Abstract
OBJECTIVE
Glucagon-like peptide-1 (7-36)amide (GLP-1) is cleaved by dipeptidyl peptidase-4 (DPP-4) to GLP-1 (9-36)amide. We examined whether chemical inhibition or genetic elimination of DPP-4 activity affects cardiovascular function in normoglycemic and diabetic mice after experimental myocardial infarction.
RESEARCH DESIGN AND METHODS
Cardiac structure and function was assessed by hemodynamic monitoring and echocardiography in DPP-4 knockout (Dpp4−/−) mice versus wild-type (Dpp4+/+) littermate controls and after left anterior descending (LAD) coronary artery ligation–induced myocardial infarction (MI). Effects of sustained DPP-4 inhibition with sitagliptin versus treatment with metformin were ascertained after experimental MI in a high-fat diet–streptozotocin model of murine diabetes. Functional recovery from ischemia-reperfusion (I/R) injury was measured in isolated hearts from Dpp4−/− versus Dpp4+/+ littermates and from normoglycemic wild-type (WT) mice treated with sitagliptin or metformin. Cardioprotective signaling in the murine heart was examined by RT-PCR and Western blot analyses.
RESULTS
Dpp4−/− mice exhibited normal indexes of cardiac structure and function. Survival post-MI was modestly improved in normoglycemic Dpp4−/− mice. Increased cardiac expression of phosphorylated AKT (pAKT), pGSK3β, and atrial natriuretic peptide (ANP) was detected in the nonischemic Dpp4−/− heart, and HO-1, ANP, and pGSK3β proteins were induced in nonischemic hearts from diabetic mice treated with sitagliptin or metformin. Sitagliptin and metformin treatment of wild-type diabetic mice reduced mortality after myocardial infarction. Sitagliptin improved functional recovery after I/R injury ex vivo in WT mice with similar protection from I/R injury also manifest in hearts from Dpp4−/− versus Dpp4+/+ mice.
CONCLUSIONS
Genetic disruption or chemical inhibition of DPP-4 does not impair cardiovascular function in the normoglycemic or diabetic mouse heart.
Type 2 diabetes is associated with an increased risk of cardiovascular disease, hence there is considerable interest in strategies that reduce cardiovascular morbidity and mortality in diabetic subjects. Although aggressive treatment of blood pressure and dyslipidemia reduces cardiovascular events in both nondiabetic and diabetic patients, whether reduction in blood glucose alone reduces cardiac events in subjects with established diabetes remains controversial (1). Moreover, pharmacotherapy of diabetes using agents with unique antidiabetic mechanisms may be associated with differential and occasionally unexpected adverse effects on cardiovascular outcomes, independent of effects on glucose control (2,3). Therefore, a detailed understanding of the unique cardiovascular benefits and risks of each antidiabetic drug used to treat diabetes seems prudent.
The two most recently approved drug categories for the treatment of type 2 diabetes, GLP-1 receptor (GLP-1R) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors, exert their antidiabetic actions largely through potentiation of GLP-1R activation (4). Because these agents have only been used clinically for several years, there are scant data on cardiovascular outcomes associated with these incretin-based therapies. GLP-1 improves endothelial function in subjects with type 2 diabetes (5), and transient GLP-1 administration improves cardiovascular outcomes in subjects with myocardial infarction (MI) or congestive heart failure (CHF) (6,7). Moreover, preclinical data demonstrate that GLP-1 is cardioprotective when administered before the induction of ischemia (8–10). Furthermore, therapy with the GLP-1R agonists exenatide and liraglutide is associated with blood pressure reduction in the majority of treated subjects (11,12). Hence, although limited, the available data support the hypothesis that antidiabetic therapy with GLP-1 may be associated with beneficial effects on cardiovascular outcomes. Nevertheless, as GLP-1R agonists may produce weight loss (13), the extent to which the salutary effects of GLP-1R activation on the cardiovascular system in vivo reflect the beneficial consequences of weight loss remains unclear.
In contrast, much less is known about the cardiovascular biology of DPP-4. Unlike therapy with GLP-1R agonists, the use of sitagliptin, saxagliptin, or vildagliptin has not been associated with weight loss or sustained improvement in lipid profiles (4). Moreover, inhibition of DPP-4 enzyme activity modulates the activity of cardioactive peptides such as brain natriuretic peptide, neuropeptide Y, and stromal cell–derived factor-1 (SDF-1) (14) via non–GLP-1 mechanisms of action. More recently, GLP-1 (9-36), a peptide metabolite derived from native GLP-1 (7-36)amide after cleavage by DPP-4, has been shown to exert cardioprotective actions in rodents (15,16) and improve cardiovascular function in dogs with CHF (17). Accordingly, to delineate the importance of DPP-4 for cardiovascular biology in vivo, we studied cardiovascular function in Dpp4−/− mice in vivo, in isolated perfused Dpp4+/+ and Dpp4−/− hearts ex vivo, and in wild-type diabetic mice subjected to experimental MI and treated with the DPP-4 inhibitor sitagliptin.
