Abstract
Although it has been known for some time that estrogen exerts a profound influence on brain development a definitive demonstration of the role of the classical estrogen receptor (ERα) in sexual differentiation has remained elusive. In the present study we used a sexually dimorphic population of dopaminergic neurons in the anteroventral periventricular nucleus of the hypothalamus (AVPV) to test the dependence of sexual differentiation on a functional ERα by comparing the number of tyrosine hydroxylase (TH)-immunoreactive neurons in the AVPV of wild-type (WT) mice with that of mice in which the ERα had been disrupted by homologous recombination (ERKOα). Only a few ERα-immunoreactive neurons were detected in the AVPV of ERKOα mice, and the number of TH-immunoreactive neurons was three times that of WT mice, suggesting that disruption of the ERα gene feminized the number of TH-immunoreactive neurons. In contrast, the AVPV contains the same number of TH-immunoreactive neurons in testicular feminized male mice as in WT males, indicating that sexual differentiation of this population of neurons is not dependent on an intact androgen receptor. The number of TH-immunoreactive neurons in the AVPV of female ERKOα mice remained higher than that of WT males, but TH staining appeared to be lower than that of WT females. Thus, the sexual differentiation of dopamine neurons in the AVPV appears to be receptor specific and dependent on the perinatal steroid environment.
Keywords: anteroventral periventricular nucleus, hypothalamus, sexual dimorphism, knockout mice, gonadotropin secretion
From an evolutionary perspective, the most adaptive physiological responses are those that ensure successful reproduction. Coordinated sex-specific behaviors and physiological mechanisms have evolved to facilitate reproduction and it is now clear that most are determined by the central action of steroid hormones during brain development. For example, only female rats display a massive surge in gonadotropin secretion in response to treatment of gonadectomized animals with estrogen and progesterone, and exposure of genetic females to high levels of gonadal hormones such as testosterone near the time of birth results in defeminization of the sexually dimorphic pattern of luteinizing hormone (LH) secretion that triggers ovulation (1–4). Abundant evidence from a variety of animal models indicates that steroid hormones secreted by the gonads during development cause profound sex-specific changes in the structure and neurochemistry of certain forebrain regions, including sexually dimorphic regions in the hypothalamus thought to play key roles in mediating the preovulatory secretion of LH (4–7). A particularly important and sexually dimorphic part of neural circuits that regulate the secretion of LH is the anteroventral periventricular nucleus of the preoptic region (AVPV). The AVPV has been shown to play a critical role in transducing hormonal feedback on LH secretion and is required for spontaneous ovulation (8, 9). Consistent with this functional role, the AVPV contains high densities of neurons that express receptors for estrogen and progesterone (10) and implants of antiestrogens cause significant changes in LH secretion (11). Moreover, the results of recent neuroanatomical studies suggest that the AVPV provides direct projections to a subpopulation of gonadotropin releasing hormone neurons in the preoptic region that are thought to participate in the initiation of the preovulatory LH surge (12). The AVPV is unusual among sexually dimorphic nuclei because it is larger in female animals and contains a greater number of dopaminergic neurons in female rats, relative to that of males (13). Treatment of neonatal female rats with testosterone, or lesioning of the AVPV, blocks hormonal induction of an LH surge; testosterone exposure also reduces the number of dopaminergic neurons in the AVPV to that characteristic of male animals (8, 14, 15).
