Abstract
Monocytes, macrophages, and inflammation play a key role in the process of neointimal proliferation and restenosis. The present study evaluated whether systemic and transient depletion of monocytes could be obtained by a single intravenous (IV) injection of simvastatin liposomes, for the inhibition of neointima formation. Balloon-injured carotid artery rats (n = 30) were randomly assigned to treatment groups of free simvastatin, simvastatin in liposomes (3 mg/kg), and saline (control). Stenosis and neointima to media ratio (N/M) were determined 14 days following single IV injection at the time of injury by morphometric analysis. Depletion of circulating monocytes was determined by flow cytometry analyzes of blood specimens. Inhibition of RAW264.7, J774, and THP-1 proliferation by simvastatin-loaded liposomes and free simvastatin was determined by the 3-(4, 5-dimethylthiazolyl-2)-2, 5- diphenyltetrazolium bromide assay. Simvastatin liposomes were successfully formulated and were found to be 1.5-2 times more potent than the free drug in suppressing the proliferation of monocytes/macrophages in cell cultures of RAW 264.7, J774, and THP-1. IV injection of liposomal simvastatin to carotid-injured rats (3 mg/kg, n = 4) resulted in a transient depletion of circulating monocytes, significantly more prolonged than that observed following treatment with free simvastatin. Administration to balloon-injured rats suppressed neointimal growth. N/M at 14 days was 1.56 ± 0.16 and 0.90 ± 0.12, control and simvastatin liposomes, respectively. One single systemic administration of liposomal simvastatin at the time of injury significantly suppresses neointimal formation in the rat model of restenosis, mediated via a partial and transient depletion of circulating monocytes.
Key words: drug delivery systems, liposomes, monocytes, restenosis, statins
INTRODUCTION
Restenosis, a major unfavorable outcome of percutaneous coronary intervention (PCI, angioplasty and stenting) warrants repeated revascularization in up to 35-40% of PCI patients (1). Systemic pharmacological approaches to reduce restenosis have not been successful in clinical use. Local treatment with drug-eluting stent (DES) demonstrated significant reduction in restenosis rate and the subsequent need for revascularization (2). Nevertheless, DES does not resolve all the problems arising from PCI and may be associated with an increased risk for late stent thrombosis (3–7).
Recent experimental and clinical data indicate that the innate immunity and inflammation are of major importance in vascular repair and are significant determinants of the PCI outcome (1,8). Monocyte adhesion and infiltration occur immediately after vascular injury, and macrophages accumulated in the vessel wall activate smooth muscle cells (SMC) proliferation by the secretion of cytokines, metalloproteinases, and growth factors (9–11). The bisphosphonates (BPs), bone-seeking drugs, and osteoclasts inhibitors deplete circulating monocytes and inhibit macrophages when administered in a particulated dosage form, which is avidly phagocytosed by these cells. Recently, we have shown that partial and transient systemic inactivation of monocytes by polymeric nanoparticles (NP) and liposome-encapsulated BPs suppresses neointimal formation and reduces experimental restenosis (12–16).
Statins are extensively used in medical practice and have been shown to improve survival in patients with cardiovascular disease (17,18). In the early 1990s, experimental studies have suggested that statins might reduce restenosis after balloon angioplasty (19). Several preclinical studies have demonstrated that multiple systemic administration of statins before and after surgery attenuates neointimal formation (20,21). Furthermore, accumulating clinical evidence points to the favorable effects of statins on restenosis following stent deployment (22–24). However, all clinical studies (25–28), with a single exception (29), have reported a lack of significant effect for statins in preventing restenosis after PCI. Statins, similar to nitrogen-containing bisphosphonates (nBPs), are inhibitors of the mevalonate pathway blocking the prenylation of small GTPases, such as Ras, Rho, and Rac (22). These proteins regulate a variety of cell processes important for monocyte/macrophage function, including cell morphology, membrane ruffling, and trafficking of endosomes (30,31). In contrast to nBPs, which accumulate in bone, statins accumulate in the liver affecting hepatocytes. Recent in vitro and in- vivo findings indicate that statins, in addition to their lipid-lowering effects, possess certain anti-inflammatory properties such as inhibiting the production of pro-inflammatory cytokines (e.g., TNF-α and IL-1ß), C-reactive protein, cellular adhesion molecules (e.g., ICAM-1, P-selectin) and chemotaxic molecules (MCP-1; 32,33). Furthermore, in vitro and in vivo studies have documented that statins induce macrophage/monocyte apoptosis (34,35).
In contrast to the biodistribution of drugs following parenteral administration of a solution, ‘conventional’ liposomes (charged and not of ultra-small size) accumulate in the mononuclear phagocytic system (MPS, formerly known as the reticuloendothelial system; 36–43). A preferential uptake of intact ‘conventional’ liposomes containing serotonin by circulating monocytes has been demonstrated recently (44). We hypothesized that encapsulation of statins in a suitable liposomal formulation, similarly to liposomal BPs (14–16,42,44–46), will divert the statins from their main target (hepatocytes) to circulating monocytes resulting in systemic inactivation of monocytes and consequently, attenuation of neointimal formation. To the best of our knowledge, there are no reports in the literature on targeting circulating monocytes by a liposomal delivery system of statins for preventing restenosis. Furthermore, the inhibitory effect of liposomal statins on monocyte for attenuating inflammation has not yet been studied.