RESEARCH DESIGN AND METHODS
Animal models and drug treatments.
Experimental procedures adhered to approved protocols of the University Health Network and Mt. Sinai Hospital Animal Care Committees. Mice were housed under pathogen-free conditions in micro-isolator cages and maintained on a 12-h light (0700)/dark (1900) cycle with access to standard rodent food and water ad libitum, except where noted. All experiments used age- and sex-matched littermates. Dpp4−/− mice were inbred on the C57BL/6 background (18). Experimental animals were derived by crosses between heterozygous Dpp4+/− mice to generate Dpp4−/− and Dpp4+/+ littermate mice. All genotypes were confirmed by PCR analyses of tail DNA.
C57BL/6 mice, 4 weeks old, were purchased from Taconic (Germantown, NY) and housed as described above, but placed on a high-fat diet (HFD: 45% kcal from fat; Research Diets, New Brunswick, NJ). After 5 weeks of HFD, mice were fasted for 5 h and then injected with a single dose of streptozotocin (STZ; Sigma) (75 mg/kg i.p.) as a freshly prepared solution in 0.1 mmol/l sodium citrate, pH 5.5. Mice were then randomized to treatment with either HFD alone, HFD plus a DPP-4 inhibitor (sitagliptin, 250 mg · kg−1 · day−1) (19), or HFD plus metformin (450 mg · kg−1 · day−1). The dose of metformin was chosen based on related studies (20) and following pilot studies demonstrating optimal antidiabetic actions without significant effects on food intake or body weight. This dose of sitagliptin does not affect food intake, yet results in 90% inhibition of DPP-4 (19,21). Sitagliptin and metformin were supplied by Merck Research Labs (Rahway, NJ).
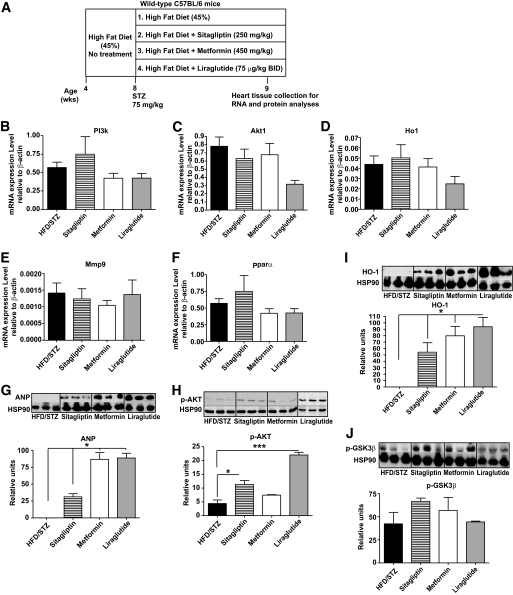
For RNA and protein analyses by real-time quantitative PCR and Western blot, respectively, heart tissue was obtained from separate groups of normoglycemic Dpp4+/+ or Dpp4−/− mice fed regular food, or wild-type C57BL/6 mice exposed to HFD for 4 weeks, given a single dose of STZ (75 mg/kg), and then treated with either HFD alone, or HFD plus 1) metformin, 2) sitagliptin, or 3) the GLP-1R agonist liraglutide (Novo Nordisk, Novo Alle, Bagsvaerd, Denmark) (10), 75 μg/kg i.p. twice daily for an additional 7 days. All animals were killed by exposure to carbon dioxide.
Isolated heart preparations were from 12-week-old male Dpp4−/− and Dpp4+/+ littermates or separate groups of wild-type C57BL/6 mice. Only isolated mouse hearts exhibiting a heart rate >350 bpm were used. Wild-type C57BL/6 mice were treated with an intraperitoneal injection of either sitagliptin (20 mg/kg) or metformin (125 μg/kg) or saline at 24 h and 1 h before heart excision.
Metabolic measurements.
Oral glucose tolerance tests were performed in sitagliptin- or metformin-treated wild-type C57BL/6 mice and untreated controls. Mice were fasted for 16 h and administered glucose (1.5 mg/g) via oral gavage. A1C and blood glucose levels were measured on whole blood using the DCA 2000+ Analyzer and a Glucometer (both Bayer, Toronto, ON, Canada). GLP-1 (7-36)amide levels were measured by a Meso Scale Discovery (MSD) metabolic sandwich immunoassay (MSD, Gaithersburg, MD) in plasma from mice fasted for 5 h.
Coronary artery ligation.
