface MT1-MMP activity, a phenotypic epithelial-mesenchymal transition is observed, characterized by enhanced migration and collagen invasion, whereas growth within three-dimensional collagen gels is inhibited. These data support an EGFR-dependent mechanism for regulation of the transition between invasive and expansive growth of ovarian carcinoma cells via modulation of MT1-MMP cell surface dynamics.
Keywords: epidermal growth factor receptor, matrix metalloproteinase, MT1-MMP, ovarian cancer, collagen, invasion, metastasis, endocytosis
INTRODUCTION
The epidermal growth factor receptor (EGFR) plays a prominent role in both physiological and pathological processes. In a ligand-dependent manner, the EGFR mediates the effects of epidermal growth factor (EGF), transforming growth factor-α, and amphiregulin [1–4]. The classical function of the EGFR is that of a receptor tyrosine kinase that catalyzes the phosphorylation of multiple substrates and downstream effector molecules at key tyrosine residues and thereby activates intracellular signaling networks related to cell growth and survival [4,5], including those involving Src family kinases [6, 7], Ras/MAPK [8, 9], and Jak/Stat [10–12]. The functional consequences of EGFR mediated signal transduction include enhanced proliferation, migration, and cellular differentiation [6, 7, 13, 14].
The ability of the EGFR to promote cellular responses that contribute to cancer pathology underscores its overexpression in the vast majority of carcinomas [15–19]. EGFR is expressed in 10–70% of ovarian cancer cases with an average reported expression of 48% [4, 20]. In the normal ovary, the EGFR is preferentially expressed in actively proliferating cells, including the ovarian surface epithelium [4, 21–23]. Tumors arising from the ovarian epithelium account for over 90% of ovarian malignancies. While women diagnosed with early stage disease have a favorable outcome, detection following secondary metastasis beyond the ovaries results in poor patient prognosis. Evidence suggests that EGFR expression may be an early event in ovarian cancer development as well as in progression and metastasis [4, 24–26]. Activated (phosphorylated) EGFR is found in 35% of primary ovarian cancers and is often elevated in peritoneal metastases relative to primary tumors from the same patient [27, 28]. A recent meta-analysis revealed a relationship between EGFR status and decreased survival [29], supporting the conclusion that aberrant EGFR status is a factor in ovarian cancer outcome. With an estimated 15,000 mortalities expected each year (NCI), ovarian cancer is the leading cause of death from gynecologic malignancy among women in the United States. As abrogation of EGFR signaling retards tumor progression [30, 31], a more detailed understanding of cellular events mitigated by this receptor is warranted.
Emerging data support a link between EGFR activation and the regulation of matrix degrading proteinases in ovarian carcinoma. Metastatic ovarian carcinoma cells require proteolytic activity for invasion of the mesothelial monolayer, intra-peritoneal anchoring in the sub-mesothelial interstitial collagen (types I and III) stroma, and for tumor angiogenesis [32–37]. Matrix metalloproteinases (MMPs) facilitate matrix remodeling and play a key role in ovarian cancer pathobiology [38], as ovarian carcinomas display high levels of MT1-MMP (MMP-14), MMP-2, MMP-9 and TIMP-2 [27, 32–33,39–43]. MT1-MMP is essential to matrix remodeling during physiological processes [44], and key to acquisition of a metastatic phenotype in a variety of tumor cells [45–48]. Acquisition of MT1-MMP expression promotes cell migration [49, 50], invasion of three-dimensional collagen [41, 48, 51–53], and three-dimensional growth [53], key cellular processes that drive ovarian pathology. Consequently, high levels of MT1-MMP correlate with poor survival of women with ovarian cancer [39]. Given the central role of MT1-MMP in cancer progression and metastasis, multiple mechanisms have evolved for transcriptional and post-translational regulation of MT1-MMP enzymatic activity. EGFR activation has been shown to induce MT1-MMP expression at both mRNA and protein levels, and lack of the EGFR is correlated with decreased MT1-MMP function [42, 54–56]. Conversely, recent work demonstrates a role for MT1-MMP in the trans-activation of the EGFR, suggesting potential mechanisms for reciprocal regulation [57].
While there is an expansive body of literature outlining the central role of the EGFR in the architecture of intracellular signaling networks, less in known about its ability to regulate transmembrane protein trafficking. EGFR signaling may modify the molecular landscape of the cell surface by regulating the trafficking, and consequently the function, of specific cell surface proteins, highlighting an underappreciated outcome of EGFR activation. In the present study, the role of EGFR activation in MT1-MMP membrane trafficking was investigated. The current data support an EGFR-dependent mechanism for regulation of the transition between invasive and expansive growth of ovarian carcinoma cells via modulation of MT1-MMP cell surface dynamics.
RESULTS
EGF stimulates MT1-MMP internalization through a mechanism distinct from EGFR
