Abstract
BACKGROUND
The role of bone marrow (BM)-derived cells in pancreatic β-cell regeneration remains unresolved. Here we examined whether BM-derived cells are recruited to the site of moderate pancreatic injury and contribute to β-cell regeneration.
METHODS
Low-dose streptozotocin (STZ) treatment was utilized to induce moderate pancreatic damage and hyperglycemia. Enhanced green fluorescent protein-positive (EGFP+) BM chimeras were evaluated for β-cell regeneration after STZ treatment.
RESULTS
To test the hypothesis that pancreatic tissue injury induces an SDF-1 gradient to chemoattract the stem cells, we evaluated the expression of mRNA for SDF-1 in damaged pancreatic tissue. SDF-1 was significantly increased in the pancreas after damage, peaking day 10. The majority of BM cells expressing mRNA for pancreatic development markers were detected in the subpopulation of CD45−/Sca-1+/Lin− very small embryonic-like (VSEL) cells. VSEL cells mobilized from BM to peripheral blood (PB) in response to pancreatic damage, peaking in PB at day 5, and were highly enriched in the pancreas 10–15 days after STZ treatment. To confirm a role for BM-derived cells in pancreatic β-cell regeneration, we prepared EGFP+→ B6 chimeras. In the EGFP+ chimeras, EGFP+ cells were detected around duct and islets and were positive for insulin after STZ treatment. However, STZ-induced hyperglycemia was reduced only transiently (49 to 77 days) after pancreatic injury.
CONCLUSIONS
These data suggest that VSEL cells are mobilized into injured pancreatic tissue and contribute to β-cell regeneration. Transplantation of BM-derived cells improves the function of injured pancreas, although the response is not sufficient to restore sustained normoglycemia.
Keywords: Bone marrow, stem cells, transplantation, pancreatic β cell regeneration
INTRODUCTION
Evidence suggests that bone marrow (BM) harbors a pool of stem cells capable of differentiating into multiple tissue types. Bone marrow-derived cells have the potential to transdifferentiate into multiple lineage cells, including liver, brain, lung, gastrointestinal tract, and skin (1–3). Until now, the search for specific pancreatic stem cells has focused on pancreatic ductular cells (4), pre-existing β cells (5), and embryonic stem cells (6). Very small embryonic-like (VSEL) stem cells are CD45−/Sca-1+/Lin− cells that reside in bone marrow (BM) and express markers characteristic of embryonic stem cells, epiblast stem cells, and primordial germ cells (7). VSEL cells have the potential to differentiate into cells from all three germ layers (8). In the present study, we evaluated whether VSEL cells are candidate pancreatic stem cells that may play a pivotal role in regeneration of damaged pancreatic β cell mass.
A number of studies have shown that regeneration of damaged β cells can be induced. Prediabetic non-obese diabetic (NOD) mice rendered BM chimeras are cured of their autoimmunity (9,10), and a recent report suggested that regeneration of β cells in the damaged islets occurred (11). The evidence for β cell regeneration in mixed chimerism was established in NOD mice even late in the progression of the autoimmunity, but it remains to be shown whether BM-derived cells contributed directly to β cell regeneration. A recent study showed that BM cells have the capacity to differentiate into functionally competent pancreatic endocrine β cells in vivo without evidence of cell fusion (12). Moreover, syngeneic BM cells restored normoglycemia in mice rendered hyperglycemic from moderate pancreatic injury from streptozotocin (STZ) (13). However, several other studies have contradicted these findings and showed no evidence for BM-derived cells differentiating into pancreatic β cell (14,15). Other studies showed that BM transplantation results in a proliferation of recipient pancreatic β cells that produce insulin and reduce hyperglycemia in STZ-induced diabetic mice (16,17), and in spontaneously diabetic animals (18,19). Homing of donor BM-derived cells in BM and subsequent mobilization into the damaged pancreas is required for BM-induced regeneration of recipient pancreatic β cells (17). Whether BM-derived cells contribute directly to regenerate β cell mass remains unresolved.
In the present study, we found that VSEL cells are significantly enriched for pancreatic developmental markers and are recruited to the site of moderate islet injury. The level of mRNA for SDF-1 was significantly increased in the pancreas after STZ treatment, peaking on day 10. In EGFP+ bone marrow chimeras treated with STZ, hyperglycemia improved for up to 77 days, but this was not sustained. EGFP+/insulin+ cells were found surrounding islet and ductal structures in these chimeras at 42 days. Collectively, our data indicate that bone marrow cells can mediate β cell regeneration/repair but additional stimuli are required for more durable and robust long-term outcomes.
MATERIALS AND METHODS
Animals
Five to six-week-old male C57BL/6J (B6, H-2b) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Four to five-week-old male C57BL/6-Tg (ACTB-EGFP) 10sb/J (EGFP+) mice were generated through in-house breeding. Animals were housed in a barrier animal facility at the Institute for Cellular Therapeutics and cared for according to specific National Institute of Health animal care guidelines.
Dose titration of STZ in induction of hyperglycemia
B6 mice were injected intraperitoneally with 35 mg or 45 mg/kg STZ (Sigma-Aldrich, St. Louis, MO) daily for 5 or 6 days. STZ was dissolved in sodium citrate buffer (pH 4.5) before injection (16). Blood glucose was monitored weekly between 9:00 and 10:00 a.m. from day 0 to day 106 using a glucometer (Walgreens blood glucose monitor).
EGFP+ chimera preparation
Whole BM cells were harvested from EGFP+ B6 mice as previously described (20). 5 × 106 EGFP+ whole BM cells were transplanted by tail-vein injection into B6 recipients conditioned with 950 cGy total body irradiation (TBI). One month after transplantation, analysis of donor chimerism was performed by using flow cytometry.