Experimental MI was induced by left anterior descending (LAD) artery ligation as described (22). Briefly, 12-week-old male and female Dpp4−/− and Dpp4+/+ mice were anesthetized using 1% isoflurane, intubated, and ventilated with room air using a positive-pressure respirator (model 680; Harvard, South Natick, MA). Left thoracotomy was performed via the fourth intercostal space; the lungs were retracted to expose the heart, and the pericardium was opened. The LAD was ligated with an 8–0 silk suture near its origin between the pulmonary outflow tract and the edge of the left atrium. Acute myocardial ischemia was considered successful when the anterior wall of the left ventricle (LV) turned pale. The lungs were inflated by an increase in positive end-expiratory pressure, and the thoracotomy was closed. Animals were kept on the ventilator until awake. Sham operation differed only in that the 7–0 suture was passed under the coronary artery and then removed.
Ultrasound biomicroscopy in mice after LAD artery ligation.
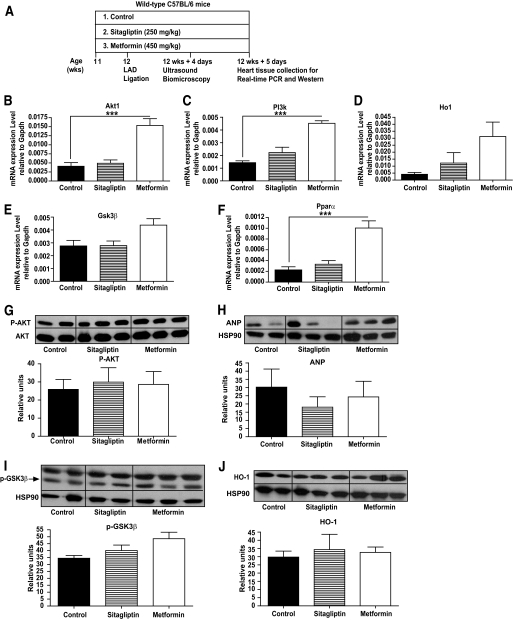
Male C57BL/6 mice, 10 weeks old, from Taconic (Germantown, NY) were housed under pathogen-free conditions in micro-isolator cages and maintained on a 12-h light (0700)/dark (1900) cycle. At 11 weeks of age, mice were placed on either a control diet (Research Diets) or a diet containing sitagliptin (250 mg · kg−1 · day−1) or metformin (450 mg · kg−1 · day−1) for 1 week before LAD ligation. High-frequency ultrasound imaging was carried out on day 4 post-MI. Mice were killed on day 5 after coronary artery ligation, and hearts were collected for RNA and protein analyses.
Isolated heart preparations.
After administration of heparin (1,000 IU/kg s.c.) and sodium pentobarbital (200 mg/kg i.p.), hearts were excised, cannulated through the aorta, and perfused at 80 mmHg in a Langendorff apparatus with gassed (95% O2, 5% CO2) Krebs-Hensleit buffer (mmol/l: 118 NaCl, 4.7 KCl, 11 glucose, 1.2 MgSO4, 25 NaHCO3, 1.2 KH2PO4, and 2.5 CaCl2) maintained at 37°C (Harvard Apparatus, Holliston, MA) as described (15). For measurement of isovolumetric pressures, a small plastic balloon was inflated in the LV via a small left atrial incision. Both end systolic (LVESP) and end diastolic (LVEDP) pressures were monitored, the latter maintained between 4 and 8 mmHg throughout the experiment. LV developed pressure (LVDP) was calculated as LVESP − LVEDP.
Ischemia reperfusion protocol.
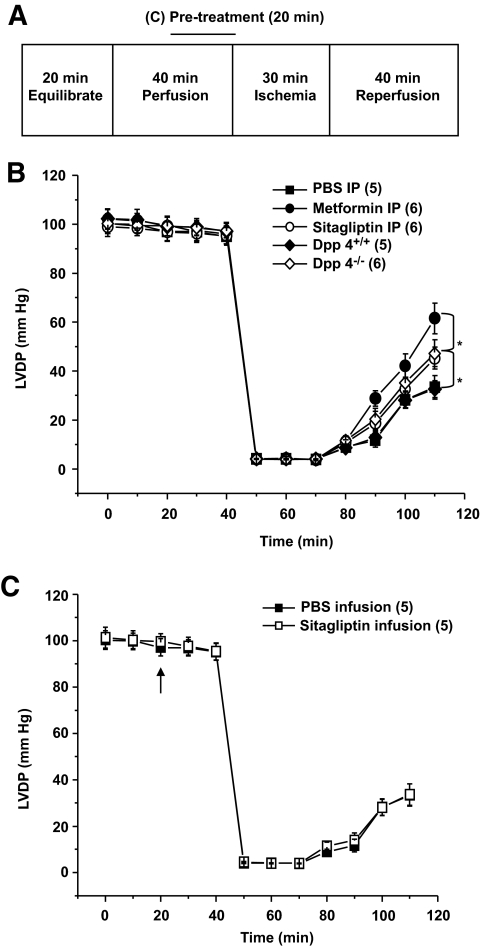
Hearts underwent 20- and 40-min equilibrium and perfusion phases, respectively, during which hemodynamic parameters were recorded. Global ischemia and reperfusion phases were produced by clamping and restoring inflow for 30 and 40 min, respectively. For direct infusion of sitagliptin (5 μmol/l) (versus PBS), the agent was added to the perfusion buffer for the final 20 min of the perfusion phase. Recovery of LVDP was measured at the end of reperfusion and expressed as % of LVDP at the end of perfusion (i.e., before ischemia) (15).