The present study investigated the antiproliferative effects and cytotoxicity of liposomal simvastatin in cell cultures. Liposomal delivery system of simvastatin was successfully formulated and characterized. In addition, we have examined the effects of a single systemic administration of simvastatin encapsulated in liposomes (a single intravenous (IV) injection at the time of angioplasty) on circulating monocytes, and on neointimal formation in a rat model of restenosis, in comparison to IV free simvastatin administration.
METHODS
Liposome Preparation
For preparing liposomes distearoylphosphatidylcholine (DSPC), distearoyl phosphatidyl glycerol (DSPG; Lipoid GmbH, Ludwigshafen, Germany) and cholesterol were used (Sigma, St. Louis, MO, USA). The liposomal formulations were prepared by a modified thin film hydration method. Phospholipids, cholesterol, and simvastatin (DSPC:DSPG:CHOL:simvastatin, 7:3:1:1, molar ratio) were dissolved in tert-butanol and lyophilized to produce a film. Eight milliliters of Modified Earle's salt solutions/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) and 2-(N-morpholino)ethansulphonic acid (MES) buffer pH 7.2 (50 mM MES, 50 mM HEPES, 75 mM NaCl) was added to the film, and the liposomes thus obtained were homogenized to 200 nm by means of an extruder (Lipex Biomembranes, Vancouver, Canada). To remove non-encapsulated drug, the liposomes were passed through a Sephadex G-50 column and eluted with MES/HEPES buffer. The formulation volume was adjusted to 8.0 ml.
Effect on Cell Growth
Murine macrophage cell lines, RAW 264.7 (ATCC, Rockville, MD, USA) and J774A.1 (ECACC, CAMR, England), and peripheral human blood monocytes cell line, THP-1 (ATCC, Rockville, MD, USA) were utilized. RAW 264 and J774A were grown in DMEM supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 units/ml penicillin and 100 µg/ml streptomycin in 8% CO2 atmosphere at 37°C. THP-1 cells were grown in RPMI supplemented with 10% FCS, 2 mM l-glutamine, 10 mM HEPES, 100 units/ml penicillin, 0.1 mg/ml streptomycin, adjusted to contain 4.5 g/L glucose, and 1.0 sodium pyruvate in 8% CO2 atmosphere at 37°C. THP-1 cells were additionally supplemented with 0.005 mM 2- mercaptoethanol (90% solution).
The cells were plated at 4 × 104 cells per well in 24-well plates and allowed to grow overnight. Unless otherwise noted, all materials for cell cultures were purchased from Biologic Industries (Beit Haemek, Israel) and from Sigma. Inhibition of RAW264.7, J774 and THP-1 proliferation by simvastatin loaded liposome and free simvastatin was determined by incubating the cells for 48 h after treatment followed by the 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide assay (47).
Rat Balloon Injury Model
Male Sabra rats (n = 30, Harlan Laboratories), weighing 300-350 g were utilized. Animal care and procedures were in accordance with the standards for care and use of laboratory animals of the Hebrew University of Jerusalem. Animals were fed standard laboratory chow and tap water ad libitum, and experiments were performed under general anesthesia achieved by an IP injection of ketamin (80 mg/kg, Fort Dodge Animal Health, USA) and xylazine (5 mg/kg, V.M.D. NV, Belgium). The rat carotid injury model was performed as described previously (48).
The left common carotid artery was denuded of endothelium by the intraluminal passage of a 2 F balloon catheter (Baxter, Irvine, CA, USA) introduced through the external carotid artery. The balloon was sufficiently distended with saline to generate a slight resistance and passed three times. Animals were randomly assigned to treatment and control groups, and an investigator blinded to the type of experimental group performed the experiments. Immediately after surgery (day 0), animals were injected IV with saline, simvastatin in solution (3 mg/kg), and liposomal simvastatin (3 mg/kg). Animals were killed 14 days after injury by ether suffocation. Arteries were perfusion-fixed in situ with 150 ml of 4% formaldehyde solution (pH 7.4) for morphometric analysis. The arterial segments were embedded in paraffin and cut at 8-10 sites (600 µm apart). Sections of 6 µm were mounted and stained with Verhoeff’s elastin stain (48).
Morphometric Analysis
Eight to ten sections in each slide were analyzed by means of computerized morphometric analysis (NIH Image) by an investigator blinded to the type of the experimental group. The section with the greatest luminal narrowing by neointima was analyzed as previously described (49). The residual lumen, the area bounded by the internal elastic lamina (original lumen), and the area circumscribed by the external elastic lamina (total arterial area) were measured directly. The degree of neointimal thickening was expressed as the ratio between the area of the neointima and the original lumen (percent stenosis) and as the ratio between the neointimal area to the area of the media.
