Abstract
At present, eszopiclone and zolpidem are the most commonly prescribed drugs for treating insomnia. Despite the established relationship between sleep disturbance and anxiety, it remains unknown whether targeted treatment for insomnia may affect acute anxiety. Therefore, the objective of this study was to examine the effects of three different doses (1, 3, and 10 mg/kg) of eszopiclone and zolpidem on the states of sleep and wakefulness, levels of anxiety-like behavior, and long-term contextual memory in footshock-induced anxious rats. The results of this study demonstrated that the administration of eszopiclone and zolpidem both were equally effective in attenuating footshock stressor-induced suppression of slow-wave sleep (SWS). The administration of eszopiclone at 1 mg/kg or zolpidem at 1 and 3 mg/kg doses showed a tendency for attenuating stressor-induced suppression of REM sleep. However, the REM sleep attenuating effects of these drugs disappeared when they were administered at higher doses. The administration of eszopiclone at 3 and 10 mg/kg doses and zolpidem at all three doses reduced the power of electroencephalographic theta band frequencies during wakefulness. In addition, the administration of eszopiclone at 1 and 3 mg/kg doses suppressed stressor-induced anxiety-like behavior. The administration of zolpidem at 1, 3, or 10 mg/kg doses was not effective in attenuating stressor-induced anxiety-like behavior. Contextual memory after administration of eszopiclone at 1 mg/kg dose had no effects, but was reduced significantly with increased dosage. Contextual memory after administration of zolpidem, at all three doses, was severely disrupted. The results of this study suggest that eszopiclone at a low dose could be used effectively to control anxiety and anxiety-induced insomnia.
Keywords: Eszopiclone, Zolpidem, REM sleep, Slow-wave sleep, Wakefulness, Anxiety, Contextual memory, Rat
1. Introduction
Insomnia and generalized anxiety disorder (GAD) are highly prevalent conditions with significant associated distress and morbid consequences [11, 50, 91, 93]. These conditions commonly coexist and have considerable symptomatic overlap. The majority of patients with GAD have at least one form of comorbid sleep disturbance [66, 73, 94, 96]. Additionally, GAD is one of the most common psychiatric comorbidities in individuals with insomnia [61, 65]. Most objective clinical studies of sleep disturbances and anxiety have focused primarily on long-term effects of anxiety [50] and have noted an increase in sleep latency, lower percentage of deep sleep and decreased rapid eye movement (REM) density [31, 67, 85, 94]. The immediate effects of anxiety on sleep architecture are considerably more elusive. Insomnia may predispose individuals to develop anxiety disorders [30] or may develop subsequently after the onset of anxiety [44, 65, 80, 81]. Despite an established relationship between insomnia and anxiety, it remains relatively unknown whether targeted treatment of insomnia may affect anxiety.
To study the immediate effects of anxiety, many researchers utilize the rodent model as a convenient method of analyzing behavioral and pharmacological responses to stress. The elevated plus-maze (EPM) is the most extensively used behavioral paradigm to measure anxiety in the rodent model [9, 42, 53]. Anxiety-related behavior reflects a conflict between the rodent’s desire to explore a novel environment and its innate preference to protected areas [106]. The open arm of the EPM is considered anxiogenic, or anxiety-inducing; thus, rats exhibiting greater anxiety-related behavior will enter and spend less time in the open arms of the maze [3, 70]. The percentage of time spent on the open arm is increased and decreased with anxiolytic and anxiogenic substances, respectively [3, 70, 78]. When used appropriately, the EPM is the most effective and popular animal model to observe and quantify anxiety within the rodent model [53, 77].
The rodent model is also frequently employed to measure the changes in sleep-wake architecture after experimental manipulation of fear-inducing stress [41, 43, 53, 56, 99]. Classical fear conditioning, the most common paradigm, utilizes an unconditioned foot-shock presented with a conditioned stimulus to measure the acquisition, expression and/or extinction of fear [43, 53, 68, 99]. Neuroimaging techniques, such as fMRI, have afforded researchers the opportunity to examine the similarities between structures involved in the acquisition and expression of fear in humans and rodents [25]. A number of studies have demonstrated that homologous structures in the human and rat are involved in fear acquisition, an important aspect of anxiety behavior [7, 112]. The contextual fear conditioning model is also a popular model to study acquisition, consolidation, and retrieval of memory in rodents [5, 16, 29]. Numerous other paradigms employ a range of stressors including immobilization, corticosterone administration and open field exposure [98, 100, 103]. All of these techniques and manipulations have significant effects on sleep-wake behavior, but the degree of behavioral stress is often assumed or unknown.
Benzodiazepines (BZs) have long been used as anxiolytics, and these drugs have also found widespread use in the treatment of a variety of sleep disorders [49, 52, 62]. BZs work by binding to specific allosteric BZ sites on GABA-A receptors, thereby modulating the function of these receptors [74, 75, 83, 108, 109]. Mammalian GABA-A receptors in the central nervous system are pentameric structures consisting of distinct subunits, which can include α1–6, β1–3, and γ1–3 or δ [90]. Classical BZs bind equally well to GABA-A receptors containing all of the α subunit isoforms except α4 and α6 [38, 74, 108]. In recent years, the use of BZs has been demonstrated to have a number of deleterious side effects such as anterograde amnesia and the potential to build tolerance, dependence, and withdrawal pathologies [27, 49, 59, 62, 76]. Awareness of these undesirable effects of BZs resulted in the development of a new generation of nonbenzodiazepine hypnotics, including zolpidem (Zol; Ambien; Sanofi-aventis, Bridgewater, NJ) and eszopiclone (Esz; Lunesta; Sepracor, Marlborough, MA). zolpidem has a high affinity for GABA-A receptors containing the α1 subunit, low affinity for α2- and α3-containing receptors, and no significant affinity for α5-containing receptors [2, 38, 74, 108]. In contrast, eszopiclone exhibits considerable activity at GABA-A receptors containing α1, α2, α3, and α5 subunits [2, 13, 23, 36, 38, 84, 92].
In recent years, a number of studies in both humans [24, 28, 40, 47–49, 62, 63, 82] and animals [10, 34, 35, 54, 64, 95, 110, 111] have tested the hypnotic effects of eszopiclone and zolpidem. These studies have suggested that both eszopiclone and zolpidem are nearly equally effective hypnotics with very few or no adverse side effects. One animal study, suggested that eszopiclone might have some anxiolytic action in naïve (not yet subjected to experimental stress) rats [8]. While a number of reports have described the hypnotic effects of eszopiclone and zolpidem in humans and animals, there have been no animal or human studies that have systematically tested and/or compared the effects of eszopiclone and zolpidem on acute anxiety and anxiety-induced changes in the sleep-wake cycle and memory. The changes in the power of delta frequency waves in the cortical EEG is considered to be a physiological indicator of changes in the quality of sleep during slow-wave sleep and intensity of homeostatic sleep pressure during wakefulness [4, 43, 89]. Similarly, the changes in the power of theta frequency waves in the cortical EEG is considered to be a physiological indicator of changes in the contextual memory processing [16, 72]. The present study therefore examines the effects of an intraperitoneal injection of eszopiclone and zolpidem on stressor-induced anxiety-like EPM behavior, changes in the sleep-wake architecture, delta and theta frequency powers in the cortical EEG, and contextual memory in chronically-instrumented freely moving rats.
2. Materials and methods
2.1. Subjects and housing
Experiments were performed on 56 adult male Wistar rats (Charles River Laboratories, Wilmington, MA) weighing between 300 and 375 grams. The rats were housed individually at 24°C with ad libitum access to food and water. Lights were on from 7:00 a.m. to 7:00 p.m. (light cycle) and off from 7:00 p.m. to 7:00 a.m. (dark cycle). The principles for care and use of laboratory animals in research, as outlined by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (1996), were strictly followed. Additional care was taken to ensure that any potential discomfort and the number of animals used were minimized. To reduce additional stress that might be imposed by the experimental handling, animals were handled daily for 15–20 min between 09:00 a.m. and 10:00 a.m. This habituation handling began one week prior to surgery and continued for the duration of the experiment.
2.2. Surgical procedures for electrode implantation
All surgical procedures were performed stereotaxically under aseptic conditions and were in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (protocol AN-14829). Animals were anesthetized with sodium pentobarbital (40 mg/kg, i.p.; Ovation Pharmaceuticals, Deerfield, IL), placed in the stereotaxic apparatus, and secured using blunt rodent ear bars as described previously [69]. The appropriate depth of anesthesia was judged by the absence of palpebral reflexes and a response to tail pinch. Core body temperature was maintained at 35 ± 1°C with a thermostatic heating pad and a rectal thermister probe. A scalp incision was made, and the skin was retracted. The skull surface was cleaned in preparation for electrode implantation. After completion of the surgical procedure, ampicillin (50 mg per rat, s.c. Bristol-Myers Squibb, Princeton, NJ ) was administered to control any potential postsurgical infection. Potential postoperative pain was controlled with buprenorphine (0.03 mg/kg, s.c.; Ben Venue Laboratories, Bedford, OH).
To record vigilance states, cortical electroencephalogram (EEG), dorsal neck muscle electromyogram (EMG) and hippocampal EEG (to record theta wave activity) recording electrodes were chronically implanted as described previously [14, 18]. All electrodes were secured to the skull with dental acrylic. Electrodes were crimped to miniconnector pins and brought together in a plastic connector. Immediately after surgery, animals were placed in recovery cages and monitored for successful recovery from anesthesia and surgery. Successful recovery was gauged by the return of normal postures, voluntary movement, and grooming.
2.3. Habituation to sleep-wake recording conditions
After a postsurgical recovery period of 7–10 days, rats were habituated to a polygraphic sleep-wake recording cage under a freely moving recording conditions for 7 days as described in previous publications [15, 21]. During their recovery, habituation, and free-moving recording conditions, all rats experienced the same 12-h light/dark cycle with free access to food and water. These 6-h habituation sessions (from 10:00 a.m. to 4:00 p.m.) were also considered to be the baseline recording sessions for electrode testing and monitoring daily variations in the percentages of wake/sleep stages.
2.4. Drugs
Eszopiclone (Sepracor Inc., Marlborough, MA) or zolpidem (Sigma-Aldrich Co., St. Louis, MO) were used in this study. The compounds were dissolved in 50 mM acetate buffer. Control subjects were injected with an identical vehicle. The doses of both drugs were 1, 3, and 10 mg/kg. These doses were predetermined based on previous studies to determine the effects of zopiclone, eszopiclone, and zolpidem on sleep-wake behavior in guinea pigs [110, 111] and rats [34, 35]. All drugs were injected i.p. in a final volume of 1.0 ml.
2.5. Contextual fear conditioning and memory testing apparatus and procedures
This apparatus is an automated freezing behavior testing chamber (30 × 24 × 21 cm; Standard modular test chamber, ENV-008; Sound attenuating cubicle, ENV-022MD; Med Associates, St. Albans, VT). The chamber is constructed from aluminum (side walls) and Plexiglas (rear wall, ceiling, and hinged front door) and is situated in a sound-attenuating cabinet located in a brightly lit and isolated room. The floor of the chamber consists of 19 stainless-steel rods (4 mm in diameter) spaced 1.5 cm apart (center-to-center). Rods are wired to a shock source and solid-state grid scrambler for the delivery of foot-shocks. A speaker is mounted outside a grating in one wall of the chamber for the delivery of acoustic stimuli (tone). One small light bulb is mounted next to the speaker in the wall of the chamber for the delivery of light stimuli. A closed circuit video camera is mounted inside on a hinged front door of the cabinet to videotape the behavior of the rat inside the chamber. A ventilation fan in the cabinet provides background noise (65 db). A 15 W house light is mounted on the ceiling of the cabinet for illumination. Stimulus presentations are controlled by a custom written computer program using MED-PC (Med Associates, St. Albans, VT). Video freeze responses are also recorded and analyzed online using a customized computer program (Med Associates, St. Albans, VT).
In the present study, a contextual fear conditioning model was used for the induction of anxiety. For the contextual fear conditioning, naïve rats were taken from their housing room and transported to the behavioral testing room in a clear plastic cage. Five minutes after arrival, rats were placed inside the freezing behavior testing chamber at around 9:40 a.m. and were given 2 min of adaptation prior to foot-shock trials. After adaptation, all but the control group of rats were subjected to 10 trials of shock exposure. These trials consisted of unannounced (no light or tone) 1 s scrambled foot-shocks (1.0 mA) with variable intervals (0.5 to 2.0 min). One min after the end of the last trial, rats were removed from the behavioral testing chamber. The procedure for the control group was identical, except no shock was administered. For this procedure, all rats were inside the freezing behavior testing chamber for a total period of 15 min (between 9:40 a.m. and 9:55 a.m.). As soon as animals were removed from the chamber, they were injected (i.p., 1 mL) with control vehicle (50 mM acetate buffer) or three different doses (1, 3, and 10 mg/kg body weight) of eszopiclone (Sepracor Inc., Marlborough, MA) or zolpidem (Sigma-Aldrich Co., St. Louis, MO). For the shock and no-shock exposures, animals were selected randomly. Similarly, for the injection of vehicle control and one of the three different doses of two different drugs, shock-exposed animals were selected randomly. Immediately after the injection, these rats were connected to the polygraphic recording system and sleep-wake activities were recorded for 6 h (10:00 a.m. to 4:00 p.m.). Before and after each use, the behavior testing chamber was cleaned and disinfected with soapy water and chlorine dioxide disinfectant (MB-10, Quip laboratories, Wilmington, DE).