Flt3-ligand (FL) and granulocyte colony stimulating factor (G-CSF) treatment
Recombinant human FL and G-CSF were kindly provided by Amgen, Thousand Oaks, CA). FL was diluted in 0.1% mouse serum albumin in saline (Sigma) at a concentration of 100 μg/mL. G-CSF was diluted in saline at a concentration of 75 μg/mL, and mice were injected once daily subcutaneously. Mice received 10 μg FL from day 1 to 10, and 7.5 μg G-CSF from day 4 to 10. Control animals were injected with saline only.
Cell sorting
CD45+/Sca-1+/Lin− hematopoietic stem cells (HSC) or VSEL cells were isolated from B6 BM by multiparameter live sterile cell sorting (FACSAria or FACSVantage SE; Becton Dickinson, Mountainview, CA). BM was isolated and collected in single cell suspension at a concentration of 100 × 106 cells/ml in sterile cell sort media (CSM), containing sterile 1x Hank’s Balanced Salt Solution without phenol red (GIBCO, Grand Island, New York), 2% heat-inactivated fetal calf serum (FCS; GIBCO), 10 mM/ml HEPES buffer (GIBCO), and 30 μl/ml Gentamicin (GIBCO). CD45+/Sca-1+/Lin− or CD45−/Sca-1+/Lin− cells sorting experiments used the following mAbs: stem cell antigen-1 (Sca-1) phycoerythrin (PE; E13-161.7; rat IgG2a), ), CD8α fluorescein isothiocyanate (FITC; 53-6.7; rat IgG2a), Mac-1 FITC (M1/70; rat IgG2b), B220 FITC (RA3-6B2; rat IgG2a), Gr-1 FITC (11-26c.2a; rat IgG2a), β-TCR FITC (H57-597; Armenian hamster IgG), and CD45 allophycocyanin (APC; 30-F11; Rat IgG2b). Directly labeled mAbs were added at saturating concentrations and the cells were incubated for 30 min on ice and washed twice with CSM. Cells were resuspended in CSM at a concentration of 2.5 × 106 cells/ml.
Bone marrow transplantation of STZ-induced hyperglycemic mice
Whole BM cells were harvested form donor EGFP+ B6 mice. B6 recipients were treated with 45 mg/kg streptozotocin for 6 days (−6 day to −1 day). 2 × 106 BM cells were transplanted by tail-vein injection into STZ-treated B6 mice conditioned with 350 cGy of TBI on day 10. This TBI dose was based on the animal model use by Hess (16).
Real time reverse transcriptase-polymerase chain reaction (RT-PCR)
Total mRNA was isolated from cells with the RNeasy Mini Kit (Quiagen Inc., Valencia, CA). mRNA was reverse-transcribed with TaqMan Reverse Transcription Reagents (Applied Biosystems, Foster City, CA). Detection of SDF-1, hepatocyte growth factor (HGF), leukemia inhibitory factor (LIF), Nkx 6.1, Pdx1, Ptf1 and β2-microglobulin mRNA levels was performed by real-time RT-PCR using an ABI PRISM® 7000 Sequence Detection System (Applied Biosystems). A 25 μl reaction mixture contains 12.5 μl SYBR Green PCR Master Mix, 10 ng of cDNA template, forward and reverse primers. Primers were designed with Primer Express software (supplementary data). The threshold cycle (Ct), i.e., the cycle number at which the amount of amplified gene of interest reached a fixed threshold, was determined subsequently. Relative quantization of SDF-1, HGF, LIF, Nkx 6.1, Pdx1, and Ptf1 mRNA expression was calculated with the comparative Ct method. The relative quantification value of target, normalized to an endogenous control β2-microglobulin gene and relative to a calibrator, is expressed as 2−ΔΔCt (fold difference), where ΔCt = Ct of target genes - Ct of endogenous control gene, and ΔΔCt = ΔCt of samples for target gene-ΔCt of calibrator for the target gene.
To avoid the possibility of amplifying contaminating DNA, the following measures were taken: 1) all the primers for real time RT-PCR were designed with an intron sequence inside cDNA to be amplified; 2) reactions were performed with appropriate negative controls (template-free controls); 3) a uniform amplification of the products was rechecked by analyzing the melting curves of the amplified products (dissociation graphs); 4) the melting temperature (Tm) was 57–60 °C, the product Tm was at least 10°C higher than primer Tm; and finally, 5) gel electrophoresis was performed to confirm the correct size of the amplification and the absence of nonspecific bands.
Histological evaluation and Immunofluorescence staining
Fresh pancreata were embedded in OCT embedding medium (Tissue-Tek® OCT compound 4853, Electron Microscopy Science, Hatfield, PA) by using dry ice-cooled isopentane (277258, Sigma) bath. Serial sections (5 μm) were obtained and mounted on superfrost plus slides (12-550-15, Fisher Scientific, Pittsburgh, PA). For histological evaluations, sections were stained with hematoxylin and eosin to evaluate morphology of pancreatic. For immunofluorescence staining, sections were fixed for 15 min in 4 % paraformaldehyde diluted in PBS (pH = 7.4). Sections were then permeabilized with 0.5% saponin in PBS (pH = 7.4) for 10 min at room temperature and blocked with 10% goat serum containing 1 % bovine serum albumin in PBS (pH = 7.4) for 1 h. Sections were simultaneously incubated at 4°C overnight with the mixture of the primary antibody. Antibody dilution factors were as follows: guinea pig anti-insulin 1:200 (ab7842, Abcam, Cambridge, MA), and chicken anti-EGFP 1:200 (AB16901, Millipore, Billerica, MA). After three washes with PBS, sections subsequently incubated at room temperature for 1 h with the mixture of second antibody. Secondary antibodies and dilution factors were as follows: goat anti-guinea pig Texas Red conjugate 1:400 (ab6906, Abcam), and goat anti chicken FITC conjugate 1:200 (103095155, Jackson ImmunoResearch, West Grove, PA,). Finally, after counterstaining nuclei with DAPI slides (D3571, Invitrogen, Carlsbad, CA), slides were mounted in Vectorshield Antifade medium (Vector Laboratory, Burlingame, CA) and imaged using a Leica SP5 confocal microscope system (Leica Microsystems Inc., Exton, PA).
