Abstract
Alzheimer disease is characterized by extracellular β-amyloid (Aβ) plaques and intracellular inclusions containing neurofibrillary tangles of phospho-Tau and intraneuronal Aβ associated with neuronal cell death. We generated a novel gene transfer animal model using lentiviral Aβ1–42 that resulted in intracellular but not extracellular Aβ accumulations in the targeted rat primary motor cortex. Expression of intracellular Aβ1–42 led to pathological changes seen in human Alzheimer disease brains, including cell death, inflammatory signs, activation of two Tau kinases, and Tau hyperphosphorylation. Promoting clearance of lentiviral Aβ1–42 reversed these effects, demonstrating that intraneuronal Aβ1–42 is a toxic peptide that lies upstream of Tau modification. These studies reveal the role of intracellular Aβ1–42 in a novel gene transfer animal model, which is a useful tool to study intraneuronal Aβ1–42-induced pathology in the absence of extracellular plaques. Targeted delivery of Aβ will allow speedy delineation of pathological mechanisms associated with specific neurodegenerative lesions.
Keywords: Alzheimer Disease, Amyloid, Gene Transfer, Lentivirus, Neurodegeneration, Parkin
Introduction
Alzheimer disease (AD)2 is the leading cause of dementia in the aging population. AD is characterized by widespread degeneration in the association cortices and the limbic system (1) accompanied by β-amyloid (Aβ) deposition (2–4), the formation of intracellular neurofibrillary tangles of the Tau protein (5), and neuronal loss (6). Aβ1–40 and Aβ1–42 are produced intracellularly, and intraneuronal Aβ1–42 accumulates in the brain of individuals with AD (7–11). Both intracellular Aβ and extracellular oligomeric Aβ have been implicated in AD pathology, but intracellular oligomeric species may act in the earlier stages of disease (12, 13). Aβ is produced intracellularly via the endosomal system and secretory pathways (14, 15). In AD, endosomes in the pyramidal neurons are significantly bigger than control (16), and endocytic alterations can even happen before clinical symptoms and accumulation of extracellular Aβ deposits (17). In primary cultures of neurons overexpressing β-amyloid precursor protein, accumulation of intraneuronal Aβ induces neuronal apoptotic cell death (18). These findings suggest a crucial role for intracellular Aβ in the early stages of AD.
Some studies showed that Tau is required for Aβ1–42 to mediate detrimental effects, including toxicity in cell culture (19) and learning and memory impairments in amyloid precursor protein mice (20). Intracellular Aβ1–42 accumulation precedes Tau pathology in triple transgenic mice exhibiting both plaque and tangle pathologies (13, 21), whereas β-amyloid pathology alone exacerbates Tau pathology (22). Removal of Aβ1–42 significantly reduces Tau hyperphosphorylation in rodents (21, 23). These findings suggest a complex relationship between Tau and Aβ pathology in AD and other tauopathies.
AD neuropathological changes also include astrogliosis and microglial cell proliferation (24–27). Astrocytes and microglia are activated in areas of the brain affected by amyloid plaques and Tau pathology accompanied by alteration of the levels of pro- and anti-inflammatory mediators, cytokines, chemokines, oxygen free radicals, and other inflammatory molecules (27–30). Pro-inflammatory cytokines, tumor necrosis factor (TNF-α) and interleukin-1β, are elevated in microglia of brains from AD patients (31, 32) and transgenic mice (33). These various inflammatory responses may contribute to the clinical symptoms of AD.
We generated a novel and simple animal model using lentiviral expression of Aβ1–42. This model demonstrates intraneuronal Aβ1–42, and we tested the in vivo effects of this particular AD pathology. We hypothesized that intracellular Aβ1–42 lies upstream of other major histopathological changes such as degenerative cell death and Tau phosphorylation.
EXPERIMENTAL PROCEDURES
Stereotaxic Injection
Lentiviral constructs were used to generate the animal models as explained in Ref. 34. The identity of the Aβ species generated was confirmed by mass spectrometry (34). Stereotaxic surgery was performed to inject the lentiviral constructs encoding parkin and Aβ into the M1 primary motor cortex of Sprague-Dawley rats weighing between 170 and 220 g. All animals were anesthetized (50 mg/kg of body weight) with a mixture of ketamine and xylazine (50:8). The stereotaxic coordinates for the primary motor cortex were 2.8 mm lateral, 3.2 mm ventral, 2.7 mm posterior. Viral stocks were injected through a microsyringe pump controller (Micro4) using total pump (World Precision Instruments, Inc.) delivery of 6 μl at a rate of 0.2 μl/min. The needle remained in place at the injection site for an additional minute before slow removal over a period of 2 min. Animals were injected into one side of M1 cortex with 1) a lentiviral LacZ vector at 2 × 1010 MOI; 2) 1 × 1010 MOI lentiviral parkin and 1 × 1010 MOI lentiviral LacZ; 3) 1 × 1010 MOI lentiviral Aβ1–42 and 1 × 1010 MOI lentiviral LacZ; or 4) 1 × 1010 MOI lentiviral Aβ1–42 and 1 × 1010 MOI lentiviral parkin.
