Abstract
The theoretical framework proposed that cortisol and saliva alpha amylase (sAA) reactivitiy are vulnerabilities for antisocial behaviour. These indices of hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenal-medulary (SAM) components of the stress system, respectively, were considered vulnerabilities that also interact with the putative stressful transition of timing of puberty to predispose adolescents toward antisocial behaviour. The sample consisted of 8- to-13-year-old boys and girls (N=135) and a parent. For boys, timing of puberty moderated the association between cortisol and sAA reactivity and antisocial behaviour. Higher cortisol reactivity in later timing boys was related to a composite index of antisocial behaviour and rule-breaking behaviour problems. In contrast, lower sAA reactivity and earlier timing of puberty in boys was related to rule breaking and conduct disorder symptoms. The interaction between timing of puberty and HPA or SAM regulation and timing of puberty in boys suggests that reproductive, neuroendocrine mechanisms may be involved in the extensively documented adverse consequences of off-time pubertal development.
Keywords: Cortisol, salivary alpha amylase, aggressive behavior
1. Introduction
Aggressive and antisocial behaviours may increase in some adolescents for a host of reasons including neuroendocrine and physical maturational changes, increasingly complex social roles, peer influences, and asynchronies between brain development and emotional and behavioural regulation. Recent studies suggest that these problems begin in later childhood and the early adolescent years (Andrews et al., 2003) and become more problematic in mid to late adolescence. The problem is that neuroendocrine developmental transitions are rarely examined as influences on behaviour problems even though major neuroendocrine changes are the mechanisms responsible for pubertal development. Early adolescence is considered an especially stressful and vulnerable period for the expression of antisocial behaviour problems because of the rapid, neuroendocrine, puberty-related changes that are differentially timed for males and females. A promising mechanism linking early vulnerabilities and antisocial behaviour is the putative stress of differential timing of puberty. The purpose of this report was to test hypotheses regarding psychobiological, stress-system vulnerabilities and the interaction between these vulnerabilities and timing of puberty. Antisocial behaviour includes parent reports of externalizing behaviour problems and conduct disorder and oppositional defiant disorder symptoms. The theoretical perspective upon which the study is based integrates multi-level processes: reactivity of the hypothalamic-pituitary-adrenal (HPA) and sympathetic-adrenal-medullary (SAM) response to stress, timing of puberty as indexed by pubertal physical maturation, and antisocial behaviour.
1.1. Psychobiological vulnerabilities and timing of puberty
The specific heuristic model guiding this report proposes, first, that certain psychological risks (vulnerabilities) predispose adolescents to behaviour problems during puberty. This diathesis-stress model assumes that most individuals have some level of predisposing risk factors, or diatheses, for psychosocial problems (Richters & Weintraub, 1990; Abela & D’Alessandro, 2002). The tendency to develop psychosocial problems varies depending on the interaction between the degree to which risk factors are present and the degree of stress experienced by the individual (Richters & Weintraub, 1990; Monroe & Simons, 1991). We expanded this perspective by including HPA and SAM vulnerabilities as risks given the documented relation between these systems and antisocial behaviour (McBurnett et al., 1991; 2001; Raine, 2005; Shirtcliff et al., 2005; Gordis et al., 2006; Granger et al., 2006; 2007). Gordis and colleagues (Gordis, et al., 2006) showed the importance of including both HPA and SAM stress regulation in understanding aggressive behaviour.
Second, the heuristic model includes the concept of transitional stress and describes the stress imposed by ambiguity related to individualization and destandardization of roles that accompany periods of change like puberty. Transitional stress with regard to puberty is directly linked to neuroendocrine and physical-maturational changes and predicts that an increase in adrenal and gonadal hormones and the physical changes of maturation lead to an increase in antisocial behaviours (Graber et al., 1997, 2004; Angold et al., 1999; Stoff & Susman, 2005; Ge et al., 2006). Transitional stress is especially acute if there is an asynchrony between the timing of the physical changes relative to same age peers. Specifically, an earlier or later pubertal transition biases vulnerable individuals toward behaviour problems (Stattin & Magnusson, 1990; Caspi & Moffitt, 1991) in some but not all adolescents.
1.2 Biological Vulnerabilities
A recent theoretical perspective (Bauer et al., 2002) suggests that arousal and recovery from reactivity, as reflected in reactivity to stressors entailing novelty and uncertainty, is representative of how individuals generally regulate their arousal. Difficulty in arousal regulation, including both high and/or sustained responsivity, is considered a vulnerability for behaviour problems (Kagan et al., 1994). Bauer et al., (2002) go on to suggest that differences in arousal may explain differences in susceptibility to the adverse effects of stressors. Variations in arousal, as indexed herein by cortisol and salivary alpha amylase (sAA) reactivity, reflect coordinated, yet distinct axes of the stress response system. Central components of the stress system are the corticotrophin-releasing-hormone (CRH) neurons of the endocrine HPA axis system and the locus coeruleus, norepinephrine system (LC/NE), and the sympathetic-adrenal-medullary (SAM) system (Chrousos & Gold, 1992). Individual differences in both HPA and SAM reactivity are expected to be related to antisocial behaviour.
1.1.1 Cortisol
From the prenatal period onward, regulatory patterns of cortisol are related to psychological functioning. For instance, low basal, maternal prenatal cortisol levels predicted difficult infant temperament in three-year-old children (Susman et al, 2001). At the older end of the life span, higher basal cortisol levels were related to cognitive declines (Seeman et al., 1997; Kudielka et al., 2004) and exaggerated cortisol reactivity (Préville et al., 2008). In addition, non-invasively collected salivary cortisol and its links to emotions and behaviours have been extensively validated in laboratory settings (Kirschbaum et al., 1992; Schwartz et al., 1998; Dickerson & Kemeny, 2004). With regard to antisocial behaviour, lower basal salivary cortisol levels are characteristic of individuals exhibiting disruptive behaviour problems (McBurnett et al., 1991; Susman et al., 1997; McBurnett et al., 2000; Oosterlaan et al., 2005), including oppositional defiant behaviour (van Goozen et al., 1998), conduct disorder (Vanyukov et al., 1993; Pajer et al., 2001;), habitual violence (Virkkunen, 1985), and abuse of others (Bergman & Brismar, 1994). Furthermore, low basal cortisol is both a concurrent correlate and risk factor for future alcohol use (Moss et al., 1995) and externalizing behaviour problems (Shirtcliff et al., 2005). With regard to cortisol reactivity, hyperreactivity characterized the HPA axis response to stressors in some studies (Susman et al., 1997), whereas hyporeactivity was characteristic of antisocial boys in other studies (Fairchild et al., 2008). The precise mechanisms whereby inconsistencies occur are unknown (Dickerson & Kemeny, 2004). Nonetheless, age, dysfunction in the serotonin system, developmental differences between children and adults, composition of the sample and outcome measures are explanations of the inconsistencies (Van Goozen et al., 2007), Given the similarity of our methods with those of previous studies that used a cognitive and social evaluative stressor, we expected that higher cortisol reactivity would be associated with antisocial behaviour.
1.1.2 Salivary Alpha Amylase
A new marker of stress reactivity, sAA is considered a surrogate marker of SAM activity (Granger et al. 2007; Stroud et al, 2009). SAA is an enzyme produced in the oral mucosa and is an assumptive marker of the adrenergic component of the stress response. SAA levels are associated with SAM activity and increase under stressful conditions that are also associated with increases in plasma catecholamines, heart rate, systolic blood pressure, preinjection period (PEP), and cardiac output (Chatterton et al., 1996; Skosnik et al., 2000). However, sAA can be elevated in response to a stressor independently of serum catecholamines and may reflect a general marker of SAM activity (van Stegeren et al., 2006). The validity of salivary sAA as an index of SAM activity additionally is supported by the suppression of sAA secretion after experiencing periods of emotionally charged stressors by the adrenergic blocker propranolol (van Stegeren et al., 2006). Increases in sAA also are evident in response to laboratory stressors that include adolescents (Nater et al. 2005; Gordis et al. 2006; 2008; Stroud et al. 2009). In addition, in four independent studies intra-individual sAA responses to challenge differed distinctly from salivary cortisol responses, particularly, in associations with social behaviour, negative affectivity, cognitive problems, and cardiovascular activity. Similarly, Gordis et al. (2006) show that sAA levels increased post stressor in adolescents. If stress reactivity in the SAM system is parallel to that of HPA reactivity, we expected to see an increase in sAA post stressor to be associated with antisocial behavior.