Internalization of MT1-MMP is recognized as a mechanism to regulate surface expression, and as a result, peri-cellular proteolytic activity. This process has been shown to occur through both clathrin- and caveolae-dependent processes in response to temperature shift or stimulation with concanavilin A [58–62]. Although physiologic mechanisms that regulate MT1-MMP trafficking have not been characterized, recent data show an EGF-induced change in MT1-MMP-dependent pericellular proteolytic activity [63]. To evaluate the effect of EGF stimulation on MT1-MMP trafficking, OVCA 433 cells expressing GFP-tagged MT1-MMP were treated with 25nm EGF and monitored via live cell imaging. Images were acquired at one minute intervals over 45 minutes. No evidence for enhanced MT1-MMP exocytosis was observed in this time frame. MT1-MMP is rapidly internalized from the cell surface following EGF stimulation [Suppl. Fig. 1]. In unstimulated cells, MT1-MMP co-localization with EGFR is observed (Fig. 1A), while ligand stimulation results in dissociation of MT1-MMP and EGFR fluorescent signals (Fig. 1A). These data suggest that MT1-MMP is internalized via a pathway distinct from the EGFR. Previous studies have shown that prior to internalization, EGF receptors are concentrated in caveolae [64–67; Suppl. Fig2A). Following activation, EGFR dimers migrate to the bulk plasma membrane [55,66–67; Suppl. Fig. 2B] and subsequently localize to clathrin coated pits, initiating endocytosis from the cell surface [1, 68, 69]. In contrast, MT1-MMP immunoreactivity is co-localized with caveolin-1 both prior to and following EGF stimulation (Fig. 1B) and endocytosed MT1-MMP colocalizes with the caveosomal markers 58K and GRP94, but not Rab7 (Fig. 1C). This is supported by quantitative analysis of surface protein levels following EGFR activation using fluorescence activated cell sorting. A rapid loss of cell surface EGFR is observed following ligand activation (Fig. 2A, closed circles). The kinetics of EGFR turnover are not altered by pre-treatment of cells with methyl-β-cyclodextrin (Fig. 2A, open circles), which causes cholesterol depletion, and as a result, caveolar and lipid raft disruption [70, 71]. Previous studies demonstrate that methyl-β-cyclodextrin treatment shifts caveolar EGFR to non-caveolar membrane [72]. Thus, while cholesterol depletion affects the structure of caveolae, it does not influence the ability of EGFR to exit caveolae and undergo endocytosis in response to EGF treatment. In contrast to EGFR, endocytosis of MT1-MMP is dependent on caveolar integrity. Pre-treatment of cells with methyl-β-cyclodextrin disrupts EGF-induced MT1-MMP endocytosis (Fig. 2B, open circles), resulting in accumulation of MT1-MMP on the cell surface. Together these data support a caveolae-dependent mechanism for EGF-induced MT1-MMP endocytosis in ovarian cancer cells.
Figure 1. EGFR activation modulates MT1-MMP surface dynamics.
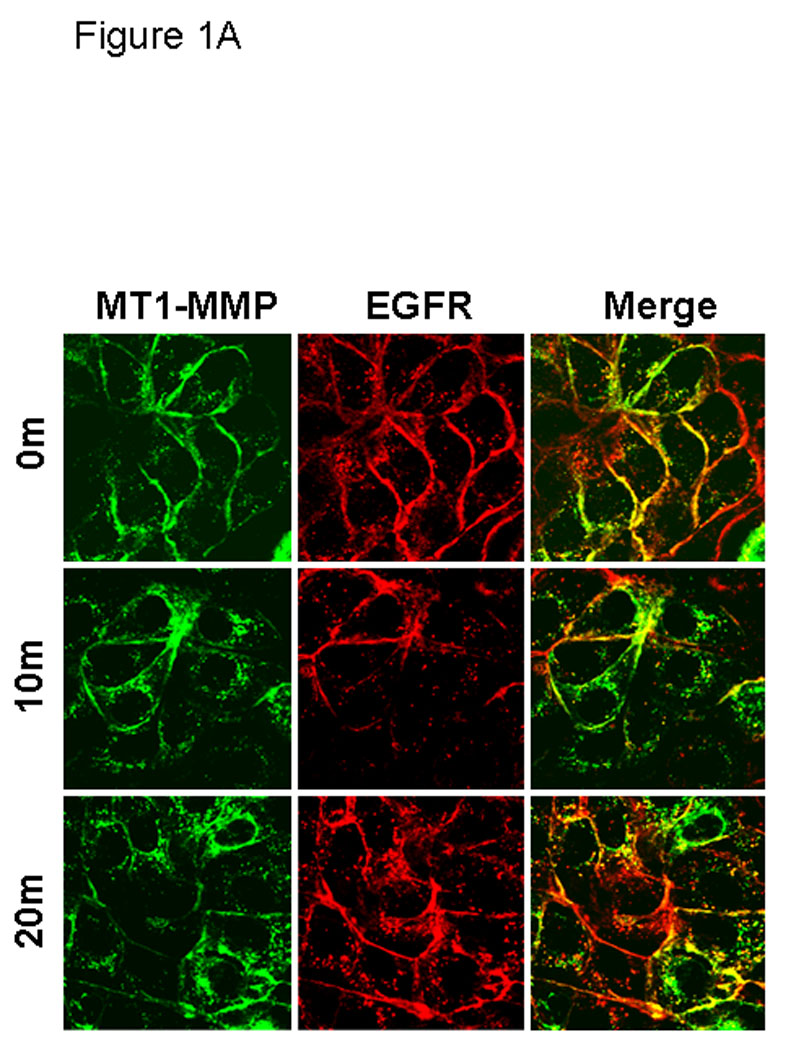
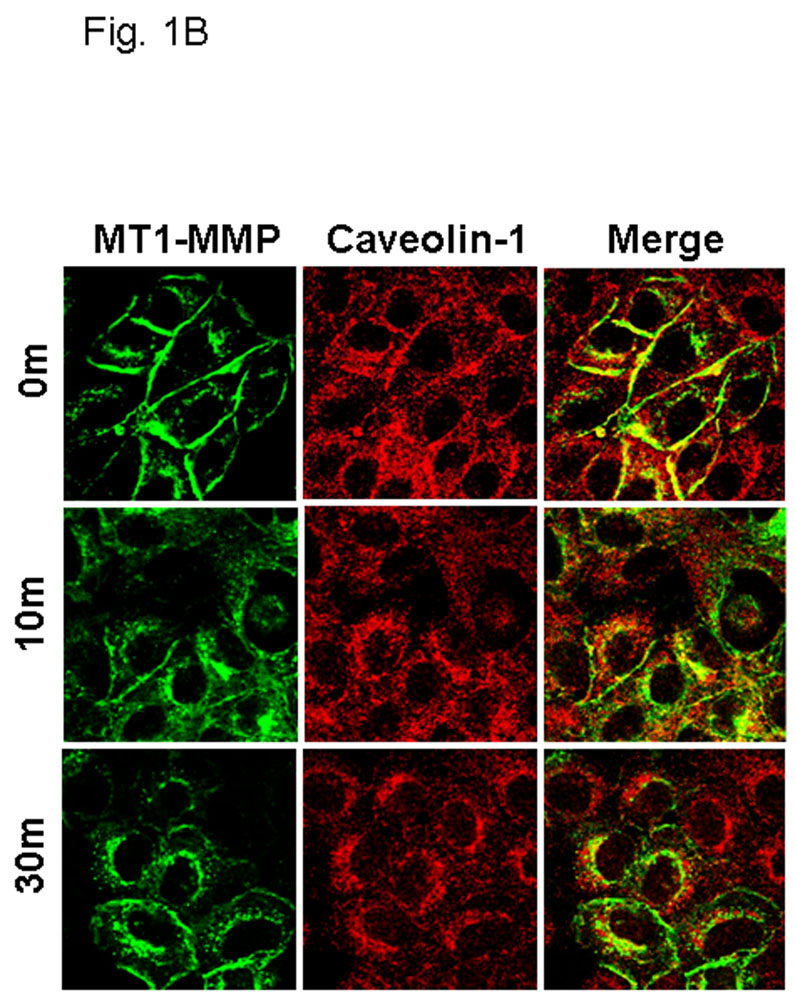
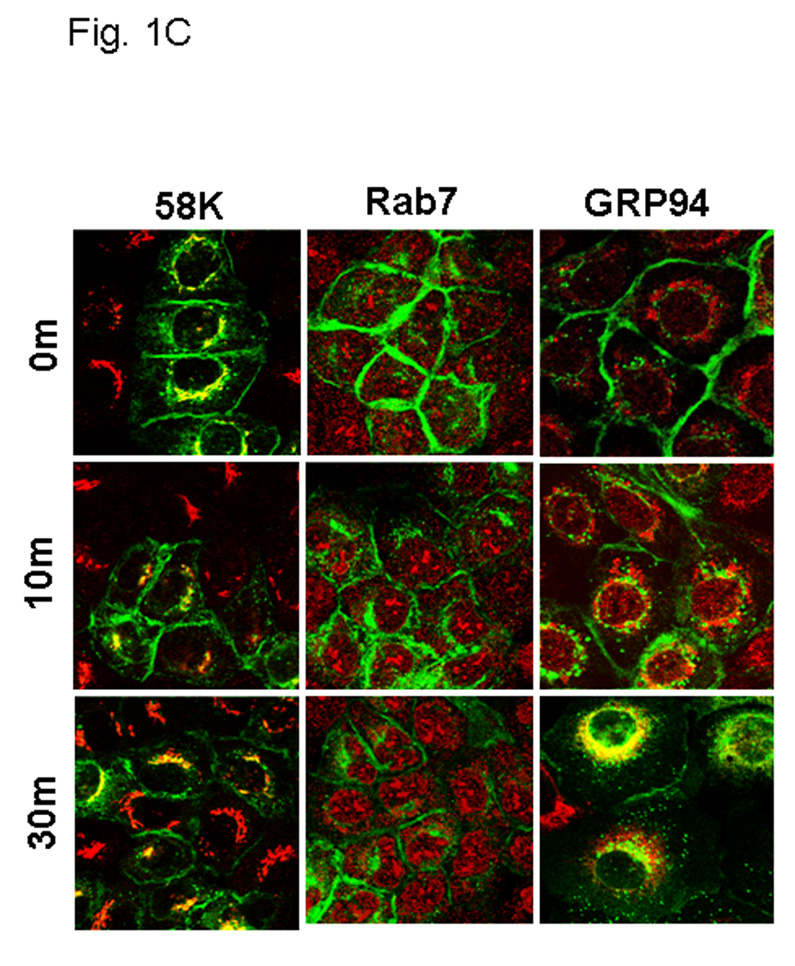
(A) Cells expressing GFP-tagged MT1-MMP were serum starved overnight and treated with EGF (25nm) for the indicated times in serum free medium and processed for staining with anti-EGFR (red) as described in Experimental Procedures. All images were viewed using the 63 × 1.4 oil immersion objective. (B) Cells were treated as in A, and processed for staining with anti-caveolin-1 (red) antibodies as described in Experimental Procedures. (C) GFP-tagged MT1-MMP-expressing cells were treated as in A and processed for staining with either Rab7, 58K or GRP96 (red) as indicated. Only merged images are shown.
Figure 2. EGFR activation alters surface presentation.
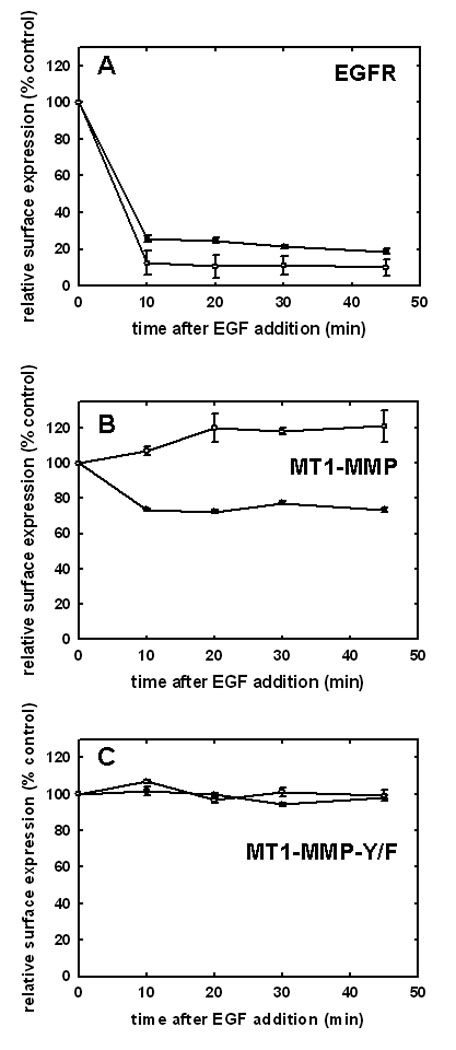
Surface levels of (A) EGFR, (B) MT1-MMP or (C) MT1-MMP-Y573F were quantified at the indicated time points using flow cytometry following treatment with EGF (25 nM) in control (closed circles) or methyl-β-cyclodextrin-treated cells (open circles). Cells were serum starved, treated with EGF, and trypsinized on ice at the indicated time points prior to processing for flow cytometry using anti-Flag antibody (1:100) to detect MT1-MMP or anti-EGFR antibody (1:100) to detect EGFR. Alexa Fluor 488-conjugated goat anti-mouse IgG (1:200) was used as a secondary antibody. Data are normalized such that surface expression in the absence of EGF treatment is designated as 100%. Assays were performed with 3–6 replicates.
Altered EGF-induced trafficking in MT1-MMP-Y573F
The cytoplasmic tail of MT1-MMP undergoes reversible Src-dependent phosphorylation at tyrosine 573 (Y573) in response to the platelet-derived chemoattractant sphingosine-1-phosphate [73, 74]. As EGFR downstream signaling can also activate the non-receptor tyrosine kinase Src, the contribution of Y573 phosphorylation to MT1-MMP trafficking was evaluated. Using site directed mutagenesis, Y573 in wild type GFP-tagged MT1-MMP was converted to the non-phosphorylatable homolog Phe to generate GFP-tagged MT1-MMP-Y573F. Following transfection and G418 selection, stable cell populations of OvCa433 cells expressing either wild type- or MT1-MMP-Y573F were isolated by FACS sorting and shown to have equivalent MT1-MMP total and surface expression under basal conditions (Fig. 3A). Whereas treatment of serum-starved cells with EGF induced phosphorylation of wild-type MT1-MMP, phospho-Tyr signal was not detected in cells expressing MT1-MMP-Y573F (Fig. 3B). Altered trafficking of MT1-MMP-Y573F in response to EGF was also observed. Whereas approximately 30% of wild type MT1-MMP is internalized in response to EGF (Fig. 2B), no internalization of the mutant was detected following EGF treatment in either control or methyl-β-cyclodextrin-treated cells (Fig. 2C). Persistence of cell surface localization of MT1-MMP-Y573F was also shown by confocal microscopy, as after 30 min of EGFR stimulation, little detectable loss of surface MT1-MMP-Y573F staining was observed (Fig. 3C). Lack of significant changes in co-localization with endocytic markers following EGF stimulation (Fig. 3D) further underscores the role of Y573 phosphorylation in mediating internalization of MT1-MMP in response to EGF treatment.
Figure 3. Altered surface dynamics in MT1-MMP-Y573F-expressing cells.