Western Blot Analysis
Either 2 or 4 weeks after injection, brain tissues were homogenized in 1× STEN buffer (50 mm Tris (pH 7.6), 150 mm NaCl, 2 mm EDTA, 0.2% Nonidet P-40, 0.2% bovine serum albumin, 20 mm phenylmethylsulfonyl fluoride, and protease mixture inhibitor) and centrifuged at 10,000 × g for 20 min at 4 °C, and the supernatant containing the soluble fraction of proteins was collected. The supernatant was analyzed by Western blot on SDS NuPAGE 4–12% Bis-Tris gel (Invitrogen). Protein estimation was performed using the Bio-Rad protein assay (Bio-Rad Laboratories Inc.). Parkin and Aβ1–42 were immunoprobed as indicated (34). Total AKT was probed with monoclonal (1:1000) antibody (BIOSOURCE), and p-AKT at serine 473 was probed (1:1000) with polyclonal antibody (BIOSOURCE). Total GSK-3β was probed (1:1000) with monoclonal antibody (BIOSOURCE), and p-GSK-3β at tyrosine 216/279 was probed (1:1000) with polyclonal antibody (BIOSOURCE). CDK5 was probed (1:1000) with monoclonal antibody (BIOSOURCE), and p-CDK5 at tyrosine 15 was probed (1:1000) with polyclonal antibody (Santa Cruz Biotechnology, Inc.). Total Tau was probed (1:1000) with Tau-15 monoclonal antibody (Chemicon), and phosphorylated Tau was probed (1:1000) with epitopes against polyclonal serine 396 (Chemicon), polyclonal AT8 (1:1000) serine 199/202 (BIOSOURCE), polyclonal (1:1000) serine 262 (Affinity BioReagents), AT180 polyclonal (1:1000) threonine 231(BIOSOURCE), and AT270 polyclonal (1:1000) threonine 181 (BIOSOURCE). β-Actin was probed (1:1000) with polyclonal antibody (Cell Signaling Technology). TNF-α was probed with (1:1000) anti-TNF-α rabbit antibody (Serotec), and iNOS was probed with (1:1000) anti-iNOS/NOS rabbit antibody (BD Transduction Laboratories).
Western blots were quantified by densitometry using Quantity One 4.6.3 software (Bio-Rad). Densitometry was obtained as arbitrary numbers measuring band intensity. At least n = 4 was used in each group and the data were analyzed as mean ± S.D. and statistical comparison of variables was obtained by ANOVA with Newman Keuls multiple comparison test, p < 0.05. The ratio of phosphorylated to total proteins in LacZ-treated animals was standardized to 1, and other treatments were compared relative to LacZ.
Immunocytochemical and Histological Analysis of Brain Sections
Immunohistochemistry was performed on 20-μm-thick brain sections and compared between different treatment conditions. Aβ1–42 was probed (1:200) with rabbit polyclonal specific anti-Aβ1–42 antibody (Zymed Laboratories Inc.) followed by diaminobenzidine staining. Active caspase-3 was probed (1:200) with polyclonal antibody (Millipore Corp.). Glial fibrillary acidic protein (GFAP) was probed (1:200) with monoclonal antibody (Millipore Corp.), and microglia was probed (1:200) with IBA-1 polyclonal antibody (Wako). Further staining was performed to assess neural disintegrative degeneration in animal models using the FD NeuroSilverTM staining kit II (FD NeuroTechnologies, Inc., Baltimore, MD), which provides high contrast and rapid silver staining for the microscopic detection of neuronal and fiber degeneration in vivo. Total cell counts in cortical subfields were obtained by a blinded investigator using unbiased stereology analysis (Stereologer, Stereology Resource Center, Chester, MD). A minimum of six 20-μm sections were analyzed from 400 μm in each direction from the injection site in the ipsilateral area and an equivalent size area within the same region in the contralateral area. The multilevel sampling design in the Stereologer software, based on the optical fractionator sampling method, was used to estimate positive cell numbers (detected by cells positive for GFAP, IBA-1, silver, and caspase-3).
