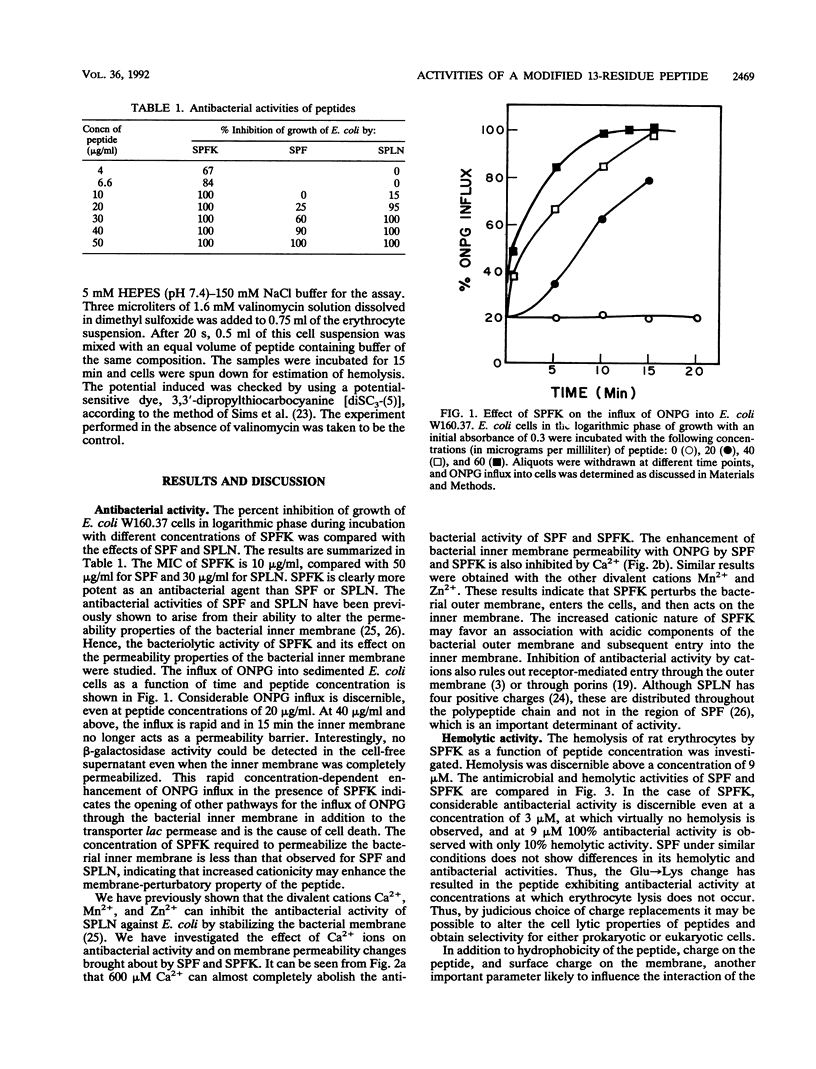
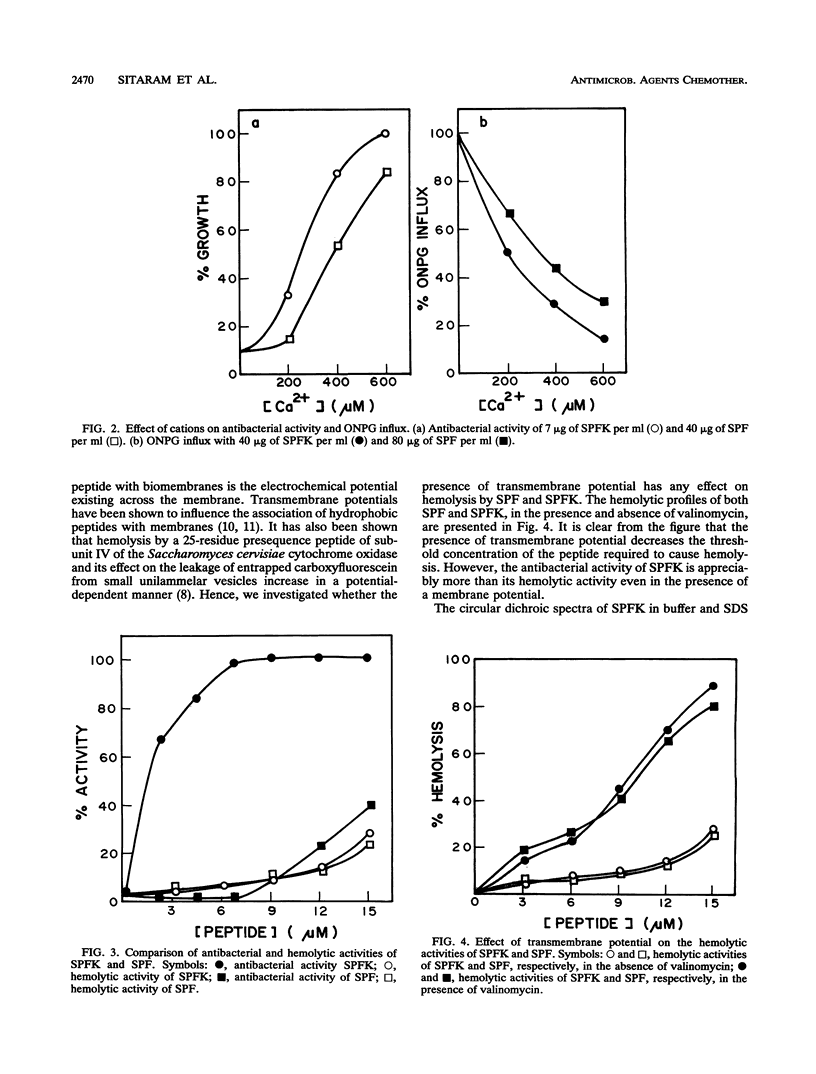
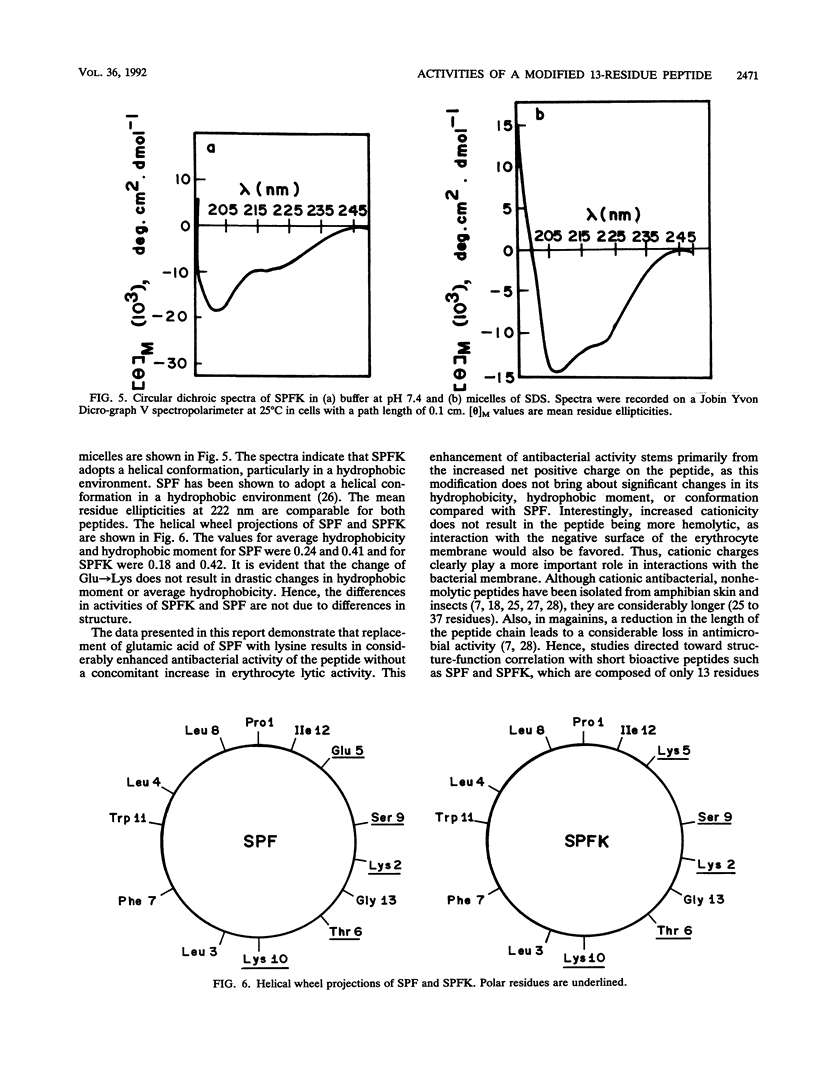
Abstract
A 13-residue peptide corresponding to a hydrophobic segment of the antimicrobial 47-residue peptide seminalplasmin, PKLLETFLSKWIG (SPF), has been shown to have antibacterial and hemolytic activities (N. Sitaram and R. Nagaraj, J. Biol. Chem. 265:10438-10442, 1990). In an effort to get an insight into the structural and charge requirements for these biological activities, an analog of SPF in which Glu has been replaced with Lys has been synthesized and its antibacterial and hemolytic properties have been examined. It has been demonstrated that the analog, SPFK, exhibits potent antibacterial activity at concentrations at which hemolysis does not occur.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu D., Ubach J., Boman A., Wåhlin B., Wade D., Merrifield R. B., Boman H. G. Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992 Jan 20;296(2):190–194. doi: 10.1016/0014-5793(92)80377-s. [DOI] [PubMed] [Google Scholar]
- Argiolas A., Pisano J. J. Bombolitins, a new class of mast cell degranulating peptides from the venom of the bumblebee Megabombus pennsylvanicus. J Biol Chem. 1985 Feb 10;260(3):1437–1444. [PubMed] [Google Scholar]
- Benedetti H., Lazdunski C., Lloubès R. Protein import into Escherichia coli: colicins A and E1 interact with a component of their translocation system. EMBO J. 1991 Aug;10(8):1989–1995. doi: 10.1002/j.1460-2075.1991.tb07728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondelle S. E., Houghten R. A. Hemolytic and antimicrobial activities of the twenty-four individual omission analogues of melittin. Biochemistry. 1991 May 14;30(19):4671–4678. doi: 10.1021/bi00233a006. [DOI] [PubMed] [Google Scholar]