Depletion of Blood Monocytes
Flow cytometry (fluorescence-activated cell sorting, FACS) analyzes were performed by means of a FACScan Flowcytometer (Becton Dickinson, USA). A separate group of rats (n = 12) were subjected to balloon injury and treatment. Animals were randomly assigned to treatment of 3 mg/kg liposomal simvastatin, 3 mg/kg free simvastatin and saline (control) group, injected concurrently to surgery. Blood specimens were taken prior to and 48, 96, and 168 h after injections. No sampling was done in the second week following injury to allow recovery. At the specified time points, blood specimens (500 ml) were drawn from the retro orbital sinus by capillary tube under isofluran anesthesia (Minrad International, USA) and collected in ethylenediaminetetraacetic acid tubes (Vacutainer; BD, NJ, USA). Each blood sample (100 ml) was incubated for 30 min (37°C, in the dark) with mouse anti-rat fluorescein isothiocyanate-conjugated anti-CD3, and mouse anti-rat PE- conjugated anti-CD4 (BD). A red blood cell lysing solution (Erythrolyse, 1:20 dilution, AbD Serotec) was added to the mixture, which was incubated for additional 10 min. The residual cells were washed twice (x1500 rpm, 5 min, 37°C) in the FACS medium (PBS, 1% BSA and 0.02% sodium azide) and were suspended in 1 ml FACS medium for flow cytometry. The population of white blood cells (WBC) was gated according to forward and side scattering. In order to distinguish monocytes from other WBC, the gated WBC was analyzed using anit-CD3 and anti- CD4 staining: the proportion of monocytes was determined by an investigator blinded to the experimental group, based on the expression of CD4 and lack of CD3 (CD3− CD4+).
Statistical Analysis
Comparisons among treatment groups were made by the one way analysis of variance followed by Dunnett or Student Newman-Keuls multiple comparisons test. Differences were determined statistically significant with p < 0.05.
RESULTS
Characterization of the Liposomal Formulations
The encapsulation yield was 61 ± 5%. The concentration of encapsulated simvastatin and lipids in the liposome was 0.70 ± 0.04 mg/ml and 11.6 ± 1.8 mg/ml, respectively. The liposomes obtained had a mean diameter of 164 ± 25 nm, with a polydispersity index below 0.1, and a negative zeta potential of −14.3 ± 0.3 mV.
Inhibition of Cell Growth
To evaluate the inhibitory effect of free and liposomal simvastatin on phagocytic cells, three different types of monocyte/macrophage cell lines, J774, RAW264.7, and THP-1, were utilized. The growth-inhibitory properties (IC50) of liposomal simvastatin in comparison to free simvastatin and liposomal alendronate (positive control) are summarized in Table I. The rank of sensitivity of these cells to both forms of simvastatin was  . Simvastatin liposomes were found to be 1.5-2 times more potent than the free drug in suppressing the proliferation of RAW 264.7, J774, and THP-1. Moreover, liposomal simvastatin was found more potent in inhibiting RAW 264.7 cells in comparison to liposomal alendronate (3.0 ± 1.5 µM and 25.0 ± 3.2 µM, respectively), and tenfold more potent in THP-1 cell culture.
. Simvastatin liposomes were found to be 1.5-2 times more potent than the free drug in suppressing the proliferation of RAW 264.7, J774, and THP-1. Moreover, liposomal simvastatin was found more potent in inhibiting RAW 264.7 cells in comparison to liposomal alendronate (3.0 ± 1.5 µM and 25.0 ± 3.2 µM, respectively), and tenfold more potent in THP-1 cell culture.
Table I.
The Inhibition of Monocytes/Macrophages Proliferation in Cell Cultures by Free and Liposomal Simvastatin. The Results are Presented as mean ± SD
| IC50 (µM) | |||
|---|---|---|---|
| Cell line | RAW264.7 | J774 | THP-1 |
| Treatment | |||
| Free simvastatin | 6.0 ± 1.7 | 25.0 ± 6.2 | 46.0 ± 1.9 |
| Liposomal simvastatin | 3.0 ± 1.5 | 15.0 ± 1.3 | 28.0 ± 3.0 |
| Liposomal alendronate | 25.0 ± 3.2 | 14.0 ± 2.9 | 300.0 ± 6.0 |
Depletion of Blood Monocytes
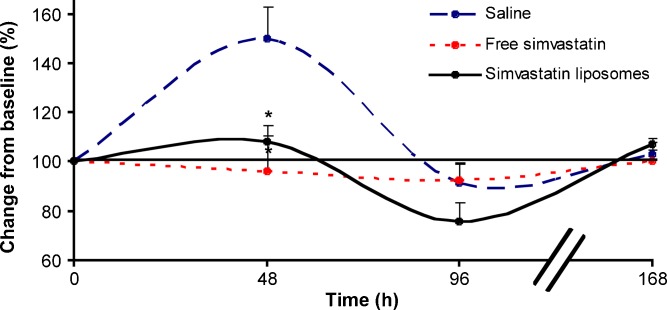
An increase in monocyte count was observed 48 h after vascular injury (43.9 ± 13.0% change from baseline, n = 4), returning to baseline levels after 96 h. Treatment with simavastatin reduced the monocytic response to injury. Liposomal simvastatin administration (3 mg/kg, n = 4) resulted in a transient depletion of circulating monocytes; significantly more prolonged than that observed following treatment with free simvastatin, −24.1 ± 7.8 and −7.9 ± 6.8% change from baseline after 96 h, respectively (Fig. 1). Monocyte levels returned to baseline levels 168 h following treatment in both free and liposomal simvastatin treatment groups.