For assessing contextual fear memory the next morning at 9:40 a.m., the rats were again brought to the behavioral testing room. At 9:45 a.m. rats were placed in the freezing behavior chamber for 5-min testing sessions without any shock. During this 5-min testing session rat behavior was recorded by a video camera (located inside the sound-attenuating cabinet) and freezing response was analyzed online using video freeze software (sample rate: 30 frame/sec; min freeze duration: 15 frame; % freeze bin duration/5 min). Freezing behavior was defined as the absence of movement with the exception of breathing. Rats exhibiting higher percentages of freezing behavior are considered to have higher levels of contextual memory.
2.6. Elevated plus-maze (EPM) and assessment of anxiety-like behavior
An elevated plus-maze (EPM; Stoelting Co., Wood Dale, IL) test was used to measure the level of anxiety [9, 53, 70]. The EPM was constructed of heavy polyurethane plastic and consisted of two opposed open arms (50 × 10 cm) and two opposed enclosed arms (50 × 10 × 40 cm) with open roof. The maze was elevated 50 cm from the ground. This EPM testing room was illuminated with a red light bulb (40 W). Immediately after 6 h of polygraphic recordings, the rats were transferred to the EPM testing room and placed in a box (60 × 30 × 90 cm; open roof) with a smooth polyurethane plastic surface (identical to the EPM surface) for 5 min of adaptation. This 5 min period of adaptation was intended for rats to practice walking on a surface similar to the EPM surface under red light. In our preliminary experiments, we have seen that the natural exploratory walking activity of the naïve rats on the EPM is 30% more in rats with this adaptation than the rats without adaptation (unpublished study of our lab). Immediately after this 5 min adaptation session, rats were placed into the maze in the center of the cross, facing an enclosed arm, and allowed to freely roam for five minutes while being digitally recorded for behavior. Digital records of these EPM tests were scored in 1-min intervals as described in our earlier publication [53]. Each rat was tested only once for a duration of 5 min. Prior to each individual rat test, the elevated plus-maze was cleaned and disinfected with soapy water and chlorine dioxide disinfectant.
2.7. Experimental groups
A total of 56 rats were divided into 8 different groups based on the shock and drug treatments: (1) non-shocked injected with vehicle control (n = 7 rats; C-NS group), (2) shocked and injected with vehicle control (n = 7 rats; C-S group), (3) shocked and injected with 1 mg dose of zolpidem (n = 7 rats; S-Zol-1), (4) shocked and injected with 3 mg dose of zolpidem (n = 7 rats; S-Zol-3), (5) shocked and injected with 10 mg dose of zolpidem (n = 7 rats; S-Zol-10), (6) shocked and injected with 1 mg dose of eszopiclone (n = 7 rats; S-Esz-1), (7) shocked and injected with 3 mg dose of eszopiclone (n = 7 rats; S-Esz-3), (8) shocked and injected with 10 mg dose of eszopiclone (n = 7 rats; S-Esz-10).
2.8. Analysis of behavioral states and cortical EEG spectral
For the purpose of determining possible effects on sleep and wakefulness, polygraphic data were captured on-line with a computer using Gamma software (Grass product group; Astro-Med, West Warwick, RI). From this captured data, three behavioral states were distinguished and scored visually using Rodent Sleep Stager software (SleepSign for Animal; Kissei Comtec Co., LTD, Tokyo, Japan). These three states were W, SWS, and REM sleep. The physiological criteria for the identification of these wake sleep states were described in detail previously [21, 22]. The behavioral states of W, SWS and REM sleep were scored in successive 5-second epochs. The polygraphic measures provided the following dependent variables that are quantified for each animal: (1) percentage of recording time spent in W, SWS, and REM sleep; (2) latencies to onset of SWS and REM sleep; and (3) total number of REM sleep episodes. The effect of different treatments on the percentages of W, SWS, and REM sleep were statistically analyzed using a two-way ANOVA with time as a repeated-measure variable (six levels corresponding to six 1 h epochs after injection) and treatment as a between-subjects variable (8 levels corresponding to the eight different treatments). After two-way ANOVA, post-hoc Bonferroni post tests were done to determine the individual levels of significant difference between the vehicle control-injected shocked and non-shocked groups of rats. Post-hoc tests were also used among the shocked animals to determine significant differences between those injected with vehicle control and those treated with six different groups of drugs. The SWS and REM sleep latencies and total number of REM sleep episodes were analyzed using one-way ANOVA followed by Bonferroni’s multiple comparison test.
For power spectral analysis, cortical EEG signals were amplified and bandpass filtered (0.2–100 Hz) with a polygraph and Rodent Sleep Stager software (Grass product group; Astro-Med, West Warwick, RI). The amplified and filtered data were digitized at a sampling frequency of 200 Hz and subjected to a Fast Fourier transformation (SleepSign for Animal; Kissei Comtec Co., LTD, Tokyo, Japan) to calculate the cortical EEG power during the total 6 hour recording. Analysis focused on two frequency ranges: delta (0.2–4.0Hz) and theta (5–10Hz). The power of each frequency band was averaged and expressed as a percent of the total power within the frequency range of 0.2–40Hz. These two variables were analyzed using two-way ANOVA followed by Bonferroni post tests. All statistical tests were performed using Prism software (GraphPad Software, Inc., La Jolla, CA).
2.9. Analysis of EPM and freezing response
Anxiety-like behavior was measured using the EPM data. For statistical comparisons, the level of anxiety was assessed using the percentage of time spent in the open arm of the EPM and number of entries in the open arm. A high percentage of time on the open arm indicated of a low level of anxiety; correspondingly, a low percentage of time on the open arm indicated a high level of anxiety. Similarly, a high number of entries on the open arm indicated a low level of anxiety. Since the C-NS group received no stressor (shock), the percentage of time spent and number of entries on the open arm were considered to be the baseline EPM values for normal rats. The main effects of different treatment groups on the percentage of time spent and number of entries on the open arm were evaluated using a one-way ANOVA followed by post-hoc Bonferroni’s multiple comparison test. To determine the drug effects on contextual memory, the total percentages of time in freezing were analyzed using one-way ANOVA followed by Bonferroni’s multiple comparison test. All of these statistical tests were performed using Prism software.
3. Results
3.1. Effects on sleep-wake architecture
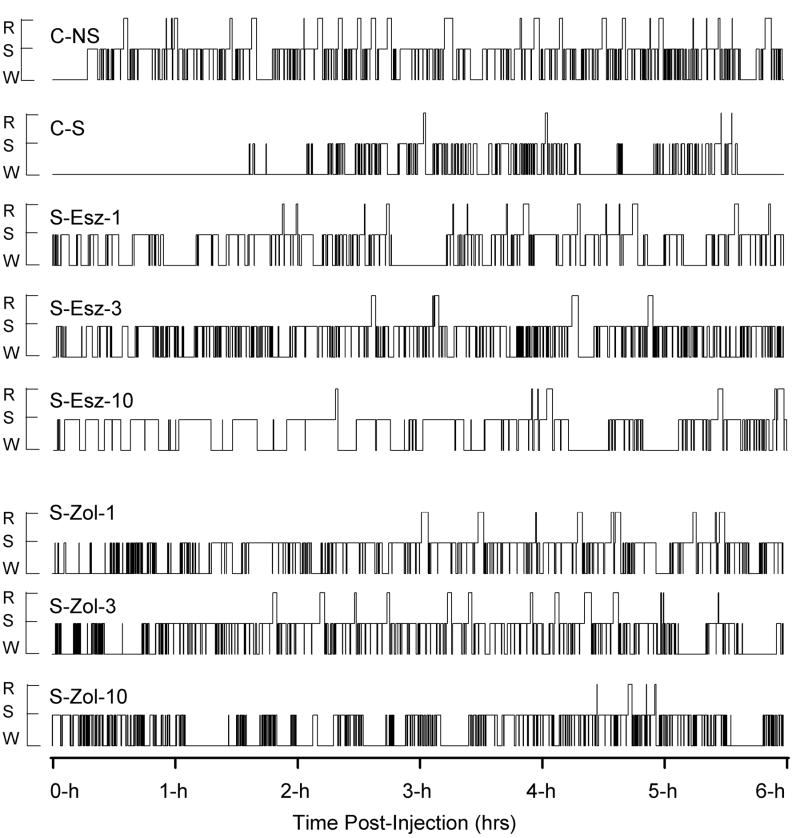
Figure 1 shows the representative sleep-wake architectures for the 6-h postinjection recording sessions (10:00 AM to 4:00 PM) starting immediately after each of eight different types of treatments. These sleep-wake architectures show that foot-shocked rats (C-S) after vehicle control injection remained awake for a long period of time, as compared to non-shocked vehicle-injected rat (C-NS). In the C-S animal, the latency between the end of injection and the first episode of REM sleep was much longer, and fewer REM sleep episodes occurred compared to the C-NS animal. These differences in sleep-wake architecture between the C-S and C-NS animals were the effects of inescapable foot-shock (stressor). The latencies of SWS were much shorter in the animals that received injections of drugs after the stressor (S-Esz-1, S-Esz-3, S-Esz-10, S-Zol-1, S-Zol-3, and S-Zol-10) than the animals receiving vehicle control injections after the stressor (C-S). These SWS latencies of foot-shocked drug-injected rats were also shorter than the non-shocked vehicle-injected rat (C-NS). The REM sleep latencies of S-Esz-1 and S-Zol-3 rats were much longer than the C-NS rat. In the S-Esz-10 and S-Zol-10 rats, the REM sleep latencies were much longer than the C-S and C-NS rats. These sleep-wake architectures indicate that a 1 mg dose of eszopiclone and 3 mg dose of zolpidem are somewhat more effective in attenuating the stressor-induced changes in the sleep-wake architecture.
Figure 1.
Examples of sleep-wake architecture for the 6-h period starting immediately after eight different types of treatment in various rats. The treatments were: non-shocked vehicle-injected (C-NS), shocked and vehicle-injected (C-S), shocked and 1 mg/kg dose of eszopiclone-injected (S-Esz-1), shocked and 3 mg/kg dose of eszopiclone-injected (S-Esz-3), shocked and 10 mg/kg dose of eszopiclone-injected (S-Esz-10), shocked and 1 mg/kg dose of zolpidem-injected (S-Zol-1), shocked and 3 mg/kg dose of zolpidem-injected (S-Zol-3), and shocked and 10 mg/kg dose of zolpidem-injected (S-Zol-10). These eight 6-h continuous step histograms plot occurrence and duration of polygraphically and behaviorally defined wakefulness (W), slow-wave sleep (S), and REM sleep (R).
3.2. Effects on wakefulness
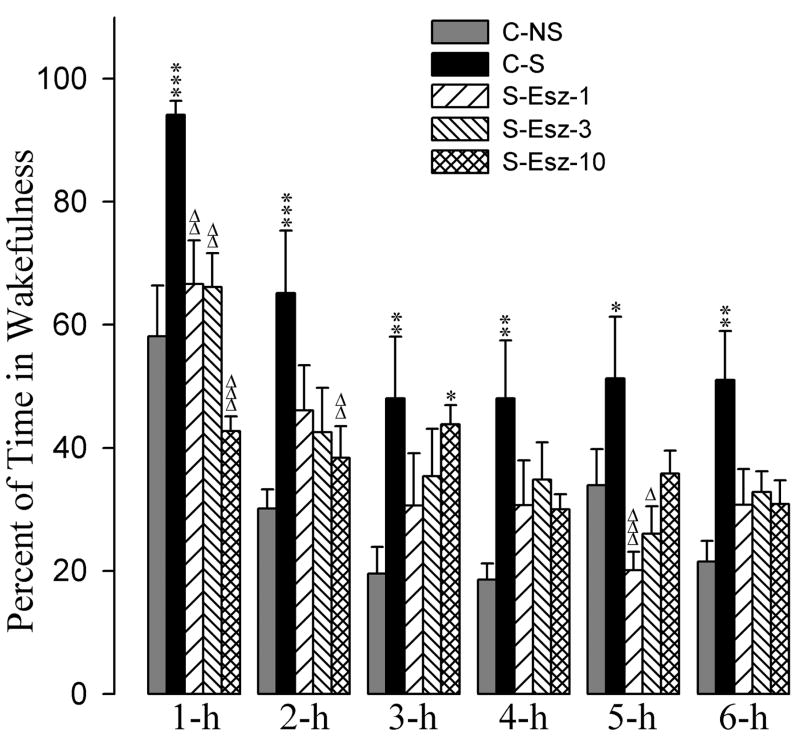
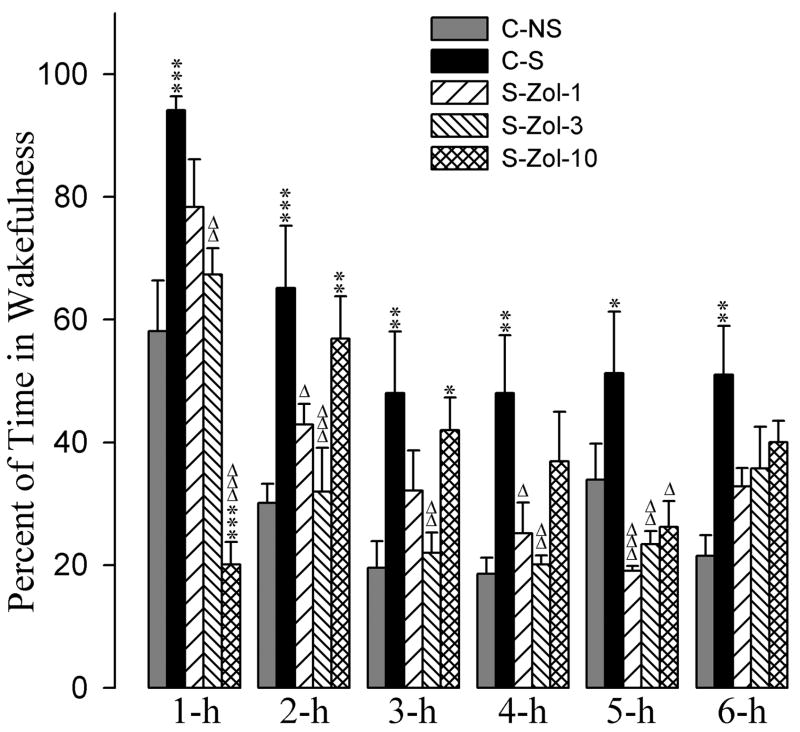
The effects of the eszopiclone and zolpidem administrations on the stressor-induced changes in the percentages of time spent in W are summarized in figures 2 and 3. A two-way ANOVA indicated a significant main effect of treatment [F(7,288) = 19, p<0.001], time [F(5,288) = 34, p<0.001], and a treatment × time interaction [F(35,288) = 3.3, p<0.001] on total percentages of time spent in W. The detailed results of post-hoc analysis (Bonferroni posttest) on the total percentages of time spent in W are presented in figures 2 and 3. Post-hoc analyses (Bonferroni posttest) revealed that the C-S animals spent significantly more time in W compared with the C-NS animals (Fig. 2). These results suggest that the stressor used in this study increased percentages of time spent in W and that this effect lasted through the 6-h period of experimental recording session.