Graphs and Statistics
All graphs and statistical analyses were performed in GraphPad Prism software (GraphPad Software, Inc.). All statistics were performed using ANOVA with Newman Keuls multiple comparison test and p < 0.05 statistically significant. All studies were performed with n = 4.
RESULTS
Distribution of Aβ1–42 Expression
We generated a lentivirus for expression of Aβ1–42 to study the role of intracellular Aβ1–42 on rat brain pathology. This lentivirus contains the Aβ1–42 sequence fused to a signal peptide (Fig. 1A). We injected this lentivirus into the M1 rat primary motor cortex (34), and the animals were sacrificed 2 weeks after gene delivery. To delineate the distribution and expression of lentiviral Aβ1–42, we examined ∼3.6 mm on either side of the injection area (Fig. 1, B and C), using immunohistological assessment of Aβ1–42 expression in serial brain sections. Strong Aβ1–42 staining was observed around the injection site within the cerebral cortex with no detectable Aβ1–42 in the corpus callosum or other brain regions (Fig. 1A). Robust staining was still observable at ∼2 mm away from either side of the injection area (Fig. 1, C and D). Staining completely faded at ∼3 mm in either direction of the injection site (Fig. 1, E and F), indicating that the lentiviral Aβ1–42 is expressed within an ∼4–5-mm range of the cerebral cortex. Expression of lentiviral Aβ1–42 was observed in the ipsilateral cortex (Fig. 1H) but completely absent from the contralateral cortex (Fig. 1G). We did not observe extracellular Aβ deposits 2–4 weeks after injection of this Aβ lentivirus.
FIGURE 1.
Distribution of lentiviral Aβ expression in the rat brain. A, sequence of signal peptide and Aβ1–42 gene followed by a stop codon. B, staining of 20-μm-thick rat brain sections with Aβ1–42 and diaminobenzidine around the point of injection with lentiviral Aβ1–42. C and D, sections stained ∼2 mm anterior to the injection point toward the bregma (C) and ∼2 mm rostral to the injection point (D). E and F show staining ∼3.5 mm on either side of the injection area. G and H show staining with Aβ1–42 on the contralateral hemisphere (G) and in the ipsilateral cortex (H).
Lentiviral Aβ1–42 Expression Causes Neuronal Degeneration
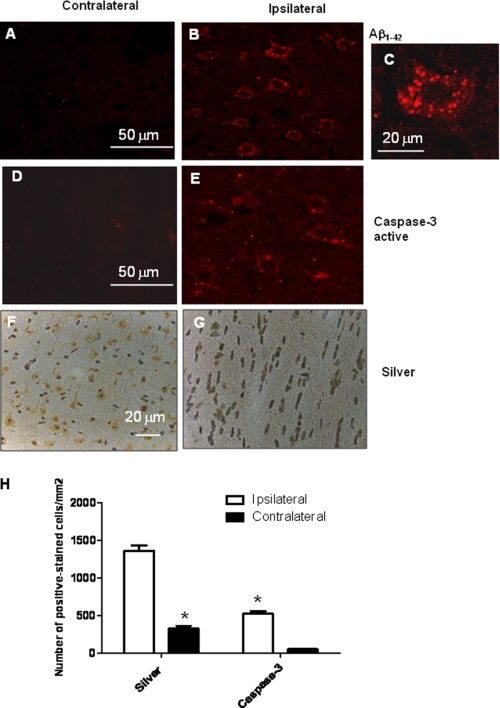
Accumulation of intracellular and extracellular Aβ is associated with neuronal death in experimental models and AD brains. To examine whether intracellular Aβ1–42 causes cell death, we analyzed the injected cortex for active caspase-3 and silver staining of degenerating neurons. Expression of Aβ1–42 was intraneuronal in the ipsilateral cortex (Fig. 2B) when compared with the contralateral cortex (Fig. 2A). A higher magnification of intraneuronal Aβ1–42 revealed a punctuate pattern of Aβ (Fig. 2C). Expression of Aβ1–42 2 weeks after injection was associated with caspase-3 activation in the ipsilateral cortex (Fig. 2E) when compared with the contralateral cortex (Fig. 2D). Accumulation of intracellular Aβ1–42 was paralleled by silver-stained neurons in the ipsilateral (Fig. 2G) but not the contralateral cortex (Fig. 2F), which indicate degenerative cell death (Burns et al. (34)). Quantification of positive stains using stereological counting methods revealed a significant 4-fold increase (p < 0.05) in both silver-stained and active caspase-3 neurons (Fig. 2H). These data demonstrate that intracellular Aβ1–42 may promote caspase-3 activation and degenerative cell death.