Despite the numerous sexually dimorphic brain structures identified in the rodent forebrain, the molecular mechanisms underlying sexual differentiation remain unclear. Testosterone can influence neural development directly by acting through the androgen receptor (AR), but because testosterone can be converted in the brain to estradiol by the enzyme aromatase, which is itself locally regulated by sex steroid hormones (16), estradiol may contribute to the developmental actions of perinatal androgen in a target region specific way. Moreover, treatment of neonatal rats with estrogen is as effective as testosterone in defeminizing the pattern of gonadotropin secretion, and treatment of female rats with antiestrogen protects female rats from the masculinizing effects of androgen treatment on gonadotropin secretion (2, 17, 18). In addition, administration of estradiol during the first 5 days of life is as effective as testosterone in reducing (defeminizing) the volume of the AVPV (19). The classical estrogen receptor (ERα) is thought to mediate the effects of estrogen on neuronal morphology, survival, or neurotransmitter expression, and during development it is expressed abundantly in sexually dimorphic regions such as the AVPV (refs. 20 and 21 and R.B.S., unpublished observations). By using a variety of pharmacological approaches evidence for ERα-mediated effects of sex steroids on neuronal development has been obtained, but the interpretation of the results of these experiments is complicated by the mixed specificity of the compounds used, their access to the brain, or their differential metabolism in various brain regions (22). Furthermore, both the ERα and AR appear to be expressed in several sexually dimorphic nuclei raising the possibility that sex steroids may contribute to sexual differentiation in such regions by acting through both receptors. Recently a second ER has been isolated and was designated ERβ (23). This new receptor for estrogen is expressed in several sexually dimorphic nuclei, including the AVPV (24), and therefore may be responsible for some of the effects of estradiol on the development of these regions.
That administration of androgen causes a defeminization of gonadotropin secretion was first shown in mice by Barraclough and Leathem (25). Thus, an alternative approach to addressing the problem of steroid specificity in sexual differentiation is to use mice that lack a functional receptor for either androgen or estrogen. The AR gene of the testicular feminized male mouse (Tfm) contains a point mutation that causes a frameshift resulting in a nonfunctional receptor (26). These animals have been used to evaluate the receptor-specific effects of androgen on development and expression of reproductive behavior, but have been less useful in investigating the role of estrogen in sexual differentiation (27). Recently, a line of mice was developed in which the ERα gene was disrupted through homologous recombination (28). These ERα knockout mice (ERKOα) are infertile and show altered reproductive behavior and neuroendocrinological responses, as well as changes in behavior (29–31). Although hormonal regulation of the LH surge has not been examined in ERKOα mice, females tonically secrete elevated levels of LH (32, 33). In addition, immunohistochemical studies indicate that female ERKOα mice have absent or greatly reduced levels of ER immunoreactivity in the hypothalamus (34, 35). In the present study we used male and female ERKOα mice to determine whether the ERα played a role in the sexual differentiation of dopaminergic neurons in the AVPV. Further, we also examined dopaminergic neurons in the AVPV of Tfm mice to test whether the AR is involved in the development of this sexually dimorphic population of neurons.
METHODS
Animals.
Before sacrifice all animals were housed under standard lighting conditions (14:10) with free access to food and water. Three separate experiments were carried out. Experiment 1: Three groups (n = 4) of intact adult mice (8–10 weeks old) were perfused on the same day. Groups included male C57BL/6J mice, female C57BL/6J mice (The Jackson Laboratory), and an age-matched group of ERKOα male mice raised at Taconic Farms that were genotyped by PCR (33) and shipped to the Oregon Regional Primate Center 1 week before perfusion. The ERKOα animals are mixed C57BL/6J and 129/J background; details of the gene disruption have been described in detail (29). Experiment 2: Intact adult female ERKOα mice (n = 3 for each group) were perfused together with wild-type (WT) (+/+) male and female littermates (10–15 weeks old). Experiment 3: The brains of three intact male C57BL/6J mice and four intact Tfm mice (8–10 weeks old; The Jackson Laboratory; C57BL/6J background) were processed for TH immunohistochemistry.
Histochemistry.
Each animal was deeply anesthetized with tribromoethanol and perfused transcardially with a solution of 4% paraformaldehyde in 0.1 M sodium borate buffer (pH 9.5). The brains were processed for immunohistochemistry as described (15) by using an antiserum directed against tyrosine hydroxylase (TH; EugeneTech, Allendale, NJ), which was localized with an affinity-purified goat anti-rabbit IgG conjugated with fluorescein isothiocyanate (BioSource International, Camarillo, CA). Adjacent sections from some of the animals were stained for ER-immunoreactivity (H222 generously provided by Abbott) by using a variation of the avidin-biotin complex system (36, 37) and a commercially available kit (ABC Elite Kit, Vector Laboratories). In addition, we used in situ hybridization, as described previously (38) to compare the number of neurons in the AVPV that express preproenkephalin (PENK) mRNA in WT and ERKOα male and female mice.