1.3 Transitional Stress: Timing of puberty
Young adolescents experience multiple changing social contexts (Stroud et al. 2009) as well as rapid neuroendocrine risks. The early years of puberty entail transitioning from a childlike to youthful appearance that is accompanied by changing social demands, role changes and expectations for more mature behaviour. Timing of puberty is primarily under genetic and neuroendocrine control. Those genetic factors that are associated with timing of puberty also may be associated with antisocial behaviour (Comings et al., 2002). Although under genetic control there is a great deal of developmentally significant variability in timing around a normative age. Earlier or later puberty has implications for multiple dimensions of adjustment and behaviour during adolescence and beyond (Stattin & Magnusson, 1990; Ge et al., 1996; 2003; 2006; Graber et al. 2004;). In general, girls who mature earlier than their peers are at elevated risk for emotional and behaviour problems (e.g. Stattin & Magnusson, 1990), whereas for boys, effects of early maturation are variable across studies with some positive (e.g. popularity, self confidence) and some negative outcomes (e.g. delinquency) (Huddleston & Ge, 2003; Collins & Steinberg, 2006; Steinberg et al. 2006). Off time puberty is assumed to be a transitional stressor given that being asynchronous with peers in morphological characteristics can be emotionally arousing.
Shirtcliff and Essex (2008) were the first to show that stressors associated with a developmental transition are associated with different patterns of cortisol reactivity prior to and after the transition. In stable, nonschool transition years, reflected in the cross-sectional analyses, hypoarousal at 5th grade predicted increasing severity of mental health problems and hyperarousal at 7th grade after the school transition had occurred. The diathesis-stress model in the current instance suggests that HPA and SAM vulnerabilities, specifically, cortisol reactivity, will interact with the transitional stressor of timing of puberty to place individuals at risk for antisocial behaviour problems.
Specifically, we hypothesized that earlier or later puberty in combination with higher cortisol or sAA reactivity would be related to antisocial behaviour in young adolescents. It was expected that: (1) Higher reactivity of the stress system based on cortisol or sAA independently would be related to antisocial behaviour. (2) However, timing of puberty was expected to moderate the relation between cortisol and sAA reactivity and antisocial behaviour such that adolescents with high reactivity and off-time maturation would exhibit the most antisocial behaviour, although these relations may vary for boys and girls.
2. Methods
2.1 Sample
Participants were 135 healthy children and adolescents and a parent or caregiver (88.2% mother, 9.6% father and 1.5% grandmother). Girls were age 8, 10 or 12 years (N = 69, M = 10.06 years, SD = 1.64) and boys were age 9, 11, or 13 years (N= 66, M =10.94 years, SD = 1.61). The age difference was designed to include boys and girls at similar stages of pubertal development since girls mature earlier than boys. Day-in-menstrual cycle was controlled as the girls were assessed between day five to nine of their cycle. The racial/ethnic composition of the adolescents appears in Table 1 along with demographic characteristics of the sample. The study was approved by an Institutional Review Board and all procedures were carried out with the adequate understanding and written consent or assent of the parents and adolescents. Parents signed a written consent for themselves and their adolescents. Adolescents signed an assent form.
Table 1.
Means and standard deviations, and percent for demographic characteristics of the sample.
| Girls (69) | Boys (66) | |
|---|---|---|
| Mean (SD) | Mean (SD) | |
| Age | 10.06 (1.644) | 10.94 (1.607) |
| Family SES | 46.2(10.6) | 47.2(10.6) |
| Race | Count(%) | Count(%) |
| White | 62 (89.9) | 59 (89.4) |
| African American | 2 (2.9) | 1 (1.5) |
| Hispanic White/Latino | 3 (4.3) | 2 (3.0) |
| Asian | 1 (1.4) | 1 (1.5) |
| Korean | 1 (1.5) | |
| White, Part Thai (Asian) | 1 (1.5) | |
| White, Arab, Black | 1 (1.5) | |
| White & African American | 1 (1.4) | |
| Tanner Stage | ||
| 1 | 25 | 10 |
| 2 | 13 | 22 |
| 3 | 23 | 17 |
| 4 | 3 | 9 |
| 5 | 2 | 2 |
| Refused | 3 | 6 |
Note: SES was determined by the Hollingshead Scale.
The recruitment strategy consisted of obtaining a list of children’s names from designated ZIP codes from the American Student List (ASL), a commercial enterprise that provided names, addresses, ages and phone numbers of school age children. The comprehensive list of names was generated by ASL from ZIP codes supplied by the investigator. The ZIP codes were chosen to include low income neighborhoods from the county in which the research lab was located and adjacent counties that had easy accessibility to the lab. A letter was mailed to the families on the list. The parents either called the lab to ask about the study or families were telephoned to inquire if the adolescent was interested in participating in the study.
The sample was heterogeneous with regard to occupational status and the employment and education of the participants was representative of the counties from which the sample was drawn. Eligibility criteria were: boys age 9 -, 11 -, or 13 - years; girls age 8 -, 10 -, or 12 - years; not on medications that would interfere with hormone levels (e.g. oral steroids); and free from chronic physical (e.g. diabetes) or serious mental health problems. Names were chosen at random from the list until all names were exhausted. Eighty-five children were enrolled from the ASL list. The remaining participants were obtained from flyers distributed throughout the community and from parental telephone response to e-mails distributed to non faculty, staff at a large university.
2.2 Measures
2.2.1 Vulnerabilities: Cortisol and sAA Reactivity
Stress reactivity was assessed using change in salivary cortisol level and sAA after completion of the Trier Social Stress Test for Children (TSST-C) (Buske-Kirschbaum et al., 1997). The TSST-C is a common method used to elicit an HPA stress response in a laboratory environment and includes both a cognitive and social evaluative challenge. It consists of a story completion task in the presence of two confederate judges. The adolescents were told that the judges would evaluate their story performance in relation to the story of other children their age. Participants are then asked to complete an age-graded serial subtraction, mental arithmetic task. The TSST-C has proven to be a reliable stressor across different age groups and populations (Kirschbaum & Hellhammer, 1994; Young & Nolen-Hoeksema, 2001; Dorn et al., 2003).
Participants were instructed not to eat, drink (except water), or vigorously exercise in the two hours preceding the TSST-C in the lab session. Interview sessions were conducted at M = 3:38 pm, SD 58 minutes. Participants passively drooled into a 5 ml tube for five minutes. Samples were transferred to a −70 °C ultra low freezer until assay.
Cortisol
All samples were assayed using a highly sensitive enzymeimmunoassay (ELISA) specifically designed for use with saliva (Cat. No.1-0102/1-0112 Salimetrics, PA). The test has a range of sensitivity from 0.007 to1.8 µg/dl. Average intra- and interassay coefficients of variation (CV) were 5.34% and 9.86%, respectively. The standard curve was highly reproducible (mean R2= .998). Samples were assayed in duplicate and the average used in analyses.
A total of five cortisol saliva samples was collected, two prior to the TSST-C, and three post-TSST-C. Sample 1 was obtained at Time (T) - 20 minutes; Sample 2 at T - 5 minutes; Sample 3 immediately post TSST-C, T 0; Sample 4 at T + 10 minutes post TSST-C, and Sample 5 at T + 20 minutes post TSST-C. Stress reactivity was determined using area under the curve cortisol in response to the TSST-C using the method of Pruessner and colleagues (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). This score incorporates information across samples into one score allowing for maximizing information while sacrificing few degrees of freedom. The area between ground and the first measure is removed for all time points to capture change from the ground measurement.
Salivary Alpha Amylase
A total of three samples from the five collected and described above were assayed for sAA: Sample 2 (T − 5), Sample 3 (T + 0) and Sample 4 (T + 10). The rationale for not assaying T + 20 was that AA was expected to have returned to baseline (Gordis et al., 2006). Following Granger et al (2006), samples were assayed for sAA using a commercially available kinetic reaction assay without modification to the manufacturer’s protocol (Salimetrics, State College PA). Results are computed in U/mL of sAA. Intrassay variation (CV) computed for the mean of 30 replicate tests was less than 7.5%. Inter-assay variation computed for the mean of average duplicates for 16 separate runs was less than 6%. The slope was used as the index of reactivity of the SAM system, as the SAM system response is relatively short-lived in comparison to the HPA axis, which has a longer sustained activation.
