Abstract
Sparfloxacin (50 mg/kg of body weight given subcutaneously each day), alone or in combination with ethambutol (50 mg/kg given subcutaneously each day), was examined for its therapeutic efficacy against experimental infection induced with the Mycobacterium avium complex in normal C57BL/6 mice. In addition, the potential anti-infective role of RU-40 555 (100 mg/kg given intraperitoneally each day), a drug that inhibits the cortisol receptors, was examined in the same model. Treatments were started 24 h after intravenous bacterial challenge and were continued for 21 days. Compared with controls, sparfloxacin or ethambutol decreased the CFU counts in spleens and lungs (P < 0.001). The sparfloxacin plus ethambutol combination was more effective than sparfloxacin alone in spleens (P < 0.001) but not in lungs. The sparfloxacin plus ethambutol plus RU-40 555 combination was more effective than the sparfloxacin plus ethambutol combination in spleens and lungs (P < 0.001). Thus, in this model, RU-40 555 enhanced the antibacterial activities of the antibiotics tested. Results of the study showed that normal C57BL/6 mice infected with the M. avium complex can be used for the evaluation of antimicrobial agents.
Full text
PDF
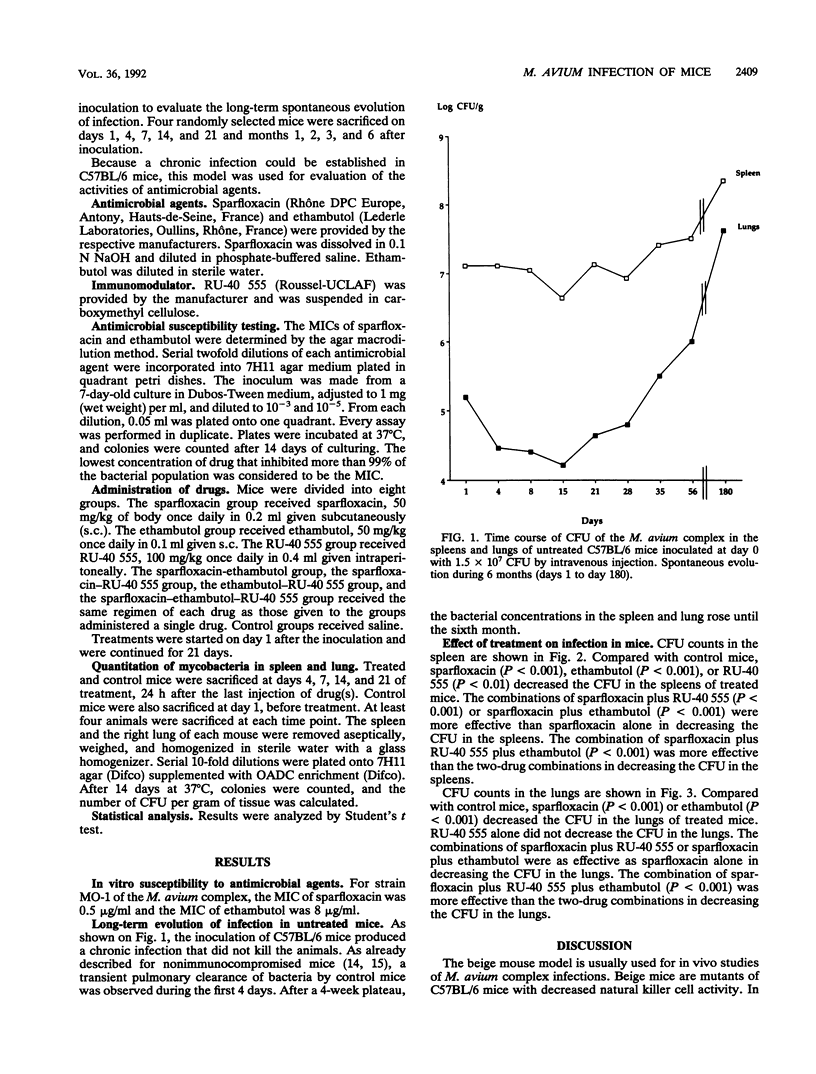
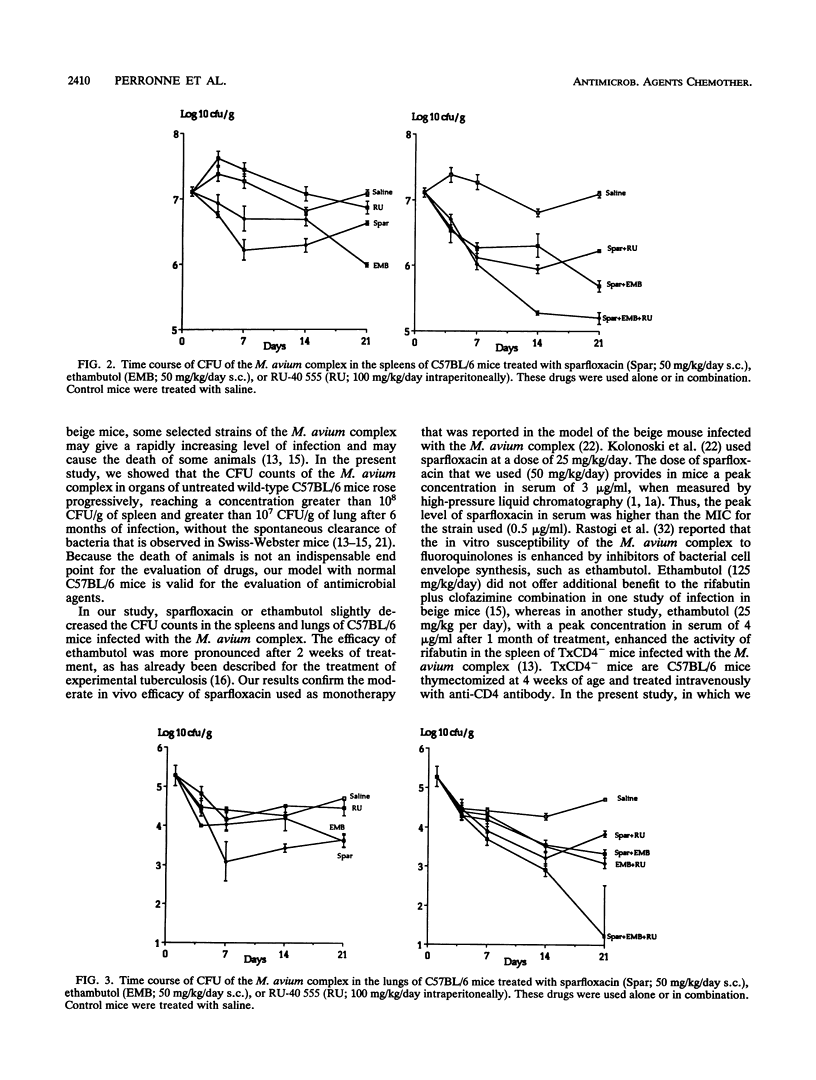


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baulieu E. E. Contragestion and other clinical applications of RU 486, an antiprogesterone at the receptor. Science. 1989 Sep 22;245(4924):1351–1357. doi: 10.1126/science.2781282. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Stevens P., Kolonoski P., Wu M., Young L. S. Treatment of experimental disseminated Mycobacterium avium complex infection in mice with recombinant IL-2 and tumor necrosis factor. J Immunol. 1989 Nov 1;143(9):2996–3000. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Activities of amikacin, roxithromycin, and azithromycin alone or in combination with tumor necrosis factor against Mycobacterium avium complex. Antimicrob Agents Chemother. 1988 Aug;32(8):1149–1153. doi: 10.1128/aac.32.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton C., Flower R. J. The effects of the anti-glucocorticoid RU 38486 on steroid-mediated suppression of experimental allergic encephalomyelitis (EAE) in the Lewis rat. Life Sci. 1989;45(1):97–104. doi: 10.1016/0024-3205(89)90441-4. [DOI] [PubMed] [Google Scholar]