Fig. 1.
The effect of liposomal simvastatin (IV, 3 mg/kg) on peripheral blood monocytes in the rat restenosis model. Representative flow cytometry analysis demonstrate the gated monocytes population (CD3− CD4+), 48, 96, and 168 h after treatment with liposomal simvastatin in comparison to the free drug and saline. The treatment effect on circulating monocytes number (+SEM) over time is expressed as the percentage of total WBC (n = 4 at each group, *p < 0.05)
Effect of Liposomal Simvastatin on Neointimal Formation
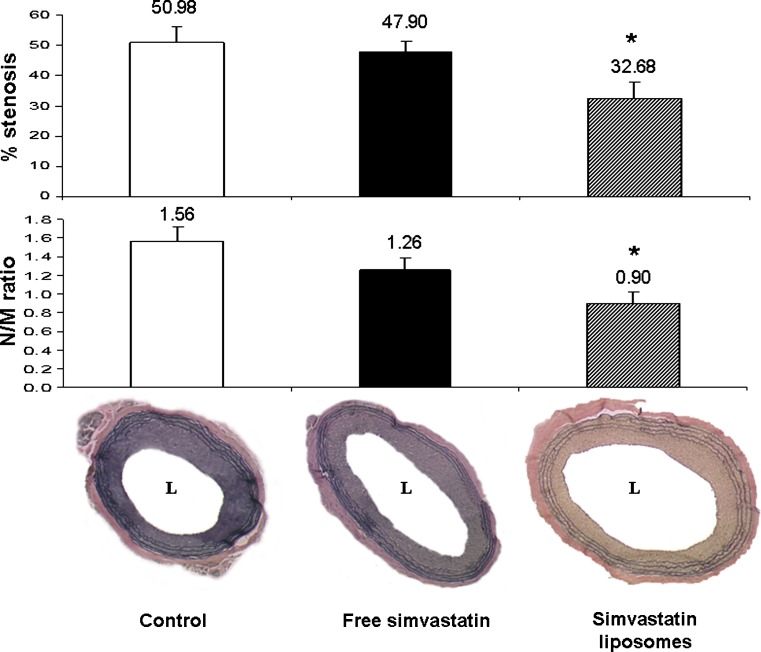
Marked neointimal formation (51.0 ± 5.5%) and decreased luminal area were observed in control animals. Liposomal simvastatin treatment resulted in suppressed intimal growth following a single intravenous injection on day 0. N\M ratio and % stenosis 14 days after angioplasty were significantly reduced following liposomal simvastatin treatment in comparison to control animals, 0.90 ± 0.12 and 32.7 ± 5.3%, and 1.56 ± 0.16 and 51.0 ± 5.5%, respectively (Fig. 2). A single IV injection of free simvastatin with the same dose resulted in a mild, nonsignificant reduction of N\M ratio and % stenosis, 1.26 ± 0.12 and 47.9 ± 3.6%, respectively. Three rats (one in each group) died during surgery probably due to surgical complications. No overt infection or side effects were noted in all animals.
Fig. 2.
Bar graph depicting reduced stenosis (upper) and neointima/media (N/M) ratio (lower) in liposomal simvastatin treated rats (3 mg/kg, IV, n = 9) 14 days after injury, in comparison to treatments with simvastatin solution (3 mg/kg, IV, n = 9, free drug) and saline (IV, n = 9, control). Below the bar graph are photomicrographs of Verhoeff’s tissue elastin staining of full size sections of control, free simvastatin, and liposomal-simvastatin-treated animals (*p < 0.05)
DISCUSSION
The major findings of the present study are that liposomal simvastatin significantly suppresses neointimal formation following arterial balloon injury in rats, most probably via the inhibition of circulating monocytes.
A liposomal formulation of simvastatin has been successfully formulated in the nano range size (164 ± 25 nm), negatively charged and with a homogeneous size distribution. Particulated dosage forms are endocytosed by the MPS (37–41). The preferable physicochemical properties of monocyte-targeted liposomes are those of the so called ‘conventional’ liposomes, neither hydrophilic nor neutral membrane, and not of ultra-small size (36,40–42). A charged membrane, particularly negative, enhances the internalization of liposomes into phagocytic cells through adsorptive endocytosis (36,50), in part following opsonization (39,51). The optimal size of monocyte-targeted liposomes is around 200 nm, for maximizing both efficacy and safety. Large particles (>500 nm) accumulate in the lungs causing thrombosis (52), and particles smaller than 80 nm are, on the other hand, prone to be transported to non-phagocytic cells escaping the MPS (40,41,51,53,54). It should also be noted that vesicles under ∼250 nm can be readily filter sterilized. Simvastatin’s lipophilic nature resulted in high encapsulation efficiency (61 ± 5%). Lipophilic drugs such as simvastatin are incorporated in the liposomal bilayer up to their solubility and hence are effectively entrapped.