Currently, there is no standard method for measuring change pre-and post stressor in cortisol or sAA and the measure of change used may yield different results. Therefore, all analyses first were run using area under the curve, increase (AUC)I (Pruessner, et al., 2003) and sAA slope as indices of change. In preliminary analyses, the strongest and most consistent findings across models were identified using AUCI for cortisol and slope for sAA. Cortisol values were log transformed but raw values are reported in the tables. Outliers in the distribution (N = 7) were reassigned a value equaling 2 S.D. above or below the mean following the procedure of Kertes and Gunnar (2004). Slope of change was estimated using a measure of dynamic change by regressing the cortisol values on the time of sampling during the stress challenge. Finally, when the interaction between the timing of puberty and cortisol AUCI or sAA was significant or even marginally significant (i.e., p<.10), follow-up testing was conducted to illuminate the directionality of the interaction. This p level was judged appropriate given the documented difficulty in detecting interactions in nonexperimental designs (McClelland & Judd, 1993).
2.2.2. Timing of Puberty
Stage of puberty was assessed by a pediatric research nurse using Tanner criteria of genital and pubic hair stage for boys and breast and pubic hair stage for girls (Marshall & Tanner, 1969, 1970). The nurse first explained the five stages of puberty and showed the adolescent and parent pictures of the stages of sexual maturity. Adolescents and a parent then independently rated the adolescents’ stage of pubertal development based on the pictures. The nurse next did a physical exam for Tanner stage, which included breast palpation as recommended by Kaplowitz and others (Kaplowitz, Oberfield, & the Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society, 1999). If the adolescent did not consent to the physical exam (N = 8), the adolescents’ self rating of his/her stage of pubertal development was substituted for the nurse rating. The correlation between nurse rating and adolescent rating of breast (girls) or genital stage (boys) Tanner stage was, r = .76, p < .01. Breast or genital stage was used in the analysis given that these growth parameters are a direct reflection of gonadal, target tissue exposure to estrogen or testosterone, respectively. Pubertal timing was established by regressing pubertal stage (genital or breast) on chronological age within age and sex groups and calculating a residual timing score for each adolescent. The residuals were used in the analyses (See also Dorn, Susman et al., 2003). A residual indicates how far an adolescent’s stage of pubertal development lies above or below the regression line representing the expected value for his/her age; thus, a negative value indicates later timing (i.e., development less than expected) and a positive value indicates earlier timing. National norms were not used to calculate timing as methods for evaluating Tanner stage vary across studies
2.2.3. Antisocial Behaviour
Child Behaviour Checklist
Externalizing behaviour problems were assessed using the well-standardized Child Behaviour Checklist (CBCL), a norm-referenced rating scale completed by parents (Achenbach, 2001). Parents rate their child's behaviour on a three-point scale for 113 behavioural and emotional problems. Externalizing problem subscales were used in the current analysis: rule breaking (α=0.98, 15 items), aggressive behaviour (α=0.98, 18 items), attention (α=0.80, 8 items), and social problems (α=0.98, 11 items). Raw scales were used in the analyses, although the results were identical when T-scores were used.
Diagnostic Interview Schedule for Children (DISC-IV) (parent)
The DISC-IV is a structured interview used to assess symptoms of psychiatric disorders in children and adolescents in accordance with DSM-IV criteria (APA, 2000; Shaffer et al., 2000). The DISC is a completely preconstructed, computerized interview. Parent reports of DISC-IV symptoms are used in the current analysis given the higher reported correlation of parent scores across time (Piacentini et al., 1999). As psychiatric disorders are infrequently diagnosed in a community sample, symptom counts of oppositional defiant disorder (ODD) and conduct disorder (CD) were used in the current analysis (α ODD = 0.81, α CD = 0.64).
Composite score
A composite score of the four CBCL subscales scores and ODD and CD symptom scores also was calculated [Total Antisocial Behaviour (Total Asb)]. The CBCL subscale scores and DISC-IV symptoms were summed to form a composite score given the correlation between the subscales and symptom scores. For both boys and girls, all subscales contributed comparably to the total score. Cronbach's alpha for the Total Asb score was .74 for boys, and .78 for girls. The CBCL subscales and CD and ODD symptoms scores also were analysed separately based on previous studies showing that the stress response is differentiated in adolescents with varying types of behaviour problems. For instance, inattentive compared to hyperactive adolescents showed a decrease in cortisol post stressor (King et al., 1998; Randazzo et al., 2008).
Analysis Plan
The initial step in the analysis was to identify confounding influences on the relation between vulnerabilities, timing of puberty and antisocial behaviour. First, the effect of prescription and over-the-counter drugs on antisocial behaviour was examined (Hibel, Granger, Kivlighan, Blair, & The Family Life Project Investigators, 2006). There were no significant mean group differences between those “on” versus “off” prescription drugs (e.g. CNS stimulants, psychotropic drugs, antihistamines). In addition, there were no correlations between taking over the counter or prescription drugs and timing of puberty, cortisol or sAA. Second, the relation between the antisocial variables and SES was examined but SES was not correlated with any of the antisocial behaviours. There were no significant mean sex differences on any of the measures with the exception of age, t (df=133) = 3.67, p<.01. The girls were significantly older than the boys because of the design of the study. Tanner stage distribution and demographic characteristics of the sample appear in Table 1.
Means and standard deviations for the measures appear in Table 2. Finally, the hypotheses were tested using hierarchical regression analysis with the total and subscale antisocial behaviours entered into separate equations.
Table 2.
Means and standard deviations for Cortisol AUCI, sAA, SES, timing of puberty and antisocial behaviours for boys and girls.
| Mean | SD | Mean | SD | |
|---|---|---|---|---|
| Boys | Girls | |||
| Cortisol AUCI | 0.86 | 4.80 | 1.00 | 5.10 |
| sAA Slope | 0.67 | 43.7 | 3.66 | 48.00 |
| SES | 47.25 | 10.60 | 46.90 | 12.9 |
| Pubertal Timing (residuals) | .00 | .99 | −.02 | .99 |
| Total Antisocial Behaviour | 10.60 | 10.02 | 10.89 | 10.01 |
| CBCL Behaviour Problems |
||||
| Rule Breaking | .98 | 1.4 | 0.60 | 1.2 |
| Aggressive Problems | 3.72 | 4.40 | 3.30 | 4.10 |
| Social Problems | 1.40 | 2.50 | 1.40 | 2.30 |
| Attention Problems | 1.67 | 2.45 | 1.61 | 2.51 |
| DISC | ||||
| CD symptoms | 1.10 | 1.50 | .71 | 1.40 |
| ODD symptoms | 5.20 | 3.30 | 5.10 | 2.90 |
Note: sAA = salivary Alpha Amylase, AUCI -Area Under the Curve Increase; CD = Conduct Disorder symptoms; ODD = Oppositional Defiant Disorder symptoms
3.0 Results
3.1 Correlations
The correlations between cortisol AUCi, sAA slope, timing of puberty and the antisocial measures appear in Table 3 for boys and girls. For boys, the antisocial variables of Total asb, rule breaking, aggressive behaviour and CD symptoms were significantly and negatively related to sAA. There were moderate to strong correlations between the antisocial indices. For girls, the only significant correlations were those between the antisocial indices.
Table 3.
Correlations between Cortisol AUCi, sAA, timing of puberty and antisocial behaviour.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Cortisol AUC1 | - | −.05 | −.19 | .01 | −.01 | −.02 | −.15 | .02 | .10 | .07 |
| 2. sAA Slope | .16 | - | .25 | .25 | .18 | .15 | .20 | .21 | .22 | .22 |
| 3. Pubertal Timing | −.13 | −.01 | - | .01 | −.03 | −.01 | −.04 | .10 | −.03 | .05 |
| 4. Total Asb | .03 | −.33* | −.01 | - | .93** | .83** | .77* | .81** | .61** | −.02 |
| 5. Aggressive behaviour | .03 | −.29* | .28* | .94** | - | .78** | .67* | .73** | .45** | .03 |
| 6. Social Problems | .24 | −.25‡ | .17 | .73** | .66** | - | .67** | .59** | .34** | .42** |
| 7. Rule-breaking behaviour | −.04 | −.29* | .24 | .70** | .67** | .47** | - | .56** | .39** | .46** |
| 8. Attention Problems | −.01 | −.19 | .11 | .66** | .58** | .46** | .51** | - | .41** | .43** |
| 9. CD symptoms | .01 | −.32* | .17 | .69** | .57** | .45* | .57** | .30* | - | .57** |
| 10. ODD symptoms | −.10 | −.27* | .28* | .65** | .55** | .24 | .30* | .13 | .45** | - |
p=.01;
p=.05;
p = .10; Correlations below the diagonal are for boys (N=66) and correlations above the diagonal are for girls (N=68).