Statistics
Data are expressed as means ± SD. Statistical analysis for significance was performed using an unpaired two-tailed student’s t-test. Data were considered significant when P < 0.05.
RESULTS
CD45−/Sca-1+/Lin− bone marrow-derived VSEL express markers for pancreatic development
To evaluate whether subpopulations of BM-derived cells express transcript for pancreatic development, we performed real-time RT-PCR to analyze the expression of mRNA for Nkx6.1, Pdx1, and Ptf1 in whole BM cells, CD45+/Sca-1+/Lin− cells that are enriched for HSC, and CD45−/Sca-1+/Lin− for VSEL cells. The transcript level in the CD45−/Sca-1+/Lin− cells was increased 110 fold in Nkx6.1 expression compared to whole BM or CD45+/Sca-1+/Lin− cells (Fig. 1A; P <0.001). The CD45−/Sca-1+/Lin− cells were increased 8- and 10-fold increase in Pdx1 and Ptf1 compared to whole bone marrow and CD45+/Sca-1+/Lin−, respectively (Fig. 1A; P <0.001). These data demonstrate that the subpopulation of CD45−/Sca-1+/Lin− VSEL cells is highly enriched in mRNA for pancreatic development markers.
Figure 1. Pancreas developmental markers (Nkx 6.1, Pdx 1, and Ptf 1) are significantly increased in the CD45− fraction.
(A) BM cells were harvested from B6 mice and sorted for CD45+/Sca-1+/Lin− or CD45−/Sca-1+/Lin− cells. The total RNA was isolated from BM cells, CD45+/Sca-1+/Lin− or CD45−/Sca-1+/Lin− cells, and analyzed for transcript for NKx 6.1, Pdx 1, and Ptf 1 by real-time RT-PCR. The CD45−/Sca-1+/Lin− cell fraction was highly enriched in mRNA for markers of pancreas development. *P< 0.001 for CD45−/Sca-1+/Lin− vs. BM cells or CD45+/Sca-1+/Lin− cells. The pancreatic damage from using STZ treatment resulted in down-regulation of markers of pancreatic development (NKx6.1, Pdx1, and Ptfl) in BM (B) and upregulation of these markers in PB (C), spleen (D), and pancreas (E).
To test whether cells expressing markers for pancreatic development are mobilized into damaged pancreas, moderate pancreatic injury due to STZ was induced and the PB MNC, BM MNC, spleen, and pancreas were harvested on day 0, 5, 10, 15 after the injury. mRNA for pancreatic developmental markers was analyzed using real-time RT-PCR. The levels of mRNA for Nkx6.1 in PB MNC were increased, with a peak at day 5 (Fig. 1C). In contrast, the levels of mRNA for Nkx6.1, Pdx1, and Ptf1 in BM MNC were decreased (Fig. 1B), suggesting that cells expressing Nkx6.1, Pdx1, and Ptf1 were mobilized into the PB from the BM. In spleen, cells expressing Nkx6.1, Pdx1, and Ptf1 were increased at day 5 and day 10 after pancreas injury (Fig. 1D). In pancreas, cells expressing Nkx6.1 were increased at day 10, and cells expressing Pdx1 and Ptf1 were increased at day 15 post-STZ treatment (Fig. 1E). These data suggest that BM-derived cells which express Nkx1, Pdx1, and Ptf1 are likely mobilized into pancreas after pancreatic injury.
SDF-1 mRNA is upregulated in damaged pancreas
SDF-1 is a chemokine produced by stromal cells in the bone marrow that provides a gradient for chemoattracting HSC via the receptor CXCR4 (21). It has been shown that CD45−/Sca-1+/Lin− VSEL cells express CXCR4, c-met, and LIF-receptor, and respond robustly to SDF-1, HGF and LIF gradients (8). To test whether mobilization of cells expressing Nkx6.1, Pdx1, and Ptf1 into injured pancreas is in response to SDF-1, HGF and LIF gradients, we evaluated the expression of mRNA for SDF-1, HGF, and LIF in pancreatic tissue at day 0, 5, 10, and 15 after STZ treatment. SDF-1 and HGF were upregulated, with a peak at day 10 (Fig. 2). The kinetics of SDF-1 and HGF expression coincided with the recovery time of cells expressing markers for pancreatic development, suggesting that the SDF-1 and HGF gradients may play an important role in promoting BM-derived cell mobilization into damaged pancreas.
Figure 2. Upregulation of SDF-1, HGF mRNA in damaged pancreas.

Animals were treated with 35 mg/kg/day of STZ. The total RNA was isolated from STZ-damaged pancreatic tissue. The levels of SDF-1, HGF and LIF mRNA were analyzed by quantitative real-time RT-PCR. Data showed that the mRNA of SDF-1 and LIF were upregulated in damaged pancreatic tissue.