Histology.
Animals were anesthetized using 3% isoflurane/97% air. The chest was opened to expose the heart, where an apical injection of KCl (1 mol/l) was used to arrest the heart in diastole. The heart was then perfusion-fixed with 4% buffered formalin at physiological pressure. Hearts were postfixed in formalin, embedded in paraffin, sectioned at 6 μm, and stained with hematoxylin and eosin (H&E) or Masson's Trichrome. Cardiac morphometry was performed with LEICA QWin V3 software (2003) using digital planimetry of images obtained from mid-ventricular cross-sections. Infarcted LV area was calculated as a % of total LV area.
Quantitative RT-PCR.
Total RNA was prepared from mouse heart using Tri-Reagent (Sigma-Aldrich). First-strand cDNA synthesis used random hexamers and the Superscript II (Invitrogen) system. Real-time PCR analysis was carried out using Taqman gene expression assays and Universal PCR master mix (Applied Biosystems), using the ABI prism 7900 Sequence Detection System. Primers used included mouse Akt1 (Mm00437443_m1), Mmp9 (Mm00442991_m1), Ho1 (Mm00516005_m1), Pparα (Mm00440939_m1), Gsk3β (Mm00444911_m1), PI3K (Mm01282781_m1), β-actin (Mm00607939_s1) (Applied Biosystems), and Gapdh (Mm99999915-g1). Relative mRNA transcript levels were quantified with the 2−ΔΔCT method, using β-actin as an internal control.
Western blots.
Extracts from whole hearts were prepared as described (23). After SDS-PAGE, proteins were electrotransferred onto a Hybond-C nitrocellulose membrane (Amersham, Piscataway, NJ). Blots were incubated over night at 4°C with 1° antibody (Ab). Horseradish peroxidase–conjugated 2° Ab and enhanced chemiluminescence (Amersham, Piscataway, NJ) were used to detect proteins. Primary Abs include pGSK3β (Ser9) 1:2,000 (Cell Signaling, Danvers, MA), atrial natriuretic peptide (ANP) 1:500 (Santa Cruz, Santa Cruz, CA), HO-1 1:5,000 (Stressgen, Ann Arbor, MI), pAKT (Ser 473) and total AKT 1:1,000 (Cell Signaling), and HSP90 1:2,000 (BD Biosciences, San Jose, CA).
Statistical analysis.
Data are presented as mean + SE except where noted. Analyses were performed using Prism software (Version 4.02; GraphPad Software, San Diego, CA). Differences in the number of surviving animals were analyzed using Kaplan-Meier Survival analyses. The remaining results were analyzed using ANOVA followed by Bonferroni's post hoc tests. P < 0.05 was considered statistically significant.
RESULTS
Cardiac structure and function in Dpp4−/− mice.
We first verified that Dpp4−/− mice used in our studies retained the phenotype of improved glucose tolerance and reduced DPP-4 activity as originally described (18). Consistent with previous findings, oral glucose tolerance was improved and plasma DPP-4 activity was markedly reduced in Dpp4−/− mice (supplemental Fig. 1A–C, available in an online appendix at http://diabetes.diabetesjournals.org/cgi/content/full/db09-0955/DC1). Heart weights did not differ between 12-week-old sex-matched Dpp4−/− and Dpp4+/+ mice (Fig. 1A, P = NS). High-resolution echocardiography did not detect any differences in LV wall thicknesses, LV end systolic and end diastolic dimensions, mitral and aortic flow velocities, LV systolic and diastolic areas, LV outflow tract diameter, aortic ejection time, left atrial size, or fractional shortening between Dpp4−/− and Dpp4+/+ mice (Table 1, NS for all comparisons). Hence, genetic disruption of the Dpp4 gene in mice is not associated with baseline abnormalities in cardiac structure or function.
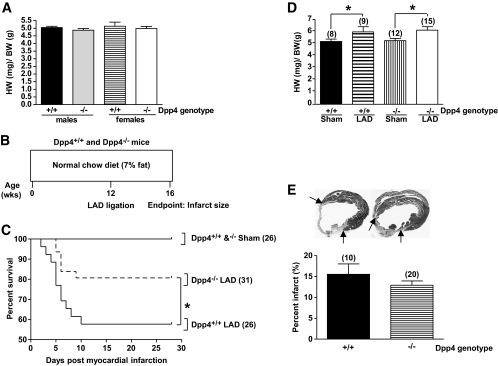
FIG. 1.