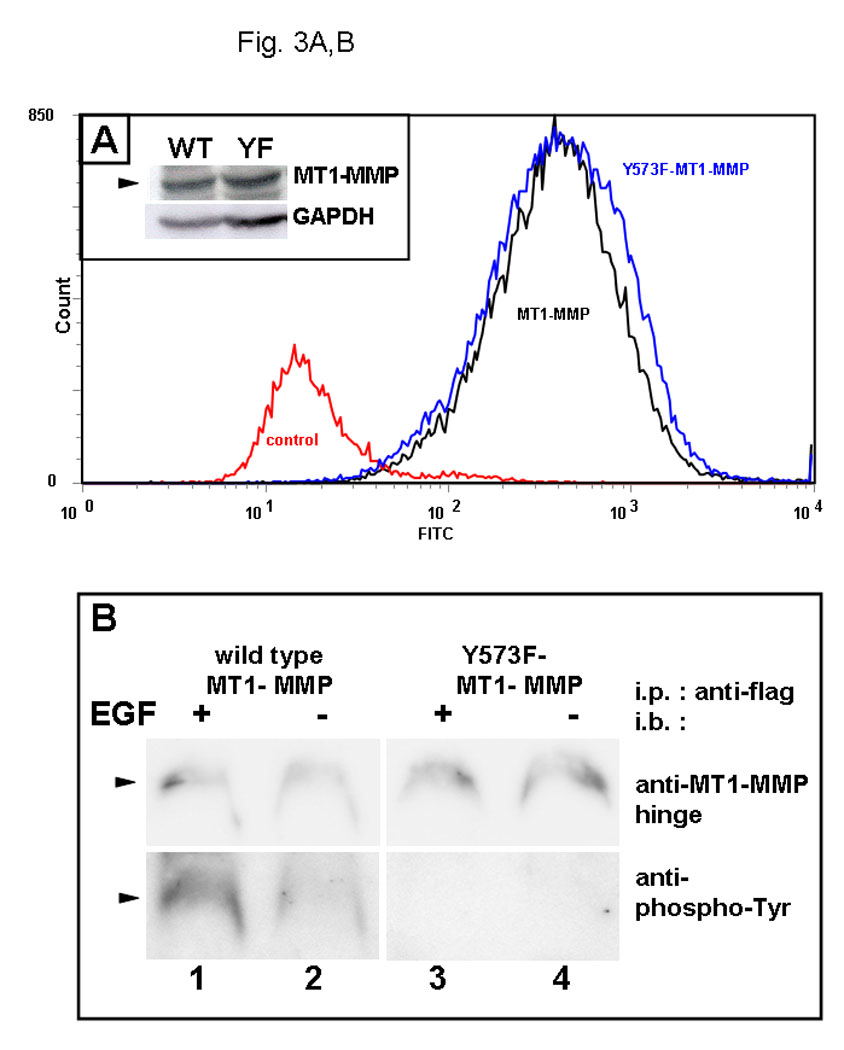
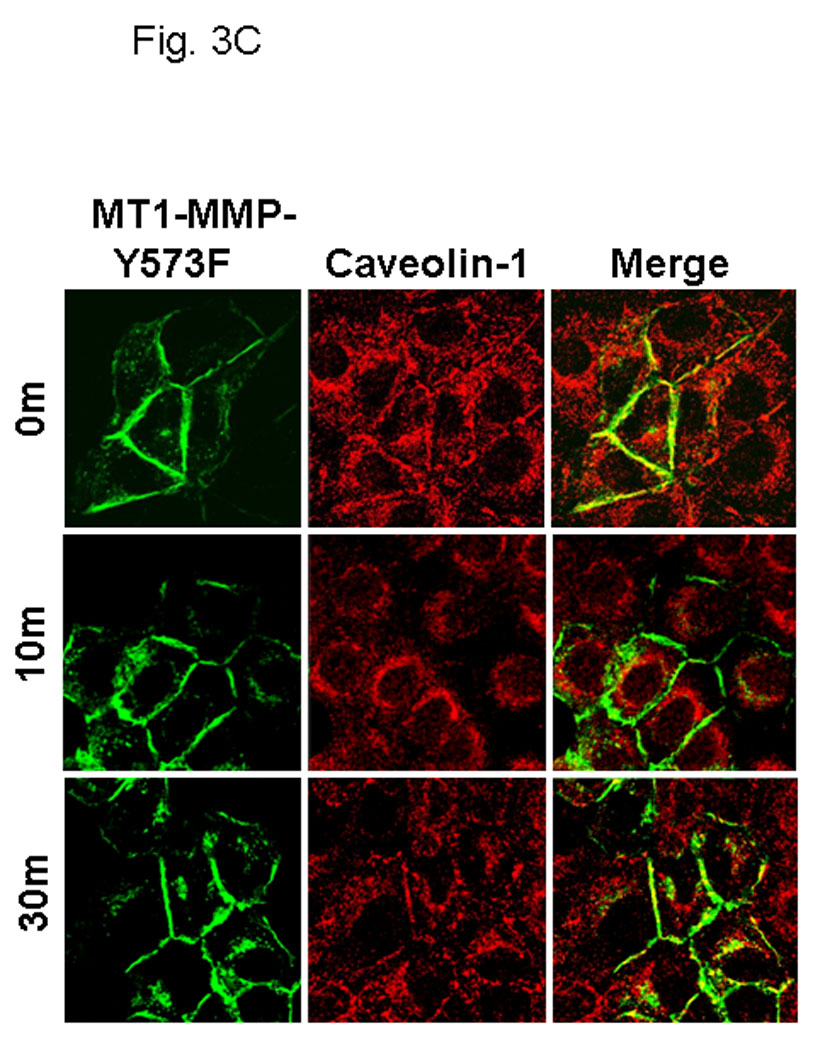
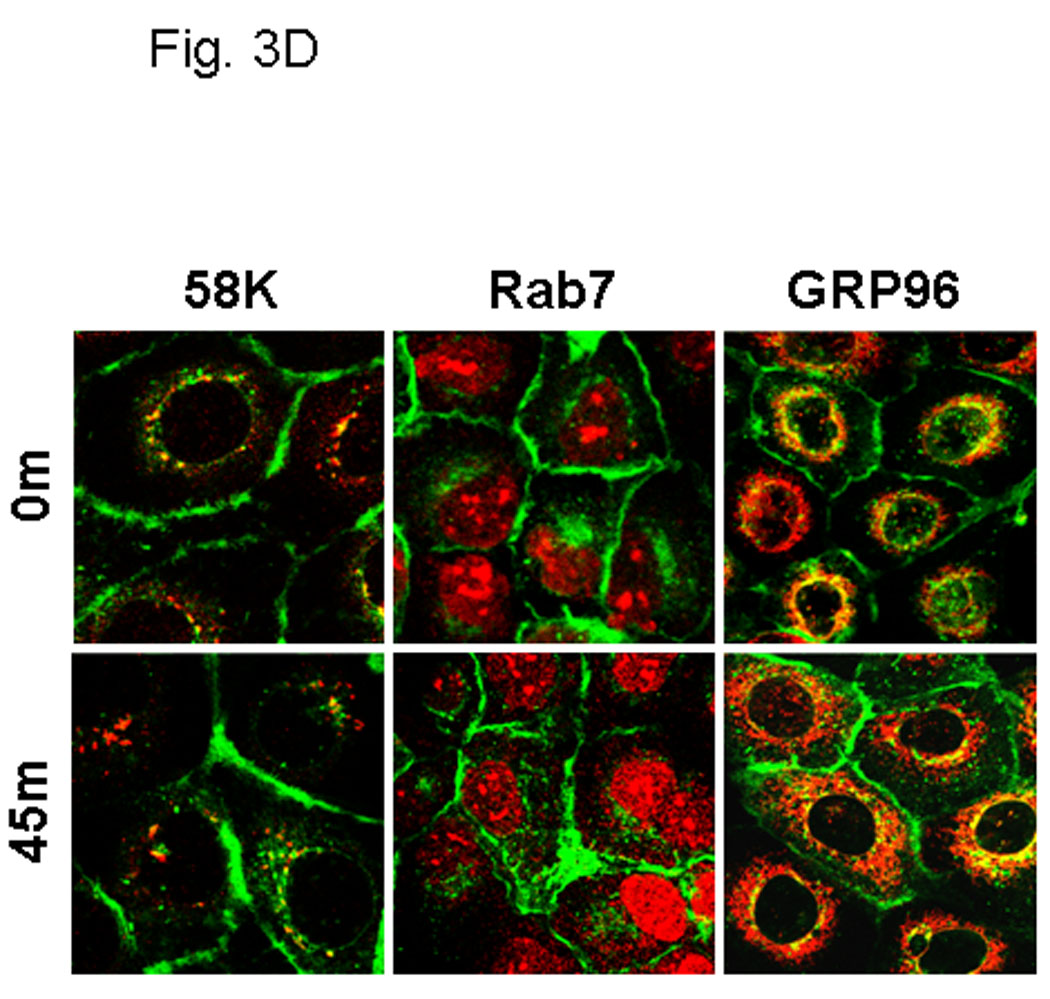
(A) Cells were analyzed by flow cytometry for expression levels of wild type MT1-MMP (black trace) or MT1-MMP-Y573F (blue trace) relative to vector controls (red trace) as described in Experimental Procedures. Inset: western blot of whole cell lysates from cells expressing wild type (WT) or mutant (YF) MT1-MMP. Blot was probed with anti-MT1-MMP (hinge antibody) or anti-GAPDH as described in Experimental Procedures. (B) Cells were cultured overnight in serum free medium prior to treatment with EGF (25 nM) for 30 min, as indicated. Following treatment, cells were lysed, immunoprecipitated using anti-FLAG M2 antibody and immunoprecipitates probed with antibodies to MT1-MMP (hinge, upper panel) or phospho-Tyr (lower panel) (C) Cells expressing GFP-tagged MT1-MMP-Y573F were serum starved overnight and treated with EGF (25nm) for the indicated times in serum free medium and processed for staining with anti-caveolin-1 (red) as described in Experimental Procedures. (D) GFP-tagged MT1-MMP-Y573F expressing cells were treated as in D and processed for staining with either Rab7, 58K or GRP96 (red) as indicated. Only merged images are shown.
Modification of Y573 in MT1-MMP alters three-dimensional collagen growth and invasion
Ovarian cancer metastasis is characterized by adhesion to and invasion of the type I collagen-rich sub-mesothelial membrane, followed by proliferation within the interstitial collagenous matrix to anchor secondary lesions [35, 75, 76]. Both in vivo and in vitro studies indicate that expression of MT1-MMP facilitates tumor cell growth within a 3-dimensional (3-D) matrix [45,52,53]. In control experiments, analysis of cell growth atop of collagen surfaces illustrated similar growth rates for cells expressing either wild type- or MT1-MMP-Y573F (Fig. 4A–C). To determine whether the Y573F modification exerts an effect on MT1-MMP-mediated 3-D growth, cells were seeded within 3-D type I collagen gels and cultured for 6 days prior to quantitation of cell proliferation. As previously reported [53], proliferation in 3-D collagen gels is largely attenuated in the absence of MT1-MMP expression or in the presence of MMP inhibitors. However, cells expressing wild type MT1-MMP exhibited reproducibly enhanced proliferation within a 3-D matrix relative to MT1-MMP-Y573F cells (Fig. 5A, 5C) relative to vector transfected cells or cells in matrices containing the metalloproteinase inhibitor TIMP-2 (not shown). These cells grew as expansive, well organized multi-cellular aggregate structures with readily apparent cell-cell interactions (Fig. 5A left panels, Fig. 5C). However in contrast, striking morphological differences were exhibited by MT1-MMP-Y573F cells, which survived in 3D collagen gels, but grew as elongated, single cell entities with a mesenchymal phenotype (Fig. 5A right panels, Fig. 5B). Enhanced invasion of 3D collagen gels (Fig. 5D) and chemotactic migration (not shown) was evident with MT1-MMP-Y573F-expressing cells, consistent with the observed morphological alterations characteristic of a migratory, invasive phenotype.
Figure 4. MT1-MMP-Y573F modification does not alter two-dimensional growth.
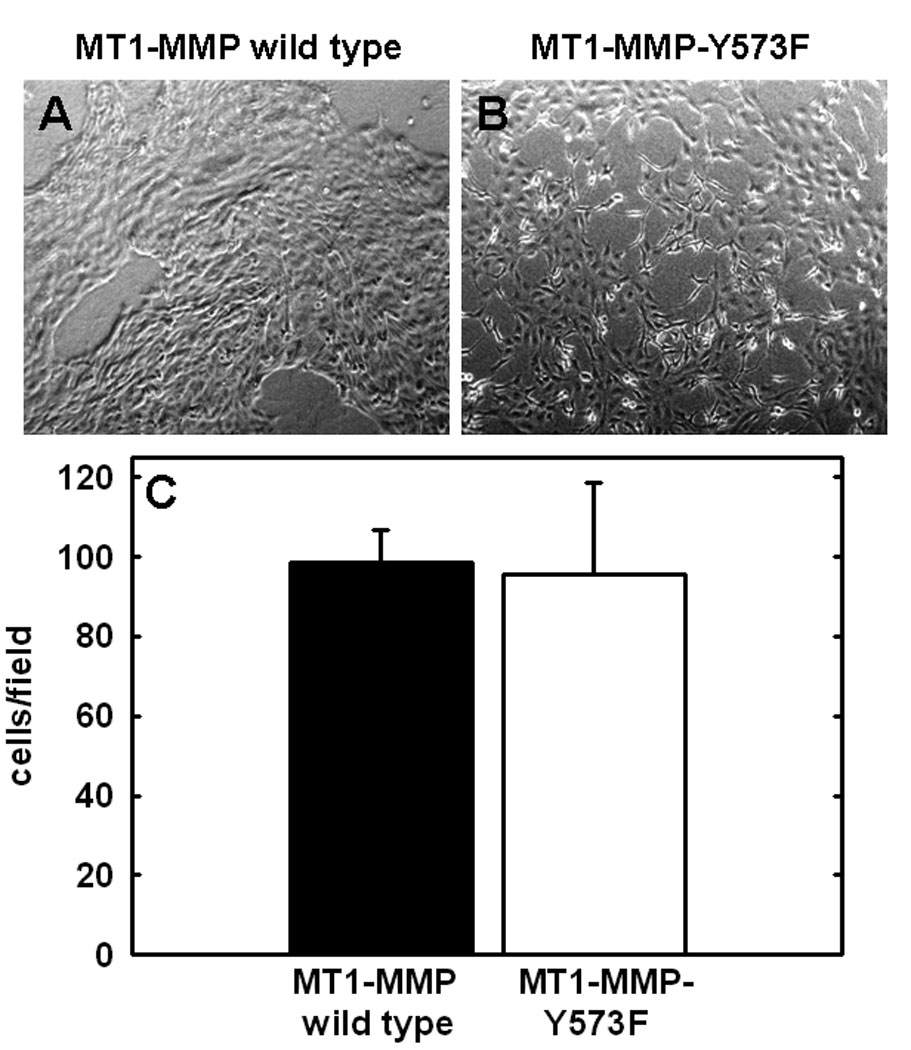
Cells expressing (A) wild type MT1-MMP or (B) MT1-MMP-Y573F, as indicated, were seeded at an initial density of 5×104 cells/well atop thin layer collagen-coated culture wells and allowed to proliferate. (C) Following incubation at 37°C for 6 days, collagen cultures were photographed prior to dissolution using bacterial collagenase (2mg/ml, Worthington) and cell number was evaluated by hemocytometry as described [52,53].