Analysis.
The slides were numerically coded to obscure experimental group. Sections stained immunohistochemically for ERα were examined for nuclear staining with conventional transmitted light microscopy. The sections stained for immunofluorescence were examined with fluorescence microscopy and the number of TH-immunoreactive neurons within the AVPV were counted in every third section through the nucleus. The total number of TH-stained neurons in the AVPV of each animal was estimated and Abercrombie’s factor (39) was used to correct for double counting errors. The Kruskal–Wallis one-way analysis of variance with a post hoc multiple comparison (40) was used to identify significant differences between groups in each experiment. A P value of ≤0.05 was defined as significant.
RESULTS
To confirm that ERα was not expressed in the AVPV we stained sections through the nucleus immunohistochemically by using an antibody that recognizes ERα (H222; Abbott). In contrast to the high density of intensely stained ERα-immunoreactive nuclei found in the AVPV of WT male and female mice, only a low density of faintly stained cells could be visualized in the AVPV of ERKOα animals; infrequently a darkly stained nucleus was found (Fig. 1). Although the H222 antibody is directed to the hormone binding domain of the ERα, which shares considerable homology to the hormone binding domain of the ERβ, only low levels of estrogen binding are detected in the AVPV of ERKOα mice (41).
Figure 1.
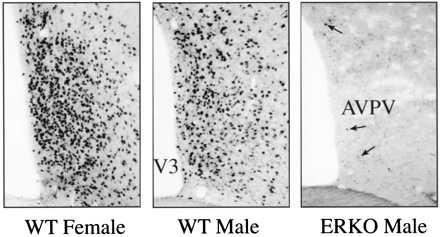
ERα expression in the AVPV: Images of immunohistochemically stained sections through the AVPV to illustrate the density and distribution of neurons that express ERα (arrows) in the AVPV of WT (+/+) female (A), WT (+/+) male (B), or ERKOα male (C) mice (×100).
The number of dopaminergic neurons in the AVPV of male and female mice was compared by counting the number of neurons that were immunoreactive for TH, the rate limiting enzyme for catecholamine biosynthesis. Similar to our previous findings in the rat, the AVPV of female mice contained approximately four times the number of TH-immunoreactive neurons relative to the number detected in the AVPV of male mice (Figs. 2 and 3). The number of TH-stained neurons in the AVPV of male ERKOα mice was 2–3 fold greater than that of WT male mice (P < 0.004) suggesting that the absence of a functional ERα prevented the hormone induced 4-fold reduction in the number of detectable TH-immunoreactive neurons that normally occurs in males during the neonatal period. However, the number of TH-immunoreactive neurons in the AVPV of ERKOα males remained significantly lower than WT females (P ≤ 0.01) suggesting that other factors independent of ERα may be required for full expression of the female phenotype. In contrast to the results obtained in ERKOα males, the number of TH-immunoreactive neurons in the AVPV of Tfm males was not different from that of WT males (Fig. 4), indicating that the differentiation of these cells is not dependent on a functional AR. The number of TH-immunoreactive neurons in the AVPV of ERKOα females remained significantly higher than that of WT males (P ≤ 0.025), and tended to be lower than the number of cells in WT females (Fig. 3), but this difference was not statistically significant. In addition, the intensity of TH staining within individual cells appeared to be reduced (Fig. 2). Neither the size, nor overall morphology of TH-immunoreactive neurons appeared to be different between experimental groups.
Figure 2.