Bone marrow-derived cells contribute to β cell regeneration
To evaluate the potential capacity of BM-derived cells to regenerate β cells, we used a previously reported model of low-dose STZ to induce pancreatic injury (16). With this model, reversal of hyperglycemia was demonstrated but the end point was only 42 days (6,16). First, we titrated the dose and timing of STZ treatment. In Groups A or B, animals were injected intraperitoneally daily for 5 days with STZ 35 mg/kg, or 6 days with STZ 45 mg/kg, respectively. 77% (10/13) of animals in Group B developed hyperglycemia (blood glucose > 250 mg/dl; Fig. 3A and B). In contrast, only 29% (5/17) of animals in Group A developed hyperglycemia (Fig. 3A). Morphology of pancreatic tissue sections stained with H & E and for insulin showed that pancreatic damage by STZ was associated with the destruction of pancreatic islets (Fig. 3C) and a decrease in insulin-producing cells at day 42 after STZ treatment (Fig. 3D).
Figure 3. Dose titration of STZ treatment.
(A) % of mice with hyperglycemia after treatment with STZ for 35 mg/kg/day × 5 days (group A) or 45 mg/kg/day × 6 days (Group B). (B) Blood glucose level after STZ treatment. Blood glucose concentrations of hyperglycemic animals are shown as the mean ± SD. (C) H & E staining of representative pancreatic section. The left panel shows islet; the right panel shows the islet and the duct. Bar = 50 μm. (D) Immunofluorescence of pancreatic section at 42 days after STZ treatment. Islets in STZ-treated mice showed altered morphology such as small size, disorganized architecture, and decreased insulin secretion compared to untreated controls.
To test whether BM-derived cells contribute to β cell regeneration, BM cells were harvested from donor EGFP+ B6 mice and 5 × 106 BM cells were transplanted into B6 recipients conditioned with 950 cGy TBI (Fig. 4A). All recipients engrafted, with 86% average donor EGFP+ cells (Fig. 4B). The EGFP+ chimeras were treated for 6 days with STZ (45 mg/kg/day). On day 42 after STZ treatment, there was a marked increase of EGFP+ foci in the pancreas of the EGFP+ chimeras (Fig. 4C, right panel). Immunofluorescence staining of pancreatic tissue showed that EGFP+ BM-derived cells surrounding the ducts (Fig. 4D, top panel) and islet structures (Fig. 4D, bottom panel) were positive for insulin production.
Figure 4. Donor EGFP+ BMC contribute to regenerate.
β-cells. (A) Preparation of EGFP+ chimeras. Donor BM cells were harvested from EGFP+ B6 mice and 5 × 106 cells were transplanted into recipient B6 mice conditioned with 950 cGy TBI. (B) Donor EGFP+ chimerism was assessed 3 months using flow cytometry. (C) EGFP+ cells were observed in pancreas of EGFP+ mixed chimeras 42 days after STZ treatment. The pancreas of EGFP+ B6 and naïve B6 mice served as controls. (D) Top panel: sections were stained for EGFP (green) and insulin (red) and show co-localization of EGFP+ and insulin expressing cells in pancreatic ducts. The lower panel: sections stained for nuclei using DAPI (blue), insulin (red), and EGFP (green), and show co-localization of EGFP+ and insulin-expressing cells in the islet region at 42 days after STZ treatment.
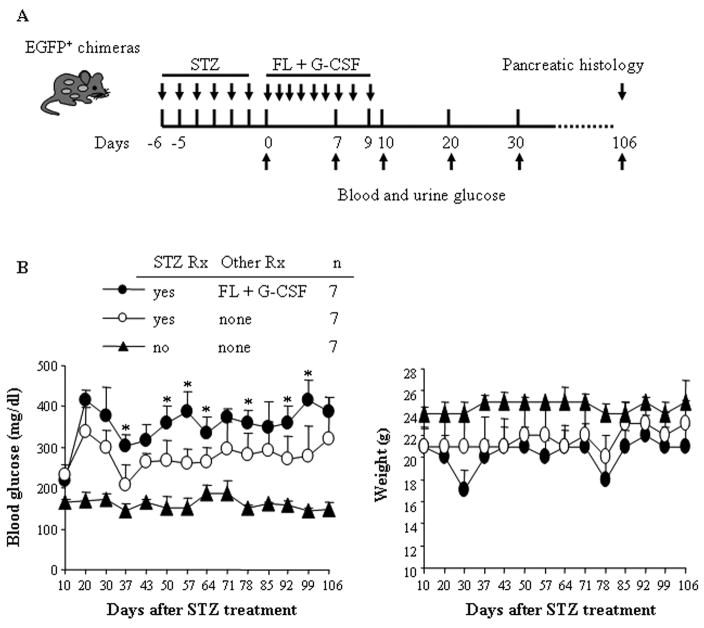
Flt3 ligand and G-CSF treatment failed to improve pancreatic function
FL and G-CSF mobilize HSC into peripheral blood (22). We previously reported that treatment of mice with myocardial ischemia/reperfusion damage with FL and G-CSF significantly limited adverse left ventricular remodeling and improved left ventricular performance by promoting cardiac regeneration (23). We therefore examined whether FL and G-CSF treatment would enhance pancreatic β cell regeneration and improve the function of the pancreas. To test this, STZ-treated EGFP+ chimeras were either given a follow-up treatment of FL and G-CSF for 10 days or no treatment (Fig. 5A). We found that STZ-treated mice receiving FL + G-CSF have a significantly higher level of blood glucose compared to those without FL + G-CSF treatment (Fig. 5B) and who also had low body weight (Fig. 5C). FL + G-CSF treatment failed to enhance β cell regeneration and improve the function of the damaged pancreas long term.
Figure 5. FL + G-CSF treatment elevated blood glucose in STZ-treated mice.