Dpp4−/− mice exhibit increased survival after myocardial ischemic injury. A: Heart weight (HW)–to–body weight (BW) ratios in 12-week-old Dpp4+/+ versus Dpp4−/− mice not exposed to LAD ligation. B: Experimental scheme for analysis of normoglycemic Dpp4−/− and Dpp4+/+ mice on a regular diet. C: MI was induced by permanent coronary artery ligation (LAD) and survival was assessed in male and female 12-week-old Dpp4−/− and Dpp4+/+ mice over the subsequent 4 weeks. D: Hypertrophy is observed in Dpp4+/+ and Dpp4−/− mice after MI. E: Infarct size was determined through quantitative histological analysis of hearts from male and female Dpp4+/+ and Dpp4−/− mice. *P < 0.05; numbers in brackets correspond to number of male and female mice (combined) per treatment.
TABLE 1.
Echocardiography-defined dimensional and functional parameters in Dpp4+/+ and Dpp4−/− mice
| Dpp4+/+ | Dpp4−/− | |
|---|---|---|
| n | 5 | 5 |
| Aortic flow | ||
| Aortic outflow velocity (cm/s) | 72.8 ± 4.4 | 63.6 ± 2.9 |
| Aortic valve ejection time (ms) | 68.5 ± 3.9 | 69.6 ± 3.6 |
| Mitral flow | ||
| Peak E velocity (cm/s) | 51.7 ± 2.3 | 47.7 ± 5.0 |
| Deceleration time (ms) | 42.5 ± 3.5 | 47.1 ± 1.3 |
| LV chamber dimensions by M-mode | ||
| Left atrium size (mm) | 1.74 ± 0.06 | 1.66 ± 0.03 |
| LV end diastolic diameter (mm) | 3.85 ± 0.22 | 3.87 ± 0.09 |
| LV end systolic diameter (mm) | 2.45 ± 0.18 | 2.62 ± 0.08 |
| LV outflow tract diameter (mm) | 1.17 ± 0.03 | 1.14 ± 0.02 |
| Anterior wall thickness (mm) | 0.74 ± 0.30 | 0.76 ± 0.01 |
| Posterior wall thickness (mm) | 0.77 ± 0.04 | 0.74 ± 0.02 |
| Fractional shortening (%) | 36.42 ± 2.17 | 32.85 ± 1.04 |
Data are means ± SE. Genetic elimination of DPP-4 activity is not associated with abnormalities in parameters of cardiovascular function. Transthoracic echocardiographic studies were carried out in male 12-week-old Dpp4+/+ and Dpp4−/− mice. Mice were lightly anesthetized using 3% isoflurane/97% oxygen. Two-dimensional and M-mode echocardiography, as well as pulsed Doppler analyses, were performed by a blinded observer using a Hewlett-Packard 5500 ultrasound device (Hewlett-Packard, Palo Alto, CA) and a 12-MHz phased array and 15-MHz Doppler probes. Three M-mode recordings of end systolic and end diastolic LV internal diameters and end diastolic LV posterior wall thickness were made. A single mean measurement was then calculated for each mouse. Analysis of data was carried out as previously described (44,45).
Myocardial infarction outcomes in normoglycemic Dpp4−/− mice.
To determine whether disruption of the Dpp4 gene modifies the response to cardiac injury, we induced MI in nondiabetic 12-week-old male and female Dpp4−/− and Dpp4+/+ mice via permanent surgical LAD ligation (Fig. 1B). At the predefined end point of 4 weeks post-MI, Dpp4−/− mice exhibited an ∼20% absolute increase in survival compared with Dpp4+/+ littermate controls (Fig. 1C). Post-MI, the hearts of Dpp4−/− and Dpp4+/+ mice underwent similar compensatory hypertrophy (Fig. 1D). Although infarct size was reduced in Dpp4−/− mice, this difference was not statistically significant (Fig. 1E).