3.2 Hypothesis testing
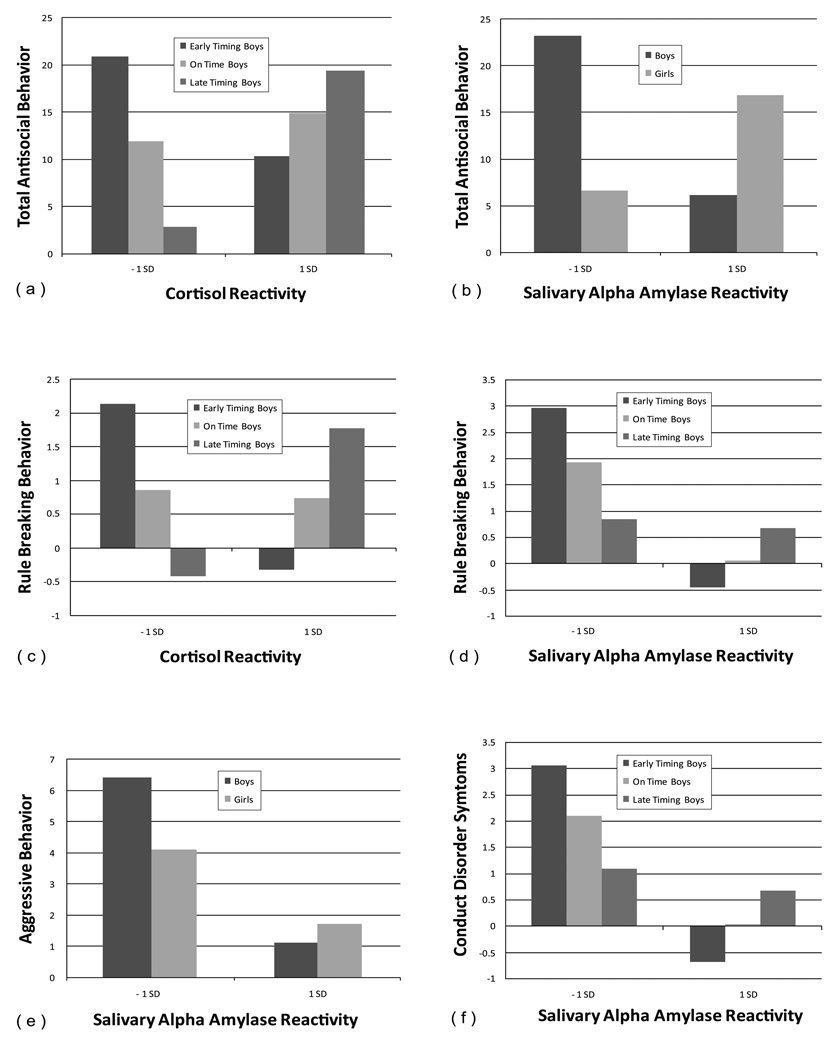
The hypotheses were tested using hierarchical regression analyses with Total Asb problems score and each CBCL subscale and DISC-IV CD and ODD scores entered into separate models. The specific model consisted of the following: Child sex was entered first, then the main effects of cortisol AUCi or sAA slope, followed by timing of puberty. Two-way interactions of pubertal timing and cortisol AUCi or sAA slope, sex and cortisol AUCi or sAA slope and sex and timing were then entered followed by the three way interaction of cortisol AUCi or sAA slope, pubertal timing and sex. The significant interactions predicting parent/guardian-reported antisocial behaviours were probed using procedures described by Aiken and West (1991). Briefly, reactivity or slope was divided into those 1 SD above and below the mean and plotted for earlier and later timing of puberty adolescents. (See also Gordis et al., 2006). The regression statistics appear in Table 4 a and b. The significant interaction plots appear in Figure 1.
Table 4.
| Table 4a. Hierarchical regression of Total ASB and Rule-breaking behaviour on sex, Cortisol AUCI,, timing of puberty, and the interactions of sAA SlopeI, and timing of Puberty and sex, the interactions of sex and timing of puberty, and the interaction of AUCI, timing of puberty and sex. | ||||||
|---|---|---|---|---|---|---|
| Total ASB | Rule-breaking Behaviour | |||||
| β | F | R2 | β | F | R2 | |
| Sex | −.03 | -0.2 | ||||
| Cortisol AUCI | .04 | .09 | ||||
| Timing of puberty | .94** | .94** | ||||
| AUCI*timing of puberty | −.79* | −.97** | ||||
| AUCI *sex | .04 | −.19 | ||||
| Sex*timing of puberty | −.80 | −.81* | ||||
| AUCI*timing*sex | .81 | .86* | ||||
| 1.92‡ | .10 | .290** | .14 | |||
| Table 4b. Hierarchical regression of antisocial behaviours on sex, sAA Slope, timing of puberty, and the interactions of sAA Slope, and timing of puberty and sex, the interactions of sex and timing of puberty, and the interaction of sAA Slope, timing of puberty and sex. | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total ASB | Aggressive Behaviour | Rule-breaking Behavior | Conduct Disorder | Oppositional Defiant Disorder | |||||||||||
| β | F | R2 | β | F | R2 | Β | F | R2 | Β | F | R2 | β | F | R2 | |
| Sex | −.13 | −.10 | −.14 | −.13 | −.06 | ||||||||||
| sAA Slope | -1.00 | −.91** | −.94** | −.98** | −.85‡ | ||||||||||
| Timing of puberty | .53‡ | .63* | .48* | .31 | .59‡ | ||||||||||
| sAA Slope*timing of puberty | −.72‡ | −.51 | −.85* | −.85* | −.14 | ||||||||||
| sAA Slope *sex | 1.06** | .93** | .97** | 1.02** | .88** | ||||||||||
| Sex*timing of puberty | -- | −.58‡ | −.47 | −.31 | −.47 | ||||||||||
| sAA Slope*timing*sex | -- | .41 | .75‡ | .72‡ | .10 | ||||||||||
| 3.07** | .17 | 2.49 | .14 | 2.97** | .16 | 2.84‡ | .16 | 1.92‡ | .11 | ||||||
Note : For Total ASB R2 = .003 for Step 1, ΔR2 = .00 for Step 2, ΔR2 ..06 for Step 3, ΔR2 .011 for Step 4 ΔR2 .003 for Step 5 ΔR2 .021 for Step 6 and ΔR2 .031 for Step 7. For Rule-breaking Behaviour ASB R2 = .010 for Step 1, ΔR2 = .009 for Step 2, ΔR2 .017 for Step 3, ΔR2 .040 for Step 4 ΔR2 .014 for Step 5 ΔR2 .020 for Step 6 and ΔR2 .034 for Step 7;
p<.01
p<.05;
P<.10.
Note : Only significant models are presented; **=p<.01 *= p<.05; = P<.10.
Note : For Total ASB R2 = .015 for Step 1, ΔR2 = .000 for Step 2, R2 =.019 for Step 3, ΔR2 = .008 for Step 4Δ R2 =.092 for Step 5, Δ R2 =.020 for Step 6 and Δ R2 =.019 for Step 7; For Aggressive Behaviour R2 = .007 for Step 1, ΔR2 = .002 for Step 2, ΔR2 =.018 for Step 3Δ̣,R2 =.013 for Step 4Δ R2 =.067 for Step 5 ΔR2 =.028 for Step 6 andΔ R2 =.008 for Step 7; For Rule-breaking Behaviour ASB R2 = .016 for Step 1, ΔR2 = ..001 for Step 2, ΔR2 =.012 for Step 3, Δ̣R2 =.016 for Step 4 ΔR2 =.075 for Step 5 ΔR2 =.018 for Step 6 and ΔR2 =.027 for Step 7; For Conduct Disorder R2 = .015 for Step 1, ΔR2 =.001 for Step 2, ΔR2 =.007 for Step 3, Δ̣R2 =.016 for Step 4Δ R2 =.090 for Step 5 ΔR2 =.009 for Step 6 and ΔR2 =.024 for Step 7; For Oppositional Defiant Disorder R2 = .003 for Step 1, ΔR2 = .000 for Step 2, ΔR2 =.033 for Step 3, ΔR2 =.001 for Step 4 ΔR2 =.058 for Step 5 ΔR2 =.019 for Step 6 and ΔR2 =.000 for Step 7;
p<.01
p<.05;
P<.10
Figure 1.