FIGURE 2.
Effects of expression of Aβ1–42 on cell survival. A and B, immunohistochemistry with anti-Aβ1–42 antibody in the contralateral (A) and ipsilateral cortex (B). C, high magnification view (×63) of Aβ1–42-stained cell. D and E, immunohistochemistry with anti-active caspase-3 antibody in the contralateral (D) and ipsilateral cortex (E). F and G, silver staining of the contralateral (F) and ipsilateral areas (G). H, quantification of silver and caspase-3 staining in the ipsilateral and contralateral areas. *, significantly different from control, ANOVA, Newman Keuls with multiple comparison, n = 4, p < 0.05. Error bars indicate S.D.
Lentiviral Aβ1–42 Promotes Inflammatory Responses
We tested whether expression of intracellular Aβ1–42 leads to changes in astrocytes and microglia, which undergo morphological changes in response to inflammatory stimuli in the central nervous system. Two weeks after injection, an increase in GFAP labeling was observed in the ipsilateral site (Fig. 3B) when compared with the contralateral area (Fig. 3A). Changes in brain microglia, as revealed by immunoreactivity to IBA-1 antibody that detects microglia (Fig. 3, C and D), were not evident. Quantification of GFAP- and IBA-1-positive cells using stereological counting methods revealed a significant (29%, p < 0.05) increase in astrocytes in the ipsilateral area (Fig. 3E), but no significant changes were observed in microglia (Fig. 3E). Assessment of inflammatory molecules, including TNF-α, showed a significant increase (110%) in TNF-α in animal brains injected with Aβ1–42 when compared with LacZ-injected brains (Fig. 3, F and G). Aβ1–42-injected brains also showed a significant increase (98%) in iNOS when compared with LacZ animals (Fig. 3, F and G). The observed changes in astrocytes and microglia demonstrate that the intraneuronal Aβ1–42 and cell death after lentiviral Aβ1–42 expression lead to increased inflammation 2 weeks after injection.
FIGURE 3.
Inflammatory response to Aβ1–42 expression. A and B, immunohistochemistry with anti-GFAP antibody in the contralateral (A) and ipsilateral cortex (B), showing the reaction of astrocytes to Aβ1–42. C and D, immunohistochemistry with anti-IBA-1 antibody in the contralateral (C) and ipsilateral cortex (D) showing microglial response to Aβ1–42. E, quantification of GFAP and IBA-1 staining using stereological methods. F, Western blot analysis of inflammatory molecules showing increased levels of TNF-α and iNOS in Aβ-injected brains. G, densitometric analysis of TNF-α and iNOS blots. *, significantly different from control, ANOVA, Newman Keuls with multiple comparison, n = 4, p < 0.05. Error bars indicate S.D.
Lentiviral Aβ1–42 Activates Signal Transduction Pathways
We tested whether lentiviral Aβ1–42 activates signal transduction pathways, involving kinases that affect cell death or survival. We analyzed brain homogenates by Western blot with antibodies against either total or phosphorylated AKT, GSK-3β, and CDK5. Phosphorylated forms of each of the enzymes indicate activated kinases. We previously demonstrated the ability of parkin to clear intraneuronal Aβ1–42 in this animal model (34), so we also tested whether clearance of Aβ1–42 would mitigate its effects on kinases (34). No significant changes in either phosphatidylinositol 3-kinase (data not shown) or phospho-AKT (Fig. 4, A and C) levels were observed in the brain of the gene transfer animal model. One month after injection, a significant increase (70%) in GSK-3β activity (Fig. 4, A and C) was detected in the brains of animals injected with lentiviral Aβ1–42 when compared with either control or parkin-injected brains (n = 4, p < 0.05). Parkin mitigated the effects of Aβ1–42 on GSK-3β activity, as the ratio of phospho-GSK-3β at Tyr-216 and total GSK-3β indicates (Fig. 4, A and C). Similarly, a significant increase in CDK5 phosphorylation at Tyr-15 (120%) was observed in Aβ1–42-injected brains (Fig. 4, B and C) when compared with either control or parkin brains (p < 0.05, n = 4). Parkin also abrogated the effects of Aβ1–42 on CDK5 phosphorylation (Fig. 4, B and C). These data indicate that lentiviral Aβ1–42 activates two independent kinase pathways, CDK5 and GSK-3β, but not the AKT pathway, whereas clearance of Aβ1–42 via a parkin-mediated pathway results in restoration of these pathways.