(A) EGFP+ mixed chimeras were treated with STZ for 6 days. Experimental mice were then treated with FL and G-CSF for 10 days. Untreated animals and STZ-treated animals served as controls. (B) Blood glucose and (C) weight were measured weekly. Blood glucose concentrations and weight were shown as the mean ± SD. *P< 0.05 for mice treated with FL + G-CSF vs. without FL + G-CSF.
Transplantation of bone marrow cells reduces blood glucose levels temporarily
To test whether transplantation of BM-derived cells into recipients improved islet function by regeneration of β cells, B6 mice were treated with STZ for 6 days. Ten days after cessation of treatment, hyperglycemic B6 mice received BM-derived cells from EGFP+ donors (n = 10, Fig. 6A). Naïve B6 mice (n = 4) or STZ-treated B6 mice receiving media alone (n = 11) served as controls. Notably, 39 days after transplantation (day 49), the levels of blood glucose were significantly decreased in hyperglycemic mice that had received BM cells compared to controls that did not (Fig. 6B). However, hyperglycemia reappeared by day 84 (Fig. 6B), suggesting that transplantation of BM-derived cells only transiently improves hyperglycemia after pancreatic injury.
Figure 6. Bone marrow transplantation temporarily reduces high blood glucose in STZ-treated mice.
(A) Experimental design for the induction of hyperglycemia and transplantation of EGFP+ B6 bone marrow. (B) Transplantation of BM-derived cells into STZ-treated B6 mice conditioned with 350 cGy of TBI (n = 10). Animals received only media (n = 11) or animals without STZ treatment (n = 4) served as controls. Blood glucose concentrations were shown as the mean ± SD. *P < 0.05 for STZ-treated mice that received BM cells or media alone vs. mice not treated with STZ.
DISCUSSION
Previous studies indicated that BM cells can develop into multiple tissue-specific cell types, including brain (2), muscle (24), liver (25), and heart (26), suggesting that adult BM harbors a pool of primitive stem cells capable of differentiating into multiple tissue types. Recent studies on chimeric animals involving the transplantation of a single EGFP+ HSC demonstrated that plasticity of circulating HSC is an extremely rare event, if it occurs at all (27). These observations provide a strong rationale for an alternative hypothesis that other types of stem cells that also reside in BM, separate from HSC, contribute to tissue regeneration. In the mouse, VSEL cells, a rare homogenous population in BM, have been shown to have similar morphology to and express several developmental markers of undifferentiated embryonic-like stem cells. These cells are also capable of differentiating into cells from all three germ-layers in vitro (8). We previously demonstrated that VSEL are a mobile pool of stem cells that are mobilized into PB following: 1) heart infarct in mice (28) and humans (29); 2) in a murine model of stroke (8); and 3) after toxic liver or skeletal muscle injury and G-CSF induced mobilization in mice (30).
VSEL cells express CXCR4 (α-chemokine Gαi-protein-coupled seven-transmembrane span receptor), c-met (tyrosine kinase receptor), and LIF-receptors, and respond robustly in migration to SDF-1, HGF, and LIF gradients (8). In the present studies, we found that developmental pancreatic transcripts Nkx6.1, Pdx1 and Ptf1 are also significantly enriched in the VSEL population compared to HSC. The kinetic changes for upregulation for mRNA for SDF-1 and HGF coincided with the recovery time of cells expressing Nkx6.1, Pdx1, and Ptf1 found in damaged pancreas after STZ treatment. These findings suggest that CXCR4/SDF-1 and c-met/HGF may play an important role in mobilization of VSEL from BM into damaged pancreatic tissue. Furthermore, we provide evidence that BM-derived cells contribute to β cell regeneration and improve pancreatic function in STZ-induced hyperglycemia. In the current study we found that VSEL cells are also mobilized into PB after moderate pancreatic damage. Taken together, these data suggest that VSEL cells are a population of stem cells that are mobilized in response to tissue injuries, possibly acting to circulate in PB to attempt to support regeneration of damaged organs.
It was previously reported that infusion of bone marrow cells improved the function of damaged pancreas in STZ-treated mice (16). Transplantation of EGFP+ whole BM cells restored blood glucose and serum insulin levels to almost normal within 7 to 42 days following low-dose STZ-damage to pancreas (16). Only 2.5% of pancreatic cells co-expressed EGFP and insulin. However, the EGFP+ cells showed an absence of Pdx1 expression, suggesting that although bone marrow-derived cells were capable of adopting a β cell phenotype (insulin positive), they did not express transcriptional factors associated with β cell development (16). The animals in this study were euthanized at day 42 and long-term follow up was not performed to assess durability of the outcome. A second publication which used slightly higher doses of STZ found that syngeneic whole BMC resulted in temporary resolution of hyperglycemia (13). However, as we observed, this was not sustained long term. Using a similar protocol, our findings confirmed that hyperglycemia was reduced for up to 77 days (Fig. 6). However, we unexpectedly found that with longer follow up, the observed reduction in hyperglycemia was not durable. One possible explanation for this result might be the lack of additional stimuli to promote β cell regeneration that resulted in failure of long-term improvement of pancreatic function in STZ-induced diabetes.