Interactions between cortisol reactivity and salivary alpha amylase and timing of puberty.
Total Asb
The three-way interaction of cortisol AUCi, pubertal timing and sex predicted unique variance in Total Asb. The post hoc tests showed that this interaction was significant for boys only. We plotted the slope of the relation between cortisol AUCi and Total Asb at 1 SD above and below the mean. At 1 SD above the mean on timing of puberty, the simple slope of the relation between timing of puberty and Total Asb slope was null whereas at one SD below the mean, the relation was significant, B=1.04, t=2.04, p=0.05. Antisocial boys were characterized by later timing of pubertal development as well as higher cortisol reactivity.
For sAA, there was a significant two-way interaction between sex and sAA. We plotted the slope of the interaction for sAA and Total Asb at 1 SD above and below the mean. At 1 SD below the mean, the relation was negative and significant, B=−10, t = 2.57, p=01. Boys with lower sAA reactivity exhibited more antisocial behaviours. For girls, the relation was not significant.
Rule Breaking
The three-way interaction of cortisol reactivity by pubertal timing by sex was significant. Further probing of these interactions indicated that the interaction was significant for boys only. We plotted the slope of the relation between cortisol AUCi and rule breaking behaviours at 1 SD above and below the mean for rule breaking behaviours. At one standard deviation below the mean the relation was negative and significant, B=−0.15, t=−2.40, p=0.02, whereas at one standard deviation above the mean the relation was positive and significant, B=−14, t = 2.29, p =.03. Adolescent boys with off-time pubertal timing exhibited more rule breaking behaviour problems. Earlier maturing boys with lower cortisol reactivity and later maturing boys with higher cortisol activity exhibited the most rule breaking behaviour.
For sAA there was a three-way interaction between sAA, timing of puberty and sex. Further probing of the interaction showed that the interaction effect was for boys only. At one standard deviation above the mean on timing of puberty the relation was positive and significant, B= −02, t =3.13, p =002 whereas at one standard deviation below the mean the relation was null. Earlier timing boys with lower sAA reactivity exhibited more rule breaking behaviour than later timing boys
Aggressive Behaviours
For cortisol, there were no main effects or significant interactions for sex, timing or AUCi for aggressive behavior problems.
For sAA there was a significant interaction of sAA slope by sex and sex by timing of puberty. We plotted the slope of the relation between sAA and aggressive behaviours at 1 SD above and below the mean for girls and boys. At one standard deviation below the mean the relation was negative and a trend to significant, B= −0.03, t= −2.24, p=0.09, whereas at one standard deviation above the mean the relation was null. Aggressive behaviour was highest in boys with lower sAA reactivity but the same was not true for girls.
For the sex by timing interaction, in boys, at 1 SD above the mean the relation between timing and aggressive behaviours was positive and significant, B= −.03, t= −2.24, p=0.03. Earlier timing boys were more aggressive than later timing boys and girls.
Attention Problems
No main or interactive effects for cortisol AUCi or sAA and aggressive behavior were found.
Social Problems
No main or interactive effects of cortisol AUCi or sAA were found.
Oppositional Defiant Disorder Symptoms
The two-way interactions of sex and sAA and sex and pubertal timing each predicted unique variance in ODD symptoms. Further probing of the two way interactions revealed non-significant slopes on the post hoc tests likely related to a weak overall interaction.
Conduct Disorder Symptoms
Cortisol reactivity was not related to conduct disorder symptoms.
For sAA the three-way interaction of sAA, pubertal timing and sex and sAA slope predicted unique variance in parent-reported conduct disorder symptoms. Further probing of the interaction indicated that this interaction was significant for boys. We plotted the slope of the relation between sAA and conduct disorder symptoms at 1 SD above and below the mean and at the mean of pubertal timing. For boys, at one SD above the mean of timing of puberty, the simple slope of the relation between timing of puberty and CD symptoms was significant, B= −0.02, t= −3.54, p= 0.001, whereas at one SD below the mean, the relation was null. Earlier timing of puberty in adolescent boys with lower sAA reactivity was associated with more conduct disorder symptoms.
4. Discussion
The psychobiological moderators involved in aggressive behaviour have been of interest for decades. We sought to advance the literature on this socially important issue by testing hypotheses based on a theoretical model of the interaction of the psychobiological vulnerabilities of cortisol and sAA and off-time puberty and antisocial behaviour. Three primary issues are highlighted by the findings. First, the relations between cortisol and sAA and antisocial behaviour were qualified by the timing of puberty. Second, the direction of the interactions between HPA and SAM activity and timing of puberty were not consistent across earlier or later timing of puberty and indices of antisocial behaviour. Third, the findings were more prominent for boys than for girls. The contribution of the current findings is that they point to the developmental transition of puberty as an important moderator of both HPA and SAM reactivity. The current findings are the first to show the moderating effects of timing of puberty on biological vulnerabilities.
The current findings show that both hypo- and hyper reactivity were related to antisocial behaviour in interaction with timing of puberty. The majority of the literature shows that hypoarousal of basal cortisol is related to delinquent and aggressive behaviour (Pajer et al., 2001; McBurnett et al, 2005; Oosterlaan et al., 2005; van Bokhoven et al. 2005). However, the relation between cortisol reactivity and antisocial behaviour tends to be mixed as discussed above. Susman et al., (1997) showed that hyperarousal after a laboratory challenge predicted aggressive behaviours one year later. In contrast, Klimes-Dougan et al., (2001) reported no hypo- or hyperarousal in HPA axis activity in children with conduct disorder symptoms. Overall, higher cortisol reactivity is considered a risk for maladjustment (Boyce & Ellis, 2005) and chronic HPA activation can lead to increasing allostatic load (McEwen, 2004) that, in turn, predisposes an individual to early morbidity or mortality. Much less is known about hypo- and hyper sAA reactivity but our findings indicate that the more antisocial adolescents are characterized by hypo reactivity in SAM activity in interaction with earlier puberty. A recent report also indicated that hypo and hyper reactivity may be influenced by the transitions in the contexts of development, specifically, the transition to a new school (Shirtcliff & Essex, 2008). In young adolescents, stress responses may not yet be canalized and biological and social changes may acutely influence arousal and recovery from stressors as the young adolescents cope with their physically changing bodies.
The findings are novel with regard to the puberty transition yet they coincide with the literature showing that timing of puberty also is moderated by contextual vulnerabilities, like family environment and adjustment problems (Duncan et al.,1985; Stattin & Magnusson, 1990; Susman et al., 1991; Caspi & Moffitt, 1991; Ge et al., 2002; 2003; Graber et al., 1997; 2005; Hayward, 2003;). With regard to cortisol, a notable difference between the current and previous psychsocial findings is that later timing and higher cortisol reactivity was more highly related to antisocial behaviour than earlier timing. Earlier timing of puberty is usually associated with maladjustment, although timing of puberty has not been examined simultaneously in relation to HPA and SAM vulnerabilities. Later maturing adolescents are smaller in size than their peers who have already begun pubertal development whereas later maturers are still childlike in appearance, which may contribute to their overall stress levels.
Of note is that different antisocial behaviours were related to cortisol and sAA. Specifically, cortisol reactivity interacted with timing of puberty to predict total antisocial behaviour and rule breaking problems but cortisol reactivity was not related to the other indices of antisocial behaviour. As cortisol reactivity increased so did antisocial behaviours and rule breaking in later timing of puberty adolescents. Later-developing boys are still of an age where appearing childlike could be highly stressful because they are treated according to their stature rather than their chronological age (Johnson & Collins, 1988). They may also be excluded from engaging in same-age peers activities (e.g. coed dancing), which may have profound sequelae for psychological development (Lindfors et al., 2007). It should also be noted that no interaction effects were found for social, attention and aggressive behaviour problems and conduct disorder symptoms. We propose that this lack of findings may be attributable to the relatively small sample size and related low power.