Figure 2.
Effects of stressor and three different doses of eszopiclone on wakefulness. Bars represent percentages (mean + SE) of wakefulness during each of the 6-h periods after following treatments: non-shocked vehicle injected (C-NS), shocked and vehicle-injected (C-S), shocked and 1 mg/kg dose of eszopiclone-injected (S-Esz-1), shocked and 3 mg/kg dose of eszopiclone-injected (S-Esz-3), and shocked and 10 mg/kg dose of eszopiclone-injected (S-Esz-10). *Comparisons with non-shocked vehicle-injected (C-NS) and Δcomparisons with shocked and vehicle-injected (S-C). * or Δ: p<0.05; ** or ΔΔ: p<0.01; *** or ΔΔΔ: p<0.001.
Figure 3.
Effects of stressor and three different doses of zolpidem on wakefulness. Bars represent percentages (mean + SE) of wakefulness during each of the 6-h periods after following treatments: non-shocked vehicle-injected (C-NS), shocked and vehicle-injected (C-S), shocked and 1 mg/kg dose of zolpidem-injected (S-Zol-1), shocked and 3 mg/kg dose of zolpidem-injected (S-Zol-3), and shocked and 10 mg/kg dose of zolpidem-injected (S-Zol-10). *Comparisons with non-shocked vehicle-injected (C-NS) and Δcomparisons with shocked and vehicle-injected (S-C). * or Δ: p<0.05; ** or ΔΔ: p<0.01; *** or ΔΔΔ: p<0.001.
Having documented that the stressor increased W, next we determined whether application of eszopiclone after the stressor has any affect in stressor-induced W. To determine this, the total percentages of W values during individual time periods in the animals subjected with the combination of the stressor with one of three different doses of eszopiclone were compared with the corresponding W values of the C-NS and with the C-S (Fig. 2). Post-hoc analysis indicated that the values of the total percentages of time spent in W among S-Esz-1, S-Esz-3, S-Esz-10, and C-NS groups were comparable, except the 3-h value of the S-Esz-10 group (Fig. 2). Similar post-hoc comparisons with the C-S group revealed that the total percentages of W values in the S-Esz-1, S-Esz-3, and S-Esz-10 were significantly less during the 1-h period. These comparisons on the total percentages of W values with the C-S group also revealed that in the S-Esz-10 group during 2-h and in the S-Esz-1 and S-Esz-3 groups during the 5-h period W values were significantly less. These results suggest that the application of all three doses of eszopiclone were effective in suppressing the stressor-induced increase in W (Fig. 2). The post-hoc comparisons on the total percentages of time spent in W values in the S-Zol-1 and S-Zol-3 groups were not significantly different compared with the C-NS group (Fig. 3). Compared to the C-NS group, in the S-Zol-10 group, the total percentage of W values were significantly less during 1-h followed by significantly higher percentages during the 2-h and 3-h periods (Fig. 3). However, compared to the C-S group, in the S-Zol-1, S-Zol-3, and S-Zol-10 groups, the total W percent values were less (Fig. 3). These results indicate that the applications of all three doses of zolpidem were effective in suppressing stressor-induced increases in total percentages of time spent in W.
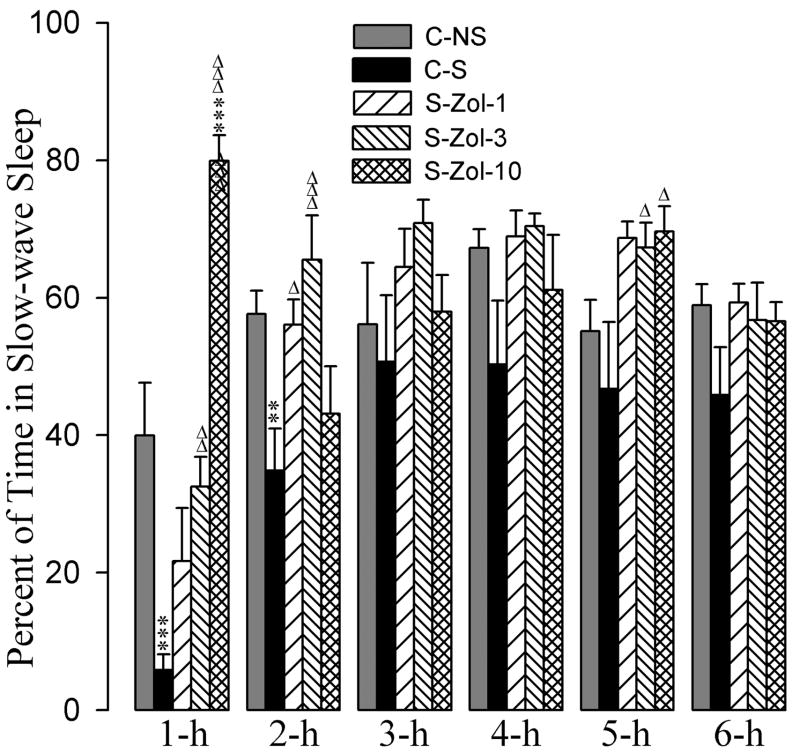
3.3. Effects on SWS
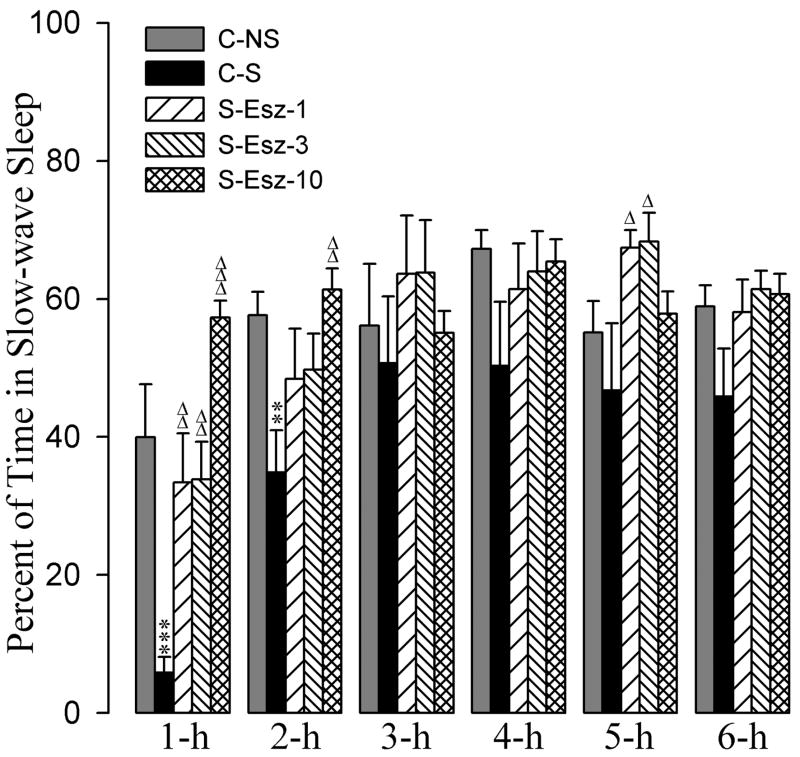
The effects of the zolpidem and eszopiclone administration on stressor-induced changes in percentages of time spent in SWS are summarized in figures 4 and 5. A two-way ANOVA indicated a significant main effect of treatment [F(7,288) = 12, p<0.001], time [F(5,288) = 21, p<0.001], and a treatment × time interaction [F(35,288) = 3.0, p<0.001] on the total percentages of time spent in SWS. Post-hoc comparisons (Bonferroni posttest) between C-S and C-NS groups revealed that the total percentages of SWS in the C-S group were significantly less during the 1-h and 2-h periods (Fig. 4). These results suggest that after a stressor event, animals spend significantly less time in SWS during the first two hours of recording.
Figure 4.
Effects of stressor and three different doses of eszopiclone on slow-wave sleep. Bars represent percentages (mean + SE) of slow-wave sleep during each of the 6-h periods after following treatments: non-shocked vehicle injected (C-NS), shocked and vehicle-injected (C-S), shocked and 1 mg/kg dose of eszopiclone-injected (S-Esz-1), shocked and 3 mg/kg dose of eszopiclone-injected (S-Esz-3), and shocked and 10 mg/kg dose of eszopiclone-injected (S-Esz-10). *Comparisons with non-shocked vehicle-injected (C-NS) and Δcomparisons with shocked and vehicle-injected (S-C). Δ: p<0.05; ** or ΔΔ: p<0.01; *** or ΔΔΔ: p<0.001.
Figure 5.
Effects of stressor and three different doses of zolpidem on slow-wave sleep. Bars represent percentages (mean + SE) of slow-wave sleep during each of the 6-h periods after following treatments: non-shocked vehicle-injected (C-NS), shocked and vehicle-injected (C-S), shocked and 1 mg/kg dose of zolpidem-injected (S-Zol-1), shocked and 3 mg/kg dose of zolpidem-injected (S-Zol-3), and shocked and 10 mg/kg dose of zolpidem-injected (S-Zol-10). *Comparisons with non-shocked vehicle-injected (C-NS) and Δcomparisons with shocked and vehicle-injected (S-C). Δ: p<0.05; ** or ΔΔ: p<0.01; *** or ΔΔΔ: p<0.001.
The detailed results of post-hoc analysis (Bonferroni posttest) on the total percentages of SWS are presented in figures 4 and 5. Post-hoc analyses revealed that the total percentages of SWS during all six 1-h periods were comparable among C-NS, S-Esz-1, S-Esz-3, and S-Esz-10 groups (Fig. 4). These results suggest that all three doses (1 mg, 3 mg, and 10 mg) of eszopiclone were effective in blocking stressor-induced suppression of SWS. Post-hoc analyses also revealed that the total percentages of time spent in SWS among the C-NS, S-Zol-1, S-Zol-3, and S-Zol-10 groups were comparable, except during the 1-h period of the S-Zol-10 (Fig. 5). During 1-h period, animals in the S-Zol-10 spent almost 80% of their time in SWS. These results suggest that the application of all three doses of zolpidem were effective in suppressing the stressor-induced reduction in SWS.
3.4. Effects on REM sleep
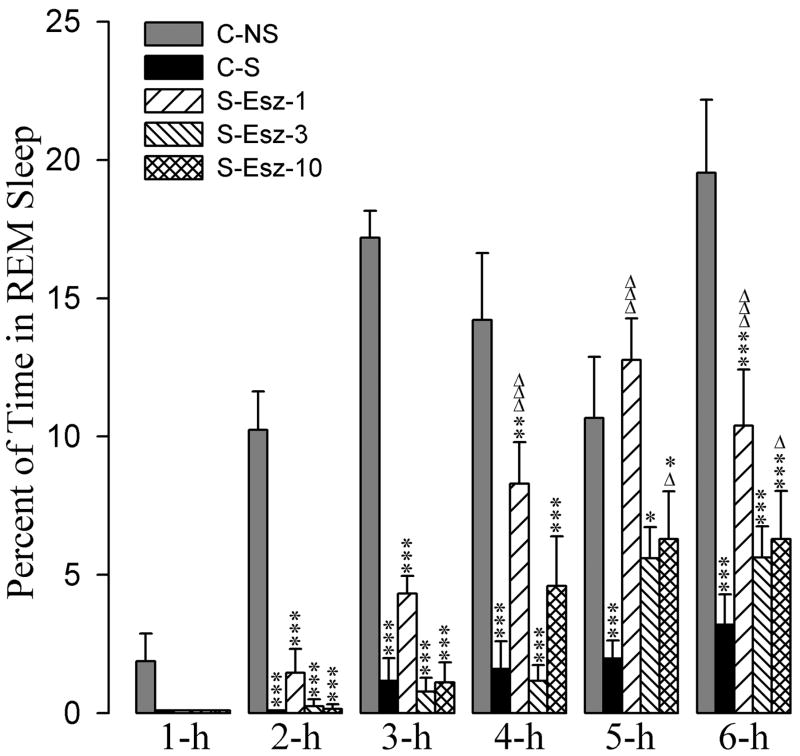
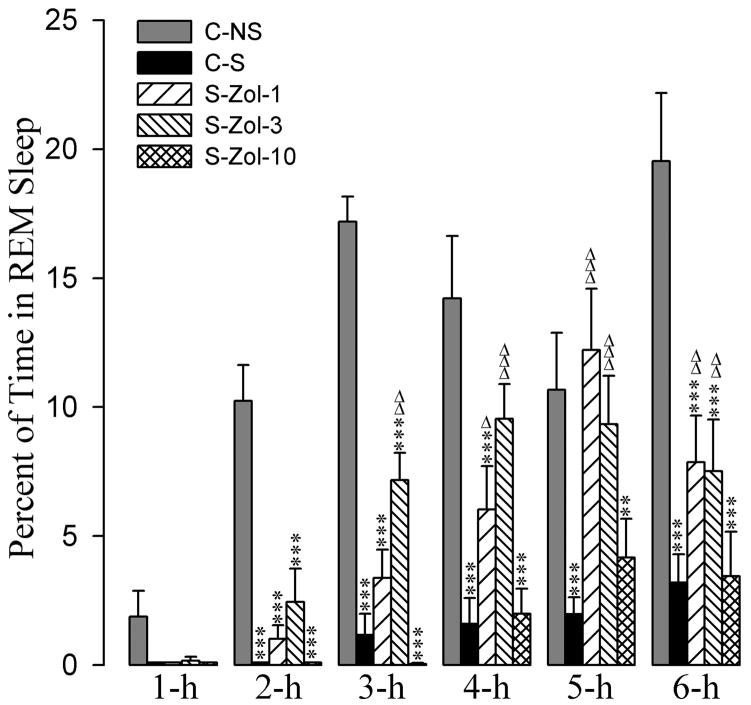
The effects of zolpidem and eszopiclone administration on the stressor-induced changes in percentages of time spent in REM sleep are summarized in figures 6 and 7. A two-way ANOVA indicated a significant main effect of treatment [F(7,288) = 58, p<0.001], time [F(5,288) = 43, p<0.001], and a treatment × time interaction [F(35,288) = 3.2, p<0.001] on the total percentages of time spent in REM sleep. Post-hoc comparisons (Bonferroni posttest) between C-S and C-NS groups revealed that in the C-S group the total percentages of REM sleep were significantly less at all six 1-hour periods (Figs. 6 and 7). These results demonstrate that the stressor used in this study suppressed REM sleep.