The potential for BM-derived cells to differentiate to form pancreatic β cells has been investigated. However, the cells responsible and mechanisms of islet neogenesis remain elusive. When EGFP+ BM cells were transplanted into neonatal or adult female NOD mice, the number of donor BM-derived ductal cells was significantly higher in the neonatal mice (4.6%) compared to adult mice (0.5%) (31). As low as 1% allogeneic chimerism was shown to reverse the progression of diabetes and autoimmune destruction of islets of Langerhans in prediabetic NOD mice. Moreover, endogenous β cell function and mass were restored (11). It has been shown that BM-derived cells have the ability to transdifferentiate to produce functioning pancreatic β cells but show no evidence of cell fusion in vivo (12). In a different mouse model for β cell regeneration after chemical ablation by the β cell toxin alloxan, rapid regeneration of β cell mass by stem/progenitor cell differentiation was observed, rather than by proliferation of surviving β cells (32). To investigate the potential role of BM-derived cells to differentiate to β cells, we prepared EGFP+ chimeras by transplanting donor EGFP+ B6 BM cells into ablated B6 recipients. The EGFP+ chimeras were treated with STZ to induce pancreatic damage. We found co-expression of donor-derived EGFP+ and insulin+ cells in the pancreatic ducts and islets (Fig. 4D). However, the donor BM-derived cells differentiated into pancreatic tissue at a very low frequency. Taken together, these findings provide strong evidence that BM-derived cells contribute to pancreatic β cell regeneration and suggest a role for host as well as donor stem cells in this process.
The transcription factors Pdx1, Nkx6.1, and Ptf1 are differentially involved in β cell development. All pancreatic cell lineages, including endocrine cells, originate in the pancreatic anlage expressing the homeobox transcription factor Pdx1 (33). Complete knockout of Pdx1 in mice results in pancreatic agenesis (34). Pdx1 expression was associated with β cell neogenesis in a rodent model of pancreatic injury (35). A recent study showed that recombinant Pdx1 treatment of mice with STZ-induced diabetes promotes β cell regeneration and leads to restoration of normoglycemia (36).
Nkx6.1 is a homeodomain transcription factor. Disruption of the Nkx6.1 gene in mutant mice leads to loss of β cell precursors and blocks β cell neogenesis specifically during secondary transition (37). The decision to differentiate into exocrine or endocrine cells requires the Notch signaling pathway acting on the expression of Ngn3 and the Ptf1 (38). Our results found that in bone marrow, VSEL cells are significantly enriched for developmental pancreatic transcripts (Nkx6.1, Pdx1 and Ptf1) compared with HSC (Fig. 1A). The data in Fig. 1B-E strongly suggest that VSEL cells were mobilized into peripheral tissue (PB, spleen, and pancreas) after STZ-induced pancreas damage.
In addition, we found that the levels of mRNA for SDF-1 and HGF were upregulated in damaged pancreatic tissue. The kinetic changes of the levels of SDF-1 and HGF mRNA were consistent with cells expressing Nkx6.1, Pdx1 and Ptf1 in damaged pancreas. SDF-1, which is secreted by BM stroma, plays an essential role in chemoattracting HSC and other stem cells expressing its receptor CXCR4 to BM (39,40). We envision that VSEL circulate between BM and peripheral tissues and if needed could take part in the regeneration of damaged organs (41–43). It has been shown that SDF-1 is expressed in and around the proliferating ductal epithelium of the regenerating pancreas of NOD mice (44). An increase in SDF-1 has been observed in other damaged organs including infarcted heart (45), limb ischemia (46), toxic liver damage (47).
β cell regeneration can be enhanced by a variety of growth factors, such as IGF-1 growth hormone, gastrin and others (6). Specific growth factors such as G-CSF, granulocyte/monocyte colony stimulating factor (GM-CSF), and FL can expand and mobilize stem cells from the BM into PB (48,49). We previously reported that treatment with FL plus G-CSF at the time of ischemic cardiac damage is associated with a highly significant improvement in cardiac function (23). Notably, EGFP+ BM-derived cells mediated repair in a mouse model for cardiac regeneration (23). In contrast, treatment of recipients with a combination of FL + G-CSF did not improve the function of STZ-injured pancreas (Fig. 5B). The level of blood glucose in mice treated with FL and G-CSF was significantly higher than in those without FL and G-CSF treatment, suggesting that FL + G-CSF treatment failed to mobilize specific pancreatic stem cells into damaged pancreas to enhance β cell regeneration and recover insulin secretion to reduce the levels of blood glucose. Previous studies have shown that inhibition of macrophage infiltration by silica treatment decreases insulitis and the development of diabetes in low-dose STZ-treated mice (50). FL treatment expands the number of lymphocytes, granulocytes, and monocytes in peripheral blood (51). It is therefore possible that FL treatment expands the number of macrophages in the islets and leads to more severe STZ-induced diabetes development. In the future, however, it would be important to evaluate the effect of CXCR4 blockade (e.g., by T140) on mobilization of VSEL cells and their subsequent contribution to pancreatic regeneration. Our recent data on G-CSF + T140 induced mobilization in mice lend support to this notion (30). We previously found that FL-administration to pre-diabetic NOD mice resulted in diabetes-prevention (52). Treatment of prediabetic NOD mice with a 10 day course of FL significantly decreased insulitis and progression to diabetes, and was associated with a significant increase in myeloid dendritic cells, plasmacytoid dendritic cells and induction of CD4+/CD25+ regulatory T cells in pancreatic lymph nodes.
In conclusion, we show here that BM-derived VSEL cells express markers for pancreatic development and are mobilized into peripheral tissues after pancreatic damage. Further, our data indicate that BM-derived cells contribute to β cell regeneration and transiently improve the function of damaged pancreas. An approach to promote and maintain β cell regeneration or limit β cell apoptosis may result in an alternative approach to treat diabetes.
Supplementary Material
Acknowledgments
The authors thank Dr. Larry D. Bozulic for review of the manuscript and helpful comments; Drs. Daoxin Wang and Bing Li, Barry Udis and Michael K. Tanner for technical assistance; Carolyn DeLautre for manuscript preparation; and the staff of the University of Louisville animal facility for outstanding animal care.