Figure 5. MT1-MMP-Y573F alters invasive versus expansive growth in three-dimensional collagen.
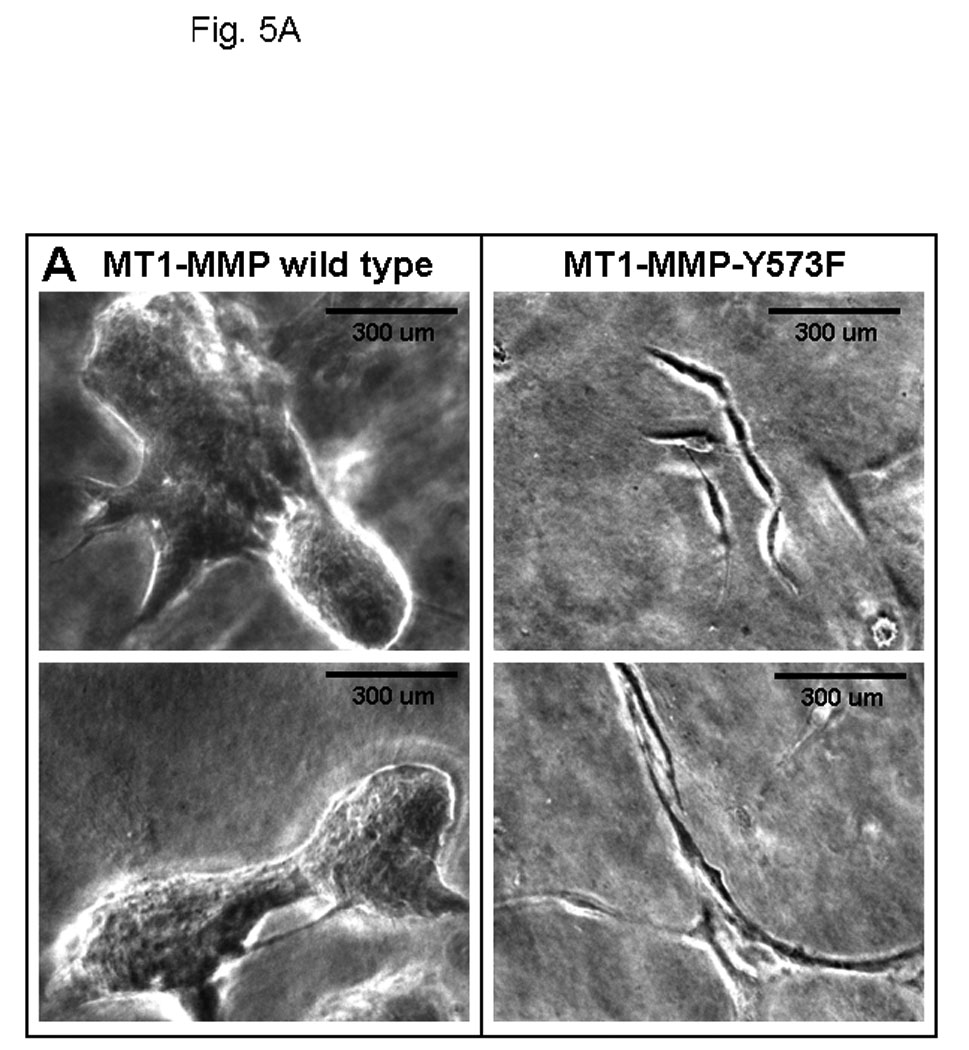
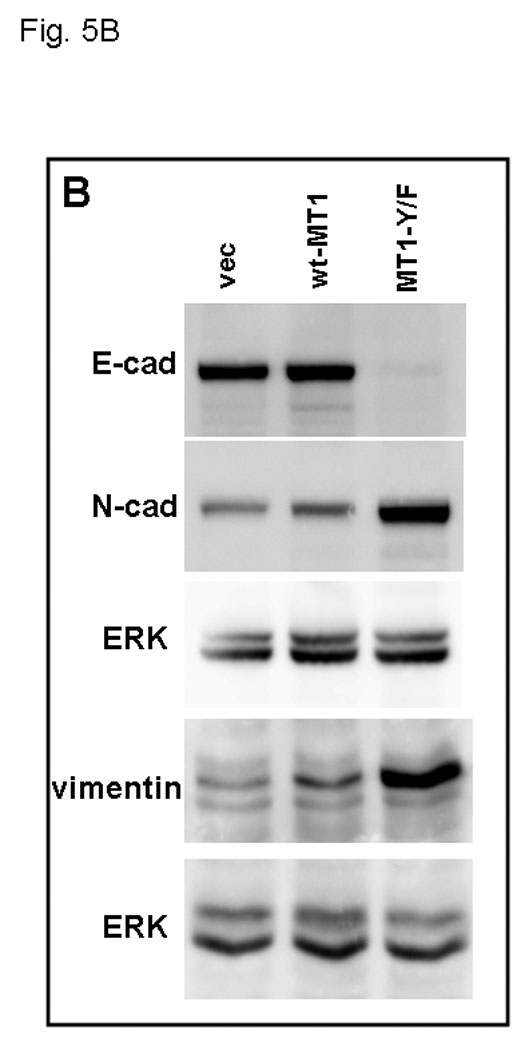
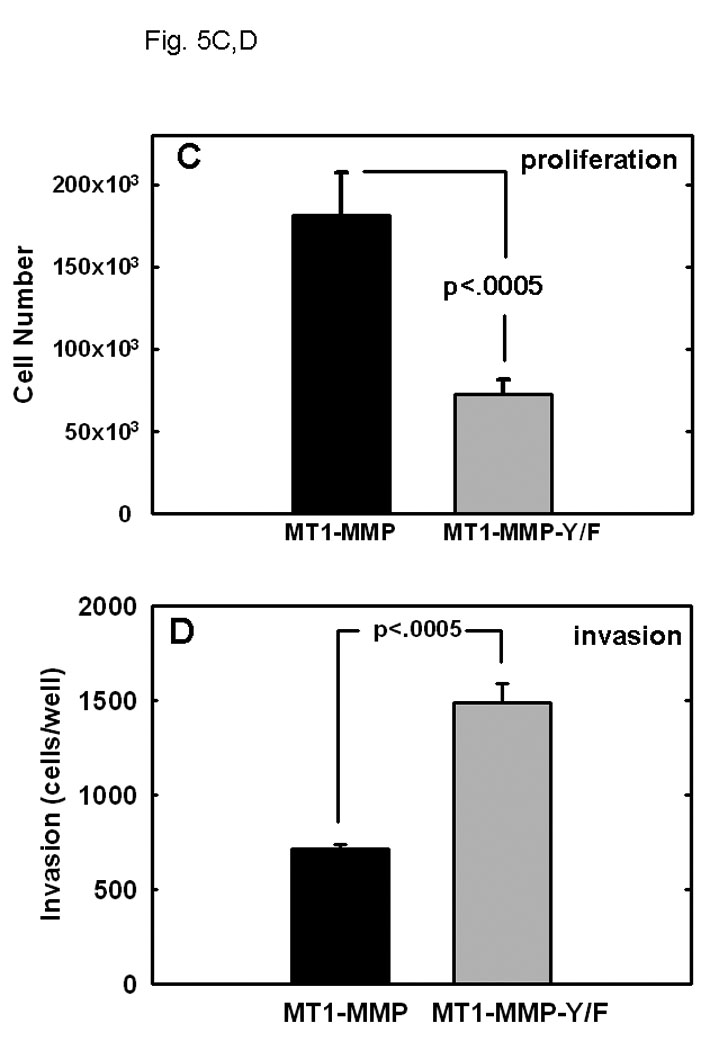
(A) An equal number of cells (5×104) expressing wild type MT1-MMP or MT1-MMP-Y573F, as indicated, were seeded at low density within three dimensional collagen cultures prepared by adding cells to type I collagen prior to solidification as described in Experimental Procedures. Two representative images of each culture are shown following incubation at 37 C for 6 days. (B) Expression of MT1-MMP-Y573F is associated with acquisition of mesenchymal markers. Cells expressing wild type MT1-MMP or MT1-MMP-Y573F were lysed, lysates electrophoresed and transferred to Immobilon. Blots were probed with antibodies directed against E-cadherin, N-cadherin, or vimentin, as indicated. GAPDH is shown as a loading control. (C) Wild type MT1-MMP enhances proliferation in three-dimensional collagen. Following incubation at 37°C for 6 days, three dimensional collagen cultures were dissolved using bacterial collagenase (2 mg/ml, Worthington) and cell number was evaluated by hemocytometry as described [53]. Experiments were repeated in triplicate. Wild-type MT1-MMP enhances proliferation relative to untransfected controls (not shown; 122,431 +/− 3519 cells/gel) and proliferation is inhibited by polymerization of TIMP-2 (5 ug/ml) within the gels (not shown; 121,293 +/− 2158 cells/gel). (D) Invasion of three-dimensional collagen gels was analyzed by incubating cells (250,000) in a Boyden chamber containing an 8 um porous filter overlaid with 100 ul of type I collagen (200ug/ml) for 24 hours at 37°Celsius. Non-invading cells were removed and invading cells were enumerated. Results are from averages of three independent experiments.
DISCUSSION
As both MT1-MMP and the EGFR are overexpressed in epithelial ovarian carcinoma [4, 27, 51], elucidating the role of EGFR signal transduction in modulating MT1-MMP activity bears potential clinical significance. A growing body of work demonstrates that acquisition of MT1-MMP expression alone promotes enhanced cell migration [49, 50], invasion of the basement membrane or three dimensional collagen gels [41,51–53,77], and three dimensional expansive growth [53]. Studies performed in multiple cell lines indicate that activation of the EGFR induces MT1-MMP protein expression [54–56]. While the ability to augment protein levels offers an effective means of influencing molecular activity, altering the surface presentation of the transmembrane proteinase MT1-MMP represents an alternative efficient regulatory mechanism for rapid changes in pericellular proteolytic potential.
Results in the present study demonstrate that activation of the EGFR regulates MT1-MMP cell surface topology, leading to internalization of MT1-MMP. While the EGFR and MT1-MMP colocalize with caveolae prior to activation, stimulation with EGF disrupts this association. While the exact function of caveolae in cell signaling is not well defined, emerging lines of evidence indicate that the caveolar structure functions in the compartmentalization of receptors and second messengers involved in signal transduction [66, 78–81]. Caveolin-1 polymerizes to form a scaffold within these compartments [78], promoting direct interaction with many of the receptors and signaling proteins that reside in caveolae [82]. Caveolar localization may thereby facilitate signaling by placing the appropriate receptors and effectors at optimal proximity to elicit efficient intermolecular action. This is underscored by the observation that although caveolae comprise only 5–10% of the plasma membrane, 40–60% of unstimulated EGFR is concentrated in these domains [66]. In addition to the EGFR [66], nerve growth factor receptors [83], vascular epidermal growth factor receptor [84], several components of the MAP kinase signaling pathway, including ERK and Ras [85, 86], protein kinase C [81, 87] and Src kinases [81, 87] are present in caveolae. While this is not a comprehensive list, the compartmentalization of entire signaling units supports a role for caveolae as mediators in cell signaling.
