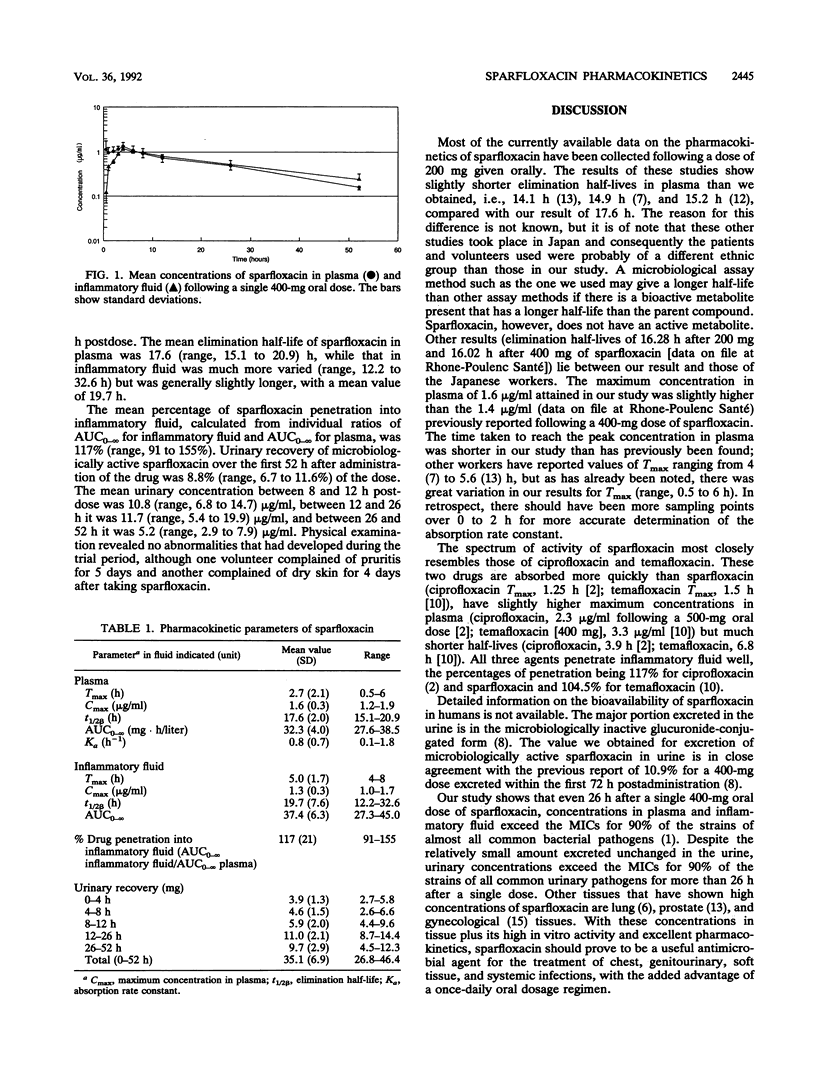
Abstract
A single 400-mg oral dose of sparfloxacin was given to each of six healthy male volunteers, and the concentrations of the drug were measured in plasma, cantharides-induced inflammatory fluid, and urine over the subsequent 52 h. The mean peak concentration in plasma of 1.6 micrograms/ml was attained at a mean time of 2.7 h postdose. The mean peak concentration in inflammatory fluid of 1.3 micrograms/ml was attained at a mean time of 5 h postdose. The mean elimination half-life in plasma was 17.6 h, and that in inflammatory fluid was 19.7 h. The overall penetration into inflammatory fluid was 117%. Urinary recovery within the first 52 h postdose was 8.8% of the administered dose. Our results indicate that a once-daily dosage of sparfloxacin should be adequate to treat systemic infections caused by most common bacterial pathogens.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper M. A., Andrews J. M., Ashby J. P., Matthews R. S., Wise R. In-vitro activity of sparfloxacin, a new quinolone antimicrobial agent. J Antimicrob Chemother. 1990 Nov;26(5):667–676. doi: 10.1093/jac/26.5.667. [DOI] [PubMed] [Google Scholar]
- Crump B., Wise R., Dent J. Pharmacokinetics and tissue penetration of ciprofloxacin. Antimicrob Agents Chemother. 1983 Nov;24(5):784–786. doi: 10.1128/aac.24.5.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomeni R. PHARM--an interactive graphic program for individual and population pharmacokinetic parameter estimation. Comput Biol Med. 1984;14(1):25–34. doi: 10.1016/0010-4825(84)90017-9. [DOI] [PubMed] [Google Scholar]
- Greenblatt D. J., Kock-Weser J. Drug therapy. Clinical Pharmacokinetics (first of two parts). N Engl J Med. 1975 Oct 2;293(14):702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Nakata K., Kurobe N., Kouno K., Sakaguchi Y., Kashimoto S., Yoshida H., Kojima T., Ohue T. In vitro and in vivo antibacterial activities of AT-4140, a new broad-spectrum quinolone. Antimicrob Agents Chemother. 1989 Aug;33(8):1167–1173. doi: 10.1128/aac.33.8.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye K., Shi Y. G., Andrews J. M., Ashby J. P., Wise R. The in-vitro activity, pharmacokinetics and tissue penetration of temafloxacin. J Antimicrob Chemother. 1989 Sep;24(3):415–424. doi: 10.1093/jac/24.3.415. [DOI] [PubMed] [Google Scholar]
- Raoult D., Bres P., Drancourt M., Vestris G. In vitro susceptibilities of Coxiella burnetii, Rickettsia rickettsii, and Rickettsia conorii to the fluoroquinolone sparfloxacin. Antimicrob Agents Chemother. 1991 Jan;35(1):88–91. doi: 10.1128/aac.35.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Gillett A. P., Cadge B., Durham S. R., Baker S. The influence of protein binding upon tissue fluid levels of six beta-lactam antibiotics. J Infect Dis. 1980 Jul;142(1):77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]