Figure 6.
Effects of stressor and three different doses of eszopiclone on REM sleep. Bars represent percentages (mean + SE) of REM sleep during each of the 6-h periods after following treatments: non-shocked vehicle injected (C-NS), shocked and vehicle-injected (C-S), shocked and 1 mg/kg dose of eszopiclone-injected (S-Esz-1), shocked and 3 mg/kg dose of eszopiclone-injected (S-Esz-3), and shocked and 10 mg/kg dose of eszopiclone-injected (S-Esz-10). *Comparisons with non-shocked vehicle-injected (C-NS) and Δcomparisons with shocked and vehicle-injected (S-C). * or Δ: p<0.05; **: p<0.01; *** or ΔΔΔ: p<0.001.
Figure 7.
Effects of stressor and three different doses of zolpidem on REM sleep. Bars represent percentages (mean + SE) of REM sleep during each of the 6-h periods after following treatments: non-shocked vehicle-injected (C-NS), shocked and vehicle-injected (C-S), shocked and 1 mg/kg dose of zolpidem-injected (S-Zol-1), shocked and 3 mg/kg dose of zolpidem-injected (S-Zol-3), and shocked and 10 mg/kg dose of zolpidem-injected (S-Zol-10). *Comparisons with non-shocked vehicle-injected (C-NS) and Δcomparisons with shocked and vehicle-injected (S-C). Δ: p<0.05; ** or ΔΔ: p<0.01; *** or ΔΔΔ: p<0.001.
The detailed results of post-hoc (Bonferroni posttest) analysis on the total percentages of REM sleep are presented in figures 6 and 7. Post-hoc analyses indicated that the total percentages of time spent in REM sleep at 2-h, 3-h, 4-h, and 6-h were significantly less in the S-Esz-1 group compared with the C-NS group. Post-hoc comparisons also revealed that the total percentages of time spent in REM sleep at all six postinjection times were significantly less in the S-Esz-3 and S-Esz-10 groups compared with the C-NS group (Fig. 6). However, compared to the C-S group, in the S-Esz-1 group the total percentages of REM sleep values were significantly higher at 4-h, 5-h, and 6-h periods of recording (Fig. 6). Similarly, compared to the C-S group, in the S-Esz-10 group, the values for the total percentages of time spent in REM sleep were significantly higher at the 5-h and 6-h periods. In the S-Esz-3 group, the values for the total percentages of time spent in REM sleep were higher during 5-h and 6-h periods, but those higher values did not reach a level of significance. (Fig. 6). These results indicate that a 1 mg dose of eszopiclone is relatively more effective for the attenuation of stressor-induced suppression of REM sleep.
Compared to the C-NS group, in the S-Zol-1 group the total percentages of time spent in REM sleep was significantly less at the 2-h, 3-h, 4-h, and 6-h recording periods (Fig. 7). Similarly, compared to the C-NS group, in the S-Zol-3 group the total percentages of time spent in REM sleep was significantly less at the 2-h, 3-h and 6-h periods. In the S-Zol-10 group, the total percentages of REM sleep are significantly less at all six recording periods. On the contrary, when the total percentages of REM sleep values in the S-Zol-1, S-Zol-3, and S-Zol-10 groups were compared with C-S group, the total percentages of REM sleep values were significantly higher in the S-Zol-1 group at 4-h, 5-h, and 6-h and in the S-Zol-3 group at 3-h, 4-h, 5-h, and 6-h (Fig. 7). Interestingly, the total percentages of REM sleep values in the S-Zol-10 group were not significantly different compared with C-S group (Fig. 7).
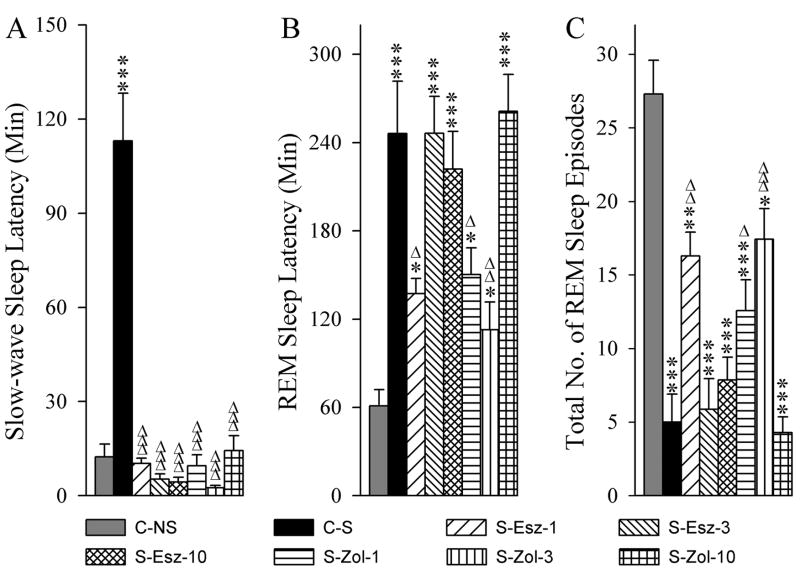
3.5. Effects on the latency of sleep and numbers of REM sleep episodes
The effects of a stressor with and without application of eszopiclone and zolpidem on sleep-wake behavior were also assessed by measuring the latency to onset of the first episode of SWS and REM sleep and number of REM sleep episodes (Fig. 8). Since the injections were made during W, there was no measure of the W latency. One-way ANOVA revealed a significant group effect on the latency of both SWS [F (7,48) = 11.46, p<0.001) and REM sleep [F (7,48) = 12.72, p<0.001]. Similarly, there was a significant group effect on the number of REM sleep episodes [F (7,48) = 18.12, p<0.001]. The mean latency of SWS in the C-S group was significantly longer (Bonferroni’s Multiple comparison test; t = 6.47, p<0.001) than the C-NS group (Fig. 8A). This result suggests that the stressor increases the latency period before the onset of SWS. The mean latencies of SWS were comparable among C-NS, S-Esz-1, S-Esz-3, S-Esz-10, S-Zol-1, S-Zol-3, and S-Zol-10 groups (Bonferroni’s Multiple comparison test). However, compared with C-S group, mean latencies were significantly shorter in the S-Esz-1 (t = 6.61, p<0.001), S-Esz-3 (t = 6.93, p<0.001), S-Esz-10 (t = 6.99, p<0.001), S-Zol-1 (t = 6.66, p<0.001), S-Zol-3 (t = 7.11, p<0.001), and S-Zol-10 (t = 6.34, p<0.001) groups. These results indicate that all three doses of both zolpidem and eszopiclone block stressor-induced increases in SWS latency.
Figure 8.
Effects of stressor and three different doses of eszopiclone and zolpidem on slow-wave sleep latency (A), REM sleep latency (B), and total number of REM sleep episodes (C). Abbreviations: C-NS, non-shocked vehicle-injected group; C-S, shocked and vehicle-injected group; S-Esz-1, shocked and 1 mg/kg dose of eszopiclone-injected group; S-Esz-3, shocked and 3 mg/kg dose of eszopiclone-injected group; S-Esz-10, shocked and 10 mg/kg dose of eszopiclone-injected group; S-Zol-1, shocked and 1 mg/kg dose of zolpidem-injected group; S-Zol-3, shocked and 3 mg/kg dose of zolpidem-injected group; S-Zol-10, shocked and 10 mg/kg dose of zolpidem-injected group. *Comparisons with non-shocked vehicle-injected group (C-NS) and Δcomparisons with shocked and vehicle-injected group (S-C). * or Δ: p<0.05; ** or ΔΔ: p<0.01; *** or ΔΔΔ: p<0.001.
Like SWS, mean latency to the onset of first REM sleep episode in the C-S group was significantly longer (Bonferroni’s Multiple comparison test; t = 6.47, p<0.001) than the C-NS group (Fig. 8B). This result suggests that the stressor increases the latency period before the onset of REM sleep. The mean latencies to the first episode of REM sleep were also significantly longer (Bonferroni’s Multiple comparison test) in the S-Esz-1 (t = 3.40, p<0.05), S-Esz-3 (t = 5.78, p<0.001), S-Esz-10 (t = 5.02, p<0.001), S-Zol-1 (t = 3.36, p<0.05), S-Zol-3 (t = 3.45, p<0.05), and S-Zol-10 (t = 6.25, p<0.001) groups compared with C-NS group. Post-hoc comparisons with C-S group revealed that the first episode of REM sleep latencies were significantly shorter in the S-Esz-1 (t = 3.40, p<0.05), S-Zol-1 (t = 3.32, p<0.05) and S-Zol-3 (t = 4.16, p<0.05) groups. Similar comparisons revealed that the first episodes of REM sleep in the S-Esz-3, S-Esz-10, and S-Zol-10 groups were comparable with C-S group. These results suggest that the application eszopiclone 1 mg dose and zolpidem 1 mg and 3 mg doses attenuate stressor-induced increased REM sleep latency. These results also suggest that 3 mg and 10 mg doses of eszopiclone and the 10 mg dose of zolpidem are unable to change the stressor-induced increases in latency of REM sleep.
Compared to the C-NS group, the mean number of REM sleep episodes in the C-S group was significantly low (Bonferroni’s Multiple comparison test; t = 8.41, p<0.001; Fig. 8C). This result suggests that the stressor decreases the number of REM sleep episodes. The numbers of REM sleep episodes were also significantly low in the S-Esz-1 (t = 4.15, p<0.01), S-Esz-3 (t = 8.09, p<0.001), S-Esz-10 (t = 7.33, p<0.001), S-Zol-1 (t = 5.57, p<0.001), S-Zol-3 (t = 3.45, p<0.05), and S-Zol-10 (t = 8.68, p<0.001) groups when compared with C-NS group. Alternatively, when compared with C-S group, the numbers of REM sleep episodes were significantly more in the S-Esz-1 (t = 4.26, p<0.01), S-Zol-1 (t = 3.38, p<0.05), and S-Zol-3 (t = 4.69, p<0.001). Similar comparisons revealed no significant differences in the S-Esz-3, S-Esz-10, and S-Zol-10 groups. These results suggest that the application of eszopiclone 1 mg dose as well as zolpidem 1 mg and 3 mg doses attenuate stressor-induced reductions in the number of REM sleep episodes. These results also suggest that the applications of 3 mg and 10 mg doses of eszopiclone and 10 mg dose of zolpidem do not change the stressor-induced reductions in the number of REM sleep episodes.
3.6. Effects on the spectral EEG power
The effects on the relative EEG power of zolpidem and eszopiclone administrations compared to a vehicle control, all following a stressor, were examined during W and SWS in the 6-hour period after the injections. During those recording periods, animals exposed to the stressor exhibited very little to no REM sleep; therefore, spectral EEG power could not be analyzed during REM sleep. A two-way ANOVA indicated a significant main effect of the treatment [F(7,288) = 30.0, p<0.001], but neither the time [F(5,288) = 1.73, p<0.13], nor the treatment × time interaction [F(35,288) = 0.36, p<1.0] were significant on the relative EEG power in the theta band during W periods. The detailed results of post-hoc analysis (Bonferroni posttest) for relative EEG power during W periods are presented in table 1. Post-hoc comparisons (Bonferroni posttest) on the relative theta powers during the six 1-hour periods did not reveal any significant differences between C-S and C-NS (Table 1). These results indicate that the stressor does not change relative power in the theta band of cortical EEG during W. Compared to the C-S group, in the S-Esz-1, S-Esz-3, S-Esz-10S-Zol-1, S-Zol-3, and S-Zol-10 groups, relative powers of the theta band in the cortical EEG during W were significantly lower (Table 1). Comparisons also revealed that this theta band power reduction in the S-Esz-1 group lasted only for an hour and in all other groups lasted for three or more hours. A two-way ANOVA revealed no significant main effect of the treatment [F(7,288) = 2.85, p<0.09], time [F(5,288) = 1.23, p<0.30], or treatment × time interaction [F(35,288) = 0.32, p<1.0] on the relative EEG power in the delta band during W periods. Similarly, a two-way ANOVA revealed no significant main effect of treatment [F(7,288) = 2.85, p<0.09], time [F(5,288) = 1.23, p<0.30], or treatment × time interaction [F(35,288) = 0.32, p<1.0] on the relative EEG power in the delta band during SWS periods.