Preliminary cell culture studies revealed that a liposomal pravastatin formulation exhibited inferior inhibiting effect on macrophages/monocytes in comparison to liposomal simvastatin and liposomal alendronate (data not shown). Therefore, simvastatin was chosen, and further research should elucidate the formulation or biologic differences between liposomal statins. Liposomes containing simvastatin were found more potent than the free drug in inhibiting various cell lines of monocytes/macrophages (Table I). Furthermore, liposomal simvastatin was found more potent than liposomal alendronate (Table I). Although liposomal simvastatin was found apparently more effective than liposomal alendronate in inhibiting macrophages in cell culture, an accurate comparison is yet to be determined in animal models of restenosis by a side-by-side study.
The inhibition of monocytes/macrophages in vitro suggested that systemic inactivation of monocytes could be obtained by IV administration of liposomal simvastatin, possibly resulting in attenuation of restenosis. Indeed, IV administration of a single dose of liposomal simvastatin (3 mg/kg) to balloon injured animals abrogated the systemic monocytic response to injury, prolonging the effect in comparison to free simvastatin (Fig. 1). The increased potency of liposomal simvastatin indicates that the liposomal delivery system targeted simvastatin to circulating monocytes. It is demonstrated, for the first time, that a single dose of simvastatin encapsulated in liposomes, administered concurrent with injury, significantly reduces luminal loss in rats (Fig. 2). The effect was significantly stronger than that observed following treatment with free simvastatin. It has been suggested that the anti-inflammatory effect of free statins is derived from extensively interfering monocytes entering the arterial wall in a process driven by chemoattractant molecules (33). Thus, the significant inhibition of stenosis in the rat model is most probably due to (1) the more potent inhibitory effect of liposomal simvastatin (exhibited in vitro and in vivo) and (2) the preferential uptake of the particulated dosage form in circulating monocytes.
Innate immunity plays a major role in vascular repair. Monocytes adhesion and infiltration occur immediately after vascular injury (55,56). Macrophage content in atherectomy tissue and the activation status of blood monocytes correlate with an increased rate of restenosis in humans (57,58). Recently, we have shown that transient and partial depletion of circulating monocytes by particulate dosage forms such as polymeric NP and liposomes-laden BPs suppresses neointimal formation and reduces experimental restenosis (12–16). It is suggested that, similarly to liposomal BPs (14–16), the inhibition of stenosis was mediated via simvastatin liposomes accumulation in circulating monocytes and their inactivation. The partial and transient depletion of circulating monocytes results in reduced number of macrophages at the injury site and consequently with a diminished inflammatory response (14–16).
After internalization, as have been demonstrated for this type of formulation (14,42,43), lysosomal action disrupts the fatty bilayer of the liposome and simvastatin is released into the cell, causing cell death. Circulating blood monocytes fully recovered 7 days after injection. The effects of liposomal simvastatin are limited to cells with phagocytic capacity with no effect on other cells, such as SMC or endothelial cells, as has been demonstrated in animals treated with liposomal BPs (12–16,42,46). Simvastatin leaking from inactivated monocytes and the delivery system would accumulate mainly in the liver. Thus, no significant adverse effects are expected after liposomal simvastatin treatment since the outcome of the liposomal formulation, as with most other liposomes and particulate drug delivery systems, is in the MPS. Long term inactivation of macrophages carries the danger of immunosuppression and infection. However, as in several studies in rabbits and rats, no overt infection was observed with partial and transient monocytes depletion (12–16,42,46,59).
Although the benefits of statins in primary and secondary prevention of atherosclerosis have been widely recognized (17,18), it is less clear whether statins can effectively prevent restenosis in the clinical settings (25,26,29). The approach of liposomal statin treatment is fundamentally different than systemic administration of the free drug in solution, which is not targeted to a specific organ/cell, and from the clinically used drug-eluting stents to inhibit restenosis (60,61). While the latter strategy is aimed to inhibit local events by a drug delivery system directed to the injured artery, the strategy of liposomal statins provides a systemic therapy to a systemic inflammatory process, regardless of the procedure and the device(s) used.
It should be noted that this approach is now under phase II clinical studies (IV injection of liposomal alendronate (62). The lipid-lowering effects of statins are derived from targeting hepatocytes and inhibiting the 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the enzyme that converts HMG-CoA into mevalonic acid (a cholesterol precursor). ‘Conventional’ liposomes as well as similarly formulated liposomes with other drugs are known to preferably accumulate in the liver as part of the MPS (15,43). It can be speculated that liposomal simvastatin, in contrast to liposomal alendronate, could have a dual effect of restenosis prevention and lowering lipids.
CONCLUSIONS
Liposomes containing simvastatin are more potent than the free drug and liposomal alendronate in inhibiting various cell lines of monocytes/macrophages. One single systemic administration of liposomal simvastatin significantly suppresses neointimal formation in the rat model of restenosis, mediated via a partial and transient depletion of circulating monocytes.
Acknowledgments
This work was supported in part by a grant from Biorest Ltd., Israel awarded to GG. GG is affiliated with the David R. Bloom Center for Pharmacy at The Hebrew University of Jerusalem.