Although cytoplasmic levels of both MT1-MMP and the EGFR increase following EGF treatment, intracellular colocalization was not detected. Following activation, punctuate staining of the EGFR is observed with clathrin and the recycling endosome Rab7 [67], both markers of clathrin dependent internalization. This is in agreement with previous data showing that activated EGFR dimers migrate to the bulk plasma membrane [5,66] and subsequently localize to clathrin coated pits, initiating endocytosis from the cell surface [1, 68, 69]. Intracellular vesicles are eventually subject to either lysosomal degradation or recycling to the cell surface [69]. In contrast, internalized MT1-MMP did not colocalize with either clathrin or Rab7, but was detected in association with GRP94 and 58K, both markers of caveolae-dependent trafficking. These findings indicate that intracellular trafficking of the EGFR and MT1-MMP occurs through two distinct mechanisms.
To our knowledge, analysis of MT1-MMP internalization in response to a biological stimulus such as EGF has not been reported. However, previous studies have evaluated MT1-MMP endocytosis in response to temperature shift or concanavilin A treatment. It has been demonstrated that MT1-MMP internalization from the surface of HT1080 fibrosarcoma cells requires dynamin [58], a GTPase required for vesicle formation from the plasma membrane during clathrin-mediated endocytosis [88]. Abrogation of MT1-MMP internalization in cells expressing a dominant negative form of dynamin (dynK44A) supported a role for clathrin mediated endocytosis in MT1-MMP trafficking induced by treatment with ConA [48]. Conversely, caveolae mediated endocytosis was also implicated as a distinct, clathrin independent mechanism of MT1-MMP internalization in response to temperature shift [89]. A physical association between MT1-MMP and caveolae has previously been demonstrated, as MT1-MMP preferentially localizes to caveolae at the surface of HT1080 and glioblastoma cells [60, 61]. Further, using the mutant EpsE_95 to specifically block clathrin-mediated endocytosis, internalization of MT1-MMP was retained in HT1080 cells [62]. It is unknown whether mutation of Tyr573 would affect MT1-MMP trafficking by these diverse mechanisms. However, the involvement of separate mechanisms for endocytosis in different cell types suggests that internalized MT1-MMP may be subject to a differential fate that is dependent upon the mode of internalization.
While the observed effect of EGFR on MT1-MMP surface presentation has not been previously reported, EGFR activation has been shown to regulate the surface receptor integrin α2, which also localizes at caveolae, in a similar fashion [67]. The ability of EGFR activation to affect the trafficking of multiple receptors that occupy caveolae suggests that the EGFR may regulate internalization of the cellular compartment itself, and as a result, alter the surface profiles of proteins occupying these domains. Although the molecular mechanism underlying caveolae function has yet to be determined, several lines of data indicate that the EGFR may function in cell signaling before exiting caveolae. The first phases of EGFR signal transduction occur in caveolae, suggesting that the entire signaling pathway is pre-organized in these compartments [80]. It is clear that following activation, the EGFR exits caveolae through a process that requires the activity of both the activated EGFR and Src kinases [66, 81]. Although endocytosis of EGFR does not occur through caveolae, it has been shown that tyrosine kinase activity mediated by Src drives caveolae internalization [81]. Caveolin-1 may act as a negative regulator of endocytosis mediated through this pathway [90, 91]. Caveolin-1 functions as a structural support for highly immobile caveolae [92]. The ability of caveolin-1 to inhibit caveolae budding may be overcome by reversible phosphorylation [93], potentially initiated by EGFR-induced Src activation [94]. It is interesting to speculate that this functions as a mechanism to initiate endocytosis of caveolar proteins including MT1-MMP. The ability of the EGFR to modify caveolin-1 via reversible tyrosine phosphorylation [93, 94] also raises the possibility that the cytoplasmic domain of MT1-MMP can serve as a substrate for kinase activity. It has been previously reported that MT1-MMP cytoplasmic tail residue Y573 undergoes reversible phosphorylation in response to sphingosine-1-phosphate [95]. This modification is catalyzed by Src kinase, which can be activated downstream of EGFR signaling, raising the hypothesis that EGFR can stimulate, either directly or indirectly, tyrosine phosphorylation of MT1-MMP. This is supported by data in the current study showing EGF-induced phopshorylation of wild type but not Y573F-mutated MT1-MMP as well as defective EGF-stimulated internalization kinetics in cells expressing MT1-MMP-Y573F.
To determine the functional consequences of prolonged surface presentation, ovarian cancer cells expressing MT1-MMP-Y573F were evaluated for the ability to invade through and proliferate within three dimensional collagen gels. Following shedding from the surface of the ovary, metastatic cells anchor, migrate and invade the mesothelium lining the organs of the peritoneum. Subsequently, ovarian cancer cells encounter the sub-mesothelial matrix, which is rich in type I collagen, and proliferate within this matrix to anchor secondary lesions [35,41,51,75]. MT1-MMP-Y573F-expressing cells exhibited enhanced invasion through type I collagen, and superior chemotactic migration relative to cells expressing wild type MT1-MMP. Similar results were obtained using MT1-MMP-Y573F-transfected MDA-MB-231 mammary carcinoma cells (N. Moss, unpublished data). As localization of MT1-MMP at invadopodia and cellular constriction rings is required for efficient cell invasion [96–100], it may be proposed that tyrosine phosphorylation of the MT1-MMP cytoplasmic tail abrogates its ability to properly localize within the migratory apparatus, resulting in impaired migration and invasion. However, these results conflict with those presented in a recent report [74] using HT1080 fibrosarcoma cells transfected with MT1-MMP-Y573F, in which collagen invasion was impaired in cells expressing the mutant proteinase. It should be noted that in addition to post-translational regulation of MT1-MMP trafficking, EGFR activation has also been shown to increase total MT1-MMP expression in ovarian cancers [42] as well as in SCC and glioma [52,53]. In addition, following siRNA-induced downregulation of MT1-MMP expression in ovarian cancer cells, residual invasive activity is observed, suggesting that MT1-MMP is not the only contributor to invasion [42]. Alternatively, the apparent discrepancy between the current study and [74] may reflect a distinction between the responses of epithelial vs mesenchymal cells to altered MT1-MMP dynamics. In this regard, it is interesting to note that ovarian cancer cells expressing MT1-MMP-Y573F undergo a striking transition to a mesenchymal phenotype, particularly apparent in 3-dimensional collagen culture. Cells expressing wild type MT1-MMP form large three-dimensional multi-cellular aggregates within collagen gels with a long axis of 200–700 um. In contrast, cells expressing MT1-MMP-Y573F display minimal cell-cell contact and, alternatively, survive in collagen gels as strikingly elongated single cells or 2–5 cell clusters with individual cells approaching 300–500 um in length. Expression of the epithelial cadherin E-cadherin is lost, while cells acquire expression of the mesenchymal markers N-cadherin and vimentin. A unique feature of epithelial ovarian carcinoma progression is that E-cadherin becomes more abundant in primary differentiated ovarian carcinomas, relative to the lack of E-cadherin expression by normal ovarian epithelium [75]. Thus, ovarian carcinomas appear to follow a mesenchymal to epithelial transition (MET) during early tumorigenesis. In advanced ovarian tumors, expression of E-cadherin is generally reduced while N-cadherin is increased, suggestive of an epithelial-mesenchymal transition (EMT) late in tumor progression [75]. While the role of dysregulated MT1-MMP activity in promotion of mesenchymal characteristics requires further investigation, previous studies have implicated MMPs in EMT via a mechanism involving induction of a Rac1 isoform that promotes snail expression and subsequent EMT [101]. However in the current study, culture of MT1-MMP-Y573F cells in the presence of the broad spectrum MMP inhibitor GM6001 for 6 days resulted in an incomplete reversal of EMT, characterized by a partial gain of E-cadherin expression (to approximately 50% wild type levels), while N-cadherin expression was retained (data not shown), suggesting alternative mechanisms of EMT regulation.
These functional data emphasize and resolve two distinct outcomes available to cells expressing MT1-MMP that encounter collagen-rich matrices: invasion vs expansion. Numerous studies have supported a requirement for MT1-MMP in collagen invasion [45,52,53,102–104]. MT1-MMP-catalyzed processing of collagen fibers results in realignment of fibers in a forward movement direction and these collagen degradation tracks are permissive for the formation of invasive cell strands [100]. MT1-MMP has also been recognized as a key component for expansive cell growth in 3-dimensional matrices [52, 53, 74,100]. In the context of serum-derived mitogens, MT1-MMP is necessary to remove physical constraints imposed by the collagenous matrix that prevent cytoskeletal alterations necessary to drive an effective proliferative response [53]. In fibroblasts, MT1-MMP drives collagen phagocytosis during tissue remodeling, and MT1-MMP is localized to sites of collagen cleavage intracellularly as well as in the pericellular space [102], suggesting that MT1-MMP internalization is necessary for tissue remodeling. Furthermore, cooperation between MT1-MMP and the endocytic receptor uPARAP/Endo180 enables endocytosis and further intracellular processing of large collagen fragments [105, 106], facilitating large scale turnover of collagen sufficient to allow aggregate proliferation. Thus, it is interesting to speculate that MT1-MMP-expressing cells capable of responding to epidermal growth factor signaling can effectively internalize MT1-MMP, as well as pericellular collagen, establishing an environment permissive for expansive growth. In contrast, lack of or inability to respond to EGFR signaling may target MT1-MMP-expressing cells to an invasion pathway favored by mesenchymal cells. While the contribution of EGFR activation to regulation of MT1-MMP interaction with collagen endocytic receptors has not been explored, the current data support the hypothesis that cellular response to growth factor signals may shift tissue dynamics of MT1-MMP-expressing cells between invasive and expansive growth. As the vast majority of women with ovarian cancer succumb due to complications of metastasis, a more detailed understanding of the factors that differentially regulate localized invasion, intraperitoneal anchoring and uncontrolled proliferation of secondary lesions is warranted.
MATERIALS AND METHODS
Cell Culture and Generation of Stable Cell Lines
The ovarian carcinoma cell line OVCA 433 was provided by Dr. Robert Bast Jr., MD Anderson Cancer Center, Houston, TX. Cells were maintained in minimal essential medium containing 10% fetal bovine serum. Cell culture media and reagents were purchased from Mediatech (Herndon, VA). For experiments involving EGF (Chemicon, Temecula, CA) OVCA 433 cell lines were serum starved for 24 hours prior to growth factor treatment. The human MT1-MMP cDNA with C-terminal FLAG tag (DYKDDDDK) was kindly provided by Dr. Duanqing Pei (University of Minnesota, Minneapolis, MN). The open reading frame (ORF) sequences of MT1-MMP cDNA were TA-subcloned into eukaryotic expression vector pCR3.1-Uni (Invitrogen, Carlsbad, CA) to obtain the wildtype construct. The wild-type construct was FLAG tagged in the stalk region as described [107]. Subsequently, the Y573F point mutation was generated on the wild type cDNA and on the tagged constructs using quick-change (Stratagene) to create MT1-MMP-Y573F. The wild type MT1-MMP coding sequence was also subcloned in a pEGFP-N1 vector (Clontech, Palo Alto, CA) to generate a fluorescent protein tag at the C-terminal using Nhe I site from pCR3.1 vector and a C terminal PCR-generated Sac II site. Transfection of cells was done using FuGENE 6 (Roche, Germany) according to manufacturer’s instructions and stable cell lines were generated using G418 selection. Cell populations were FACS-sorted using an antibody against the Flag tag (M2) in the Flow Cytometry Facility, Northwestern University Feinberg School of Medicine. To determine the effect of caveolae disruption on EGFR and MT1-MMP surface dynamics, cells were treated for 1h with 10mM methyl-β-cyclodextrin diluted in serum free 10 × MEM prior to EGF treatment and processing for confocal microscopy or FACS analysis as described below.