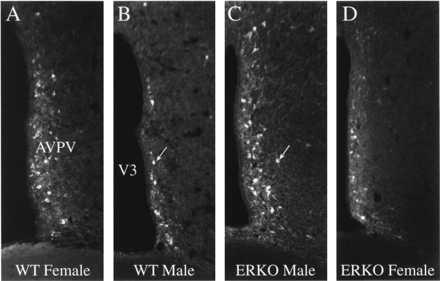
TH expression in the AVPV of the ERKOα mouse. Images of immunohistochemically stained sections through the AVPV to illustrate the density and distribution of neurons that express TH in the AVPV of WT (+/+) female (A), WT (+/+) male (B), ERKOα male (C), or ERKOα female (D) mice. WT females have significantly more TH-immunoreactive neurons relative to that of WT males. Disruption of the ERα in the male (C) prevents the development of this sexually dimorphic pattern of TH expression. Cellular staining is noticeably reduced in the AVPV of the ERKOα female (D), but the number of TH-immunoreactive neurons is similar to that of WT females (see Fig. 3; ×200).
Figure 3.
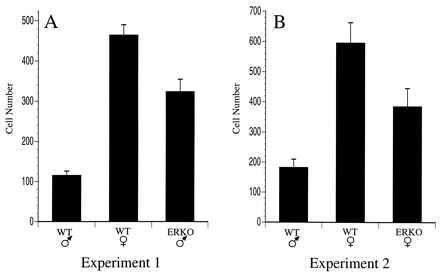
Genetic influences on TH expression in the AVPV. Graphical comparison of the number of TH-immunoreactive neurons detected in the AVPV (unilaterally) of WT and ERKOα mice. (A) The number of TH-immunoreactive neurons in WT (C57BL/6J) male and female mice were compared with that of ERKOα male mice (experiment 1). (B) In a separate experiment. TH staining was compared between WT (+/+) male or female littermates and ERKOα female mice (experiment 2).
Figure 4.
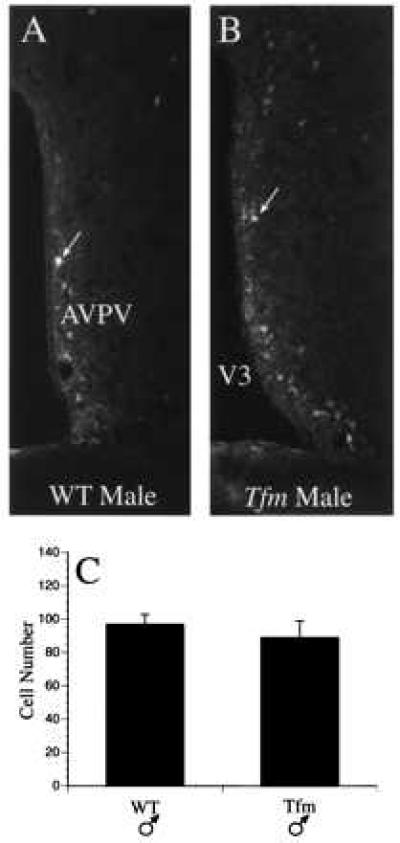
(A and B) Development of dopaminergic neurons in Tfm male mice. (C) Images to illustrate the density and distribution of TH-immunoreactive neurons in the AVPV of WT (C57BL/6J) male (A), and Tfm male (B) mice.
In rats, the number of neurons in the AVPV that express PENK mRNA is quite dimorphic with males having nearly three times as many PENK mRNA-containing neurons in the AVPV as are found in females (38). Therefore, we used in situ hybridization to compare the number of neurons in the AVPV of male and female mice that express PENK mRNA. Although the AVPV of the C57BL/6J mouse contains a high density of PENK mRNA-containing neurons, this population of cells does not appear to be sexually dimorphic, and the number of PENK mRNA-containing neurons was similar in the AVPV of WT and ERKOα animals (Fig. 5).
Figure 5.