Table 1.
Effects of stressor and combination of stressor and three different doses of eszopiclone and zolpidem on the relative power of EEG of the frontal cortex in the theta frequency band during each of the 6-h periods of wakefulness.
The Total Percentages (Mean ± SE) of Theta Frequency (5–10 Hz) Cortical EEG Waves Spectral Power During Wakefulness
| Treatment | 1-h | 2-h | 3-h | 4-h | 5-h | 6-h |
|---|---|---|---|---|---|---|
| C-NS | 41.3 ± 3.7 | 39.8 ± 3.6 | 40.5 ± 3.6 | 43.9 ± 4.9 | 39.2 ± 3.7 | 39.5 ± 4.4 |
| C-S | 38.9 ± 4.2 ns | 39.9 ± 3.7 ns | 40.4 ± 3.8 ns | 37.3 ± 1.7 ns | 38.5 ± 3.3 ns | 38.8 ± 3.7 ns |
| S-Esz-1 | 25.5 ± 4.3 ** | 28.7 ± 4.4 ns | 28.7 ± 3.9 ns | 28.7 ± 3.9 ns | 28.4 ± 3.9 ns | 35.2 ± 3.0 ns |
| S-Esz-3 | 22.5 ± 2.1 *** | 24.0 ± 2.3 ** | 25.3 ± 2.1 ** | 28.7 ± 3.9 ns | 29.6 ± 4.1 ns | 28.3 ± 2.6 ns |
| S-Esz-10 | 20.7 ± 1.6 *** | 22.7 ± 2.5 *** | 24.9 ± 2.5 ** | 28.9 ± 2.3 ns | 30.6 ± 2.6 ns | 31.6 ± 2.5 ns |
| S-Zol-1 | 20.9 ± 1.6 *** | 22.4 ± 1.6 ** | 23.5 ± 1.2 *** | 23.4 ± 1.5 ** | 23.3 ± 1.4 ** | 24.2 ± 1.1 ** |
| S-Zol-3 | 20.7 ± 0.7 *** | 24.0 ± 1.3 ** | 23.5 ± 1.0 *** | 23.1 ± 1.0 ** | 23.5 ± 1.3 ** | 22.7 ± 0.6 *** |
| S-Zol-10 | 20.1 ± 1.6 *** | 22.2 ± 3.6 *** | 24.7 ± 3.2 ** | 30.9 ± 3.1 ns | 31.7 ± 2.0 ns | 32.4 ± 3.0 ns |
Abbreviations: C-NS, non-shocked vehicle-injected group; C-S, shocked and vehicle-injected group; S-Esz-1, shocked and 1 mg/kg dose of eszopiclone-injected group; S-Esz-3, shocked and 3 mg/kg dose of eszopiclone-injected group; S-Esz-10, shocked and 10 mg/kg dose of eszopiclone-injected group; S-Zol-1, shocked and 1 mg/kg dose of zolpidem-injected group; S-Zol-3, shocked and 3 mg/kg dose of zolpidem-injected group; S-Zol-10, shocked and 10 mg/kg dose of zolpidem-injected group.
Comparisons with non-shocked vehicle-injected group (C-NS).
p<0.01;
p<0.001.
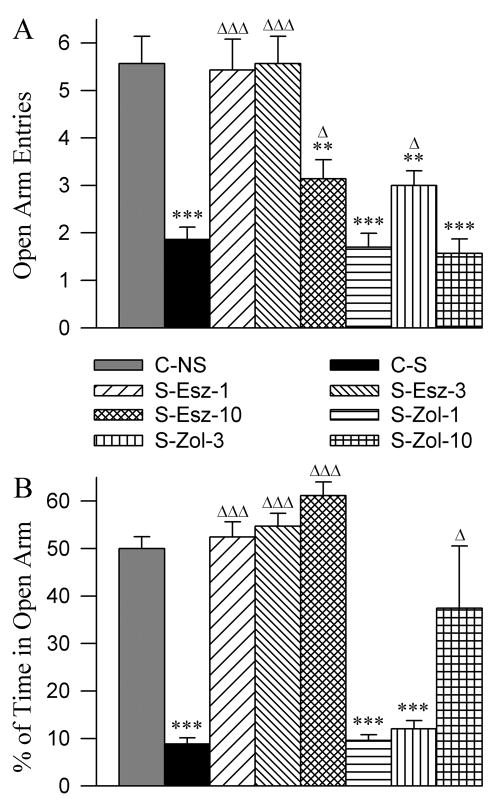
3.7. Effects on anxiety-like behavior
The EPM is the most extensively used behavioral paradigm to measure anxiety in the rodent model. The open arm of the EPM is considered anxiogenic, thus, rats exhibiting greater anxiety-related behavior will enter less frequently and spend less time in the open arms of the maze. The number of entries and total percentages of time spent on the open arm are increased and decreased with anxiolytic and anxiogenic substances, respectively. To confirm the anxiogenic-like effect of our stressor and to evaluate anxiolytic effects of eszopiclone and zolpidem application, EPM behavior was evaluated in the 8 different groups of rats. The effects of the eszopiclone and zolpidem administration on the stressor-induced changes in the EPM behaviors are summarized in figure 9. One-way ANOVA revealed a significant group effect on the number of open arm entries [F (7,48) = 16.21, p<0.001) and percentage of time spent in the open arm [F (7,48) = 18.78, p<0.001]. The mean number of entries on the open arm was significantly less in the C-S group compared with C-NS group (Bonferroni’s Multiple comparison test; t = 5.93, p<0.001; Fig. 9A). Similarly, compared to C-NS, the C-S group of animals spent significantly less percentages of time on the open arm (t = 5.67, p<0.001; Fig. 8A). These results indicate that the stressor used in this study induced a high level of anxiety-like behavior. The number of open arm entries and the percentages of time spent on the open arm in the S-Esz-1 and S-Esz-3 groups were similar compared with C-NS group (Fig. 9). These results indicate that the application of 1 mg and 3 mg doses of eszopiclone blocks stressor-induced anxiety-like behavior. Interestingly, similar comparisons revealed that in the S-Esz-10 group, the number of open arm entries was significantly less (t = 3.87, p<0.01) but the total percentages of time spent on the open arm was not significantly different compared with the C-NS group (Fig. 9). The EPM results of the S-Esz-10 group are ambiguous for the interpretation of its anxiolytic effect. Based on our observation, we believe that the 10 mg dose of eszopiclone reduces the animal’s ability to walk and move and thus, at this dose or higher the EPM paradigm may not be suitable to test this drug’s anxiolytic effect.
Figure 9.
Effects of three different doses of eszopiclone and zolpidem on elevated plus-maze (EPM) measures of stressor-induced anxiety. Bars in A represent number (mean + SE) of open arm entries and in B represent percent of times spent in the open arm. Abbreviations: C-NS, non-shocked vehicle-injected group; C-S, shocked and vehicle-injected group; S-Esz-1, shocked and 1 mg/kg dose of eszopiclone-injected group; S-Esz-3, shocked and 3 mg/kg dose of eszopiclone-injected group; S-Esz-10, shocked and 10 mg/kg dose of eszopiclone-injected group; S-Zol-1, shocked and 1 mg/kg dose of zolpidem-injected group; S-Zol-3, shocked and 3 mg/kg dose of zolpidem-injected group; S-Zol-10, shocked and 10 mg/kg dose of zolpidem-injected group. *Comparisons with non-shocked vehicle-injected group (C-NS) and Δcomparisons with shocked and vehicle-injected group (S-C). Δ: p<0.05; **: p<0.01; *** or ΔΔΔ: p<0.001.
Compared to C-NS group, the S-Zol-1 group of animals demonstrated significantly fewer entries onto the open arm (t = 6.15, p<0.001) and also spent a significantly smaller percentage of time on the open arm (t = 5.57, p<0.001). Like the S-Zol-1 group, the S-Zol-3 group of rats entered significantly fewer times (t = 4.10, p<0.01) on the open arm and also spent a smaller percentage of time (t = 5.24, p<0.001) on the open arm. These results indicate that the application of 1 mg and 3 mg doses of zolpidem do not attenuate stressor-induced anxiety-like behavior. Like S-Esz-10, the S-Zol-10 group of animals exhibited a mixed type of EPM behavior. In this group, the number of open arm entries was significantly less but the percentage of time spent in the open arm was not significantly different than compared with the C-NS group. This mixed result was due to a high dose of zolpidem that reduced the animal’s ability to walk and move. Also, we observed that both the S-Esz-10 and S-Zol-10 groups of rats had some difficulty maintaining balance while walking.
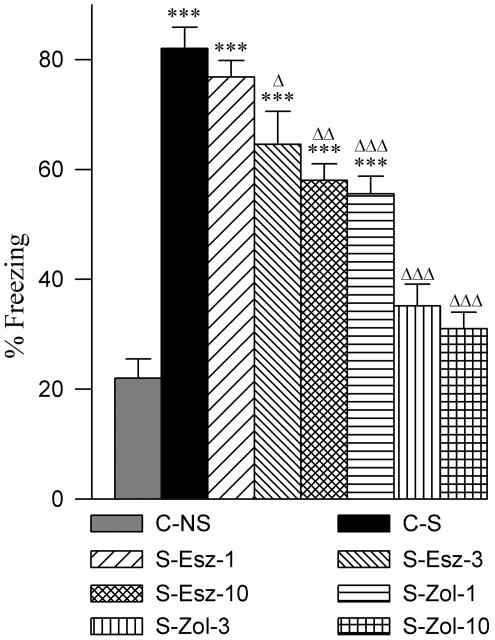
3.8. Effects on contextual memory response
To test whether application of eszopiclone and zolpidem affect memory, contextual fear memory response (freezing) was tested 24 hours later. One-way ANOVA revealed a significant group effect on the percentage of time in freezing [F (7,48) = 37.26, p<0.001). The mean percentage of freezing was significantly higher in the C-S group compared with C-NS group (post-hoc Bonferroni’s Multiple comparison test; t = 11.70, p<0.001; Fig. 10). Post-hoc analyses indicated significantly higher percentages of freezing in the S-Esz-1 (t = 10.70, p<0.001), S-Esz-3 (t = 8.30, p<0.001), S-Esz-10 (t = 8.19, p<0.001), and S-Zol-1 (t = 6.55, p<0.001) groups compared with C-NS group (Fig. 10). Similar analyses did not reveal any significant difference in the percentages of freezing in the S-Zol-3 and S-Zol-10 groups (Fig. 10). To compare the changes in the level of contextual memory after application of three different doses of eszopiclone and zolpidem, the mean percentage of freezing response in the C-S group was considered the optimum level for intact contextual memory. Post-hoc analysis revealed that the percentages of freezing in the S-Esz-1 and C-S group were comparable. This result indicates that application of the 1 mg dose of eszopiclone does not affect contextual memory. Post-hoc analyses revealed that the percentages of freezing were significantly less in the S-Esz-3 (t = 3.40, p<0.05), S-Esz-10 (t = 4.51, p<0.01), S-Zol-1 (t = 5.15, p<0.001), S-Zol-3 (t = 9.14, p<0.001), and S-Zol-10 (t = 9.95, p<0.001) groups compared with the percentage of freezing in the C-S group (Fig. 10). These results indicate that there was memory loss after applications of 3 mg and 10 mg doses of eszopiclone, and that this memory loss was greater with the 10 mg dose. These results also indicate that the levels of memory loss were almost equal after application of a 10 mg dose of eszopiclone and a 1 mg dose of zolpidem. The results suggest that with 3 mg and 10 mg doses of zolpidem there was a complete loss of contextual memory.
Figure 10.
Effects of three different doses of eszopiclone and zolpidem on contextual fear memory. Bars represent percentages (mean + SE) of freezing to the shocked context at 24 h following contextual fear conditioning training. Abbreviations: C-NS, non-shocked vehicle-injected group; C-S, shocked and vehicle-injected group; S-Esz-1, shocked and 1 mg/kg dose of eszopiclone-injected group; S-Esz-3, shocked and 3 mg/kg dose of eszopiclone-injected group; S-Esz-10, shocked and 10 mg/kg dose of eszopiclone-injected group; S-Zol-1, shocked and 1 mg/kg dose of zolpidem-injected group; S-Zol-3, shocked and 3 mg/kg dose of zolpidem-injected group; S-Zol-10, shocked and 10 mg/kg dose of zolpidem-injected group. *Comparisons with non-shocked vehicle-injected group (C-NS) and Δcomparisons with shocked and vehicle-injected group (S-C). * or Δ: p<0.05; ** or ΔΔ: p<0.01; *** or ΔΔΔ: p<0.001.
4. Discussion
The results of this study show that the contextual foot-shock (CFS) stressor increases the amount of time spent in W and decreases the amount of time spent in SWS and REM sleep. The EPM portion of this study suggests that the CFS stressor induced a high level of anxiety-like behavior. The results also demonstrate that the intraperitoneal administration of three different doses of eszopiclone and zolpidem affected CFS stressor-induced changes differently in the total percentages of time spent in the various wake-sleep stages, as well as overall level of anxiety-like behavior. Finally, the results of this study indicate that the highest dose of eszopiclone and all three doses of zolpidem applications caused a deficit in long-term contextual memory.