FIGURE 4.
Aβ1–42 causes alteration in kinase activity. A and B, Western blot analysis on 4–12% SDS-PAGE gel showing changes in the levels of AKT GSK-3β (A) and CDK5 (B). Par, parkin. C, densitometric analysis of the ratio of phosphorylated kinases to total levels. *, significantly different from control, ANOVA, Newman Keuls with multiple comparison, n = 4, p < 0.05. Error bars indicate S.D.
Disturbance of Tau Metabolism in Aβ1–42 Gene Transfer Animals
We tested whether accumulation of intracellular Aβ1–42 lies upstream of hyperphosphorylated Tau pathology. We probed brain homogenates from lentivirus-injected cortices for several Tau epitopes using Western blotting and immunohistochemistry. No changes in total Tau were observed at 2 weeks after injection (data not shown) or 4 weeks after injection (Fig. 5A). There were no changes in phosphorylation of Tau Ser-262 or Thr-181 (Fig. 5A). A significant increase (30%) in Tau phosphorylation was detected at Ser-396 (Fig. 5, A and B) in brains injected with Aβ1–42 when compared with either control or parkin brains (p < 0.05, n = 4). Parkin co-expression reversed the effects of Aβ1–42 on Tau (Fig. 5, A and B). Lentiviral Aβ1–42 also led to a significant increase in Tau phosphorylation at Thr-231 (120%) and Ser-199/202 (250%) when compared with control or parkin brains (Fig. 5, A and B). Parkin again counteracted the effects of intraneuronal Aβ1–42 on Tau modification (Fig. 5, A and B). These data indicate that intracellular Aβ1–42 lies upstream of Tau pathology in this novel animal model and leads to alteration in Tau metabolism.
FIGURE 5.
Aβ1–42 causes Tau modification, and parkin (Par) reverses this effect. A, Western blot analysis on 10% SDS-PAGE gel showing changes in the levels of Tau and its phosphorylated epitopes. B, densitometric analysis of the ratio of phosphorylated Tau epitopes to total Tau levels. *, significantly different from control, ANOVA, Newman Keuls with multiple comparison, n = 4, p < 0.05. Error bars indicate S.D.
DISCUSSION
We generated a novel gene transfer animal model that results in the accumulation of intracellular Aβ1–42. The accumulation of Aβ1–42 in this model was defined by immunohistochemistry, enzyme-linked immunosorbent assay, and Aβ1–42-derived fragments by mass spectrometry (34). We used this model to test whether accumulation of intracellular Aβ1–42, in the absence of extracellular plaque, led to other AD-like pathological changes. We observed vesicular intracellular Aβ1–42 accumulation (14, 15). The cell death we observed (detected by active caspase-3 and silver staining) as a result of Aβ1–42 expression suggests that intracellular, like extracellular, Aβ can lead to degenerative cell death, which plays a crucial role in cell death in the presence of Aβ deposits and neurofibrillary tangles in AD and other neurodegenerative diseases (12, 36, 37). Extracellular Aβ causes an increase in active caspase-3 staining in neurons in culture, involving activator p35 (38), consistent with our data showing cleaved caspase-3 and activated CDK5 in response to Aβ1–42 expression. Previous reports indicated intraneuronal Aβ1–42 aggregation in 5XFAD transgenic mouse brains, along with CDK5 activation and neurodegenerative death (39). In addition to increased neuronal death in this lentiviral model, we also observed evidence of strong astrogliosis and increased inflammation, consistent with previous findings showing strong reactive astrogliosis and neuronal loss in AD transgenic models accumulating intraneuronal Aβ1–42 in the absence of extracellular deposits (40). Taken together, these data suggest that in vivo intraneuronal Aβ1–42 is a contributing factor to cell death as well as reactive gliosis.
A significant increase in kinase activities was observed in the presence of Aβ1–42, consistent with previous reports showing that amyloid proteins increase the activity of kinases (35). Several investigators showed that Aβ activates GSK-3β through impairment of phosphatidylinositol-3 kinase/AKT signaling and induces Tau hyperphosphorylation, neurofibrillary tangle formation, neuronal death, and synaptic loss in animal models (41). No changes in the activity of either phosphatidylinositol 3-kinase or AKT were detected in this animal model, but a significant increase was observed in GSK-3β activity. This increase was paralleled by activation of CDK5, another kinase activated in AD (42, 43). CDK5 undergoes post-translational phosphorylation to become activated (44). Taken together, these data suggest that Aβ1–42 activates two independent kinase pathways, GSK-3β and CDK5, known to be activated in AD.