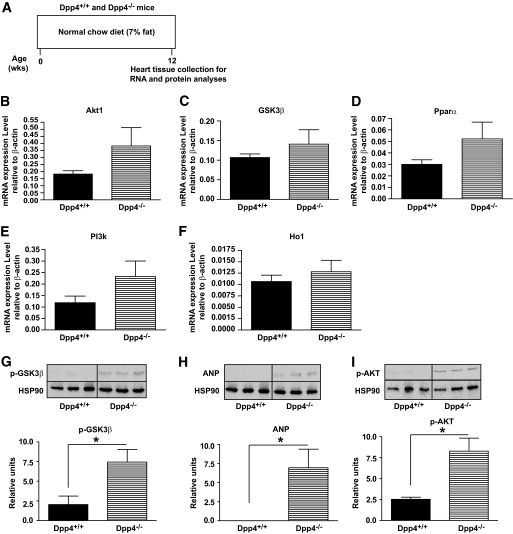
To explore mechanisms mediating the increased survival of Dpp4−/− mice post-MI, we analyzed cardiac mRNA and protein levels of known cardioprotective genes. Normoglycemic nonischemic Dpp4−/− mice exhibited small but nonsignificant increases in Akt1, Gsk3β, Pparα, PI3K, and Ho1 transcripts (Fig. 2B–F). Moreover, hearts from Dpp4−/− mice contained higher levels of phosphorylated AKT (pAKT), pGSK3β, and ANP (Fig. 2G–I), proteins known to be regulated by GLP-1R agonists (10) and associated with cardioprotection in vivo (24–27).
FIG. 2.
Dpp4−/− hearts express increased levels of proteins associated with cardiomyocyte survival. A: Experimental outline for analysis of basal RNA and protein expression in Dpp4+/+ and Dpp4−/− mice. Relative levels of mRNA transcripts for Akt1 (B), Gsk3β (C), Pparα (D), PI3k (E), and Ho1 (F) in nonischemic hearts from 12-week-old Dpp4−/− versus Dpp4+/+ mice assessed by quantitative real-time PCR and normalized to levels of β-actin transcripts in the same samples. n = 6 per group. Relative levels of pGSK3β (G), ANP (H), and pAKT1 (I) determined by Western blot analysis of protein extracts from hearts of 12-week-old Dpp4+/+ and Dpp4 −/− mice are shown. *P < 0.05, n = 3 for each genotype.
Treatment of diabetic mice with metformin or sitagliptin pre- and post-MI.
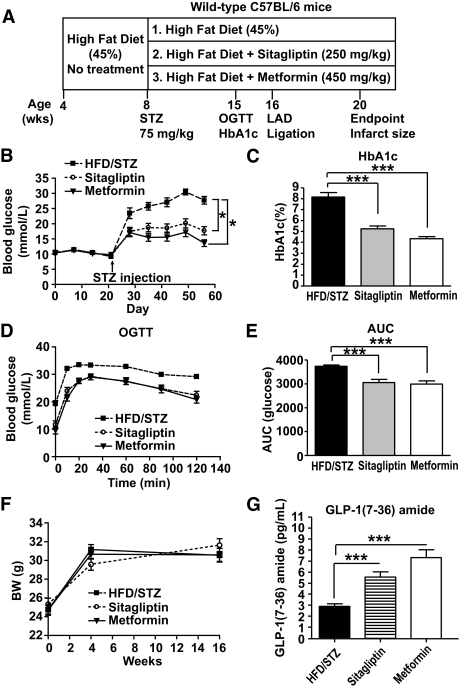
Because Dpp4−/− mice are resistant to the development of STZ-induced diabetes (28), we assessed whether reduction of DPP-4 activity is cardioprotective in diabetic Dpp4+/+ mice. Wild-type mice were placed on an HFD for 4 weeks, rendered diabetic with STZ, and maintained for an additional 12 weeks on HFD alone or on HFD plus either sitagliptin or metformin (Fig. 3A). After 8 weeks on drug treatment or HFD alone, mice were subjected to LAD ligation and observed for an additional 4 weeks (Fig. 3A). Random blood glucose (Fig. 3B), levels of A1C (Fig. 3C), and oral glucose tolerance (Fig. 3D–E) were improved to a similar extent with body weights remaining comparable (Fig. 3F) in mice treated with sitagliptin or metformin. Moreover, plasma levels of active GLP-1 (7-36)amide were increased to a similar extent in sitagliptin- versus metformin-treated mice (Fig. 3G).
FIG. 3.
Sitagliptin and metformin reduce blood glucose levels and increase plasma GLP-1 (7-36)amide levels in diabetic mice. Male C57BL/6 mice were placed on HFD (45% fat) for 4 weeks (A). At the start of week 5, mice were injected with a single dose of STZ (75 mg/kg) and then randomized into three treatment groups: 1) HFD/STZ alone, 2) HFD/STZ + sitagliptin (250 mg/kg), or 3) HFD/STZ + metformin (450 mg/kg) for an additional 8 weeks. At week 12, mice underwent LAD ligation or control sham surgery. At week 16, surviving mice were killed and infarct size was measured. Levels of random fed blood glucose (B) and A1C (C) were significantly reduced in mice treated with sitagliptin or metformin. Oral glucose tolerance (D and E) was significantly improved in sitagliptin-treated (n = 29) or metformin-treated (n = 23) mice compared with HFD alone (n = 23). F: Body weight (BW) in mice treated with HFD/STZ alone, sitagliptin, or metformin (n = 22–23 per treatment). Plasma active GLP-1 is increased in mice treated with sitagliptin or metformin (n = 14 per treatment) (G). *P < 0.05, *** P < 0.001 vs. the untreated HFD/STZ group. AUC, area under the curve.