References
- 1.Toutouzas K, Colombo A, Stefanadis C. Inflammation and restenosis after percutaneous coronary interventions. Eur Heart J. 2004;25(19):1679–87. doi: 10.1016/j.ehj.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 2.Dobesh PP, Stacy ZA, Ansara AJ, Enders JM. Drug-eluting stents: a mechanical and pharmacologic approach to coronary artery disease. Pharmacotherapy. 2004;24(11):1554–77. doi: 10.1592/phco.24.16.1554.50955. [DOI] [PubMed] [Google Scholar]
- 3.Bavry AA, Kumbhani DJ, Helton TJ, Bhatt DL. Risk of thrombosis with the use of sirolimus-eluting stents for percutaneous coronary intervention (from registry and clinical trial data) Am J Cardiol. 2005;95(12):1469–72. doi: 10.1016/j.amjcard.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Bavry AA, Kumbhani DJ, Helton TJ, Borek PP, Mood GR, Bhatt DL. Late thrombosis of drug-eluting stents: a meta-analysis of randomized clinical trials. Am J Med. 2006;119(12):1056–61. doi: 10.1016/j.amjmed.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, et al. Pathology of drug-eluting stents in humans: delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48(1):193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 6.McFadden EP, Stabile E, Regar E, Cheneau E, Ong AT, Kinnaird T, et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364(9444):1519–21. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- 7.Nilsen DW, Melberg T, Larsen AI, Barvik S, Bonarjee V. Late complications following the deployment of drug-eluting stents. Int J Cardiol. 2006;109(3):398–401. doi: 10.1016/j.ijcard.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 8.Rogers C, Edelman ER, Simon DI. A mAb to the beta2-leukocyte integrin Mac-1 (CD11b/CD18) reduces intimal thickening after angioplasty or stent implantation in rabbits. Proc Natl Acad Sci USA. 1998;95(17):10134–9. doi: 10.1073/pnas.95.17.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayes-Genis A, Campbell JH, Carlson PJ, Holmes DR, Jr, Schwartz RS. Macrophages, myofibroblasts and neointimal hyperplasia after coronary artery injury and repair. Atherosclerosis. 2002;163(1):89–98. doi: 10.1016/S0021-9150(01)00771-7. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Schwartz D, Brogi E, Tanaka H, Clinton SK. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation. 1992;86(6 Suppl):III47–52. [PubMed] [Google Scholar]
- 11.Welt FG, Tso C, Edelman ER, Kjelsberg MA, Paolini JF, Seifert P, et al. Leukocyte recruitment and expression of chemokines following different forms of vascular injury. Vasc Med. 2003;8(1):1–7. doi: 10.1191/1358863x03vm462oa. [DOI] [PubMed] [Google Scholar]
- 12.Cohen-Sela E, Dangoor D, Epstein H, Danenberg HD, Golomb G, Gao J. Nanospheres of bisphosphonates attenuate intimal hyperplasia. J Nanosci Nanotech. 2006;6:3226–34. doi: 10.1166/jnn.2006.428. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Sela E, Rosenzweig O, Gao J, Epstein H, Gati I, Reich R, et al. Alendronate- loaded nanoparticles deplete monocytes and attenuate restenosis. J Controlled Rel. 2006;113(1):23–30. doi: 10.1016/j.jconrel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Danenberg HD, Fishbein I, Epstein H, Waltenberger J, Moerman E, Monkkonen J, et al. Systemic depletion of macrophages by liposomal bisphosphonates reduces neointimal formation following balloon injury in the rat carotid artery. J Cardiovasc Pharmacol. 2003;42(5):671–9. doi: 10.1097/00005344-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R, Gati I, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. doi: 10.1161/01.CIR.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 16.Danenberg HD, Golomb G, Groothuis A, Gao J, Epstein H, Swaminathan RV, et al. Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation. 2003;108(22):2798–804. doi: 10.1161/01.CIR.0000097002.69209.CD. [DOI] [PubMed] [Google Scholar]
- 17.Doggrell SA. Statins in the 21st century: end of the simple story? Expert Opin Investig Drugs. 2001;10(9):1755–66. doi: 10.1517/13543784.10.9.1755. [DOI] [PubMed] [Google Scholar]
- 18.Liao JK. Beyond lipid lowering: the role of statins in vascular protection. Int J Cardiol. 2002;86(1):5–18. doi: 10.1016/S0167-5273(02)00195-X. [DOI] [PubMed] [Google Scholar]
- 19.Horlitz M, Sigwart U, Niebauer J. Fighting restenosis after coronary angioplasty: contemporary and future treatment options. Int J Cardiol. 2002;83(3):199–205. doi: 10.1016/S0167-5273(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 20.Indolfi C, Cioppa A, Stabile E, Di Lorenzo E, Esposito G, Pisani A, et al. Effects of hydroxymethylglutaryl coenzyme A reductase inhibitor simvastatin on smooth muscle cell proliferation in vitro and neointimal formation in vivo after vascular injury. J Am Coll Cardiol. 2000;35(1):214–21. doi: 10.1016/S0735-1097(99)00526-4. [DOI] [PubMed] [Google Scholar]