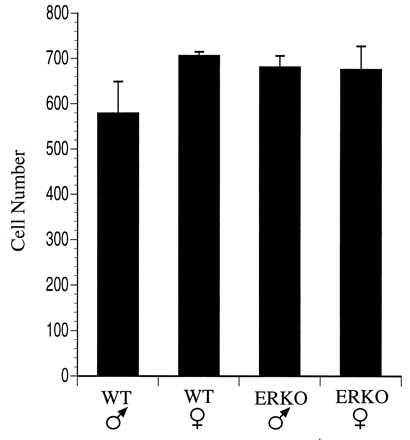
Development of PENK mRNA-containing neurons in the AVPV of mice. Graphical comparison of the number of neurons detected in the AVPV (unilaterally) that express PENK mRNA. Neither a sex difference, nor an effect of ERα gene disruption, was observed.
DISCUSSION
These results represent direct demonstration that the ERα plays an essential role in the sexual differentiation of neurons in the mammalian central nervous system. Moreover, the observation that development of dopaminergic neurons in the AVPV is feminized in male ERKOα mice, but not in Tfm mice, is consistent with the fact that estrogen treatment can defeminize the pattern of gonadotropin secretion, yet nonaromatizable androgens do not (2). Although earlier pharmacological studies have established an important role for estrogen in sexual differentiation, and provide indirect evidence for involvement of the ER in this process, the results of such experiments can be difficult to interpret (see refs. 22 and 42 for reviews). For example, treatment of neonatal mice with the nonaromatizable androgen dihydrotestosterone has been used to selectively activate ARs during the critical period for sexual differentiation, but dihydrotestosterone is rapidly metabolized to relatively ineffective compounds that are only weakly androgenic, so the absence of an effect is not strong evidence for ER action. The androgen agonist R1881 is more stable than dihydrotestosterone and binds with high affinity to ARs, but not ERs. However, R1881 is also a potent progestin that may inhibit androgen action. The use of hormone antagonists to block the actions of steroid hormone receptors is also problematic becuase these compounds may act through multiple mechanisms, and may function as agonists depending on dosage or treatment regimen (42). Another approach has been to use the aromatase inhibitor ATD to block the conversion of testosterone to estrogen, but this compound appears to compete with steroids for ARs (43). Thus, the use of genetically altered animals to dissect the potential contributions of ARs or ERs to brain development provides an alternative experimental approach that complements earlier pharmacological studies. Furthermore, these experiments distinguish between receptor- and nonreceptor-mediated mechanisms. However, the ERKOα mice used in the present study lack functional receptors throughout development, so it is not possible to define when the ERα acts to feminize dopamine cells in the AVPV. Previous studies in rats argue for a critical role for the ERα during the first week of life (2, 17), which corresponds to the period when the sex difference in dopamine neurons in the AVPV develops (44). Alternatively, the male phenotype may depend on a functional ERα in adulthood.
A fundamental concept of neuroendocrine sexual differentiation is that the neural control of gonadotropin secretion appears to be bipotential with respect to expression of either the male or female physiological phenotype: in the absence of high levels of perinatal testosterone the female pattern of gonadotropin secretion occurs regardless of genotype (1–4). Genetic male rats castrated at birth are capable of displaying an LH surge, and genetic females treated with either testosterone or estrogens do not. Our results support this concept because disruption of the ERα feminized the development of dopaminergic neurons in the AVPV of male mice, indicating that the normal pattern of masculine development for this population of neurons is dependent on the presence of a functional ERα. Disruption of the ERα in female mice tended to reduce the number of dopamine neurons in the AVPV, but this effect was not statistically significant. However, we consistently observed an apparent decrease in cellular levels of TH immunoreactivity in ERKOα females, which may reflect a role for the ERα in the full expression of the dopaminergic phenotype in the AVPV of females. Toran-Allerand (18) suggested an active role for estrogen in neural development of both sexes and our results suggest that the ERα may play a role in the maintenance of normal levels of TH expression in AVPV neurons. Alternatively, estradiol tends to suppress TH gene expression in the AVPV (15) and ERKOα females have ≈10-fold higher levels of circulating estradiol compared with that of WT females (31, 45), which may be responsible for the apparent decrease in TH staining intensity. However, such a hormone induced suppression of TH expression must occur in the absence of the ERα and, therefore, be mediated by either the ERβ or a transsynaptic mechanism (46). Consistent with the latter possibility is the observation that only a few dopaminergic neurons in the AVPV appear to express the ERα (47). Moreover, the cAMP response element-binding protein (CREB) plays a critical role in transcriptional control of TH gene expression (48, 49) and estrogen induces CREB phosphorylation in dopaminergic neurons of the AVPV (50).