ABBREVIATIONS
- BM
bone marrow
- CSM
cell sort media
- EGFP+
enhanced green fluorescent protein-positive
- FL
Flt3-ligand
- G-CSF
granulocyte colony stimulating factor
- HSC
hematopoietic stem cells
- HGF
hepatocyte growth factor
- LIF
leukemia inhibitory factor
- NOD
non-obese diabetic
- PB
peripheral blood
- STZ
streptozotocin
- VSEL
very small embryonic-like
Footnotes
This work was supported in part by NIH R01 DK069766 and NIH 5RO1 HL063442; JDRF 1-2005-1037 and JDRF 1-2006-146; The Department of the Navy, Office of Naval Research; The Department of the Army, Office of Army Research. (Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the Office of Army Research); the National Foundation to Support Cell Transplant Research; the Commonwealth of Kentucky Research Challenge Trust Fund; the W. M. Keck Foundation; and The Jewish Hospital Foundation.
Author contribution: Yiming Huang: Conception and design, collection and/or assembly of data, data analysis and interpretation, and manuscript writing
Magda Kucia: Conception and design, collection and/or assembly of data, data analysis, and interpretation
Lala-Rukh Hussain: collection and/or assembly of data
Yujie Wen: collection and/or assembly of data
Hong Xu: collection and/or assembly of data, data analysis and interpretation
Jun Yan: collection and/or assembly of data, data analysis and interpretation
Mariusz Z. Ratajczak: Conception and design and manuscript writing
Suzanne T. Ildstad: Conception and design, manuscript writing
Reference List
- 1.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 2.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 4.Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97:7999. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 6.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotechnol. 2005;23:857. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 7.Ratajczak MZ, Zuba-Surma EK, Machalinski B, Ratajczak J, Kucia M. Very small embryonic-like (VSEL) stem cells: purification from adult organs, characterization, and biological significance. Stem Cell Rev. 2008;4:89. doi: 10.1007/s12015-008-9018-0. [DOI] [PubMed] [Google Scholar]
- 8.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4(+) stem cells identified in adult bone marrow. Leukemia. 2006;20:857. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman CL, Li H, Ildstad ST. Patterns of hemopoietic reconstitution in nonobese diabetic mice: dichotomy of allogeneic resistance versus competitive advantage of disease-resistant marrow. J Immunol. 1997;158:2435. [PubMed] [Google Scholar]
- 10.Li H, Kaufman CL, Boggs SS, Johnson PC, Patrene KD, Ildstad ST. Mixed allogeneic chimerism induced by a sublethal approach prevents autoimmune diabetes and reverses insulitis in non-obese diabetic (NOD) mice. J Immunol. 1996;156:380. [PubMed] [Google Scholar]
- 11.Zorina TD, Subbotin VM, Bertera S, et al. Recovery of the endogenous beta cell function in the NOD model of autoimmune diabetes. Stem Cells. 2003;21:377. doi: 10.1634/stemcells.21-4-377. [DOI] [PubMed] [Google Scholar]
- 12.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wen Y, Ouyang J, Yang R, et al. Reversal of new-onset type 1 diabetes in mice by syngeneic bone marrow transplantation. Biochem Biophys Res Commun. 2008;374:282. doi: 10.1016/j.bbrc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic beta-cells in vivo. Diabetes. 2004;53:616. doi: 10.2337/diabetes.53.3.616. [DOI] [PubMed] [Google Scholar]
- 15.Taneera J, Rosengren A, Renstrom E, et al. Failure of transplanted bone marrow cells to adopt a pancreatic beta-cell fate. Diabetes. 2006;55:290. doi: 10.2337/diabetes.55.02.06.db05-1212. [DOI] [PubMed] [Google Scholar]
- 16.Hess D, Li L, Martin M, et al. Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol. 2003;21:763. doi: 10.1038/nbt841. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa Y, Ogihara T, Yamada T, et al. Bone marrow (BM) transplantation promotes beta-cell regeneration after acute injury through BM cell mobilization. Endocrinology. 2007;148:2006. doi: 10.1210/en.2006-1351. [DOI] [PubMed] [Google Scholar]
- 18.Banerjee M, Kumar A, Bhonde RR. Reversal of experimental diabetes by multiple bone marrow transplantation. Biochem Biophys Res Commun. 2005;328:318. doi: 10.1016/j.bbrc.2004.12.176. [DOI] [PubMed] [Google Scholar]
- 19.Than S, Ishida H, Inaba M, et al. Bone marrow transplantation as a strategy for treatment of non-insulin-dependent diabetes mellitus in KK-Ay mice. J Exp Med. 1992;176:1233. doi: 10.1084/jem.176.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y, Ratajczak MZ, Reca R, et al. Flt3 expression discriminates HSC subpopulations with differing engraftment-potential: identifying the most potent combination. Transplantation. 2008;85:1175. doi: 10.1097/TP.0b013e31816a89cf. [DOI] [PubMed] [Google Scholar]
- 21.Wysoczynski M, Reca R, Ratajczak J, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 22.Neipp M, Zorina T, Domenick MA, Exner BG, Ildstad ST. Effect of FLT3 ligand and granulocyte colony-stimulating factor on expansion and mobilization of facilitating cells and hematopoietic stem cells in mice: kinetics and repopulating potential. Blood. 1998;92:3177. [PubMed] [Google Scholar]