- 21.Walter DH, Rittig K, Bahlmann FH, Kirchmair R, Silver M, Murayama T, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105(25):3017–24. doi: 10.1161/01.CIR.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 22.Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203(2):325–30. doi: 10.1016/j.atherosclerosis.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 23.Stenestrand U, Wallentin L. Early statin treatment following acute myocardial infarction and 1-year survival. JAMA. 2001;285(4):430–6. doi: 10.1001/jama.285.4.430. [DOI] [PubMed] [Google Scholar]
- 24.Walter DH, Schachinger V, Elsner M, Mach S, Auch-Schwelk W, Zeiher AM. Effect of statin therapy on restenosis after coronary stent implantation. Am J Cardiol. 2000;85(8):962–8. doi: 10.1016/S0002-9149(99)00910-8. [DOI] [PubMed] [Google Scholar]
- 25.Bunch TJ, Muhlestein JB, Anderson JL, Horne BD, Bair TL, Jackson JD, et al. Effects of statins on six-month survival and clinical restenosis frequency after coronary stent deployment. Am J Cardiol. 2002;90(3):299–302. doi: 10.1016/S0002-9149(02)02467-0. [DOI] [PubMed] [Google Scholar]
- 26.Horlitz M, Sigwart U, Niebauer J. Statins do not prevent restenosis after coronary angioplasty: where to go from here? Herz. 2001;26(2):119–28. doi: 10.1007/PL00002013. [DOI] [PubMed] [Google Scholar]
- 27.Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, Sardella G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49(12):1272–8. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Weintraub WS, Boccuzzi SJ, Klein JL, Kosinski AS, King SB, 3rd, Ivanhoe R, et al. Lack of effect of lovastatin on restenosis after coronary angioplasty. Lovastatin Restenosis Trial Study Group. N Engl J Med. 1994;331(20):1331–7. doi: 10.1056/NEJM199411173312002. [DOI] [PubMed] [Google Scholar]
- 29.Mulder HJ, Bal ET, Jukema JW, Zwinderman AH, Schalij MJ, van Boven AJ, et al. Pravastatin reduces restenosis two years after percutaneous transluminal coronary angioplasty (REGRESS trial) Am J Cardiol. 2000;86(7):742–6. doi: 10.1016/S0002-9149(00)01073-0. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nature reviews. 2006;6(5):358–70. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen- containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581–9. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 32.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6(12):1399–402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 33.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4(12):977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 34.Fildes JE, Shaw SM, Mitsidou A, Rogacev K, Leonard CT, Williams SG, et al. HMG- CoA reductase inhibitors deplete circulating classical and non-classical monocytes following human heart transplantation. Transpl Immunol. 2008;19(2):152–7. doi: 10.1016/j.trim.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Kim YC, Song SB, Lee MH, Kang KI, Lee H, Paik SG, et al. Simvastatin induces caspase-independent apoptosis in LPS-activated RAW264.7 macrophage cells. Biochem Biophys Res Commun. 2006;339(3):1007–14. doi: 10.1016/j.bbrc.2005.11.099. [DOI] [PubMed] [Google Scholar]
- 36.Raz A, Bucana C, Fogler WE, Poste G, Fidler IJ. Biochemical, morphological, and ultrastructural studies on the uptake of liposomes by murine macrophages. Cancer Res. 1981;41(2):487–94. [PubMed] [Google Scholar]
- 37.Monkkonen J, Valjakka R, Hakasalo M, Urtti A. The effects of liposome surface charge and size on the intracellular delivery of clodronate and gallium in vitro. Int J Pharm. 1994;107:189–97. doi: 10.1016/0378-5173(94)90433-2. [DOI] [Google Scholar]
- 38.van Etten EW, ten Kate MT, Snijders SV, Bakker-Woudenberg IA. Administration of liposomal agents and blood clearance capacity of the mononuclear phagocyte system. Antimicrob Agents Chemother. 1998;42(7):1677–81. doi: 10.1128/aac.42.7.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patel HM, Moghimi SM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system—the concept of tissue specificity. Adv Drug Deliv Rev. 1998;32(1–2):45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 40.Moghimi SM, Hunter AC. Recognition by macrophages and liver cells of opsonized phospholipid vesicles and phospholipid headgroups. Pharm Res. 2001;18(1):1–8. doi: 10.1023/A:1011054123304. [DOI] [PubMed] [Google Scholar]
- 41.Moghimi SM, Szebeni J. Stealth liposomes and long circulating nanoparticles: critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog Lipid Res. 2003;42(6):463–78. doi: 10.1016/S0163-7827(03)00033-X. [DOI] [PubMed] [Google Scholar]
- 42.Epstein H, Gutman D, Cohen-Sela E, Haber E, Elmalak O, Koroukhov N, et al. Preparation of alendronate liposomes for enhanced stability and bioactivity: in vitro and in vivo characterization. AAPS J. 2008;10(4):505–15. doi: 10.1208/s12248-008-9060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Afergan E, Epstein H, Koroukhov N, Klein M, Litchi A, Mishani E, et al. Biodistribution and imaging studies of 67 Ga-labeled liposomes in rabbits with a vascular injury. J Drug Deliv Sci Technol. 2009;19(4):263–8. [Google Scholar]