In addition to influencing the development of dopaminergic neurons in the AVPV, sex steroid hormones also regulate TH gene expression in mature animals (see ref. 15). Because the animals used in the present study were gonadally intact it is possible that the observed differences in numbers of TH-immunoreactive neurons were caused by the different levels of circulating steroid hormones that exist between adult animals. Several lines of evidence argue against this interpretation. First, gonadectomy does not alter the quantitative sex difference in the number of TH-immunoreactive neurons in the AVPV of adult rats (14), which is consistent with the notion that sex steroids act on these neurons primarily during the perinatal period. Second, even the abnormally high levels of estradiol present in ERKOα females (31, 45) did not produce a significant reduction in the number of TH-immunoreactive neurons detected in the AVPV. Finally, the hormonal regulation of TH gene expression in the AVPV of female mice appears to be relatively subtle at the level of protein (R.B.S., unpublished observation), suggesting that the greater numbers of TH immunoreactive neurons in the AVPV of WT females and ERKOα males are caused by a greater number of neurons that display this phenotype.
Our observation that PENK mRNA containing neurons in the AVPV are not affected in the ERKOα mouse suggests that the developmental effects of ERα disruption display cellular specificity in that at least some populations of neurons in the AVPV undergo a normal pattern of development. Although it is unlikely that dopaminergic neurons in the AVPV are the only population of neurons affected in the ERKOα animals, they represent a particularly striking example of receptor-specific sexual differentiation caused by a single genetic event. It is less clear, however, that estradiol exerts its effects on development of TH neurons in the AVPV through a direct action on these neurons, or acts transsynaptically via other hormone sensitive regions that supply strong inputs to the AVPV (7). Equally unclear is whether the ER is influencing the survival of neurons in the AVPV that are capable of expressing TH in detectable levels, or is effecting a lasting change in neurotransmitter phenotype. In rats, cell death in the AVPV occurs in a sexually dimorphic pattern during the first week of life with the period of maximal cell loss being longer in females relative to that of males (19).
Sexual differentiation of gonadotropin secretion is likely because of the developmental actions of sex steroid hormones on multiple neural pathways, and the functional role of dopamine in the regulation of the preovulatory release of LH remains controversial. Nevertheless, the AVPV clearly plays a critical role in mediating the preovulatory LH surge, and the development of dopaminergic neurons in the AVPV correlates with the sexual differentiation of gonadotropin secretion and the ability of gonadotropin-releasing hormone neurons to respond to estradiol. In addition, dopamine neurons in the arcuate nucleus of the hypothalamus, which regulate prolactin secretion in female rats receive a direct sexually dimorphic projection from the AVPV (7, 51). Thus, the present demonstration of ER-dependent sexual differentiation of dopamine neurons in the AVPV may represent a particularly clear example of how sex steroids specify the numbers and transmitter phenotypes of neurons that make up the sexually dimorphic neural pathways that are responsible for coordinating distinct, sex-specific reproductive events necessary for species survival.
Acknowledgments
We thank M. Crabtree for expert technical assistance, and Ms. C. Houser for preparation of the manuscript. This work was supported by National Institutes of Health Grants MH49236, RR00163, and HD18185.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AVPV, anteroventral periventricular nucleus of the hypothalamus; ER, estrogen receptor; TH, tyrosine hydroxylase; WT, wild type; LH, luteinizing hormone; AR, androgen receptor; Tfm, testicular feminized male; ERKOα, ERα knockout; PENK, preproenkephalin.