The findings for the interaction between sAA and timing of puberty and antisocial behaviour problems are quite different from the findings for cortisol reactivity. The interaction of lower sAA reactivity and earlier timing of puberty was related to more antisocial behaviour. Few studies exist with which to compare these findings but one study with maltreated youth, showed that lower levels of sAA reactivity were related to higher aggression ratings (Gordis et al., 2006). In older adolescents and adults lower SAM stress regulation is characteristic of those who exhibit antisocial behaviour or have experienced stressful events (Musante et al. 2000; Raine, 2005). Low SAM reactivity holds across antisocial behaviour ranging from minor oppositional behaviour to criminality (Raine et al., 1990; 1997; Raine, 2005). Low SAM activity can be interpreted as low arousal potentially accompanied by low empathic or altruistic inclinations, low emotion recognition potential and the tendency to inflict harm and disinhibition from engaging in aggressive behaviour. Finally, in the case of stressful events, youth who reported experiencing stressful events showed smaller increases in SAM activity indexed by blood pressure (both systolic and diastolic) and heart rate to a car-driving simulation (Musante et al., 2000). Higher sympathetic arousal is speculated to be protective against the development of aggression (Raine, 2005). In brief, the negative direction of the relationship between sAA reactivity and antisocial behaviour in this report is consistent with a growing body of literature indicating that low SAM reactivity is related to externalizing behaviour problems (Magnusson, 1986; Raine, et al., 1990). Susman (2006) proposed the attenuation hypothesis as an explanation for the link between hypoarousal and antisocial behaviour. The attenuation hypothesis suggests that chronic stressful and traumatic experiences lead to down regulation of the stress system as an adaptational strategy to deal with metabolic parameters of chronic stressors thereby avoiding stress-system collapse.
There is not yet enough basis in the literature to say definitely the precise etiology of the inconsistencies related to hypo and hyperactivity. Heim et al. (2008) suggest that early trauma can increase sensitivity of the cortisol response leading to both hypo and hyper reactivity depending on the nature of early experiences. Van Goozen et al (2007) further suggests that parenting strategies, gender, the type of stress involved, and the timing or duration of the stressor influences both HPA and ANS activity in the direction of hypo and hyper reactivity. Inconsistencies may also occur depending on whether the aggressive behaviour is overt or covert. Our findings indicate that the development transitions is highly critical in the hypo and hyperactivity of cortisol and sAA activity. If puberty occurs at a younger age, then cortisol reactivity is accentuated whereas if puberty occurs later sAA reactivity is likely to be attenuated. It also is of note that the CBCL and DISC were both administered even though the subscales in specific domains (e.g. externalizing behaviors and conduct disorder symptoms) tend to be highly correlated. However, the interactions between cortisol and sAA and timing of puberty and antisocial behavior were not always similar across measures indicating that the two arms of the stress system are antisocial-behavior specific. Similarly, Gordis et al. (2008) showed that cortisol and sAA reactivity are not symmetrical in their interactions across different samples.
Sex differences in the interaction between vulnerabilities and timing of puberty were noteworthy. The absence of findings for girls is not inconsistent with other studies of hormones and antisocial behaviour showing few associations in girls (e.g., Susman et al., 2007). Even gonadal hormones are more weakly associated with antisocial behaviour in girls than in boys (Susman et al., 1987; Shirtcliff et al., 2005;). But lack of findings for girls are not unique to this study as sex differences in cortisol reactivity are equivocal in humans (Kudielka et al., 2004 a, 2004 b; Kudielka & Kirschbaum, 2005; Uhart et al., 2006). Nonetheless, there was a significant two-way interaction between sex and sAA with boys being higher than girls on aggressive behaviour problems and ODD symptoms. Recently discovered sexual dimorphism within developing brain structures, such as the amygdala and hippocampus, may account for sex differences in both stress reactivity and timing of puberty (Giedd et al., 1997). Thus, a sex-specific analysis appears essential for identifying psychobiological mechanisms involved in antisocial behaviour.
A limitation of the study is that our sample is relatively small, the R2 is relatively small. Significant results pertaining to HPA axis activity were mainly found for rule breaking and conduct disorder symptoms. Hence, we show several negative findings indicating that the few positive regressions models may be chance findings. Given the small sample size we had little power to detect the hypothesized interactions as interactions require more power than main effects. However, given the novelty of the research on the psychobiology of timing of puberty, it is prudent to balance the probability of Type 1 and II errors. Finally, the sample was not acquired through random selection of adolescents making it difficult to replicate the findings. The use of flyers to recruit adolescents adds to the problem of replicating the findings. These findings need to be replicated in a larger sample but they are promising in terms of identifying stress-related HPA and SAM biomarkers as moderators of the timing of puberty.
We propose that regulation of externalizing antisocial behaviour is inherently associated with regulation of stress responses. Higher cortisol reactivity and externalizing behaviour in the later timing adolescents and lower SAA reactivity in the earlier timing adolescents are interpreted as difficulty in cortisol and sAA regulation as a consequence of the stress of off-time pubertal maturation. Adolescents then act out as an adaptational strategy for dealing with unfamiliar emotions, peer pressures and adult expectation that exceed underdeveloped competencies. In conclusion, studies that examine the role of cortisol reactivity and sAA in behaviour will be enriched by the inclusion of timing of puberty as a critical moderator of antisocial behavior.
Acknowledgment
This research was supported by National Institute of Mental Health Grant RO1 58393-03; National Institutes of Health, General Clinical Research Center, Grant M01 RR 10732; and the Shibley Endowment, The Pennsylvania State University.
Role of Funding Source.
Support for the research was provided by the National Institute of Mental Health, RO1 58393-03, the National Institutes of Health, General Clinical Research Center (M01 RR 10732), and the Jean Phillips Shibley Endowment, The Pennsylvania State University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
All authors contributed to and have approved the final manuscript.
Dr. Susman designed the study and wrote the first draft of the majority of the manuscript.
Dr. Dockray did the majority of the statistical analyses.
Dr. Granger provided the expertise for completing the assay and assisted with writing the manuscript.
Dr. Blades assisted with the statistical analyses.
Ms. Heaton collected data and assisted with writing the manuscript.
Mr. Randazzo collected data and assisted with the statistical analyses.
Dr. Dorn assisted with designing the study and writing of the manuscript.
Conflict of Interest.
Dr. Douglas Granger is President and Founder of Salimetrics, LLC (State College, PA). All other authors declare that they have no conflicts of interest.
Contributor Information
Elizabeth J. Susman, Department of Biobehavioral Health The Pennsylvania State University 314 Health and Human Development East University Park, PA 16802 Office:(814) 863-2281 FAX: (814) 863-7525 esusman@psu.edu
Samantha Dockray, Epidemiology & Public Health, University College London 1-10 Torrington Place London WC1E 6BT Office+44 (0)20 7679 1805 Fax +44 (0)20 7813 0242 s.dockray@ucl.ac.uk.
Douglas A Granger, Department of Biobehavioral Health The Pennsylvania State University 314 Health and Human Development East University Park, PA 16802.
Keeva T. Blades, Department of Biobehavioral Health The Pennsylvania State University 314 Health and Human Development East University Park, PA 16802
William Randazzo, 387 Townhouse The Pennsylvania State University College of Medicine/Milton S Hershey Medical Center, Hershey, PA 17033, USA.