- C J., P J. Hormone antagonist with broad potential. Science. 1989 Sep 22;245(4924):1322–1322. doi: 10.1126/science.245.4924.1322. [DOI] [PubMed] [Google Scholar]
- Dautzenberg B., Truffot C., Legris S., Meyohas M. C., Berlie H. C., Mercat A., Chevret S., Grosset J. Activity of clarithromycin against Mycobacterium avium infection in patients with the acquired immune deficiency syndrome. A controlled clinical trial. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):564–569. doi: 10.1164/ajrccm/144.3_Pt_1.564. [DOI] [PubMed] [Google Scholar]
- Dietrich J. B., Chasserot-Golaz S., Beck G., Bauer G. Antagonism of glucocorticoid induction of Epstein-Barr virus early antigens by different steroids in Daudi lymphoma cells. J Steroid Biochem. 1986 Jan;24(1):417–421. doi: 10.1016/0022-4731(86)90093-2. [DOI] [PubMed] [Google Scholar]
- Durant S., Homo-Delarche F., Duval D., Papiernik M., Smets P., Zalisz R. Opposite effects of a glucocorticoid and an immunostimulating agent on prostaglandin production by two different cell types. Int J Tissue React. 1985;7(2):117–122. [PubMed] [Google Scholar]
- Ellner J. J., Goldberger M. J., Parenti D. M. Mycobacterium avium infection and AIDS: a therapeutic dilemma in rapid evolution. J Infect Dis. 1991 Jun;163(6):1326–1335. doi: 10.1093/infdis/163.6.1326. [DOI] [PubMed] [Google Scholar]
- Emilie D., Galanaud P., Baulieu E. E., Dormont J. Inhibition of in vitro immunosuppressive effects of glucocorticosteroids by a competitive antagonist RU-486. Immunol Lett. 1984;8(4):183–186. doi: 10.1016/0165-2478(84)90075-0. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Hardy D. J., McDaniel D., Hanson C. W., Swanson R. N. In vitro and in vivo activities of clarithromycin against Mycobacterium avium. Antimicrob Agents Chemother. 1989 Sep;33(9):1531–1534. doi: 10.1128/aac.33.9.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formstecher P., Lefebvre P., Dautrevaux M. RU 486 stabilizes the glucocorticoid receptor in a non-transformed high molecular weight form in intact thymus cells under physiological conditions. J Steroid Biochem. 1988 Oct;31(4B):607–612. doi: 10.1016/0022-4731(88)90012-x. [DOI] [PubMed] [Google Scholar]