References
- 1.Harris G W. Philos Trans R Soc London. 1970;259:165–177. doi: 10.1098/rstb.1970.0056. [DOI] [PubMed] [Google Scholar]
- 2.Barraclough C A. Adv Biosci. 1979;25:433–450. [Google Scholar]
- 3.Gorski R A. Prog Brain Res. 1973;39:149–163. doi: 10.1016/S0079-6123(08)64073-X. [DOI] [PubMed] [Google Scholar]
- 4.Gerall A A, Givon L. In: Handbook of Behavioral Neurobiology. Gerall A A, Moltz H, Ward I L, editors. New York: Plenum; 1992. pp. 313–354. [Google Scholar]
- 5.Gorski R A. Can J Physiol Pharmacol. 1985;63:577–594. doi: 10.1139/y85-098. [DOI] [PubMed] [Google Scholar]
- 6.Fink G. Q J Exp Physiol. 1988;73:257–293. doi: 10.1113/expphysiol.1988.sp003145. [DOI] [PubMed] [Google Scholar]
- 7.Simerly, R. B. (1997) Behav. Brain Res., in press.
- 8.Wiegand S J, Terasawa E. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- 9.Popolow H B, King J C, Gerall A A. Physiol Behav. 1981;27:855–861. doi: 10.1016/0031-9384(81)90053-6. [DOI] [PubMed] [Google Scholar]
- 10.Simerly R B, Carr A M, Zee M C, Lorang D. J Neuroendocrinol. 1996;8:45–56. doi: 10.1111/j.1365-2826.1996.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 11.Peterson S L, Barraclough C A. Brain Res. 1989;484:279–289. doi: 10.1016/0006-8993(89)90371-5. [DOI] [PubMed] [Google Scholar]
- 12.Gu G B, Simerly R B. J Comp Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- 13.Simerly R B, Swanson L W, Gorski R A. Brain Res. 1985;330:55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- 14.Simerly R B, Swanson L W, Handa R J, Gorski R A. Neuroendocrinology. 1985;40:501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- 15.Simerly R B. Mol Brain Res. 1989;6:297–310. doi: 10.1016/0169-328x(89)90075-2. [DOI] [PubMed] [Google Scholar]
- 16.Roselli C E, Resko J A. J Steroid Biochem Mol Biol. 1993;44:499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- 17.Gorski R A, Jacobson C D. In: Clinics in Andrology. Kogan S J, Hafez E S E, editors. The Hague: Martinus Nijhoff; 1981. pp. 109–134. [Google Scholar]
- 18.Toran-Allerand C D. Prog Brain Res. 1984;61:63–98. doi: 10.1016/s0079-6123(08)64429-5. [DOI] [PubMed] [Google Scholar]
- 19.Arai Y, Nishizuka M, Murakami S, Miyakawa M, Machida M, Takeuchi H, Sumida H. In: The Development of Sex Differences and Similarities in Behavior. Haug M, Whalen R E, Aron C, Olsen K L, editors. Dordrecht, The Netherlands: Kluwer; 1993. pp. 311–323. [Google Scholar]
- 20.MacLusky N J, Brown T J. In: Neural Control of Reproductive Function. Lakoski J M, Perez-Polo J R, Rassin D K, editors. New York: Liss; 1989. pp. 45–59. [Google Scholar]
- 21.DonCarlos L L, Handa R J. Dev Brain Res. 1994;79:283–289. doi: 10.1016/0165-3806(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 22.Olsen K L. In: The Development of Sex Differences and Similarities in Behavior. Haug M, Whalen R E, Aron C, Olsen K L, editors. Dordrecht, The Netherlands: Kluwer; 1993. pp. 255–278. [Google Scholar]
- 23.Kuiper G G J M, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-A. Proc Natl Acad SciUSA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shughrue P J, Komm B, Merchenthaler I. Steroids. 1996;61:678–681. doi: 10.1016/s0039-128x(96)00222-x. [DOI] [PubMed] [Google Scholar]
- 25.Barraclough C A, Leathem J H. Proc Soc Exp Biol Med. 1954;85:673–674. doi: 10.3181/00379727-85-20989. [DOI] [PubMed] [Google Scholar]
