Abstract
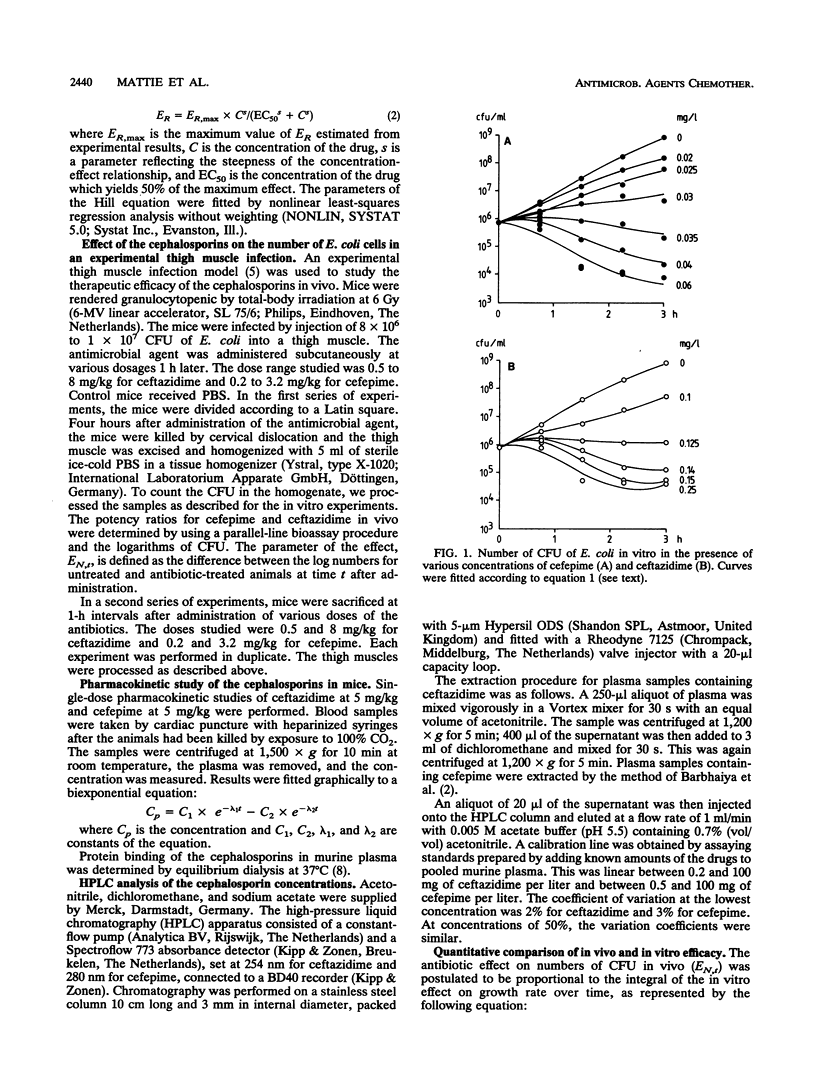
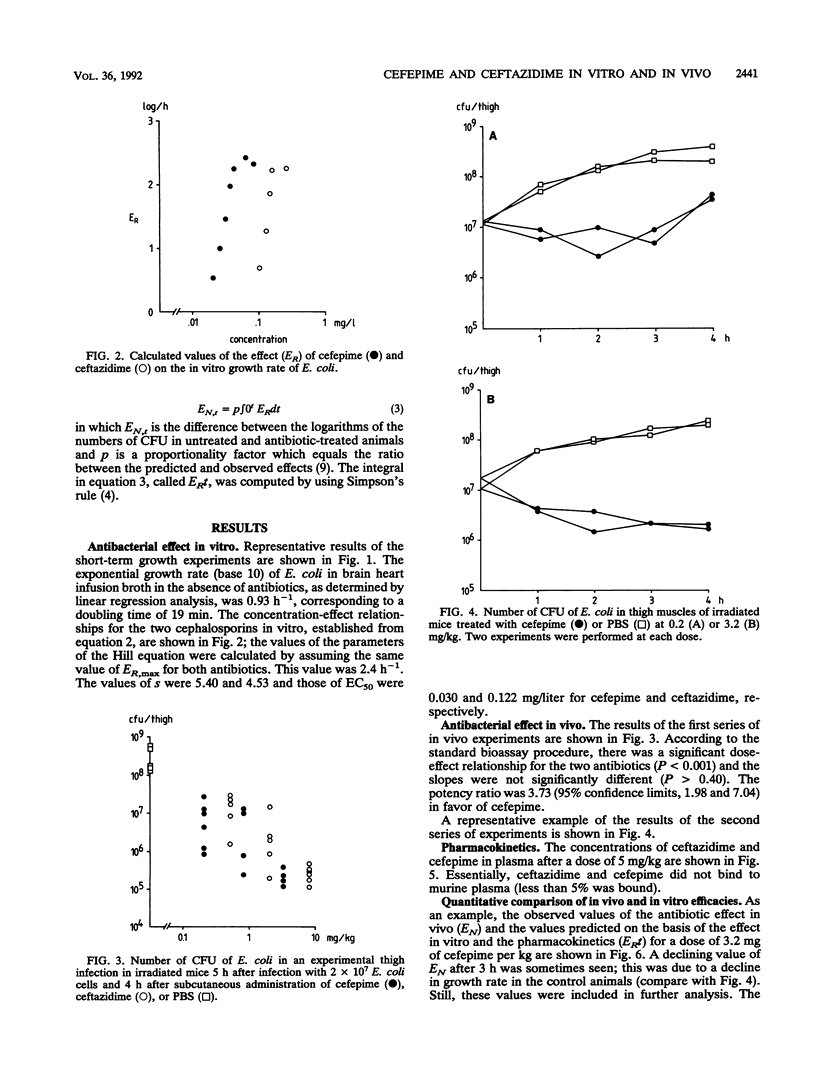
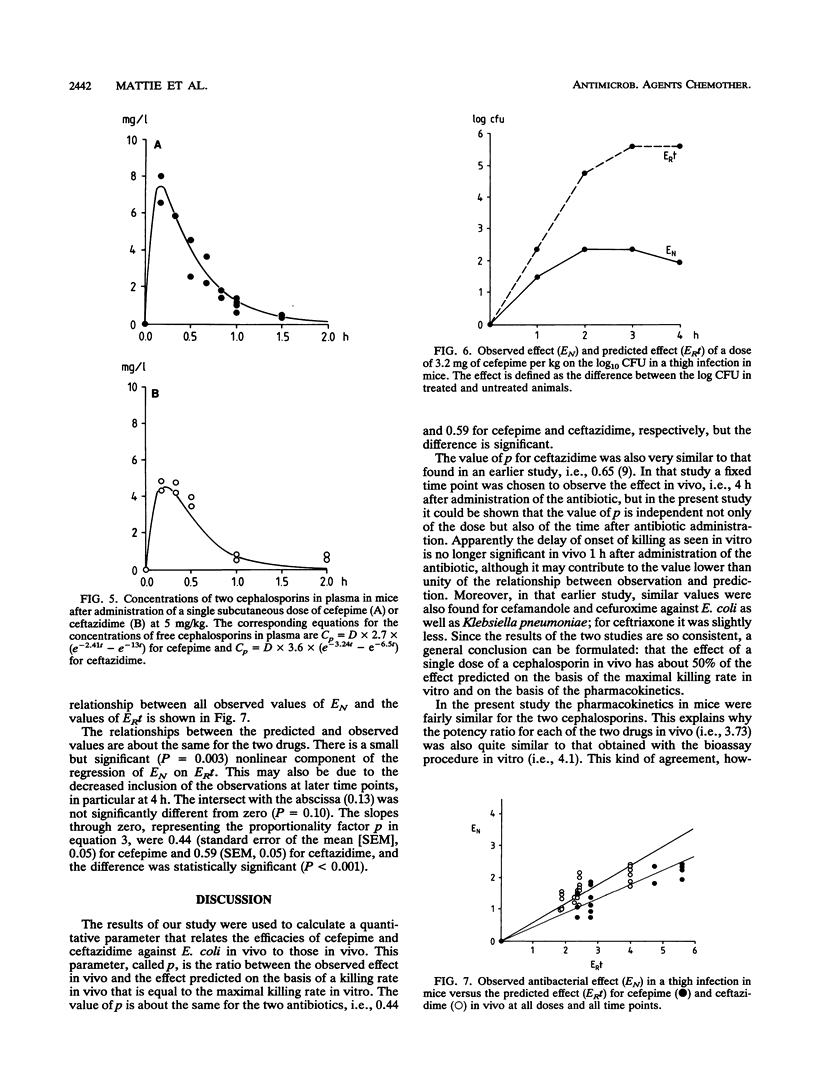
The efficacies of cefepime and ceftazidime in an experimental Escherichia coli infection in granulocytopenic mice were related to their in vitro activities and their pharmacokinetic profiles. Cefepime had a higher intrinsic activity in vitro than ceftazidime, and it had a different pharmacokinetic profile, resulting in higher peak concentrations in plasma and a longer elimination half-life. To predict the antibacterial efficacy in vivo on the basis of in vitro activity and pharmacokinetics, we applied a mathematical model in which the in vitro effect is expressed as the difference in growth rate between control cultures and cultures grown in the presence of the antibiotic (ER), whereas the in vivo effect is given by the difference in the number of CFU between controls and antibiotic-treated animals (EN). The integral of ER over time, called ERt, was calculated by using in vivo concentrations. A significant linear relationship was found between EN and ERt for different doses at various times up to 4 h after administration, although the slope of this relationship was slightly but significantly less for cefepime (0.44) than for ceftazidime (0.59).