4.1. The effects of CFS stressor on wake-sleep states
In humans, disrupted sleep has been reported in a variety of psychological disorders, notably PTSD [30, 32, 39, 80, 81, 96, 97]. Similarly, normal sleep is also critical for psychological and emotional well-being (reviewed in [19]). Thus, evaluating changes in sleep-wake behavior in post-stressor exposure could elucidate diagnostic criteria and symptoms for specific disorders. In the animal model, various well-established techniques are used to induce stress and the subsequent differences in sleep architecture are often assessed. As a result, a CFS paradigm was utilized in the current study as a comparison of rodent and human behavior to evaluate anxiety and sleep-wake behavior. The present study demonstrates that after exposure to the CFS stressor rats spent a significantly high amount of time in W for the entire 6 h recording period. This CFS stressor also delayed the initiation of sleep and ultimately reduced the total amount of time spent in SWS over an approximately 2 h period. Following the CFS stressor, REM sleep remained suppressed for about 3 h and then heavily reduced for the next 3 h. This reduction in the amount of time spent in REM sleep was primarily due to (1) the increased latency from the beginning of recording to the appearance of the first episode of REM sleep and (2) a decreased number of REM sleep episodes. These data are consistent with earlier studies that have shown that an inescapable foot-shock stressor disturbs sleep-wake architecture and reduces sleep [43, 53, 56, 68, 86]. In this study, we analyzed cortical EEG spectral power in W and SWS episodes for 6 h beginning immediately after vehicle control or one of the three doses (1, 3, and 10 mg/kg) of two different drugs (eszopiclone and zolpidem). To our surprise, unlike changes in the sleep-wake stages, no significant changes were discovered in the EEG delta and theta powers during W or SWS periods. Collectively, these results demonstrate that this CFS paradigm could be a useful animal model to screen and/or identify novel drugs for treating physical and/or mental stress-induced insomnia.
4.2. The effects of eszopiclone and zolpidem on CFS stressor-induced insomnia
The results of this study demonstrate that the administration of 1, 3, and 10 mg/kg doses of eszopiclone blocks CFS stressor-induced increases in W. Likewise, the administration of zolpidem at 1 and 3 mg/kg doses also blocks stressor-induced increases in W. Paradoxically, in the CSF-exposed rats the application of 10 mg/kg dose of zolpidem caused a marked reduction in the amount of time spent in W in a 1 h period, but then at 2 h and 3 h subsequent to injection the total amount of time spent in W increased significantly. It is important to note here that after administration of the 10 mg/kg dose of both eszopiclone and zolpidem animals exhibited signs of difficulty in walking and maintaining balance. This effect lasted for at least 6 hours from the time of application. We did not observe similar walking difficulties in rats that received 1 or 3 mg/kg doses of eszopiclone or zolpidem.
This study demonstrates that the CFS stressor decreased the amount of SWS and this effect lasted for about two hours. The administration of 1, 3, and 10 mg/kg doses of eszopiclone in the CFS stressor-exposed rats increases SWS. Similarly, the administration of 1, 3, and 10 mg/kg doses of zolpidem in the CFS stressor-exposed rats also increases SWS. These results suggest that both eszopiclone and zolpidem are effective in blocking the CFS stressor-induced SWS suppression effect. Administration of both eszopiclone and zolpidem in the CFS-stressor-exposed rats also reduced the latency of SWS. Therefore, the administration of both eszopiclone and zolpidem were effective in normalizing the SWS suppression effect of the CFS stressor.
The results of this study demonstrate that the CFS stressor decreases the amount of time spent in REM sleep during all six 1 h periods of recording. The administration of eszopiclone at doses of 1, 3, and 10 mg/kg did not attenuate this CFS stressor-induced REM sleep suppression effect during the first three 1 h periods of recordings. Likewise, the administration of zolpidem at doses of 1, 3, and 10 mg/kg did not attenuate the CFS stressor-induced REM sleep suppression effect during the same periods of recording. During the 4 h, 5 h, and 6 h periods of recording a tendency to recover from CFS stressor-induced REM sleep suppression was observed after administration of eszopiclone at 1 mg/kg dose and after 1 and 3 mg/kg doses of zolpidem. This effect of eszopiclone and zolpidem at such doses was mainly due to their attenuating the CFS stressor-induced increased latency and decreased number of REM sleep episodes. These results suggest that the administration of eszopiclone at 3 and 10 mg/kg doses and zolpidem at 10 mg/kg dose have a tendency to suppress REM sleep. This REM sleep suppressing effect of eszopiclone and zolpidem was mainly due to increased latency and decreased number of REM sleep episodes.
While no previous reports have compared the effects of eszopiclone and/or zolpidem on sleep-wake states in the CFS stressed rats or in any other animal species, the present results are generally consistent with findings from separate studies in rats and guinea pigs. For example, in adult rats, intraperitoneal administration of zopiclone in doses ranging from 2.5 to 10 mg/kg was found to decrease W, decrease latency of SWS, increase total amount of time spent in SWS, increase the latency of REM sleep, and decrease the total amount of time spent in REM sleep [34, 35, 95]. Similarly in the guinea pigs, intraperitoneal administration of 1, 3, and 10 mg/kg doses of eszopiclone were shown to decrease W and the latency of SWS and to increase the total amount of time spent in SWS [110, 111]. These two studies reported that the administration of eszopiclone increases the latency of REM sleep but did not reduce the total amount of time spent in REM sleep over a period of 2 h. Like these animal studies, Zopiclone has been shown in humans to shorten sleep latency and increase total sleep [40, 87]. Similarly, human studies have also reported that eszopiclone in doses between 1 and 3.5 mg reduces the latency to persistent sleep, increased sleep efficiency, and total sleep in both healthy subjects and subjects with insomnia [47, 79]. Our results from zolpidem administration in the CFS-exposed rats are consistent with the data of previous studies that have tested zolpidem effects in the normal rat. For example, some studies have shown that the intraperitoneal administration of zolpidem at 3 or 10 mg/kg doses increases SWS in normal rats [10, 26, 54]. Another study in the normal adult rat reported that the latency to SWS was reduced after administration of zolpidem at doses of 2.5, 5, and 7.5 mg/kg [29]. The same study further reported that the administration of zolpidem at 7.5 mg/kg dose increased latency and decreased amount of time spent in REM sleep. However, in adult guinea pigs intraperitoneal administration of zolpidem at 3 and 10 mg/kg doses was found to decrease the amount of time spent in W and increase the amount of time spent in SWS but not the REM sleep [110, 111]. Like these animal studies, studies in human subjects have shown that zolpidem, in doses between 5 and 20 mg, shortens the latency to non-REM sleep and also increases the total amount of non-REM sleep [1, 88, 107]. One study showed that the administration of zolpidem prolonged latency to REM sleep and decreased total amount of REM sleep in healthy humans [6]. Although the present study was never designed to understand the mechanisms of these two drugs’ action, it is reasonable to suggest based on the existing literature on mechanisms of REM sleep regulation [19] that both eszopiclone and zolpidem suppress REM sleep by acting at the level of the pedunculopontine tegmentum. Indeed, studies have shown GABAergic agonists suppress REM sleep by inhibiting pedunculopontine tegmental cholinergic cell activity [17, 20, 102].
The results of our spectral analyses demonstrated that the CFS stressor did not cause changes in the relative power of the delta and theta frequency bands in the cortical EEG during W and SWS periods. When eszopiclone and zolpidem were administered in the CFS-stressed rats the relative power of the theta frequency band was decreased in the cortical EEG only during W, but not during SWS. It is interesting to note that both the level and the duration of these theta power reductions were more pronounced after administration of zolpidem than after eszopiclone. Of the three different doses of eszopiclone and zolpidem administration, 1 mg/kg dose of eszopiclone had the least effect on theta power reduction. Since there is a strong positive relationship between cortical theta frequency activity and hippocampal memory processing [16, 72], it is likely that the deficit in contextual memory response in eszopiclone and zolpidem treated rats could have been caused by the reduction of cortical theta frequency power during W. Application of these two drugs did not change the relative power of delta frequency bands in the cortical EEG during W or SWS periods in the CFS-stressed rats. These results suggested that injections of eszopiclone and zolpidem did not change the sleep inertia during W or the intensity of sleep during SWS [4, 43, 89]. Some other animal and human studies have also reported the effects of eszopiclone and zolpidem on the cortical EEG power. For example, in guinea pigs, administration of eszopiclone at 1 and 3 mg/kg doses was reported to increase spectral power in the delta band and to decrease in the theta band during SWS, but not during the W or REM sleep [110, 111]. These studies have also reported that the application of 1, 3, and 10 mg doses of zolpidem do not change the power of delta or theta bands in the cortical EEG during W, SWS, or REM sleep periods. Studies of young, healthy humans report that the administration of zopiclone (a cyclopyrrolone derivative and pharmaceutical predecessor to eszopiclone) produces a significant decrease in EEG theta power and a minor increase in EEG delta power only during non-REM sleep [46]. The most likely explanation for the differences in spectral power is the species difference. Since eszopiclone is the (S)-enantiomer of zopiclone [8], spectral power differences may be attributable to the drugs’ chemical differences. Alternatively, it is possible that the treatment caused the difference in effect; in the present study animals received CFS stressor and then received eszopiclone or zolpidem but in other studies, described above, guinea pigs and humans were not subjected to this type of stressor.
4.3. The effects of eszopiclone and zolpidem on CFS stressor-induced anxiety-like behavior
A number of recent studies suggested that measuring EPM behavior after stressor exposure results in a more effective measure of individual anxiety [9, 12, 53, 57, 58, 106]. The use of EPM measures to assess anxiety has also been validated by the behaviorally selective, anti-anxiety effects of muscimol, a GABA-A receptor agonist [77]. The results of the present study demonstrate that the CFS stressor significantly decreases the number of entries and percent of time spent in the open arms of EPM, indicating a high level of anxiety-like behavior. More interestingly, this high level of anxiety-like behavior is observed 6 h after the end of CFS stressor. This finding is important because it demonstrates for the first time that a high level of anxiety persists even 6 h after the CFS stressor exposure. The results provide evidence suggesting that this CFS paradigm could be used as an animal model to study the pathophysiological mechanisms of anxiety and also to screen anxiolytic drugs. Interestingly, the EPM data also supports our earlier interpretation that the EPM measures of anxiety are not simply the noxious and/or unpleasant effects of foot-shock; rather it is a psychological affect [53].
The animals treated with 1, 3, or 10 mg/kg doses of eszopiclone after CFS stressors spent significantly more time in the open arms of the EPM than control groups. The number of entries into the open area was also significantly increased after administration of eszopiclone at 1 and 3 mg/kg doses. The percentages of time spent in the open arms and entries in the open arm were comparable between CFS-stressed animals treated with 1 and 3 mg/kg eszopiclone and non-shocked vehicle treated animals. These results indicate that 1 and 3 mg/kg doses of eszopiclone exerted anxiolytic-like activity. On the contrary, the animals treated with 1, 3 mg/kg doses of zolpidem after CFS stressors spent significantly less time in the open arms of the EPM. The number of entries in the open arm was also significantly less after administration of zolpidem at 1 and 3 mg/kg doses. The percentages of time spent in the open arms and number of entries in the open arm were comparable between CFS-stressed animals treated with 1 and 3 mg/kg zolpidem and CFS-stressed animals treated with vehicle control. These results suggest that the administration of zolpidem at 1 and 3 mg/kg doses do not attenuate CFS stressor-induced anxiety-like behavior. The results of eszopiclone are consistent with another study showing that a 5 mg/kg dose of (S)-zopiclone increased percent of time spent in the open arm of the plus maze [8]. The administration of eszopiclone and zolpidem at the 10 mg/kg dose both produced a mixed type of results. The administration of eszopiclone and zolpidem at 10 mg/kg dose both disrupted animal’s ability to maintain their balance while walking, so these animals spent most of the EPM testing time in the open arm where they were placed in the beginning of testing. Therefore, in these animals the result on the percentage of time spent in the open arm is somewhat false-positive. Indeed, in another study, it has been shown that the administration of (S)-zopiclone at 10 mg/kg dose disrupted the animal’s ability to successfully perform coordinated motor movements [8].
4.4. The effects of eszopiclone and zolpidem on contextual memory response
In the present study to test the effects of eszopiclone and zolpidem on memory, we used contextual fear conditioning paradigm. Contextual fear conditioning is a form of associative learning in which rodents are trained to associate a specific box or cage (neutral stimulus) with an aversive stimulus (in this case, mild foot-shock). Rodents display their fear with freezing behavior upon exposure to the training context during a subsequent retrieval test [29, 45, 71]. This is also one of the most popular experimental paradigms for studying the mechanisms of learning and memory in rodents [55]. It has been demonstrated that this type of learning and memory depends on the normal functioning of both the hippocampus and the amygdala [45, 51, 71]. The results of the present study demonstrate that the administration of eszopiclone at 1 mg/kg dose immediately after the contextual fear conditioning does not change the percent of time in freezing when those animals were tested 24 h later, indicating that this dose of eszopiclone did not disrupt these animals’ contextual memory. However, when eszopiclone dose was increased to 3 and 10 mg/kg, there was a dose-dependent deficit in the contextual memory. Administration of zolpidem at 1 mg/kg dose also disrupted this contextual memory and surprisingly the level of this deficit was slightly more than the 10 mg/kg dose of eszopiclone. Administration of zolpidem at 3 and 10 mg/kg doses caused an almost complete loss of contextual memory. These results are interesting because the cortical EEG power spectral data of this study showed that both eszopiclone and zolpidem reduce the power of theta wave activity in a dose-dependent manner. It is already known that theta wave activity is generated by the hippocampal activity and that theta wave activity is critical for memory consolidation [16, 72]. Since theta-wave activity was more heavily suppressed after the application of zolpidem than after eszopiclone, it is therefore no surprise that the memory deficits resulting from zolpidem administration were more severe than those of the eszopiclone.