- Furney S. K., Roberts A. D., Orme I. M. Effect of rifabutin on disseminated Mycobacterium avium infections in thymectomized, CD4 T-cell-deficient mice. Antimicrob Agents Chemother. 1990 Sep;34(9):1629–1632. doi: 10.1128/aac.34.9.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangadharam P. R., Edwards C. K., 3rd, Murthy P. S., Pratt P. F. An acute infection model for Mycobacterium intracellulare disease using beige mice: preliminary results. Am Rev Respir Dis. 1983 May;127(5):648–649. doi: 10.1164/arrd.1983.127.5.648. [DOI] [PubMed] [Google Scholar]
- Gangadharam P. R., Perumal V. K., Jairam B. T., Rao P. N., Nguyen A. K., Farhi D. C., Iseman M. D. Activity of rifabutin alone or in combination with clofazimine or ethambutol or both against acute and chronic experimental Mycobacterium intracellulare infections. Am Rev Respir Dis. 1987 Aug;136(2):329–333. doi: 10.1164/ajrccm/136.2.329. [DOI] [PubMed] [Google Scholar]
- Grumbach F. Experimental "in vivo" studies of new antituberculosis drugs: capreomycin, ethambutol, rifampicin. Tubercle. 1969 Mar;50(Suppl):12–21. [PubMed] [Google Scholar]
- Hirschelmann R., Klingner E., Schmidt K., Bekemeier H. Dexamethasone antagonism by RU 38,486 in inflammatory reactions of the rat and mouse. Part 1: Degree of inflammation. Pharmazie. 1988 Mar;43(3):219–220. [PubMed] [Google Scholar]
- Hirschelmann R., Schade R., Klingner E., Bekemeier H. Dexamethasone antagonism by RU 38486 in inflammatory reactions of the rat. Part 2: Biochemical parameters: RNA content of inflammation cells and acute phase reactants of the blood. Pharmazie. 1988 May;43(5):370–371. [PubMed] [Google Scholar]
- Horsburgh C. R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991 May 9;324(19):1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- Laue L., Kawai S., Brandon D. D., Brightwell D., Barnes K., Knazek R. A., Loriaux D. L., Chrousos G. P. Receptor-mediated effects of glucocorticoids on inflammation: enhancement of the inflammatory response with a glucocorticoid antagonist. J Steroid Biochem. 1988 Jun;29(6):591–598. doi: 10.1016/0022-4731(88)90156-2. [DOI] [PubMed] [Google Scholar]