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Forgue S. T., Gleason C. R., Knupp C. A., Pittman K. A., Weidler D. J., Martin R. R. Safety, tolerance, and pharmacokinetic evaluation of cefepime after administration of single intravenous doses. Antimicrob Agents Chemother. 1990 Jun;34(6):1118–1122. doi: 10.1128/aac.34.6.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbhaiya R. H., Forgue S. T., Shyu W. C., Papp E. A., Pittman K. A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987 Jan;31(1):55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Ho D. H., LeBlanc B. In vitro studies of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 Feb;27(2):265–269. doi: 10.1128/aac.27.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst M. W., Mattie H. Cefazolin and cephradine: relationship between antibacterial activity in vitro and in mice experimentally infected with Escherichia coli. J Infect Dis. 1978 Apr;137(4):391–402. doi: 10.1093/infdis/137.4.391. [DOI] [PubMed] [Google Scholar]
- Mattie H., Hoogeterp J. J., Hermans J. The relation between plasma and tissue concentrations of antibiotics. Description of a method. J Pharmacokinet Biopharm. 1987 Apr;15(2):191–202. doi: 10.1007/BF01062343. [DOI] [PubMed] [Google Scholar]
- Mattie H., van Dokkum A. M., Brus-Weijer L., Krul A. M., van Strijen E. Antibacterial activity of four cephalosporins in an experimental infection in relation to in vitro effect and pharmacokinetics. J Infect Dis. 1990 Sep;162(3):717–722. doi: 10.1093/infdis/162.3.717. [DOI] [PubMed] [Google Scholar]
- Mattie H., van der Voet G. B. The relative potency of amoxycillin and ampicillin in vitro and in vivo. Scand J Infect Dis. 1981;13(4):291–296. doi: 10.3109/inf.1981.13.issue-4.10. [DOI] [PubMed] [Google Scholar]
- Peetermans W. E., Hoogeterp J. J., Hazekamp-van Dokkum A. M., van den Broek P., Mattie H. Antistaphylococcal activities of teicoplanin and vancomycin in vitro and in an experimental infection. Antimicrob Agents Chemother. 1990 Oct;34(10):1869–1874. doi: 10.1128/aac.34.10.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]