Antibodies
Anti-FLAG monoclonal M2 antibody, monoclonal anti-58K (catalog number G 2404), anti-matrix metalloproteinase-14 (hinge region), monoclonal anti-vimentin (clone VIM-13.2) and rabbit polyclonal anti-Rab7 (catalog number R4779) were purchased from Sigma (Saint Louis, MO). The mouse monoclonal EGFR, rabbit polyclonal antibodies against the extracellular domain of EGFR, ERK1 antibodies (sc-93), the mouse monoclonal anti-GRP 94 (catalog number sc-53929), and mouse monoclonal anti caveolin-1 (catalog number sc-53564) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The mouse monoclonal anti-clathrin (catalog number ab2731) antibody was purchased from AbCam Inc. (Cambridge, MA). Anti-phosphotyrosine (clone PY20) was purchased from Millipore and monoclonal antibodies directed againse N-cadherin (clone 3B9) and E-cadherin (clone HECD-1) were obtained from Zymed. The mouse monoclonal antibody against CD71/Transferrin Receptor (catalog number 555534) was purchased from BD Pharmingen (San Jose, CA). The Alexa Fluor 555 cholera toxin subunit B conjugate (catalog number C34776) was purchased from Molecular Probes (Carlsbad, CA). Alexa Fluor 546 goat anti-mouse IgG (catalog number A11030), Alexa Fluor 488 goat anti-mouse IgG (catalog number A11029), Alexa Fluor 546 goat anti-rabbit IgG (catalog number A11035), Alexa Fluor 488 goat anti-rabbit (catalog number A11008) IgG were purchased from Molecular Probes (Carlsbad, CA). EGF labeled with Alexa Fluor 546 was purchased from Molecular Probes (Carlsbad, CA).
Live Cell Imaging
To acquire live cell images, cells expressing GFP-tagged MT1-MMP were seeded onto 35mm Becton glass bottom dishes at a density of 106 and serum starved overnight. Cells were treated with EGF (25 nM) and images were acquired using a Nikon TE2000 inverted microscope (Nikon Inc., Melville, NY), and analyzed using Metamorph Software (Molecular Devices, Downingtown, PA). Images were taken in 1min increments for 60m with a 60× oil immersion objective with numerical aperture 1.40. The experiment was repeated in triplicate.
Immunofluorescence and Confocal Microscopy
Following treatment with 25nM EGF, OVCA 433 cells were fixed with 4% paraformaldehyde in PBS containing MgCl2 and CaCl2, for 10min at room temperature, rinsed with PBS, permeabilized with 0.1% triton X-100 for 5min, and blocked with 10% BSA/PBS for 1 hr at 37°C. For dual staining, fixed GFP tagged MT1-MMP- or MT1-MMP-Y573F-expressing OVCA 433 cells grown on glass coverslips were stained with primary antibodies directed against EGFR, transferrin receptor, clathrin, GRP-94, 58K, caveolin-1 or Rab7 for 30 minutes at 37°C. After three washes with PBS, samples were incubated with Alexa Fluor 546 goat anti-mouse or Alexa Fluor 546 goat anti-rabbit for 30 minutes at 37°C. For dual staining for EGFR and other antigens, samples were treated with anti-EGFR (rabbit or mouse), and anti-CD71/transferin receptor, anti-clathrin, anti-caveolin-1, anti-GRP 94, anti-58K, or anti-Rab7. Confocal microscopy was conducted at the Cell Imaging Facility, Northwestern University Feinberg School of Medicine. Images were attained using a Zeiss LSM510 system equipped with HeNe lasers for excitation at 488 nm (green) and 543 nm (red) and analyzed using Metamorph. All images were viewed using the 63 × 1.4 oil immersion objective. All experiments examining MT1-MMP or EGFR dynamics were performed a minimum of three times.
Flow Cytometry
FACS analysis was used to quantify surface dynamics of MT1-MMP or EGFR in response to EGF treatment. OVCA 433 cells expressing flag-tagged wild type- or Y573F mutant MT1-MMP (106 cells) were seeded onto tissue culture dishes for each experimental time point. Prior to analysis, cells were serum starved for 24h, then treated with EGF (25 nM). At each time point, cells were trypsinized and incubated on ice with anti-flag monoclonal M2 primary antibody (to detect MT1-MMP, 1:100 dilution) or with monoclonal anti-EGFR antibody (1:100) for 30min, washed thrice, and incubated on ice with Alexa Fluor 488 goat anti mouse IgG (1:200 dilution, 30 min). Cells were washed thrice and analyzed using a Becton-Dickinson FACScan flow cytometer. Assays were performed with 3–6 replicates. Data are normalized such that surface expression in the absence of EGF treatment is designated as 100%.
Western Blot and Immunoprecipitation
For western blotting of whole cell lysates, cells were lysed using 50 mM Tris pH 7.5, 150 mM NaCl, 1% Triton X-100 and the protein concentration of lysates was analyzed using the Bio-Rad DC detection kit and bovine albumin standards. Cell lysates (50 µg) were electrophoresed on 9% SDS-polyacrylamide gels, transferred to polyvinylidene difluoride membrane, and blocked with 3% BSA in 50mM Trizma (Tris base) (pH 7.5), 300 mM NaCl, 0.2% Tween 20 (TBST). Membranes were incubated for 1 h at room temperature with a 1:1000 dilution of antibodies directed against the FLAG epitope tag (FLAG M2 monoclonal antibody), MT1-MMP hinge , E-cadherin, N-cadherin, vimentin, or GAPDH in 3% BSA/TBST. Immunoreactive bands were visualized with a peroxidase-conjugated anti-(rabbit-IgG) (1:4000 in 3% BSA/TBST) and enhanced chemiluminescence. For immunoprecipitation analyses, cells were serum starved in the presence of the broad spectrum MMP inhibitor GM6001 (Chemicon, Temecula, CA), treated with EGF (25 nM) for 30 min, collected with lysis buffer (above) and subjected to immunoprecipiation using anti-MT1-MMP (hinge antibody, 1:4000 dilution) and protein G beads. Immunoprecipitates were electrophoresed on 9% polyacrylamide gels and subjected to western blotting using anti-MT1-MMP catalytic domain (1:4000) or anti-phospho-tyrosine antibodies (1:1000) followed by ECL detection as described above.
Growth in 3-Dimensional Collagen Gels
Three-dimensional (3D) cultures were prepared by diluting type I rat tail collagen (BD Biosciences) with 10 × MEM (Gibco) to a final concentration of 1.5 mg/ml. Cells (5 × 104 ) were added to the collagen mixture prior to solidification. In parallel control experiments, the MMP inhibitor TIMP-2 was included at a final concentration of 5ug/ml [53]. Following growth for 6 days in 3D collagen, gels were dissolved using bacterial collagenase (2 mg/ml, Worthington) and cell number was evaluated by hemocytometry as described [41]. Experiments were repeated in triplicate.
Collagen Invasion
Type I collagen was dissolved in 0.5 M acetic acid at a concentration of 2 mg/ml. For invasion experiments, the collagen stock was neutralized with 100 mM Na2CO3 (pH 9.6) to a final concentration of 0.4 mg/ml. Transwell inserts (0.8 µm, Becton Dickinson, Bedford, MA) were coated on the underside with 500 µl of collagen diluted to a concentration of 100 µg/ml at 37 °C for 1 h. Collagen gels were prepared in the inner well by adding 50 µl of collagen (20 µg) at room temperature and allowing gels to air-dry overnight. Collagen-coated inserts were then washed with minimum essential medium three times to remove salts and used immediately. Cells were trypsinized, washed with serum-free medium, and 1 × 105 cells were added to the inner invasion chamber in a volume of 200 µl. The outer wells contained 400 µl of culture medium. Cells were allowed to invade for 24 h; non-invading cells were removed from inner wells using a cotton swab, and invading cells adherent to the bottom of membrane were fixed and stained using a Diff-Quick staining kit (DADE AG, Miami, FL). Invading cells were enumerated by dividing membranes into 4 quadrants and counting the number of cells in 3 distinct areas for each quadrant under a 10× objective using an ocular micrometer. Assays were performed in triplicate.
Supplementary Material
Cells expressing GFP-tagged MT1-MMP were serum starved overnight prior to treatment with EGF (25nm). Images were acquired at 1min intervals with a 60× oil immersion objective with numerical aperture 1.40 and representative time points are shown. The experiment was repeated in triplicate. Left panel – MT1-MMP-GFP fluorescence; right panel – corresponding phase image.
(A) Cells were serum starved overnight, treated with EGF (25 nM) for the indicated times in serum-free medium, and processed for staining with anti-EGFR (green) or anti-caveolin-1 (red) as described in Experimental Procedures. All images were viewed using the 63 × 1.4 oil immersion objective. (B) Cells were treated as in A, and processed for staining with anti-EGFR (green) and anti-clathrin (red) antibodies as described in Experimental Procedures.
Acknowledgements
This work was supported by Research Grants RO1 CA86984 (M.S.S.), CA86984-S1 (N.M.M.), and CA109545 (M.S.S. & L.G.H.) from the National Institutes of Health/National Cancer Institute.