Jodi A. Heaton, Department of Biobehavioral Health The Pennsylvania State University 314 Health and Human Development East University Park, PA 16802
Lorah D. Dorn, Division of Adolescent Medicine University of Cincinnati College of Medicine 3333 Burnet Avenue Cincinnati, OH 45229-3039
References
- Abela JR, D'Alessandro DU. Beck's cognitive theory of depression: a test of the diathesis-stress and causal mediation components. Br. J. Clin. Psychol. 2002;41:111–128. doi: 10.1348/014466502163912. [DOI] [PubMed] [Google Scholar]
- Achenbach TM. ASEBA, Child Behaviour Checklist for Ages 4–18 (CBCL/4–18) Burlington, VT: University of Vermont; 2001. [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA: US: Sage Publications, Inc; 1991. [Google Scholar]
- Andrews JA, Tildesley E, Hops H, Duncan SC, Severson HH. Elementary School Age Children's Future Intentions and Use of Substances. J. Clin. Child Adolesc. Psychol. 2003;32:556–567. doi: 10.1207/S15374424JCCP3204_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman C. Pubertal changes in hormone levels and depression in girls. Psychol. Med. 1999;29:1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bauer AM, Quas JA, Boyce WT. Associations between physiological reactivity and children's behaviour: Advantages of a multisystem approach. J. Dev. Behav. Pediatr. 2002;23:102–113. doi: 10.1097/00004703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Bergman B, Brismar B. Hormone levels and personality traits in abusive and suicidal male alcoholics. Alcohol Clin. Exp. Res. 1994;18:311–316. doi: 10.1111/j.1530-0277.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Boyce W, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev. Psychopathol. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Psych D, Wustmans A, Kirschbaum C, Rauh W, Hellhammer D. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom. Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Individual differences are accentuated during periods of social change: The sample case of girls at puberty. J. Pers. Soc. Psychol. 1991;61:157–168. doi: 10.1037//0022-3514.61.1.157. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Vogelsong KM, Lu YC, Ellman AB, Hudgens GA. Salivary alpha-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16:433–448. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. J. Am. Med. Assoc. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Collins WA, Steinberg L. Adolescent development in interpersonal context. In Interpersonal and Societal Contexts (Smetana, J. G., Campione-Barr, N., and Metzger, A.) Defining features of adolescence and psychosocial development. Annu. Rev. Psychol. 2006;57:255–284. doi: 10.1146/annurev.psych.57.102904.190124. [DOI] [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Johnson JP, MacMurray JP. Parent-daughter transmission of the androgen receptor gene as an explanation of the effect of father absence on age of menarche. Child Dev. 2002;73:1046–1051. doi: 10.1111/1467-8624.00456. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute Stressors and Cortisol Responses: A Theoretical Integration and Synthesis of Laboratory Research. Psychol. Bull. 2004;130:355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Campo JC, Thato S, Dahl RE, Lewin D, Chandra R, Di Lorenzo C. Psychological comorbidity and stress reactivity in children and adolescents with recurrent abdominal pain and anxiety disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2003;42:66–75. doi: 10.1097/00004583-200301000-00012. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Ponirakis A. Pubertal timing and adolescent adjustment and behaviour: Conclusions vary by rater. J Youth Adolesc. 2003;32:157–167. [Google Scholar]
- Duncan P, Ritter P, Dornbusch S, Gross R, Carlsmith J. The effects of pubertal timing on body image, school behaviour, and deviance. J. Youth Adolesc. 1985;14:227–236. doi: 10.1007/BF02090320. [DOI] [PubMed] [Google Scholar]
- Fairchild G, van Goozen SH, Stollery SJ, Brown J, Gardiner J, Herbert J, Goodyer IM. Cortisol Diurnal Rhythm and Stress Reactivity in Male Adolescents with Early-Onset or Adolescence-Onset Conduct Disorder. Biol. Psychiatry. 2008;64:599–606. doi: 10.1016/j.biopsych.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Conger RD, Elder GH., Jr. Coming of age too early: Pubertal influences on girls' vulnerability to psychological distress. Child Dev. 1996;67:3386–3400. [PubMed] [Google Scholar]
- Ge X, Brody GH, Conger RD, Simons RL, Murry V. Contextual amplification of pubertal transitional effect on African American children's problem behaviours. Dev. Psychol. 2002;38:42–54. doi: 10.1037//0012-1649.38.1.42. [DOI] [PubMed] [Google Scholar]
- Ge X, Kim IJ, Brody GH, Conger RD, Simons RL, Gibbons FX, Cutrona CE. It's about timing and change: Pubertal transition effects on symptoms of major depression among African American youths. Dev. Psychol. 2003;39:430–439. doi: 10.1037/0012-1649.39.3.430. [DOI] [PubMed] [Google Scholar]
- Ge X, Brody GH, Conger RD, Simons RL. Pubertal Maturation and African American Children’s Internalizing and Externalizing Symptoms. J Youth Adolesc. 2006;35:528–537. [Google Scholar]
- Giedd J, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacol. Biol. Psychiatry. 1997;21(8):1185–1201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Gordis E, Granger D, Susman EJ, Trickett P. Symmetry between Salivary Cortisol and a-Amylase Reactivity to Stress: Relation to Aggressive Behaviour in Adolescents. Psychoneuroendocrinology. 2006;31:976–987. doi: 10.1016/j.psyneuen.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm Behav. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JA, Brooks-Gunn J, Archibald AB. Links Between Girls' Puberty and Externalizing and Internalizing Behaviours: Moving from Demonstrating Effects to Identifying Pathways. In: Stoff DM, Susman EJ, editors. Developmental psychobiology of aggression. New York: Cambridge University Press; 2005. pp. 87–113. [Google Scholar]
- Graber JA, Lewinsohn PM, Seeley JR, Brooks-Gunn J. Is psychopathology associated with the timing of pubertal development? J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:1768–1776. doi: 10.1097/00004583-199712000-00026. [DOI] [PubMed] [Google Scholar]
- Graber JA, Seeley JR, Brooks-Gunn J, Lewinsohn PM. Is Pubertal Timing Associated With Psychopathology in Young Adulthood? J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:718–726. doi: 10.1097/01.chi.0000120022.14101.11. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Blair C, El-Sheikh M, Mize J, Lisonbee JA, Buckhalt JA, Stroud LR, Handwerger K, Schwartz EB. Integrating the measurement of salivary α-amylase into studies of child health, development, and social relationships. J. Personal Soc. Relationships. 2006;23:267–290. [Google Scholar]
- Granger DA, Kivlighan KT, El-Sheikh M, Gordis E, Stroud LR. Salivary α-Amylase in Biobehavioral Research. Ann. N.Y. Acad Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Hayward C. Gender differences at puberty. New York: Cambridge University Press; 2003. [Google Scholar]
- Heim CD, Newport J, Mletzko T, Miller AH, Nermroff CB. The link between childhood trauma and depression: Insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Kivlighan KT, Blair C The Family Life Project Investigators. Individual differences in salivary cortisol: Effects of common over the counter and prescription medications in infants and their mothers. Horm. Behav. 2006;50:293–300. doi: 10.1016/j.yhbeh.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Huddleston J, Ge X. Boys at puberty: Psychosocial implications. In: Hayward C, editor. Gender Differences at Puberty. Cambridge: Cambridge University Press; 2003. pp. 113–134. [Google Scholar]
- Johnson BM, Collins WA. Perceived maturity as a function of appearance cues in early adolescence: Ratings by unacquainted adults, parents, and teachers. J. Early Adolesc. 1988;8:357–372. [Google Scholar]
- Kagan J, Snidman N, Arcus D, Reznick JS. Galen's prophecy: Temperament in human nature. New York: Basic Books Inc; 1994. [Google Scholar]
- Kaplowitz PB, Oberfield SE the Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: Implications for evaluation and treatment. Pediatrics. 1999;104:936–941. doi: 10.1542/peds.104.4.936. [DOI] [PubMed] [Google Scholar]
- Kertes DA, Gunnar M. Evening Activities as a Potential Confound in Research on the Adrenocortical System in Children. Child Dev. 2004;75:193–204. doi: 10.1111/j.1467-8624.2004.00663.x. [DOI] [PubMed] [Google Scholar]
- King JA, Barkley RA, Barrett S. Attention-deficit hyperactivity disorder and the stress response. Biol. Psychiatry. 1998;44:72–74. doi: 10.1016/s0006-3223(97)00507-6. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Read GF, Hellhammer DH. Assessment of Hormones and Drugs in Saliva in Biobehavioural Research. Toronto: Hogrefe and Huber; 1992. [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: Individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev. Psychopathol. Special Issue: Stress and development: Biological and psychological consequences. 2001;13:695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. Differential heart rate reactivity and recovery after psychosocial stress (TSST) in healthy children, younger adults, and elderly adults: the impact of age and gender. Int. J. Behav. Med. 2004 a;11:116–121. doi: 10.1207/s15327558ijbm1102_8. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004 b;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Lindfors K, Elovainio M, Wickman S, Vuorinen R, Sinkkonen J, Dunkel L, Raappana A. The role of ego development in psychosocial adjustment among boys with delayed puberty. Journal of Research on Adolescence. 2007;17:601–612. [Google Scholar]