We observed that lentiviral Aβ1–42 increased Tau phosphorylation at Ser-396, Thr-231, and Ser-199/202. Kinases phosphorylate Tau on Ser-202, Thr-205, Thr-212, Thr-217, Ser-235, Ser-396, and Ser-404 (45), consistent with our results showing increased CDK5 and GSK-3β activities in this model. Tau hyperphosphorylation at Thr-212, Ser-202, Ser-396, and Ser-404 is found in paired helical filaments in AD brains (42, 45). Reduction in Aβ1–42 levels leads to GSK-3β inactivation and prevents Tau phosphorylation in vivo and in vitro (21, 23). An increase in CDK5 immunoreactivity is also observed in pretangle neurons and in neurons bearing early stages of neurofibrillary tangles, suggesting that CDK5 is involved in the formation of neurofibrillary tangles at a relatively early stage in AD pathogenesis (46, 47).
The array of pathological changes seen in our lentiviral Aβ1–42 model has led us to hypothesize that intracellular Aβ1–42 pathology causes kinase activation, hyperphosphorylation of Tau, neuronal loss, and glial activation. Removal of β-amyloid deposits has been extensively explored in transgenic AD models, and development of treatments has focused on increasing Aβ clearance (48–50). Several strategies have been used to degrade or remove Aβ, including immunization with Aβ42 (49) and insulin treatment (48), although these studies focus on extracellular Aβ1–42. We previously demonstrated that intracellular Aβ1–42 can be metabolized through a ubiquitin-proteasome pathway (34); co-expression of the ubiquitin ligase parkin reduced Aβ1–42, reversed the effects of β-amyloid on Tau kinases, and prevented Tau hyperphosphorylation in human SH-SY5Y neuroblastoma cells. The data suggest that strategies to remove intracellular Aβ1–42 would prevent many types of AD pathological changes. The studies demonstrate the utility of a simple lentiviral transfer model for Aβ1–42, resulting in intracellular Aβ1–42. This animal model is a very useful tool to study Aβ1–42-induced pathology in the absence of extracellular amyloid plaques. Gene delivery into targeted brain regions may be a strategic step to delineate pathological mechanisms of specific Aβ species and control gene expression and dose, as well as reverse the effects of gene expression via regulation of protein degradation or removal.
Footnotes
- AD
- Alzheimer disease
- Aβ
- β-amyloid
- TNF
- tumor necrosis factor
- GSK-3β
- glycogen synthase kinase-3β
- GFAP
- glial fibrillary acidic protein
- NOS
- nitric oxide synthase
- iNOS
- inducible NOS
- Bis-Tris
- 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol
- ANOVA
- analysis of variance
- MOI
- multiplicity of infection
- p
- phosphorylated.
REFERENCES
- 1.Hof P., Morrison J. (1994) in Alzheimer Disease (Terry R. D., Katzman R., Bick K. L. eds) pp. 197–230, Raven Press, Ltd., New York [Google Scholar]
- 2.Jellinger K. A., Bancher C. (1998) J. Neural Transm. Suppl.54, 77–95 [DOI] [PubMed] [Google Scholar]
- 3.Jellinger K. A. (2002) J. Neural Transm. 109, 813–836 [DOI] [PubMed] [Google Scholar]
- 4.Selkoe D. J. (1989) Cell 58, 611–612 [DOI] [PubMed] [Google Scholar]
- 5.Trojanowski J. Q., Lee V. M. (2000) Ann. N.Y. Acad. Sci. 924, 62–67 [DOI] [PubMed] [Google Scholar]
- 6.Terry R. D., Peck A., DeTeresa R., Schechter R., Horoupian D. S. (1981) Ann. Neurol. 10, 184–192 [DOI] [PubMed] [Google Scholar]
- 7.Greenfield J. P., Tsai J., Gouras G. K., Hai B., Thinakaran G., Checler F., Sisodia S. S., Greengard P., Xu H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 742–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gouras G. K., Tsai J., Naslund J., Vincent B., Edgar M., Checler F., Greenfield J. P., Haroutunian V., Buxbaum J. D., Xu H., Greengard P., Relkin N. R. (2000) Am. J. Pathol. 156, 15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee S. J., Liyanage U., Bickel P. E., Xia W., Lansbury P. T., Jr., Kosik K. S. (1998) Nat. Med. 4, 730–734 [DOI] [PubMed] [Google Scholar]