Although we have shown that injection of eszopiclone and zolpidem impairs contextual memory, it remains to be determined whether this memory deficit was caused by the disruption of memory consolidation, memory retrieval process, or by both processes. We suggest that the eszopiclone and zolpidem injection-induced memory deficit was caused by the disruption of memory consolidation. This suggestion is based on the following facts: 1) the initiation of memory consolidation of contextual learning begins within one hour, and this entire process is completed within the first 6 h period after training [5, 37, 104], 2) pharmacological studies have shown that the half life of eszopiclone is about 5 h and that of zolpidem is about 3 h [33, 60, 101, 105, 113]. Since the eszopiclone and zolpidem are pharmacologically effective only during the memory consolidation period, thus, it is reasonable to suggest that the eszopiclone and zolpidem injection-induced memory deficit was caused by the disruption of memory consolidation.
4.5. Conclusions
The present results demonstrate that in the rat, administration of eszopiclone and zolpidem are equally effective in normalizing stressor-induced reduction of SWS by shortening the sleep latency and increasing the amount of SWS. Both eszopiclone and zolpidem at a 10 mg/kg dose prolong REM sleep latency. Similarly, both treatments at the 10 mg/kg dose restrict animal’s movements and disrupt its ability to maintain balance while walking. In normalizing stressor-induced sleep deficits, a 1 mg/kg dose of eszopiclone is comparable to a 3 mg/kg dose of zolpidem. eszopiclone at 1 and 3 mg/kg are equally effective in suppressing stressor-induced anxiety. zolpidem at similar doses do not appear to exert this same anxiolytic effect on stressor-induced anxiety-like behavior in the rat. The anxiolytic effects of eszopiclone and zolpidem at 10 mg/kg could not be tested using the EPM because the drugs at this dose disrupt the animal’s ability to successfully perform coordinated motor movement. Eszopiclone at 1 mg/kg has no significant effect on the contextual memory but with increased doses at 3 and 10 mg/kg it disrupts contextual memory in a dose-dependent manner. zolpidem severely disrupts the animal’s contextual memory. In conclusion, the present study indicates that eszopiclone can be used not only to increase SWS but at lower doses could also be used effectively to control psychological stressor-induced anxiety-like behavior in the rat. The findings of this animal study have important clinical ramifications. These results suggest that the lower dose of eszopiclone could also be used as an anxiolytic in human subjects to alleviate acute anxiety without any cognitive side-effects. These results also suggest that the power spectral analyses of cortical EEG could be used to identify future anxiolytic drugs without cognitive side-effects.
Acknowledgments
This work was supported by research grants from the Sepracor, Inc (ESRC961) and National Institutes of Health (MH 59839).
Footnotes
Disclosure/conflict of interest statement: This study was supported by Sepracor, Inc. and National Institutes of Health. The authors have indicated no other financial conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of Sepracor or National Institutes of Health.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ancoli-Israel S, Walsh JK, Mangano RM, Fujimori M. Zaleplon, a novel nonbenzodiazepine hypnotic, effectively treats insomnia in elderly patients without causing rebound effects. Prim Care Companion J Clin Psychiatry. 1999;1:114–120. doi: 10.4088/pcc.v01n0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benavides J, Peny B, Durand A, Arbilla S, Scatton B. Comparative in vivo and in vitro regional selectivity of central omega (benzodiazepine) site ligands in inhibiting [3H]flumazenil binding in the rat central nervous system. J Pharmacol Exp Ther. 1992;263:884–896. [PubMed] [Google Scholar]
- 3.Bertoglio LJ, Carobrez AP. Previous maze experience required to increase open arms avoidance in rats submitted to the elevated plus-maze model of anxiety. Behav Brain Res. 2000;108:197–203. doi: 10.1016/s0166-4328(99)00148-5. [DOI] [PubMed] [Google Scholar]
- 4.Blanco-Centurion C, Xu M, Murillo-Rodriguez E, Gerashchenko D, Shiromani AM, Salin-Pascual RJ, Hof PR, Shiromani PJ. Adenosine and sleep homeostasis in the basal forebrain. J Neurosci. 2006;26:8092–8100. doi: 10.1523/JNEUROSCI.2181-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn & Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner DP, Dijk DJ, Munch M, Borbely AA. Psychopharmacology. Vol. 104. Berl: 1991. Effect of zolpidem on sleep and sleep EEG spectra in healthy young men; pp. 1–5. [DOI] [PubMed] [Google Scholar]
- 7.Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 8.Carlson JN, Haskew R, Wacker J, Maisonneuve IM, Glick SD, Jerussi TP. Sedative and anxiolytic effects of zopiclone’s enantiomers and metabolite. Eur J Pharmacol. 2001;415:181–189. doi: 10.1016/s0014-2999(01)00851-2. [DOI] [PubMed] [Google Scholar]
- 9.Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus-maze model 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 10.Chen HY, Kuo TB, Shaw FZ, Lai CJ, Yang CC. Psychopharmacology. Vol. 181. Berl: 2005. Sleep-related vagotonic effect of zolpidem in rats; pp. 270–279. [DOI] [PubMed] [Google Scholar]
- 11.Costa e Silva JA. Sleep disorders in psychiatry. Metabolism. 2006;55:S40–S44. doi: 10.1016/j.metabol.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 13.Damgen K, Lüddens H. Zaleplon displays a selectivity to recombinant GABA(A) receptors different from zolpidem, zopiclone, and benzodiazepines. Neurosci Res Commun. 1999;25:139–148. [Google Scholar]
- 14.Datta S. Avoidance task training potentiates phasic pontine-wave density in the rat: A mechanism for sleep-dependent plasticity. J Neurosci. 2000;20:8607–8613. doi: 10.1523/JNEUROSCI.20-22-08607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Datta S. Evidence that REM sleep is controlled by the activation of brain stem pedunculopontine tegmental kainate receptor. J Neurophysiol. 2002;87:1790–1798. doi: 10.1152/jn.00763.2001. [DOI] [PubMed] [Google Scholar]
- 16.Datta S. Activation of phasic pontine-wave generator: a mechanism for sleep-dependent memory processing. Sleep and Biological Rhythms. 2006;4:16–26. [Google Scholar]
- 17.Datta S. Activation of pedunculopontine tegmental PKA prevents GABA-B receptor activation-mediated rapid eye movement sleep suppression in the freely moving rat. J Neurophysiol. 2007;97:3841–3850. doi: 10.1152/jn.00263.2007. [DOI] [PubMed] [Google Scholar]
- 18.Datta S, Li G, Auerbach S. Activation of phasic pontine-wave generator in the rat: a mechanism for expression of plasticity-related genes and proteins in the dorsal hippocampus and amygdala. Eur J Neurosci. 2008;27:1876–1892. doi: 10.1111/j.1460-9568.2008.06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Datta S, Mavanji V, Patterson EH, Ulloor J. Regulation of rapid eye movement sleep in the freely moving rat: local microinjection of serotonin, norepinephrine, and adenosine into the brainstem. Sleep. 2003;26:513–520. doi: 10.1093/sleep/26.5.513. [DOI] [PubMed] [Google Scholar]
- 21.Datta S, Mavanji V, Ulloor J, Patterson EH. Activation of phasic pontine-wave generator prevents rapid eye movement sleep deprivation-induced learning impairment in the rat: a mechanism for sleep-dependent plasticity. Journal of Neuroscience. 2004;24:1416–1427. doi: 10.1523/JNEUROSCI.4111-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datta S, Siwek DF. Single cell activity patterns of pedunculopontine tegmentum neurons across the sleep-wake cycle in the freely moving rats. Journal of Neuroscience Research. 2002;70:611–621. doi: 10.1002/jnr.10405. [DOI] [PubMed] [Google Scholar]
- 23.Davies M, Newell JG, Derry JM, Martin IL, Dunn SM. Characterization of the interaction of zopiclone with gamma-aminobutyric acid type A receptors. Mol Pharmacol. 2000;58:756–762. doi: 10.1124/mol.58.4.756. [DOI] [PubMed] [Google Scholar]
- 24.Declerck AC, Ruwe F, O’Hanlon JF, Vermeeren A, Wauquier A. Psychopharmacology. Vol. 106. Berl: 1992. Effects of zolpidem and flunitrazepam on nocturnal sleep of women subjectively complaining of insomnia; pp. 497–501. [DOI] [PubMed] [Google Scholar]
- 25.Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Depoortere H, Granger P, Leonardon J, Terzano MG. Evaluation of the cyclic alternating pattern in rats by automatic analysis of sleep amplitude variations: effect of zolpidem. In: Terzano MG, Halasz PL, Declerck AC, editors. Phasic events and dynamic organization of sleep. New York: Raven Press; 1991. pp. 17–33. [Google Scholar]
- 27.Edgar DM, Seidel WF, Dement WC. Psychopharmacology. Vol. 105. Berl: 1991. Triazolam-induced sleep in the rat: influence of prior sleep, circadian time, and light/dark cycles; pp. 374–380. [DOI] [PubMed] [Google Scholar]
- 28.Erman MK, Zammit G, Rubens R, Schaefer K, Wessel T, Amato D, Caron J, Walsh JK. A polysomnographic placebo-controlled evaluation of the efficacy and safety of eszopiclone relative to placebo and zolpidem in the treatment of primary insomnia. J Clin Sleep Med. 2008;4:229–234. [PMC free article] [PubMed] [Google Scholar]
- 29.Fanselow MS, Bolles RC. Naloxone and shock-elicited freezing in the rat. J Comp Physiol Psychol. 1979;93:736–744. doi: 10.1037/h0077609. [DOI] [PubMed] [Google Scholar]
- 30.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 31.Fuller KH, Waters WF, Binks PG, Anderson T. Generalized anxiety and sleep architecture: a polysomnographic investigation. Sleep. 1997;20:370–376. doi: 10.1093/sleep/20.5.370. [DOI] [PubMed] [Google Scholar]
- 32.Gale C, Davidson O. Generalised anxiety disorder. BMJ. 2007;334:579–581. doi: 10.1136/bmj.39133.559282.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garrigou-Gadenne D, Burke JT, Durand A, Depoortere H, Thenot JP, Morseli PL. Phamacokinetics, brain distribution and phamaco-electrocorticographic profile of zolpidem, a new hypnotic, in the rat. J Pharmacol & Experimental Therapeut. 1989;248:1283–1288. [PubMed] [Google Scholar]
- 34.Gauthier P, Arnaud C, Stutzmann JM, Gottesmann C. Psychopharmacology. Vol. 130. Berl: 1997. Influence of zopiclone, a new generation hypnotic, on the intermediate stage and paradoxical sleep in the rat; pp. 139–143. [DOI] [PubMed] [Google Scholar]
- 35.Gottesmann C, Gandolfo G, Arnaud C, Gauthier P. The intermediate stage and paradoxical sleep in the rat: influence of three generations of hypnotics. Eur J Neurosci. 1998;10:409–414. doi: 10.1046/j.1460-9568.1998.00069.x. [DOI] [PubMed] [Google Scholar]
- 36.Graham D, Faure C, Besnard F, Langer SZ. Pharmacological profile of benzodiazepine site ligands with recombinant GABAA receptor subtypes. Eur Neuropsychopharmacol. 1996;6:119–125. doi: 10.1016/0924-977x(95)00072-w. [DOI] [PubMed] [Google Scholar]
- 37.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Leanr & Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanson SM, Czajkowski C. Structural mechanisms underlying benzodiazepine modulation of the GABA(A) receptor. J Neurosci. 2008a;28:3490–3499. doi: 10.1523/JNEUROSCI.5727-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanson SM, Morlock EV, Satyshur KA, Czajkowski C. Structural requirements for eszopiclone and zolpidem binding to the gamma-aminobutyric acid type-A (GABAA) receptor are different. J Med Chem. 2008b;51:7243–7252. doi: 10.1021/jm800889m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hemmeter U, Muller M, Bischof R, Annen B, Holsboer-Trachsler E. Psychopharmacology. Vol. 147. Berl: 2000. Effect of zopiclone and temazepam on sleep EEG parameters, psychomotor and memory functions in healthy elderly volunteers; pp. 384–396. [DOI] [PubMed] [Google Scholar]
- 41.Ho YJ, Eichendorff J, Schwarting RK. Individual response profiles of male Wistar rats in animal models for anxiety and depression. Behav Brain Res. 2002;136:1–12. doi: 10.1016/s0166-4328(02)00089-x. [DOI] [PubMed] [Google Scholar]
- 42.Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Biochem Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- 43.Jha SK, Brennan FX, Pawlyk AC, Ross RJ, Morrison AR. REM sleep: a sensitive index of fear conditioning in rats. Eur J Neurosci. 2005;21:1077–1080. doi: 10.1111/j.1460-9568.2005.03920.x. [DOI] [PubMed] [Google Scholar]
- 44.Johnson EO, Roth T, Breslau N. The association of insomnia with anxiety disorders and depression: exploration of the direction of risk. J Psychiatric Res. 2006;40:700–708. doi: 10.1016/j.jpsychires.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 46.Kim YD, Zhuang HY, Tsutsumi M, Okabe A, Kurachi M, Kamikawa Y. Comparison of the effect of zopiclone and brotizolam on sleep EEG by quantitative evaluation in healthy young women. Sleep. 1993;16:655–661. doi: 10.1093/sleep/16.7.655. [DOI] [PubMed] [Google Scholar]