- Boman H. G. Antibacterial peptides: key components needed in immunity. Cell. 1991 Apr 19;65(2):205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Boman H. G., Wade D., Boman I. A., Wåhlin B., Merrifield R. B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989 Dec 18;259(1):103–106. doi: 10.1016/0014-5793(89)81505-4. [DOI] [PubMed] [Google Scholar]
- Chen H. C., Brown J. H., Morell J. L., Huang C. M. Synthetic magainin analogues with improved antimicrobial activity. FEBS Lett. 1988 Aug 29;236(2):462–466. doi: 10.1016/0014-5793(88)80077-2. [DOI] [PubMed] [Google Scholar]
- Clague M. J., Cherry R. J. Comparison of p25 presequence peptide and melittin. Red blood cell haemolysis and band 3 aggregation. Biochem J. 1988 Jun 15;252(3):791–794. doi: 10.1042/bj2520791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruciani R. A., Barker J. L., Zasloff M., Chen H. C., Colamonici O. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3792–3796. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goessens W. H., Driessen A. J., Wilschut J., van Duin J. A synthetic peptide corresponding to the C-terminal 25 residues of phage MS2 coded lysis protein dissipates the protonmotive force in Escherichia coli membrane vesicles by generating hydrophilic pores. EMBO J. 1988 Mar;7(3):867–873. doi: 10.1002/j.1460-2075.1988.tb02886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kini R. M., Evans H. J. A common cytolytic region in myotoxins, hemolysins, cardiotoxins and antibacterial peptides. Int J Pept Protein Res. 1989 Oct;34(4):277–286. doi: 10.1111/j.1399-3011.1989.tb01575.x. [DOI] [PubMed] [Google Scholar]