- 10.Wilson C. A., Doms R. W., Lee V. M. (1999) J. Neuropathol. Exp. Neurol. 58, 787–794 [DOI] [PubMed] [Google Scholar]
- 11.Xu H., Sweeney D., Wang R., Thinakaran G., Lo A. C., Sisodia S. S., Greengard P., Gandy S. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 3748–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M., Chen L., Lee D. H., Yu L. C., Zhang Y. (2007) Prog Neurobiol 83, 131–139 [DOI] [PubMed] [Google Scholar]
- 13.Oddo S., Caccamo A., Kitazawa M., Tseng B. P., LaFerla F. M. (2003) Neurobiol. Aging 24, 1063–1070 [DOI] [PubMed] [Google Scholar]
- 14.Haass C., Schlossmacher M. G., Hung A. Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B. L., Lieberburg I., Koo E. H., Schenk D., Teplow D. B. (1992) Nature 359, 322–325 [DOI] [PubMed] [Google Scholar]
- 15.Koo E. H., Squazzo S. L. (1994) J. Biol. Chem. 269, 17386–17389 [PubMed] [Google Scholar]
- 16.Cataldo A. M., Barnett J. L., Pieroni C., Nixon R. A. (1997) J. Neurosci. 17, 6142–6151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cataldo A. M., Peterhoff C. M., Troncoso J. C., Gomez-Isla T., Hyman B. T., Nixon R. A. (2000) Am. J. Pathol. 157, 277–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Octave J. N. (2005) Bull. Mem. Acad. R. Med. Belg. 160, 445–451 [PubMed] [Google Scholar]
- 19.Rapoport M., Dawson H. N., Binder L. I., Vitek M. P., Ferreira A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 6364–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberson E. D., Scearce-Levie K., Palop J. J., Yan F., Cheng I. H., Wu T., Gerstein H., Yu G. Q., Mucke L. (2007) Science 316, 750–754 [DOI] [PubMed] [Google Scholar]
- 21.McKee A. C., Carreras I., Hossain L., Ryu H., Klein W. L., Oddo S., LaFerla F. M., Jenkins B. G., Kowall N. W., Dedeoglu A. (2008) Brain Res. 1207, 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oddo S., Caccamo A., Shepherd J. D., Murphy M. P., Golde T. E., Kayed R., Metherate R., Mattson M. P., Akbari Y., LaFerla F. M. (2003) Neuron 39, 409–421 [DOI] [PubMed] [Google Scholar]
- 23.Ma Q. L., Lim G. P., Harris-White M. E., Yang F., Ambegaokar S. S., Ubeda O. J., Glabe C. G., Teter B., Frautschy S. A., Cole G. M. (2006) J. Neurosci. Res. 83, 374–384 [DOI] [PubMed] [Google Scholar]
- 24.Beach T. G., Walker R., McGeer E. G. (1989) Glia 2, 420–436 [DOI] [PubMed] [Google Scholar]
- 25.Masliah E., Mallory M., Hansen L., Alford M., Albright T., Terry R., Shapiro P., Sundsmo M., Saitoh T. (1991) Acta Neuropathol. 83, 12–20 [DOI] [PubMed] [Google Scholar]
- 26.Rogers J., Luber-Narod J., Styren S. D., Civin W. H. (1988) Neurobiol. Aging 9, 339–349 [DOI] [PubMed] [Google Scholar]
- 27.Rogers J., Webster S., Lue L. F., Brachova L., Civin W. H., Emmerling M., Shivers B., Walker D., McGeer P. (1996) Neurobiol. Aging 17, 681–686 [DOI] [PubMed] [Google Scholar]
- 28.Eikelenboom P., Bate C., Van Gool W. A., Hoozemans J. J., Rozemuller J. M., Veerhuis R., Williams A. (2002) Glia 40, 232–239 [DOI] [PubMed] [Google Scholar]
- 29.McGeer E. G., McGeer P. L. (2003) Biol. Psychiatry 27, 741–749 [DOI] [PubMed] [Google Scholar]