- 44.Afergan E, Epstein H, Dahan R, Koroukhov N, Rohekar K, Danenberg HD, et al. Delivery of serotonin to the brain by monocytes following phagocytosis of liposomes. J Control Release. 2008;132(2):84–90. doi: 10.1016/j.jconrel.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 45.van Rooijen N, van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated suicide. Methods Enzymol. 2003;373:3–16. doi: 10.1016/S0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 46.Epstein H, Berger V, Levi I, Eisenberg G, Koroukhov N, Gao J, et al. Nanosuspensions of alendronate with gallium or gadolinium attenuate neointimal hyperplasia in rats. J Controlled Rel. 2007;117(3):322–32. doi: 10.1016/j.jconrel.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 47.Hegyesi H, Csaba G. Time- and concentration-dependence of the growth-promoting activity of insulin and histamine in Tetrahymena. Application of the MTT method for the determination of cell proliferation in a protozoan model. Cell Biol Int. 1997;21(5):289–93. doi: 10.1006/cbir.1997.0146. [DOI] [PubMed] [Google Scholar]
- 48.Golomb G, Fishbein I, Banai S, Mishaly D, Moscovitz D, Gertz SD, et al. Controlled delivery of a tyrphostin inhibits intimal hyperplasia in a rat carotid artery injury model. Atherosclerosis. 1996;125(2):171–82. doi: 10.1016/0021-9150(96)05868-6. [DOI] [PubMed] [Google Scholar]
- 49.Fishbein I, Waltenberger J, Banai S, Rabinovich L, Chorny M, Levitzki A, et al. Local delivery of platelet-derived growth factor receptor-specific tyrphostin inhibits neointimal formation in rats. Arterioscler Thromb Vasc Biol. 2000;20(3):667–76. doi: 10.1161/01.atv.20.3.667. [DOI] [PubMed] [Google Scholar]
- 50.Schroit AJ, Madsen J, Nayar R. Liposome cell interactions: in vitro discrimination of uptake mechanism and in vivo targeting strategies to mononuclear phagocytes. Chem Phys Lipids. 1986;40(2–4):373–93. doi: 10.1016/0009-3084(86)90080-0. [DOI] [PubMed] [Google Scholar]
- 51.Patel HM. Serum opsonins and liposomes: their interaction and opsonophagocytosis. Crit Rev Ther Drug Carrier Syst. 1992;9(1):39–90. [PubMed] [Google Scholar]
- 52.Rodrigueza WV, Pritchard PH, Hope MJ. The influence of size and composition on the cholesterol-mobilizing properties of liposomes in vivo. Biochim Biophys Acta. 1993;1153(1):9–19. doi: 10.1016/0005-2736(93)90270-A. [DOI] [PubMed] [Google Scholar]
- 53.Kao YJ, Juliano RL. Interactions of liposomes with the reticuloendothelial system. Effects of reticuloendothelial blockade on the clearance of large unilamellar vesicles. Biochim Biophys Acta. 1981;677(3–4):453–61. doi: 10.1016/0304-4165(81)90259-2. [DOI] [PubMed] [Google Scholar]
- 54.Sato Y, Kiwada H, Kato Y. Effects of dose and vesicle size on the pharmacokinetics of liposomes. Chem Pharm Bull (Tokyo) 1986;34(10):4244–52. doi: 10.1248/cpb.34.4244. [DOI] [PubMed] [Google Scholar]
- 55.Hanke H, Hassenstein S, Ulmer A, Kamenz J, Oberhoff M, Haase KK, et al. Accumulation of macrophages in the arterial vessel wall following experimental balloon angioplasty. Eur Heart J. 1994;15(5):691–8. doi: 10.1093/oxfordjournals.eurheartj.a060569. [DOI] [PubMed] [Google Scholar]
- 56.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, et al. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104(18):2228–35. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 57.Fukuda D, Shimada K, Tanaka A, Kawarabayashi T, Yoshiyama M, Yoshikawa J. Circulating monocytes and in-stent neointima after coronary stent implantation. J Am Coll Cardiol. 2004;43(1):18–23. doi: 10.1016/j.jacc.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 58.Moreno PR, Falk E, Palacios IF, Newell JB, Fuster V, Fallon JT. Macrophage infiltration in acute coronary syndromes. Implications for plaque rupture. Circulation. 1994;90(2):775–8. doi: 10.1161/01.cir.90.2.775. [DOI] [PubMed] [Google Scholar]
- 59.Haber E, Danenberg HD, Koroukhov N, Ron-El R, Golomb G, Schachter M. Peritoneal macrophage depletion by liposomal bisphosphonate attenuates endometriosis in the rat model. Hum Reprod. 2009;24(2):398–407. doi: 10.1093/humrep/den375. [DOI] [PubMed] [Google Scholar]
- 60.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O'Shaughnessy C, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349(14):1315–23. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 61.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. A polymer- based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350(3):221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 62.Web site: http://clinicaltrials.gov/ct2/show/NCT00739466.