REFERENCES
- 1.Carpenter G, Cohen S. Epidermal growth factor. J. Biol. Chem. 1998;265:7709–7712. [PubMed] [Google Scholar]
- 2.Wells A. EGF receptor. Int J. Biochem. Cell Bio. 1999;31:637–643. doi: 10.1016/s1357-2725(99)00015-1. [DOI] [PubMed] [Google Scholar]
- 3.Yarden Y, Sliwkowski MX. Untangling the ErbB signaling network. Nat. Rev. Mol. Cell. Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 4.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer. Biochimica et Biophysica Acta. 2008;1785:232–265. doi: 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Jorissen RN, Walker F, Pouli N, Garrett T, Ward C, Burgess A. Epidermal growth factor receptor: mechanisms of activation and signaling. Exp. Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- 6.Luttrell DK, Luttrell LM, Parsons SJ. Augmented mitogenic responsiveness to Epidermal Growth Factor in murine fibroblasts that overexpress pp6Ocsrc. Mol. Cell Biol. 1998;8:497–501. doi: 10.1128/mcb.8.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc. Natl. Acad. Sci. USA. 1995;92(15):6981–5. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi K, Okabayashi Y, Kido Y, Kimura S, Matsumura Y, Inushima K, et al. Shc phosphotyrosine-binding domain dominantly interacts with epidermal growth factor receptors and mediates Ras activation in intact cells. Mol Endocrinol. 1998;12(4):536–43. doi: 10.1210/mend.12.4.0094. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto A, Kurosaki M, Gotoh N, Shibuya M, Kurosaki T. Shc regulates epidermal growth factor-induced activation of the JNK signaling pathway. J Biol Chem. 1999;274(29):20139–43. doi: 10.1074/jbc.274.29.20139. [DOI] [PubMed] [Google Scholar]
- 10.David M, Wong L, Flavell R, Thompson SA, Wells A, Larner AC, et al. STAT activation by epidermal growth factor (EGF) and amphiregulin. Requirement for the EGF receptor kinase but not for tyrosine phosphorylation sites or JAK1. J. Biol. Chem. 1996;271:9185–9188. doi: 10.1074/jbc.271.16.9185. [DOI] [PubMed] [Google Scholar]
- 11.Park OK, Schaefer TS, Nathans D. In vitro activation of Stat3 by epidermal growth factor receptor kinase. Proc. Natl. Acad. Sci. USA. 1996;93:13704–13708. doi: 10.1073/pnas.93.24.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slaaby R, Jensen T, Hansen HS, Frohman MA, Seedorf K. PLD2 complexes with the EGF receptor and undergoes tyrosine phosphorylation at a single site upon agonist stimulation. J. Biol. Chem. 1998;273:33722–33727. doi: 10.1074/jbc.273.50.33722. [DOI] [PubMed] [Google Scholar]
- 13.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366(1):2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 14.De Luca A, Carotenuto A, Rachiglio A, Gallo M, Maiello MR, Aldinucci D, et al. The role of the EGFR signaling in tumor microenvironment. J Cell Physiol. 2008;214(3):559–67. doi: 10.1002/jcp.21260. [DOI] [PubMed] [Google Scholar]
- 15.Ekstrand AJ, James CD, Cavanee WK, Seliger B, Petterson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 16.Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 17.Fox SB, Smith K, Hollyer J, Greenall M, Hastrich D, Harris AL. The epidermal growth factor receptor as a prognostic marker: results of 370 patients and review of 3009 patients. Breast Cancer Res Treat. 1999;29(1):41–49. doi: 10.1007/BF00666180. [DOI] [PubMed] [Google Scholar]
- 18.Koenders PG, Beex LV, Kienhuis CB, Kloppenborg PW, Benraad TJ. Epidermal growth factor receptor and prognosis in human breast cancer: a prospective study. Breast Canc. Res Treat. 1993;25(1):21–27. doi: 10.1007/BF00662397. [DOI] [PubMed] [Google Scholar]
- 19.Salomon DS, Brandt R, Ciardiello F, Normanno N. Epidermal growth factor-related peptides and their receptors in human malignancies. Crit Rev Oncol Hematol. 2005;3:183–232. doi: 10.1016/1040-8428(94)00144-i. [DOI] [PubMed] [Google Scholar]
- 20.See HT, Kavanagh JJ, Hu W, Bast RC. Targeted therapy for epithelial ovarian cancer: current status and future prospects. Int J Gynecol Canc. 2003;13(6):701–34. doi: 10.1111/j.1525-1438.2003.13601.x. [DOI] [PubMed] [Google Scholar]
- 21.Berchuck A, Rodriguez GC, Kamel A, Dodge RK, Soper JT, Clarke-Pearson DL, et al. Epidermal growth factor receptor expression in normal ovarian epithelium and ovarian cancer. I. Correlation of receptor expression with prognostic factors in patients with ovarian cancer. Am J Obstet Gynecol. 1991;2:669–74. doi: 10.1016/s0002-9378(11)80044-x. [DOI] [PubMed] [Google Scholar]
- 22.Wang DP, Konishi I, Koshiyama M, Nanbu Y, Iwai T, Nonogaki H, et al. Immunohistochemical localization of c-erbB-2 protein and epidermal growth factor receptor in normal surface epithelium, surface inclusion cysts, and common epithelial tumours of the ovary. Virchows Arch A Pathol Anat Histopathol. 1992;421(5):393–400. doi: 10.1007/BF01606911. [DOI] [PubMed] [Google Scholar]
- 23.Henzen-Logmans SC, van der Burg ME, Foekens JA, Berns PM, Brussée R, Fieret JH, et al. Occurrence of epidermal growth factor receptors in benign and malignant ovarian tumors and normal ovarian tissues: an immunohistochemical study. J Cancer Res Clin Oncol. 1994;118(4):303–7. doi: 10.1007/BF01208620. [DOI] [PubMed] [Google Scholar]
- 24.Marmor MD, Skaria KB, Yarden Y. Signal transduction and oncogenesis by ErbB/HER receptors. Int J Radiat Oncol Biol Phys. 2004;58(3):903–13. doi: 10.1016/j.ijrobp.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 25.El-Rayes BF, LoRusso PM. Targeting the epidermal growth factor receptor. Br J Cancer. 2004;91(3):418–424. doi: 10.1038/sj.bjc.6601921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roskoski R., Jr The ErbB/HER receptor protein-tyrosine kinases and cancer. Biochem Biophys Res Commun. 2004;319(1):1–11. doi: 10.1016/j.bbrc.2004.04.150. [DOI] [PubMed] [Google Scholar]
- 27.Cowden Dahl KD, Symowicz J, Ning Y, Gutierrez E, Fishman DA, Adley BP, et al. Matrix metalloproteinase 9 is a mediator of epidermal growth factor-dependent e-cadherin loss in ovarian carcinoma cells. Cancer Res. 2008;68(12):4606–13. doi: 10.1158/0008-5472.CAN-07-5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hudson LG, Zeineldin R, Silberberg M, Stack MS. Activated Epidermal Growth Factor Receptor in Ovarian Cancer. Cancer Treat. Research. 2009 doi: 10.1007/978-0-387-98094-2_10. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crijns APG, Boezen HM, Schouten JP, et al. Prognostic factors in ovarian cancer: current evidence and future prospects. The ECCO 12 Educational Book Eur J Cancer. 2003;1 Suppl:127–45. [Google Scholar]
- 30.Alper O, De Santis ML, Stromberg K, Hacker NF, Cho-Chung YS, Salomon DS. Anti-sense suppression of epidermal growth factor receptor expression alters cellular proliferation, cell-adhesion and tumorigenicity in ovarian cancer cells. Int J Cancer. 2000;88(4):566–74. doi: 10.1002/1097-0215(20001115)88:4<566::aid-ijc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Brader KR, Wolf JK, Chakrabarty S, Price JE. Epidermal growth factor receptor (EGFR) antisense transfection reduces the expression of EGFR and suppresses the malignant phenotype of a human ovarian cancer cell line. Oncol Rep. 1998;5(5):1269–74. doi: 10.3892/or.5.5.1269. [DOI] [PubMed] [Google Scholar]
- 32.Sood AK, Seftor EA, Fletcher MS, Gardner LM, Heidger PM, Buller RE, et al. Molecular determinants of ovarian cancer plasticity. Am J Pathol. 2001;158(4):1279–88. doi: 10.1016/S0002-9440(10)64079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sood AK, Fletcher MS, Coffin JE, Yang M, Seftor EA, Gruman LM, et al. Functional role of matrix metalloproteinases in ovarian tumor cell plasticity. Am J Obstet Gynecol. 2004;190(4):899–909. doi: 10.1016/j.ajog.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 34.Kamat AA, Fletcher M, Gruman LM, Mueller P, Lopez A, Landen CN, Jr, et al. The clinical relevance of stromal matrix metalloproteinase expression in ovarian cancer. Clin Cancer Res. 2006;12(6):1707–14. doi: 10.1158/1078-0432.CCR-05-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenny HA, Krausz T, Yamada SD, Lengyel E. Development of an organotypic peritoneal three-dimensional culture to study peritoneal attachment of ovarian cancer cells. Int. J. Canc. 2001;121:1463–1472. [Google Scholar]
- 36.Kenny HA, Kaur S, Coussens LM, Lengyel E. The initial steps of ovarian cancer cell metastasis are mediated by MMP-2 cleavage of vitronectin and fibronectin. J Clin Invest. 2008;118(4):1367–79. doi: 10.1172/JCI33775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adley BP, Gleason KJ, Yang X, Stack MS. Expression of membrane type 1 matrix metalloproteinase (MMP-14) in epithelial ovarian cancer: High level expression in clear cell carcinoma. Gyn Oncol. 2009;112:319. doi: 10.1016/j.ygyno.2008.09.025. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghosh S, Wu Y, Stack MS. Ovarian cancer-associated proteinases. Cancer Treat Res. 2002;107:331–51. doi: 10.1007/978-1-4757-3587-1_16. [DOI] [PubMed] [Google Scholar]
- 39.Davidson B, Goldberg I, Gotlieb WH, Kopolovic J, Ben-Baruch G, Nesland JM, et al. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis. 1999;17(10):799–808. doi: 10.1023/a:1006723011835. [DOI] [PubMed] [Google Scholar]
- 40.Ellerbroek SM, Hudson LG, Stack MS. Proteinase requirements of epidermal growth factor-induced ovarian cancer cell invasion. Int J Cancer. 1998;78(3):331–7. doi: 10.1002/(SICI)1097-0215(19981029)78:3<331::AID-IJC13>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.Ellerbroek SM, Wu YI, Overall CM, Stack MS. Functional interplay between type I collagen and cell surface matrix metalloproteinase activity. J Biol Chem. 2001;276(27):24833–42. doi: 10.1074/jbc.M005631200. [DOI] [PubMed] [Google Scholar]
- 42.Cowden Dahl KD, Zeineldin R, Hudson LG. PEA3 is necessary for optimal epidermal growth factor receptor-stimulated matrix metalloproteinase expression and invasion of ovarian tumor cells. Mol Cancer Res. 2007;5(5):413–21. doi: 10.1158/1541-7786.MCR-07-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afzal S, Lalani EN, Poulsom R, Stubbs A, Rowlinson G, Sato H, et al. MT1-MMP and MMP-2 mRNA expression in human ovarian tumors: possible implications for the role of desmoplastic fibroblasts. Hum Pathol. 1998;29(2):155–65. doi: 10.1016/s0046-8177(98)90226-x. [DOI] [PubMed] [Google Scholar]
- 44.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 45.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J. Cell Biol. 2004;167:769–81. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yana I, Seiki M. MT-MMPs play pivotal roles in cancer dissemination. Clin. Exp. Metastasis. 2003;19:209–15. doi: 10.1023/a:1015527220537. [DOI] [PubMed] [Google Scholar]
- 47.Zhai Y, Hotary K, Nan B, Bosch F, Muñoz N, Weiss S, et al. Expression of Membrane Type 1 Matrix Metalloproteinase Is Associated with Cervical Carcinoma Progression and Invasion. Canc. Res. 2005;65:6543–6550. doi: 10.1158/0008-5472.CAN-05-0231. [DOI] [PubMed] [Google Scholar]
- 48.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J. Cell Phys. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 49.Kajita M, Itoh Y, Chiba T, Mori H, Okada A, Kino H, et al. Membrane-type I matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Bio. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gingras D, Bousquet-Gagnon N, Langlois S, Lachambre M, Annabi B, Beliveau R. Activation of the extracellular signal-regulated protein kinase (ERK) cascade by membrane-type-1 matrix metalloproteinase (MT1-MMP) FEBS Lett. 2001;507:231–236. doi: 10.1016/s0014-5793(01)02985-4. [DOI] [PubMed] [Google Scholar]
- 51.Barbolina MV, Adley BP, Ariztia EV, Liu Y, Stack MS. Microenvironmental regulation of membrane type 1 matrix metalloproteinase activity in ovarian carcinoma cells via collagen-induced EGR1 expression. J Biol Chem. 2007;282(7):4924–31. doi: 10.1074/jbc.M608428200. [DOI] [PubMed] [Google Scholar]