- Lazar G., Agarwal M. K. The influence of a novel glucocorticoid antagonist on endotoxin lethality in mice strains. Biochem Med Metab Biol. 1986 Aug;36(1):70–74. doi: 10.1016/0885-4505(86)90108-8. [DOI] [PubMed] [Google Scholar]
- Lelievre V., Martin B., Junien J. L., Bure J. Local anti-inflammatory activities of tixocortol 21-pivalate, inhibition of prostaglandins and leukotrienes synthesis, in carrageenin-induced pleurisy. Reversion of effects by RU 486. Agents Actions. 1988 Jun;24(1-2):172–178. doi: 10.1007/BF01968097. [DOI] [PubMed] [Google Scholar]
- Peers S. H., Moon D., Flower R. J. Reversal of the anti-inflammatory effects of dexamethasone by the glucocorticoid antagonist RU 38486. Biochem Pharmacol. 1988 Feb 1;37(3):556–557. doi: 10.1016/0006-2952(88)90230-4. [DOI] [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Pocidalo J. J., Vilde J. L. Activities of clarithromycin, sulfisoxazole, and rifabutin against Mycobacterium avium complex multiplication within human macrophages. Antimicrob Agents Chemother. 1990 Aug;34(8):1508–1511. doi: 10.1128/aac.34.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Vilde J. L., Pocidalo J. J. Activities of sparfloxacin, azithromycin, temafloxacin, and rifapentine compared with that of clarithromycin against multiplication of Mycobacterium avium complex within human macrophages. Antimicrob Agents Chemother. 1991 Jul;35(7):1356–1359. doi: 10.1128/aac.35.7.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Goh K. S., David H. L. Enhancement of drug susceptibility of Mycobacterium avium by inhibitors of cell envelope synthesis. Antimicrob Agents Chemother. 1990 May;34(5):759–764. doi: 10.1128/aac.34.5.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal S. J. Mifepristone (RU 486) N Engl J Med. 1990 Mar 8;322(10):691–693. doi: 10.1056/NEJM199003083221009. [DOI] [PubMed] [Google Scholar]
- Silvestre L., Dubois C., Renault M., Rezvani Y., Baulieu E. E., Ulmann A. Voluntary interruption of pregnancy with mifepristone (RU 486) and a prostaglandin analogue. A large-scale French experience. N Engl J Med. 1990 Mar 8;322(10):645–648. doi: 10.1056/NEJM199003083221001. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]