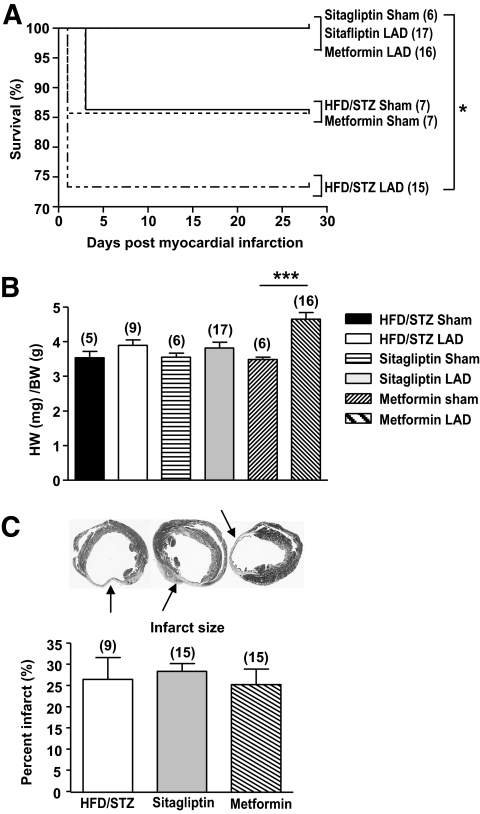
Cumulative survival assessed up to 4 weeks after LAD ligation was improved in mice treated with either sitagliptin or metformin, compared to mice on HFD/STZ alone (Fig. 4A). A significant increase in heart-to-body weight ratios post-MI was observed only in diabetic mice treated with metformin (Fig. 4B). No differences in infarct size were observed between the three groups (Fig. 4C).
FIG. 4.
Outcomes after LAD ligation in diabetic mice treated with sitagliptin or metformin. A: MI was induced by coronary artery ligation (Fig. 3A), and significant increases in survival were observed in diabetic C57BL/6 mice after treatment with either sitagliptin or metformin, compared with HFD/STZ mice alone. B: Cardiac hypertrophy was observed in metformin-treated mice after MI. C: Histological analysis reveals similar infarct size between treatment groups. *P < 0.05, ***P < 0.001, numbers in brackets correspond to number of mice per treatment.
To identify potential mechanism(s) underlying improved survival post-MI in sitagliptin- and metformin-treated diabetic mice, we assessed cardiac mRNA and protein levels of candidate pro-survival genes in separate groups of diabetic animals treated for 1 week with either sitagliptin, metformin, or the GLP-1R agonist, liraglutide. No significant changes were detected in mRNA levels of PI3k, Akt, Ho1, Mmp9, and Pparα after treatment with these antidiabetic agents (Fig. 5B–F). In contrast, sitagliptin, metformin, and liraglutide increased expression of ANP (Fig. 5G). Sitagliptin and liraglutide, but not metformin, activated the prosurvival kinase AKT (Fig. 5H), whereas all three drugs increased levels of HO-1 (Fig. 5I). A modest but nonsignificant increase in levels of phospho-GSK3β was also observed with all three antidiabetic agents (Fig. 5J).
FIG. 5.
Treatment of diabetic C57BL/6 mice with sitagliptin, metformin, or liraglutide leads to increased expression of cardioprotective proteins. Diabetes was induced in HFD-fed STZ-treated WT C57Bl/6 mice (A). Levels of mRNA transcripts in hearts from mice were treated with HFD/STZ alone, or HFD/STZ plus sitagliptin, metformin, or liraglutide, for 1 week. Relative levels of PI3k (B), Akt1 (C), Ho1 (D), Mmp9 (E), and Pparα (F) were assessed by quantitative real-time PCR and normalized to levels of β-actin transcripts in the same samples. n = 6 per group. Western blot analysis is shown for ANP (G), AKT (H), HO-1 (I), and pGSK3β (J) using heart extracts from wild-type mice on HFD/STZ alone, or HFD/STZ plus either sitagliptin, metformin, or liraglutide. *P < 0.05, ***P < 0.001, n = 3 per treatment.
We next examined whether metformin or sitagliptin treatment of HFD-fed mice produced changes in gene and protein expression in the mouse heart after LAD ligation (Fig. 6A). Levels of Ho1 and Gsk3β RNA transcripts were modestly increased (Fig. 6D and E), whereas Akt1, PI3k, and Pparα RNA transcripts were significantly increased in the post-ischemic heart after metformin treatment (Fig. 6B, C, and F). In contrast, sitagliptin treatment was not associated with significant changes in levels of cardioprotective mRNA transcripts post-MI (Fig. 6B–F). Neither metformin nor sitagliptin treatment produced detectable changes in levels of AKT, ANP, GSK3β, or HO-1 proteins assessed at day 5 post-MI (Fig. 6G–J). Similarly, although heart rate was increased in metformin-treated mice, we did not detect other significant differences in parameters of cardiac function in sitagliptin- versus metformin-treated mice after myocardial infarction (Table 2).