- 52.Hotary K, Allen E, Punturieri A, Yana I, Weiss S. Regulation of Cell Invasion and Morphogenesis in a Three-dimensional Type I Collagen Matrix by Membrane-type Matrix Metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–1323. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hotary K, Allen E, Brooks P, Datta N, Long M, Weiss SJ. Membrane type 1 matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 54.Sato T, Iwai M, Sakai T, Sato H, Seiki M, Mori Y, et al. Enhancement of membrane-type 1-matrix metalloproteinase (MT1-MMP) production and sequential activation of progelatinase A on human squamous carcinoma cells co-cultured with human dermal fibroblasts. Br. J. Cancer. 1999;80(8):1137–1143. doi: 10.1038/sj.bjc.6690477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Meter TE, Broaddus WC, Rooprai HK, Pilkington GJ, Fillmore HL. Induction of membrane-type-1 matrix metalloproteinase by epidermal growth factor-mediated signaling in gliomas. Neuro-Oncology. 2004;6(3):188–99. doi: 10.1215/S1152851703000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kheradmand F, Rishi K, Werb Z. Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci. 2002;115(4):839–48. doi: 10.1242/jcs.115.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langlois S, Nyalendo C, Di Tomasso G, Labrecque L, Roghi C, Murphy G, et al. Membrane-type 1 matrix metalloproteinase stimulates cell migration through epidermal growth factor receptor transactivation. Mol Cancer Res. 2007;5(6):569–83. doi: 10.1158/1541-7786.MCR-06-0267. [DOI] [PubMed] [Google Scholar]
- 58.Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin mediated endocytosis. Proc. Natl. Acad. Sci. USA. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274(3):1185–8. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 60.Annabi B, Lachambre MP, Bousquet-Gagnon N, Page M, Gingras D, Beliveau R. Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem J. 2001;353:547–553. doi: 10.1042/0264-6021:3530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Puyraimond A, Fridman R, Lemasle M, Arbeille B, Menashi S. MMP-2 colocalizes with caveolae on the surface of endothelial cells. Exp. Cell Res. 2001;262:28–36. doi: 10.1006/excr.2000.5069. [DOI] [PubMed] [Google Scholar]
- 62.Remacle A, Murphy G, Roghi C. Membrane type 1-matrix metalloproteinase (MT1-MMP) is internalized by two different pathways and is recycled to the cell surface. J Cell Sci. 2003;116:3905–3916. doi: 10.1242/jcs.00710. [DOI] [PubMed] [Google Scholar]
- 63.Ouyang M, Lu S, Li XY, Xu J, Seong J, Giepmans BN, et al. Visualization of polarized membrane type 1 matrix metalloproteinase activity in live cells by fluorescence resonance energy transfer imaging. J Biol Chem. 2008;283(25):17740–8. doi: 10.1074/jbc.M709872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92(22):10104–8. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271(20):11930–5. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- 66.Mineo C, Gill GN, Anderson R. Regulated migration of epidermal growth factor receptor from caveolae. J Biol Chem. 1999:30636–30643. doi: 10.1074/jbc.274.43.30636. [DOI] [PubMed] [Google Scholar]
- 67.Ning Y, Buranda T, Hudson LG. Activated epidermal growth factor receptor induces integrin alpha2 internalization via caveolae/raft-dependent endocytic pathway. J Biol Chem. 2007;282(9):6380–7. doi: 10.1074/jbc.M610915200. [DOI] [PubMed] [Google Scholar]
- 68.Beguinot L, Lyall RM, Willingham MC, Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci USA. 1984;81:2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sorkin A, Waters CM. Endocytosis of growth factor receptors. Bioessays. 1993;15:375–382. doi: 10.1002/bies.950150603. [DOI] [PubMed] [Google Scholar]
- 70.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenny JR, Anderson RG. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- 71.Ikonen E, Parton RG. Caveolins and cellular cholesterol balance. Traffic. 2000;1:212–217. doi: 10.1034/j.1600-0854.2000.010303.x. [DOI] [PubMed] [Google Scholar]
- 72.Pike LJ, Miller JM. Cholesterol depletion delocalizes phosphatidylinositol biphosphate and inhibits hormone-stimulated phosphatidylinositol turnover. J Biol Chem. 1998;273:22298–22304. doi: 10.1074/jbc.273.35.22298. [DOI] [PubMed] [Google Scholar]
- 73.Gingras D, Michaud M, Tomasso G, Béliveau E, Nyalendo C, Béliveau R. Sphingosine-1-phosphate induces the association of membrane-type 1 matrix metalloproteinase with p130Cas in endothelial cells. FEBS Lett. 2008;582(3):399–404. doi: 10.1016/j.febslet.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 74.Nyalendo C, Beaulieu E, Sartelet H, Michaud M, Fontaine N, Gingras D, et al. Impaired tyrosine phosphorylation of membrane-type 1 matrix metalloproteinase reduces tumor cell proliferation in three-dimensional matrices and abrogates tumor growth in mice. Carcinogenesis. 2008;29(8):1655–64. doi: 10.1093/carcin/bgn159. [DOI] [PubMed] [Google Scholar]
- 75.Hudson LG, Zeineldin R, Stack MS. Phenotypic plasticity of neoplastic ovarian epithelium: unique cadherin profiles in tumor progression. Clin Exp Metastasis. 2008;25(6):643–55. doi: 10.1007/s10585-008-9171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moser TL, Pizzo SV, Bafetti LM, Fishman DA, Stack MS. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the alpha2beta1 integrin. 1996;67(5):695–701. doi: 10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 77.Tsunezuka Y, Kino H, Takino T, Watanabe Y, Okada Y, Shinagawa A, et al. Expression of membrane-type matrix metalloproteinase I (MT1-MMP) in tumor cells enhances pulmonary metastasis in an experimental metastasis. Can Res. 1996;56:5678–5683. [PubMed] [Google Scholar]
- 78.Lisanti MP, Scherer P, Tang ZL, Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signaling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- 79.van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol. 2003;13(2):92–100. doi: 10.1016/s0962-8924(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 80.Mineo C, Ying YS, Chapline C, Jaken S, Anderson RG. Targeting of protein kinase C alpha to caveolae. J Cell Biol. 1998;141(3):601–10. doi: 10.1083/jcb.141.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson RGW. The caveolae membrane system. Annu. Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 82.Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002;277(44):41295–8. doi: 10.1074/jbc.R200020200. [DOI] [PubMed] [Google Scholar]
- 83.Huang CS, Zhou J, Feng AK, Lynch CC, Klumperman J, DeArmond SJ, et al. Nerve growth factor signaling in caveolae-like domains at the plasma membrane. J Biol Chem. 1999 Dec 17;274(51):36707–14. doi: 10.1074/jbc.274.51.36707. [DOI] [PubMed] [Google Scholar]
- 84.Labrecque L, Royal I, Surprenant DS, Patterson C, Gingras D, Beliveau R. Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. Mol Biol Cell. 2003;14:334–347. [Google Scholar]
- 85.Song KS, Shengwen L, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Co-purification and direct interaction of ras with caveolin, an integral membrane protein of caveolae microdomains. J. Biol. Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- 86.Furuchi T, Anderson RGW. Cholesterol depletion of caveolae causes hyperactivation of extracellular signal related kinase (ERK) J Biol Chem. 1998;273:21099–21104. doi: 10.1074/jbc.273.33.21099. [DOI] [PubMed] [Google Scholar]
- 87.Smart RJ, Graf GA, McNiven MA, Sessa WC, Engelman JA, Scherer PE, et al. Caveolins, liquid ordered domains and signal transduction. Mol Cell Biol. 1999;19:7289–7304. doi: 10.1128/mcb.19.11.7289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ahn S, Maudsley S, Luttrell LM, Lefkowitz RJ, Daaka Y. Src-mediated tyrosine phosphorylation of dynamin is required for beta2-adrenergic receptor internalization and mitogen-activated protein kinase signaling. J Biol Chem. 1999;274(3):1185–8. doi: 10.1074/jbc.274.3.1185. [DOI] [PubMed] [Google Scholar]
- 89.Uekita T, Itoh Y, Yana I, Ohno H, Seiki M. Cytoplasmic tail-dependent internalization of membrane type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le P, Guay G, Altschuler Y, Nabi IR. Caveolin-1 is a negative regulator of caveolae mediated endocytosis to the endoplasmic reticulum. J Biol. Chem. 2002;277:3371–3379. doi: 10.1074/jbc.M111240200. [DOI] [PubMed] [Google Scholar]
- 91.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, et al. Endothelial cell surface gp60 activates vesicle formation and trafficking via G (i)-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomsen P, Roepstorff K, Stahlhut M, Van Deurs B. Caveolae are highly immobile plasma membrane microdomains, which are not involved in constitutive endocytic trafficking. Mol Biol Cell. 2002;13(1):238–50. doi: 10.1091/mbc.01-06-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Parton RG, Richards AA. Lipid rafts and caveolae as portals for endocytosis: new insights and common mechanisms. Traffic. 2003;4(11):724–38. doi: 10.1034/j.1600-0854.2003.00128.x. [DOI] [PubMed] [Google Scholar]
- 94.Kim YN, Wiepz GJ, Guadarrama AG, Bertics PJ. Epidermal growth factor-stimulated tyrosine phosphorylation of caveolin-1. Enhanced caveolin-1 tyrosine phosphorylation following aberrant epidermal growth factor receptor status. J Biol Chem. 2000;275(11):7481–91. doi: 10.1074/jbc.275.11.7481. [DOI] [PubMed] [Google Scholar]
- 95.Nyalendo C, Michaud M, Beaulieu E, Roghi C, Murphy G, Gingras D, et al. Src dependent phosphorylation of Membrane-type I Matrix metallopoteinase on cytoplasmic tyrosine 573: Role in endothelial and tumor cell migration. J. Biol Chem. 2007;282:15690–9. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]
- 96.Nakahara H, Howard L, Thompson E, Sato H, Seiki M, Yeh Y, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc. Natl. Acad. Sci. USA. 1997;94:7959–7964. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen WT, Wang JY. Specialized surface protrusions of invasive cells, invadopodia and lamellipodia, have differential MT1-MMP, MMP-2, and TIMP-2 localization. Ann N Y Acad Sci. 1990;878:361–71. doi: 10.1111/j.1749-6632.1999.tb07695.x. [DOI] [PubMed] [Google Scholar]
- 98.Lehti K, Valtanen H, Wickström SA, Lohi J, Keski-Oja J. Regulation of membrane-type-1 matrix metalloproteinase activity by its cytoplasmic domain. J Biol Chem. 2000;275(20):15006–13. doi: 10.1074/jbc.M910220199. [DOI] [PubMed] [Google Scholar]
- 99.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66(6):3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 100.Wolf K, Wu YI, Liu Y, Geiger J, Tam E, Overall C, et al. Multi-step pericellular proteolysis controls the transition from individual to collective cancer cell invasion. Nat Cell Biol. 2007;9(8):893–904. doi: 10.1038/ncb1616. [DOI] [PubMed] [Google Scholar]
- 101.Radisky DC, Levy DD, Littlepage LE, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–7. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee H, Overall CM, McCulloch CA, Sodek J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol Biol Cell. 2006;17:4812–26. doi: 10.1091/mbc.E06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tam EM, Wu YI, Butler GS, Stack MS, Overall CM. Collagen binding properties of the membrane type-1 matrix metalloproteinase (MT1-MMP) hemopexin C domain. The ectodomain of the 44-kDa autocatalytic product of MT1-MMP inhibits cell invasion by disrupting native type I collagen cleavage. J Biol Chem. 2002;277(41):39005–14. doi: 10.1074/jbc.M206874200. [DOI] [PubMed] [Google Scholar]
- 104.Itoh Y, Takamura A, Ito N, Maru Y, Sato H, Suenaga N, et al. Homophilic complex formation of MT1-MMP facilitates proMMP-2 activation on the cell surface and promotes tumor cell invasion. EMBO J. 2001;20(17):4782–93. doi: 10.1093/emboj/20.17.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjøller L, et al. Extracellular Collagenases and the Endocytic Receptor, Urokinase Plasminogen Activator Receptor-associated Protein/Endo180, Cooperate in Fibroblast-mediated Collagen Degradation. J. Biol. Chem. 2007;282:27037–27045. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- 106.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, et al. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol. 2007;27(18):6309–22. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Y, Munshi HG, Sen R, Snipas S, Salvesen G, Fridman R, et al. Glycosylation Broadens the Substrate Profile of Membrane Type 1 Matrix Metalloproteinase. J. Biol. Chem. 2004;279:8728–8289. doi: 10.1074/jbc.M311870200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cells expressing GFP-tagged MT1-MMP were serum starved overnight prior to treatment with EGF (25nm). Images were acquired at 1min intervals with a 60× oil immersion objective with numerical aperture 1.40 and representative time points are shown. The experiment was repeated in triplicate. Left panel – MT1-MMP-GFP fluorescence; right panel – corresponding phase image.
(A) Cells were serum starved overnight, treated with EGF (25 nM) for the indicated times in serum-free medium, and processed for staining with anti-EGFR (green) or anti-caveolin-1 (red) as described in Experimental Procedures. All images were viewed using the 63 × 1.4 oil immersion objective. (B) Cells were treated as in A, and processed for staining with anti-EGFR (green) and anti-clathrin (red) antibodies as described in Experimental Procedures.