- 23.Dawn B, Guo Y, Rezazadeh A, et al. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson KA, Mi T, Goodell MA. Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc Natl Acad Sci USA. 1999;96:14482. doi: 10.1073/pnas.96.25.14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagasse E, Connors H, Al Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med. 2000;6:1229. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 26.Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 27.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 28.Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wojakowski W, Tendera M, Kucia M, et al. Mobilization of bone marrow-derived Oct-4+ SSEA-4+ very small embryonic-like stem cells in patients with actue myocardial infarction. J Am Coll Cardiol. 2009;53:1. doi: 10.1016/j.jacc.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kucia MJ, Wysoczynski M, Wu W, Zuba-Surma EK, Ratajczak J, Ratajczak MZ. Evidence that very small embryonic-like stem cells are mobilized into peripheral blood. Stem Cells. 2008;26:2083. doi: 10.1634/stemcells.2007-0922. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Ge S, Gonzalez I, et al. Formation of pancreatic duct epithelium from bone marrow during neonatal development. Stem Cells. 2006;24:307. doi: 10.1634/stemcells.2005-0052. [DOI] [PubMed] [Google Scholar]
- 32.Grapin-Botton A, Heimberg H, Lemaigre F. The genetic programme of pancreatic beta-cells: basic science for the development of beta-cell therapy. Workshop on programming pancreatic beta-cells. EMBO Rep. 2007;8:322. doi: 10.1038/sj.embor.7400944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev. 2002;12:540. doi: 10.1016/s0959-437x(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 35.Holland AM, Gonez LJ, Naselli G, MacDonald RJ, Harrison LC. Conditional expression demonstrates the role of the homeodomain transcription factor Pdx1 in maintenance and regeneration of beta-cells in the adult pancreas. Diabetes. 2005;54:2586. doi: 10.2337/diabetes.54.9.2586. [DOI] [PubMed] [Google Scholar]
- 36.Koya V, Lu S, Sun YP, et al. Reversal of streptozotocin-induced diabetes in mice by cellular transduction with recombinant pancreatic transcription factor pancreatic duodenal homeobox-1: a novel protein transduction domain-based therapy. Diabetes. 2008;57:757. doi: 10.2337/db07-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwitzgebel VM, Scheel DW, Conners JR, et al. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 38.Esni F, Ghosh B, Biankin AV, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 39.Geminder H, Sagi-Assif O, Goldberg L, et al. A possible role for CXCR4 and its ligand, the CXC chemokine stromal cell-derived factor-1, in the development of bone marrow metastases in neuroblastoma. J Immunol. 2001;167:4747. doi: 10.4049/jimmunol.167.8.4747. [DOI] [PubMed] [Google Scholar]
- 40.Libura J, Drukala J, Majka M, et al. CXCR4-SDF-1 signaling is active in rhabdomyosarcoma cells and regulates locomotion, chemotaxis, and adhesion. Blood. 2002;100:2597. doi: 10.1182/blood-2002-01-0031. [DOI] [PubMed] [Google Scholar]
- 41.Ratajczak MZ, Kucia M, Reca R, Majka M, Janowska-Wieczorek A, Ratajczak J. Stem cell plasticity revisited: CXCR4-positive cells expressing mRNA for early muscle, liver and neural cells ‘hide out’ in the bone marrow. Leukemia. 2004;18:29. doi: 10.1038/sj.leu.2403184. [DOI] [PubMed] [Google Scholar]
- 42.Kucia M, Ratajczak J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Tissue-specific muscle, neural and liver stem/progenitor cells reside in the bone marrow, respond to an SDF-1 gradient and are mobilized into peripheral blood during stress and tissue injury. Blood Cells Mol Dis. 2004;32:52. doi: 10.1016/j.bcmd.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Ratajczak MZ, Majka M, Kucia M, et al. Expression of functional CXCR4 by muscle satellite cells and secretion of SDF-1 by muscle-derived fibroblasts is associated with the presence of both muscle progenitors in bone marrow and hematopoietic stem/progenitor cells in muscles. Stem Cells. 2003;21:363. doi: 10.1634/stemcells.21-3-363. [DOI] [PubMed] [Google Scholar]
- 44.Kayali AG, Van Gunst K, Campbell IL, et al. The stromal cell-derived factor-1alpha/CXCR4 ligand-receptor axis is critical for progenitor survival and migration in the pancreas. J Cell Biol. 2003;163:859. doi: 10.1083/jcb.200304153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kucia M, Dawn B, Hunt G, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood following myocardial infarction. Circ Res. 2004;95:1191. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 47.Kollet O, Shivtiel S, Chen YQ, et al. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gianni AM, Bregni M, Siena S, et al. Granulocyte-macrophage colony-stimulating factor or granulocyte colony-stimulating factor infusion makes high-dose etoposide a safe outpatient regimen that is effective in lymphoma and myeloma patients. J Clin Oncol. 1992;10:1955. doi: 10.1200/JCO.1992.10.12.1955. [DOI] [PubMed] [Google Scholar]
- 49.Haas R, Ho AD, Bredthauer U, et al. Successful autologous transplantation of blood stem cells mobilized with recombinant human granulocyte-macrophage colony-stimulating factor. Exp Hematol. 1990;18:94. [PubMed] [Google Scholar]
- 50.Papaccio G, Frascatore S, Esposito V, Pisanti FA. Acta Anat. Vol. 142. Basel: 1991. Early macrophage infiltration in mice treated with low-dose streptozocin decreases islet superoxide dismutase levels: prevention by silica pretreatment; p. 141. [DOI] [PubMed] [Google Scholar]
- 51.Brasel K, McKenna HJ, Morrissey PJ, et al. Hematologic effects of flt3 ligand in vivo in mice. Blood. 1996;88:2004. [PubMed] [Google Scholar]
- 52.Chilton PM, Rezzoug F, Fugier-Vivier I, et al. Flt3-Ligand Treatment Prevents Diabetes in NOD Mice. Diabetes. 2004;53:1995. doi: 10.2337/diabetes.53.8.1995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.