- 26.Charest N J, Zhou Z-X, Lubahn D B, Olsen K L, Wilson E M, French F S. Mol Endocrinol. 1991;5:573–581. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- 27.Olsen K L. In: Sexual Differentiation: Handbook of Behavioral Neurobiology. Gerall A A, Moltz H, Ward I L, editors. Vol. 11. New York: Plenum; 1992. pp. 1–40. [Google Scholar]
- 28.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korach K S, Couse J F, Curtis S W, Washburn T F, Lindzey J, Kimbro K S, Eddy E M, Migliaccio S, Snedeker S M, Lubahn D B, Schomberg D W, Smith E P. Recent Prog Horm Res. 1996;51:159–188. [PubMed] [Google Scholar]
- 30.Ogawa S, Lubahn D B, Korach K S, Pfaff D W. Proc Natl Acad Sci USA. 1997;94:1476–1481. doi: 10.1073/pnas.94.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rissman E F, Wersinger S R, Taylor J A, Lubahn D B. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- 32.Eddy E M, Washburn T F, Bunch D O, Goulding E H, Gladen B C, Lubahn D B, Korach K S. Endocrinology. 1996;137:4796–4805. doi: 10.1210/endo.137.11.8895349. [DOI] [PubMed] [Google Scholar]
- 33.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa S, Quinones-Jenas V, Lubahn D B, Korach K S, Pfaff D W. Soc Neurosci Abstr. 1995;21:191. [Google Scholar]
- 35.Rissman E F, Early A H, Taylor J A, Korach K S, Lubahn D B. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 36.Hsu S M, Raine L. J Histochem Cytochem. 1981;29:1349–1353. doi: 10.1177/29.11.6172466. [DOI] [PubMed] [Google Scholar]
- 37.Hsu S M, Raine L, Fanger H. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 38.Simerly R B. Mol Cell Neurosci. 1991;2:473–484. doi: 10.1016/1044-7431(91)90014-f. [DOI] [PubMed] [Google Scholar]
- 39.Abercrombie M. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- 40.Conover W J. Practical Nonparametric Statistics. New York: Wiley; 1980. [Google Scholar]
- 41.Shughrue, P. J., Lubahn, D. B., Negro-Vilar, A., Korach, K. S. & Merchenthaler, I. (1997) Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 42.Etgen A M. Receptor Mediated Antisteroid Action. Berlin: de Gruyter; 1987. [Google Scholar]
- 43.Kaplan M E, McGinnis M Y. Horm Behav. 1989;23:10–26. doi: 10.1016/0018-506x(89)90071-8. [DOI] [PubMed] [Google Scholar]
- 44.Lorang D, Amara S G, Simerly R B. J Neurosci. 1994;14:4903–4914. doi: 10.1523/JNEUROSCI.14-08-04903.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Couse J F, Curtis S W, Washburn T F, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 46.Armstrong R C, Montminy M R. Annu Rev Neurosci. 1993;16:17–29. doi: 10.1146/annurev.ne.16.030193.000313. [DOI] [PubMed] [Google Scholar]
- 47.Herbison A E, Theodosis D T. Neuroscience. 1992;50:283–298. doi: 10.1016/0306-4522(92)90423-y. [DOI] [PubMed] [Google Scholar]
- 48.Lazaroff M, Patankar S, Yoon S O, Chikaraishi D M. J Biol Chem. 1995;270:21579–21589. doi: 10.1074/jbc.270.37.21579. [DOI] [PubMed] [Google Scholar]
- 49.Lewis E J, Harrington C A, Chikaraishi D M. Proc Natl Acad Sci USA. 1987;84:3550–3554. doi: 10.1073/pnas.84.11.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gu G, Rojo A A, Zee M C, Yu J, Simerly R B. J Neurosci. 1996;16:3035–3044. doi: 10.1523/JNEUROSCI.16-09-03035.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neill J D, Nagy G M. In: The Physiology of Reproduction. Knobil E, Neill J D, editors. Vol. 1. New York: Raven; 1994. pp. 1833–1860. [Google Scholar]