- Magnusson D. Antisocial conduct of boys and autonomic activity/reactivity. Reports from the Department of Psychology, Stockholm Univ. 1986;No 652:13. [Google Scholar]
- Marshall WA, Tanner JM. Variations in patterns of pubertal change in girls. Arch. Dis. Child. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch. Dis. Child. 1970;45:13–23. doi: 10.1136/adc.45.239.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, Christ MA, Hanson KA. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. J. Am. Acad. Child Adolesc. Psychiatry. 1991;30:192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behaviour. Arch Gen Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Raine A, Stouthamer-Loeber M, Loeber R, Kumar AM, Kumar M, Lahey BB. Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biol Psychiatry. 2005;57:1109–1116. doi: 10.1016/j.biopsych.2005.01.041. [DOI] [PubMed] [Google Scholar]
- McClelland GH, Judd CM. Statistical difficulties of detecting interactions and moderator effecs. Psychol Bul. 1993;114:376–390. doi: 10.1037/0033-2909.114.2.376. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N.Y. Acad. Sci. 2004;1032:1–7. doi: 10.1196/annals.1314.001. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis stress theories in the context of life stress research: Implications for the depressive disorders. Psychol. Bull. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biol Psychiatry. 1995;388:546–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Musante L, Treiber FA, Kapuku G, Moore D, Davis H, Strong WB. The effects of life events on cardiovascular reactivity to behavioural stressors as a function of socioeconomic status, ethnicity, and sex. Psychosom. Med. 2000;62:760–767. doi: 10.1097/00006842-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Gaab J, Berger S, Jud A, Kirschbaum C, Ehlert U. Human salivary alpha-amylase reactivity in a psychosocial stress paradigm. Int. J. Psychophysiol. 2005;55:333–342. doi: 10.1016/j.ijpsycho.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Oosterlaan J, Geurts HM, Knol DL, Sergeant JA. Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Res. 2005;134:1–10. doi: 10.1016/j.psychres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased morning cortisol levels in adolescent girls with conduct disorder. Arch. Gen. Psychiatry. 2001;58:297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Piacentini J, Roper M, Jensen P, Lucas C, Fisher P, Bird H, Bourdon K, Schwab-Stone M, Rubio-Stipec M, Davies M, Dulcan M. Informant-based determinants of symptom attenuation in structured child psychiatric interviews. J. Abnorm. Child Psychol. 1999;27:417–428. doi: 10.1023/a:1021923808118. [DOI] [PubMed] [Google Scholar]
- Préville M, Zarit S, Susman E, Boulenger P, Lehoux R. Response variability of salivary cortisol among older adults under psychological stress. Aging Ment. Health. 2008;12:249–257. doi: 10.1080/13607860701616499. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Raine A. The interaction of biological and social measures in the explanation of antisocial and violent behaviour. In: Stoff D, Susman E, editors. Developmental psychobiology aggression. Cambridge University Press: New York; 2005. pp. 13–42. [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: Evidence from the Mauritius Child Health Project. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Raine R, Venables PH, Williams M. Relationships between central and autonomic measures of arousal at age 15 years and criminality at age 24 years. Arch. Gen. Psychiatry. 1990;47:1003–1007. doi: 10.1001/archpsyc.1990.01810230019003. [DOI] [PubMed] [Google Scholar]
- Randazzo W, Dockray S, Susman EJ. The Stress Response in Adolescents with Inattentive Type ADHD Symptoms. Child Psychiatry Hum. Dev. 2008;39:27–38. doi: 10.1007/s10578-007-0068-3. [DOI] [PubMed] [Google Scholar]
- Richters JE, Weintraub S. Beyond diathesis: Toward an understanding of high-risk environments. In: Rolf JE, Masten AS, Cicchetti D, Nuechterlein K, Weintraub S, editors. Risk and protective factors in the development of psychopathology. New York: Cambridge University Press; 1990. pp. 67–96. [Google Scholar]
- Schwartz E, Granger DA, Susman EJ, Gunnar M, Laird B. Assessing salivary cortisol in studies of child development. Child Dev. 1998;69:1503–1513. [PubMed] [Google Scholar]
- Seeman TE, McEwen BS, Singer BH, Albert MS, Rowe JW. Increase in urinary cortisol excretion and memory declines: MacArthur studies of successful aging. J. Clin. Endocrinol. Metab. 1997;82:2458–2465. doi: 10.1210/jcem.82.8.4173. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J. Am. Acad. Child Adolesc. Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behaviour problems in youth. Dev. Psychopathol. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Essex MJ. Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev Psychobiol. 2008;50(7):690–703. doi: 10.1002/dev.20336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skosnik PD, Chatterton RT, Jr., Swisher T, Park S. Modulation of attentional inhibition by norepinephrine and cortisol after psychological stress. Int. J. Psychophysiol. 2000;36:59–68. doi: 10.1016/s0167-8760(99)00100-2. [DOI] [PubMed] [Google Scholar]
- Stattin H, Magnusson D. Pubertal maturation in female development. Paths through life. Vol. 2. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc; 1990. [Google Scholar]
- Steinberg L, Dahl R, Keating D, Kupfer DJ, Masten AS, Pine DS. The study of developmental psychopathology in adolescence: Integrating affective neuroscience with the study of context. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology, Vol 2: Developmental neuroscience. 2nd ed. Hoboken, NJ: John Wiley & Sons, Inc; 2006. pp. 710–741. [Google Scholar]
- Stoff D, Susman EJ, editors. Psychobiology of aggressive behavior. New York: Cambridge University Press; 2005. [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: performance versus peer rejection stressors. Dev. Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susman EJ, Inoff-Germain G, Nottelmann ED, Loriaux DL, Cutler GB, Jr., Chrousos GP. Hormones, emotional dispositions, and aggressive attributes in young adolescents. Child Dev. 1987;58:1114–1134. [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Chrousos GP. Negative affect and hormone levels in young adolescents: Concurrent and longitudinal perspectives. J. Youth Adolesc. 1991;20:167–190. doi: 10.1007/BF01537607. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dorn LD, Inoff-Germain G, Nottelmann ED, Chrousos GP. Cortisol reactivity, distress behaviour, behaviour problems, and emotionality in young adolescents: A longitudinal perspective. J. Res. Adolesc. 1997;7:81–105. [Google Scholar]
- Susman EJ, Schmeelk KH, Ponirakis A, Gariepy JL. Maternal prenatal, postpartum, and concurrent stressors and temperament in 3-year-olds: A person and variable analysis. Dev. Psychopathol. 2001;13:629–652. doi: 10.1017/s0954579401003121. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of Persistent Antisocial Behaviour: Stress, Early Vulnerabilities and the Attenuation Hypothesis. Neurosci. Biobehav. Rev. 2006;30:376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Susman EJ, Dockray S, Schiefelbein VL, Herwehe S, Heaton JA, Dorn LD. Morningness/eveningness, morning-to-afternoon cortisol ratio, and antisocial behavior problems during puberty. Dev. Psychol. 2007;43:811–822. doi: 10.1037/0012-1649.43.4.811. [DOI] [PubMed] [Google Scholar]
- Uhart M, Chong RY, Oswald L, Lin PI, Wand GS. Gender differences in hypothalamic-pituitary-adrenal (HPA) axis reactivity. Psychoneuroendocrinology. 2006;31:642–652. doi: 10.1016/j.psyneuen.2006.02.003. [DOI] [PubMed] [Google Scholar]
- van Bokhoven I, Van Goozen SH, van Engeland H, Schaal B, Arseneault L, Séguin JR, Nagin DS, Vitaro F, Tremblay RE. Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. J. Neural Transm. 2005;112:1083–1096. doi: 10.1007/s00702-004-0253-5. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychol Bull. 2007;133(1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol Psychiatry. 1998;43:531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- van Stegeren A, Rohleder N, Evaraerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31:137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: Associations with cortisol. Psychiatry Res. 1993;461:9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Virkkunen M. Urinary free cortisol secretion in habitually violent offenders. Acta Psychiatr. Scand. 1985;72:40–44. doi: 10.1111/j.1600-0447.1985.tb02568.x. [DOI] [PubMed] [Google Scholar]
- Young EA, Nolen-Hoeksema S. Effect of ruminations on the saliva cortisol response to a social stressor. Psychoneuroendocrinology. 2001;26:319–329. doi: 10.1016/s0306-4530(00)00059-7. [DOI] [PubMed] [Google Scholar]