- 30.Akiyama H., Barger S., Barnum S., Bradt B., Bauer J., Cole G. M., Cooper N. R., Eikelenboom P., Emmerling M., Fiebich B. L., Finch C. E., Frautschy S., Griffin W. S., Hampel H., Hull M., Landreth G., Lue L., Mrak R., Mackenzie I. R., McGeer P. L., O'Banion M. K., Pachter J., Pasinetti G., Plata-Salaman C., Rogers J., Rydel R., Shen Y., Streit W., Strohmeyer R., Tooyoma I., Van Muiswinkel F. L., Veerhuis R., Walker D., Webster S., Wegrzyniak B., Wenk G., Wyss-Coray T. (2000) Neurobiol. Aging 21, 383–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dickson D. W., Lee S. C., Mattiace L. A., Yen S. H., Brosnan C. (1993) Glia 7, 75–83 [DOI] [PubMed] [Google Scholar]
- 32.Griffin W. S., Sheng J. G., Royston M. C., Gentleman S. M., McKenzie J. E., Graham D. I., Roberts G. W., Mrak R. E. (1998) Brain Pathol. 8, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benzing W. C., Wujek J. R., Ward E. K., Shaffer D., Ashe K. H., Younkin S. G., Brunden K. R. (1999) Neurobiol. Aging 20, 581–589 [DOI] [PubMed] [Google Scholar]
- 34.Burns M. P., Zhang L., Rebeck G. W., Querfurth H. W., Moussa C. E. (2009) Hum. Mol. Genet. 18, 3206–3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moussa C. E. (2009). J. Mol. Neurosci. 37, 25–36 [DOI] [PubMed] [Google Scholar]
- 36.Sheng J. G., Mrak R. E., Griffin W. S. (1998) J. Neuropathol. Exp. Neurol. 57, 323–328 [DOI] [PubMed] [Google Scholar]
- 37.Sheng J. G., Zhou X. Q., Mrak R. E., Griffin W. S. (1998) J. Neuropathol. Exp. Neurol. 57, 714–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Utreras E., Maccioni R., González-Billault C. (2009) Neuroscience 161, 978–987 [DOI] [PubMed] [Google Scholar]
- 39.Oakley H., Cole S. L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., Vassar R. (2006) J. Neurosci. 26, 10129–10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Casas C., Sergeant N., Itier J. M., Blanchard V., Wirths O., van der Kolk N., Vingtdeux V., van de Steeg E., Ret G., Canton T., Drobecq H., Clark A., Bonici B., Delacourte A., Benavides J., Schmitz C., Tremp G., Bayer T. A., Benoit P., Pradier L. (2004) Am. J. Pathol. 165, 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takashima A. (2006) J. Alzheimers Dis. 9, 309–317 [DOI] [PubMed] [Google Scholar]
- 42.Imahori K., Uchida T. (1997) J. Biochem. 121, 179–188 [PubMed] [Google Scholar]
- 43.Lee M. S., Tsai L. H. (2003) J. Alzheimers Dis. 5, 127–137 [DOI] [PubMed] [Google Scholar]
- 44.Fu W. Y., Chen Y., Sahin M., Zhao X. S., Shi L., Bikoff J. B., Lai K. O., Yung W. H., Fu A. K., Greenberg M. E., Ip N. Y. (2007) Nat. Neurosci. 10, 67–76 [DOI] [PubMed] [Google Scholar]
- 45.Anderton B. H., Betts J., Blackstock W. P., Brion J. P., Chapman S., Connell J., Dayanandan R., Gallo J. M., Gibb G., Hanger D. P., Hutton M., Kardalinou E., Leroy K., Lovestone S., Mack T., Reynolds C. H., Van Slegtenhorst M. (2001) Biochem. Soc. Symp. 67, 73–80 [DOI] [PubMed] [Google Scholar]
- 46.Augustinack J. C., Sanders J. L., Tsai L. H., Hyman B. T. (2002) J. Neuropathol. Exp. Neurol. 61, 557–564 [DOI] [PubMed] [Google Scholar]
- 47.Pei J. J., Grundke-Iqbal I., Iqbal K., Bogdanovic N., Winblad B., Cowburn R. F. (1998) Brain Res. 797, 267–277 [DOI] [PubMed] [Google Scholar]
- 48.Gasparini L., Gouras G. K., Wang R., Gross R. S., Beal M. F., Greengard P., Xu H. (2001) J. Neurosci. 21, 2561–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., Kholodenko D., Lee M., Liao Z., Lieberburg I., Motter R., Mutter L., Soriano F., Shopp G., Vasquez N., Vandevert C., Walker S., Wogulis M., Yednock T., Games D., Seubert P. (1999) Nature 400, 173–177 [DOI] [PubMed] [Google Scholar]
- 50.Xu H., Gouras G. K., Greenfield J. P., Vincent B., Naslund J., Mazzarelli L., Fried G., Jovanovic J. N., Seeger M., Relkin N. R., Liao F., Checler F., Buxbaum J. D., Chait B. T., Thinakaran G., Sisodia S. S., Wang R., Greengard P., Gandy S. (1998) Nat. Med. 4, 447–451 [DOI] [PubMed] [Google Scholar]