- 47.Krystal AD, Walsh JK, Laska E, Caron J, Amato DA, Wessel TC, Roth T. Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep. 2003;26:793–799. doi: 10.1093/sleep/26.7.793. [DOI] [PubMed] [Google Scholar]
- 48.Lader M. Psychopharmacology. Vol. 108. Berl: 1992. Rebound insomnia and newer hypnotics; pp. 248–255. [DOI] [PubMed] [Google Scholar]
- 49.Lancel M. Role of GABA-A receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- 50.Lavie P. Sleep disturbances in the wake of traumatic events. N Engl J Med. 2001;345:1825–1832. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- 51.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 52.Leonard BE. Drug treatment of insomnia. In: Leonard BE, editor. Fundamentals of Psychopharmacology. Chichester: John Wiley and Sons; 1992. pp. 113–122. [Google Scholar]
- 53.Maclean RR, Datta S. The relationship between anxiety and sleep-wake behavior after stressor exposure in the rat. Brain Res. 2007;1164:72–80. doi: 10.1016/j.brainres.2007.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mailliet F, Galloux P, Poisson D. Psychopharmacology. Vol. 156. Berl: 2001. Comparative effects of melatonin, zolpidem and diazepam on sleep, body temperature, blood pressure and heart rate measured by radiotelemetry in Wistar rats; pp. 417–426. [DOI] [PubMed] [Google Scholar]
- 55.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 56.Mavanji V, Siwek DF, Patterson EH, Spoley EE, Datta S. Effects of passive-avoidance training on sleep-wake state-specific activity in the basolateral and central nuclei of the amygdala. Behav Neurosci. 2003;117:751–759. doi: 10.1037/0735-7044.117.4.751. [DOI] [PubMed] [Google Scholar]
- 57.Mechiel Korte S, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- 58.Mechiel Korte S, De Boer SF, Bohus B. Stress. Vol. 3. Luxembourg: 1999. Fear-pontentiation in the elevated plus-maze test depends on stressor controllability and fear conditioning; pp. 27–40. [DOI] [PubMed] [Google Scholar]
- 59.Meltzer LT, Serpa KA. Assessment of hypnotic effects in the rat: influence of the sleep-awake cycle. Drug Dev Res. 1988;14:151–159. [Google Scholar]
- 60.Mirza NR, Rodgers RJ, Mathiasen LS. Comparative cue generalization profiles of L-834,417, SL651498, zolpidem, CL218,872, Ocinaplon, Bretazenil, Zopiclone, and various benzodiazepines in chlordiazepoxide and zolpidem drug discrimination. J Pharmacol & Experimental Therapeut. 2006;316:1291–1299. doi: 10.1124/jpet.105.094003. [DOI] [PubMed] [Google Scholar]
- 61.Monti JM, Monti D. Sleep disturbance in generalized anxiety disorder and its treatment. Sleep Med Rev. 2000;4:263–276. doi: 10.1053/smrv.1999.0096. [DOI] [PubMed] [Google Scholar]
- 62.Morin AK. Strategies for treating chronic insomnia. Am J Manag Care. 2006;12:S230–245. [PubMed] [Google Scholar]
- 63.Noble S, Langtry HD, Lamb HM. Zopiclone. An update of its pharmacology, clinical efficacy and tolerability in the treatment of insomnia. Drugs. 1998;55:277–302. doi: 10.2165/00003495-199855020-00015. [DOI] [PubMed] [Google Scholar]
- 64.Noguchi H, Kitazumi K, Mori M, Shiba T. Electroencephalographic properties of zaleplon, a non-benzodiazepine sedative/hypnotic, in rats. J Pharmacol Sci. 2004;94:246–251. doi: 10.1254/jphs.94.246. [DOI] [PubMed] [Google Scholar]
- 65.Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37:9–15. doi: 10.1016/s0022-3956(02)00052-3. [DOI] [PubMed] [Google Scholar]
- 66.Papadimitriou GN, Kerkhofs M, Kempenaers C, Mendlewicz J. EEG sleep studies in patients with generalized anxiety disorder. Psychiatry Res. 1988;26:183–190. doi: 10.1016/0165-1781(88)90073-x. [DOI] [PubMed] [Google Scholar]
- 67.Papadimitriou GN, Linkowski P. Sleep disturbance in anxiety disorders. Int Rev Psychiatry. 2005;17:229–236. doi: 10.1080/09540260500104524. [DOI] [PubMed] [Google Scholar]
- 68.Pawlyk AC, Jha SK, Brennan FX, Morrison AR, Ross RJ. A rodent model of sleep disturbances in posttraumatic stress disorder: the role of context after fear conditioning. Biol Psychiatry. 2005;57:268–277. doi: 10.1016/j.biopsych.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 69.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- 70.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 71.Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 72.Poe GR, Nitz DA, McNaughton BL, Barnes CA. Experience-dependent phase-reversal of hippocampal neuron firing during REM sleep. Brain Res. 2000;855:176–180. doi: 10.1016/s0006-8993(99)02310-0. [DOI] [PubMed] [Google Scholar]
- 73.Pollack M, Kinrys G, Krystal A, McCall WV, Roth T, Schaefer K, Rubens R, Roach J, Huang H, Krishnan R. eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch Gen Psychiatry. 2008;65:551–562. doi: 10.1001/archpsyc.65.5.551. [DOI] [PubMed] [Google Scholar]
- 74.Pritchett DB, Seeburg PH. Gamma-aminobutyric acidA receptor alpha 5-subunit creates novel type II benzodiazepine receptor pharmacology. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 75.Pritchett DB, Sontheimer H, Shivers BD, Ymer S, Kettenmann H, Schofield PR, Seeburg PH. Importance of a novel GABAA receptor subunit for benzodiazepine pharmacology. Nature. 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 76.Ramakrishnan K, Scheid DC. Treatment options for insomnia. Am Fam Physician. 2007;76:517–526. [PubMed] [Google Scholar]
- 77.Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 78.Rodgers RJ, Lee C, Shepherd JK. Psychopharmacol. Vol. 106. Ber: 1992. Effects of diazepam on behavioural and antinociceptive responses to the elevated plus-maze in male mice depend upon treatment regimen and prior maze experience; pp. 102–110. [DOI] [PubMed] [Google Scholar]
- 79.Rosenberg R, Caron J, Roth T, Amato D. An assessment of the efficacy and safety of eszopiclone in the treatment of transient insomnia in healthy adults. Sleep Med. 2005;6:15–22. doi: 10.1016/j.sleep.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol Psychiatry. 1994;35:195–202. doi: 10.1016/0006-3223(94)91152-5. [DOI] [PubMed] [Google Scholar]
- 81.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146:697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 82.Roth T, Walsh JK, Krystal A, Wessel T, Roehrs TA. An evaluation of the efficacy and safety of eszopiclone over 12 months in patients with chronic primary insomnia. Sleep Med. 2005;6:487–495. doi: 10.1016/j.sleep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Rudolph U, Mohler H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu Rev Pharmacol Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- 84.Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 85.Saletu-Zyhlarz G, Saletu B, Anderer P, Brandstatter N, Frey R, Gruber G, Klosch G, Mandl M, Grunberger J, Linzmayer L. Nonorganic insomnia in generalized anxiety disorder. 1. Controlled studies on sleep, awakening and daytime vigilance utilizing polysomnography and EEG mapping. Neuropsychobiology. 1997;36:117–129. doi: 10.1159/000119373. [DOI] [PubMed] [Google Scholar]
- 86.Sanford LD, Silvestri AJ, Ross RJ, Morrison AR. Influence of fear conditioning on elicited ponto-geniculo-occipital waves and rapid eye movement sleep. Arch Ital Biol. 2001;139:169–183. [PubMed] [Google Scholar]
- 87.Scharf M, Erman M, Rosenberg R, Seiden D, McCall WV, Amato D, Wessel TC. A 2-week efficacy and safety study of eszopiclone in elderly patients with primary insomnia. Sleep. 2005;28:720–727. doi: 10.1093/sleep/28.6.720. [DOI] [PubMed] [Google Scholar]
- 88.Scharf MB, Mayleben DW, Kaffeman M, Krall R, Ochs R. Dose response effects of zolpidem in normal geriatric subjects. J Clin Psychiatry. 1991;52:77–83. [PubMed] [Google Scholar]
- 89.Shea JL, Mochizuki T, Sagvaag V, Aspevik T, Bjorkum AA, Datta S. Rapid eye movement (REM) sleep homeostatic regulatory processes in the rat: changes in the sleep-wake stages and electroencephalographic power spectra. Brain Res. 2008;1213:48–56. doi: 10.1016/j.brainres.2008.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 91.Silber MH. Clinical practice. Chronic insomnia N Engl J Med. 2005;353:803–810. doi: 10.1056/NEJMcp043762. [DOI] [PubMed] [Google Scholar]
- 92.Smith BJ, Bickel WK. Effects of alprazolam, caffeine, and zolpidem in humans trained to discriminate triazolam from placebo. Drug Alcohol Depend. 2001;61:249–260. doi: 10.1016/s0376-8716(00)00145-9. [DOI] [PubMed] [Google Scholar]
- 93.Stein MB. Public health perspectives on generalized anxiety disorder. J Clin Psychiatry. 2004;65:3–7. [PubMed] [Google Scholar]
- 94.Stein MD, Mellman TA. Anxiety disorders. In: Kryger M, Guilleminault C, editors. Principles and practice of sleep medicine. Baltimore, MD: Elsevier Saunders; 2005. pp. 1297–1310. [Google Scholar]
- 95.Stutzmann JM, Eon B, Lucas M, Blanchard JC, Laduron PM. RP 62203, a 5-hydroxytryptamine2 antagonist, enhances deep NREM sleep in rats. Sleep. 1992;15:119–124. doi: 10.1093/sleep/15.2.119. [DOI] [PubMed] [Google Scholar]
- 96.Szelenberger W, Soldatos C. Sleep disorders in psychiatric practice. World Psychiatry. 2005;4:186–190. [PMC free article] [PubMed] [Google Scholar]
- 97.Tan TL, Kales JD, Kales A, Soldatos CR, Bixler EO. Biopsychobehavioral correlates of insomnia. IV: Diagnosis based on DSM-III. Am J Psychiatry. 1984;141:357–362. doi: 10.1176/ajp.141.3.357. [DOI] [PubMed] [Google Scholar]
- 98.Tang X, Xiao J, Liu X, Sanford LD. Strain differences in the influence of open field exposure on sleep in mice. Behav Brain Res. 2004;154:137–147. doi: 10.1016/j.bbr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 99.Tang X, Yang L, Sanford LD. Rat strain differences in freezing and sleep alterations associated with contextual fear. Sleep. 2005;28:1235–1244. doi: 10.1093/sleep/28.10.1235. [DOI] [PubMed] [Google Scholar]
- 100.Tang X, Yang L, Sanford LD. Interactions between brief restraint, novelty, and footshock stress on subsequent sleep and EEG power in rats. Brain Res. 2007;1142:110–118. doi: 10.1016/j.brainres.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thenot JP, Hermann P, Durand A, Burke JT, Allen J, Garrigou D, Vajta S, Albin H, Thebault JJ, Olive G, Warrington SJ. Pharmacokinetics and metabolism of zolpidem in various animal species and in humans. In: Sauvanet JP, Langer Sz, Morselli PL, editors. Imidazopyridines in Sleep Disorders. Raven Press; New York: 1988. pp. 139–153. [Google Scholar]
- 102.Ulloor J, Mavanji V, Saha S, Siwek DF, Datta S. Spontaneous REM sleep is modulated by the activation of the pedunculopontine tegmental GABAB receptors in the freely moving rat. J Neurophysiol. 2004;91:1822–1831. doi: 10.1152/jn.01104.2003. [DOI] [PubMed] [Google Scholar]
- 103.Vazquez-Palacios G, Velazquez-Moctezuma J. Effect of electric footshock, immobilization, and corticosterone administration on the sleep-wake pattern in the rat. Physiol & Behav. 2000;71:23–28. doi: 10.1016/s0031-9384(00)00285-7. [DOI] [PubMed] [Google Scholar]
- 104.Vecsey CG, Baillie GS, Jaganath D, Havekes R, Daniels A, Wimmer M, Huang T, Brown KM, Li X-Y, Descalzi G, Kim SS, Chen T, Shang Y-Z, Zhuo M, Houslay MD, Abel T. Sleep deprivation impairs cAMP signaling in the hippocampus. Nature. 2009;461:1122–1125. doi: 10.1038/nature08488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Visser SAG, Wolters FLC, VanDerGraaf PH, Peletier LA, Danhof M. Dose-dependent EEG effects of zolpidem provide evidence for GABA-A receptor subtype selectivity in vivo. J Pharmacol & Experimental Therap. 2003;305:1251–1257. doi: 10.1124/jpet.102.044859. [DOI] [PubMed] [Google Scholar]
- 106.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Walsh JK, Soubrane C, Roth T. Efficacy and safety of zolpidem extended release in elderly primary insomnia patients. Am J Geriatr Psychiatry. 2008;16:44–57. doi: 10.1097/JGP.0b013e3181256b01. [DOI] [PubMed] [Google Scholar]
- 108.Wieland HA, Luddens H. Four amino acid exchanges convert a diazepam-insensitive, inverse agonist-preferring GABAA receptor into a diazepam-preferring GABAA receptor. J Med Chem. 1994;37:4576–4580. doi: 10.1021/jm00052a019. [DOI] [PubMed] [Google Scholar]
- 109.Wingrove PB, Safo P, Wheat L, Thompson SA, Wafford KA, Whiting PJ. Mechanism of alpha-subunit selectivity of benzodiazepine pharmacology at gamma-aminobutyric acid type A receptors. Eur J Pharmacol. 2002;437:31–39. doi: 10.1016/s0014-2999(02)01279-7. [DOI] [PubMed] [Google Scholar]
- 110.Xi M, Chase MH. Effects of eszopiclone and zolpidem on sleep and waking states in the adult guinea pig. Sleep. 2008;31:1043–1051. [PMC free article] [PubMed] [Google Scholar]
- 111.Xi M, Chase MH. Psychopharmacology. Vol. 205. Berl: 2009. The impact of age on the hypnotic effects of eszopiclone and zolpidem in the guinea pig; pp. 107–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yehuda R, LeDoux J. Response variation following trauma: a translational neuroscience approach to understanding PTSD. Neuron. 2007;56:19–32. doi: 10.1016/j.neuron.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 113.Zammit GK, McNabb LJ, Caron J, Amato DA, Roth T. Efficacy and safety of eszopiclone across 6-weeks of treatment for primary insomnia. Curr Med Res & Opin. 2004;20:1979–1991. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]