- Kordel M., Benz R., Sahl H. G. Mode of action of the staphylococcinlike peptide Pep 5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988 Jan;170(1):84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989 Aug;84(2):553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein A. K., Ganz T., Nguyen T. M., Selsted M. E., Lehrer R. I. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J Immunol. 1988 Apr 15;140(8):2686–2694. [PubMed] [Google Scholar]
- Mor A., Nguyen V. H., Delfour A., Migliore-Samour D., Nicolas P. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry. 1991 Sep 10;30(36):8824–8830. doi: 10.1021/bi00100a014. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Nakae T. The outer membrane of Gram-negative bacteria. Adv Microb Physiol. 1979;20:163–250. doi: 10.1016/s0065-2911(08)60208-8. [DOI] [PubMed] [Google Scholar]
- Ojcius D. M., Young J. D. Cytolytic pore-forming proteins and peptides: is there a common structural motif? Trends Biochem Sci. 1991 Jun;16(6):225–229. doi: 10.1016/0968-0004(91)90090-i. [DOI] [PubMed] [Google Scholar]
- Oropeza-Wekerle R. L., Muller S., Briand J. P., Benz R., Schmid A., Goebel W. Haemolysin-derived synthetic peptides with pore-forming and haemolytic activity. Mol Microbiol. 1992 Jan;6(1):115–121. doi: 10.1111/j.1365-2958.1992.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Novotny M. J., Morris W. L., Tang Y. Q., Smith W., Cullor J. S. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992 Mar 5;267(7):4292–4295. [PubMed] [Google Scholar]
- Sims P. J., Waggoner A. S., Wang C. H., Hoffman J. F. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells and phosphatidylcholine vesicles. Biochemistry. 1974 Jul 30;13(16):3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Sitaram N., Krishnakumari V., Nagaraj R. The antibacterial peptide seminal plasmin alters permeability of the inner membrane of E. coli. FEBS Lett. 1992 Jun 1;303(2-3):265–268. doi: 10.1016/0014-5793(92)80535-o. [DOI] [PubMed] [Google Scholar]
- Sitaram N., Kumari V. K., Bhargava P. M. Seminalplasmin and caltrin are the same protein. FEBS Lett. 1986 Jun 9;201(2):233–236. doi: 10.1016/0014-5793(86)80615-9. [DOI] [PubMed] [Google Scholar]
- Sitaram N., Nagaraj R. A synthetic 13-residue peptide corresponding to the hydrophobic region of bovine seminalplasmin has antibacterial activity and also causes lysis of red blood cells. J Biol Chem. 1990 Jun 25;265(18):10438–10442. [PubMed] [Google Scholar]
- Wade D., Boman A., Wåhlin B., Drain C. M., Andreu D., Boman H. G., Merrifield R. B. All-D amino acid-containing channel-forming antibiotic peptides. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4761–4765. doi: 10.1073/pnas.87.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M., Martin B., Chen H. C. Antimicrobial activity of synthetic magainin peptides and several analogues. Proc Natl Acad Sci U S A. 1988 Feb;85(3):910–913. doi: 10.1073/pnas.85.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kroon A. I., de Gier J., de Kruijff B. The effect of a membrane potential on the interaction of mastoparan X, a mitochondrial presequence, and several regulatory peptides with phospholipid vesicles. Biochim Biophys Acta. 1991 Sep 30;1068(2):111–124. doi: 10.1016/0005-2736(91)90199-i. [DOI] [PubMed] [Google Scholar]