FIG. 6.
Gene and protein expression after LAD occlusion in hearts from wild-type mice treated with metformin or sitagliptin. Nondiabetic 10-week-old mice were treated with sitagliptin or metformin for 1 week (A), and cardiac levels of mRNA transcripts for Akt1 (B), PI3k (C), Ho1 (D), Gsk3β (E), and Pparα (F) were determined by real-time PCR, normalized to the values of Gapdh mRNA transcripts in the same sample. Western blot analysis was used to ascertain levels of pAKT (G), ANP (H), pGSK3β (I), and HO-1 (J) 5 days after LAD ligation. HSP90 was used as an internal control protein. ***P < 0.001, n = 5 mice per group.
TABLE 2.
Ultrasound biomicroscopy-defined cardiac hemodynamic, functional, and dimensional parameters in mice treated with either control, sitagliptin, or metformin on day 4 post-LAD ligation
| Control | Sitagliptin | Metformin | |
|---|---|---|---|
| n | 6 | 6 | 5 |
| Aortic flow | |||
| Heart rate (bpm) | 441 ± 17 | 423 ± 19 | 526 ± 23* |
| VTImax (cm) | 2.64 ± 0.14 | 2.40 ± 0.08 | 2.39 ± 0.30 |
| Aortic orifice diameter (mm) | 1.04 ± 0.04 | 1.06 ± 0.03 | 1.04 ± 0.03 |
| LV stroke volume (μl) | 22.38 ± 1.24 | 21.10 ± 1.33 | 20.34 ± 3.07 |
| Cardiac output (ml/min) | 9.82 ± 0.46 | 8.95 ± 0.71 | 10.70 ± 1.66 |
| Mitral flow | |||
| Heart rate (bpm) | 447 ± 13 | 433 ± 16 | 792 ± 33 |
| Peak E velocity (cm/s) | 67.6 ± 3.0 | 62.6 ± 2.4 | 53.4 ± 6.2 |
| Peak A velocity (cm/s) | 48.0 ± 49 | 36.0 ± 40 | 54.6 ± 73 |
| Peak E/A ratio | 1.47 ± 0.15 | 1.89 ± 0.29 | 0.99 ± 0.08 |
| LV chamber dimensions by M-mode | |||
| Heart rate (bpm) | 493 ± 35 | 458 ± 15 | 541 ± 18 |
| LV end diastolic diameter (mm) | 4.26 ± 0.15 | 4.28 ± 0.19 | 4.43 ± 0.16 |
| LV end systolic diameter (mm) | 3.54 ± 0.27 | 3.68 ± 0.21 | 3.89 ± 0.22 |
| LV fractional shortening (%) | 17.3 ± 3.5 | 14.2 ± 1.2 | 12.4 ± 2.4 |
Data are means ± SE. The 12-week-old C57Bl/6 mice treated with control, sitagliptin, or metformin for 1 week before experimental cardiac ischemic injury were subjected to high-frequency ultrasound imaging. VTImax, velocity-time integral of Doppler flow waveform by tracing the maximal velocity. In mitral inflow, the peak E velocity represents the maximal velocity of the early diastolic wave caused by active left ventricular relaxation. The peak A velocity represents the maximal velocity caused by atrial contraction in late diastole. Acquisition of images and analysis of data were carried out as previously described (44,45).
*P < 0.05 vs. control.
Ischemia-reperfusion injury and metformin versus DPP-4 inhibition in normoglycemic mouse hearts.
To determine whether cardioprotective actions of sitagliptin are observed in the normoglycemic murine heart, we acutely administered sitagliptin, metformin, or saline (PBS) to nondiabetic wild-type mice in vivo before assessing recovery of LVDP after I/R injury to their hearts ex vivo (15). Parallel experiments included I/R injury in hearts from normoglycemic Dpp4−/− and Dpp4+/+ animals, as well as testing cardioprotective actions of an acute ex vivo sitagliptin infusion (20 min) versus placebo (PBS) immediately before wild-type hearts undergoing I/R injury (Fig. 7A).
FIG. 7.
Functional recovery after I/R injury in the murine heart. A: Experimental protocol for I/R injury in isolated mouse hearts. B: Intraperitoneal (IP) injections of sitagliptin and metformin in 12-week-old wild-type mice in vivo reduced I/R injury of their hearts ex vivo. The 12-week-old Dpp4−/− mice display significantly greater improvement in functional recovery after I/R compared with Dpp4+/+ littermate controls. C: Direct 20-min infusion of sitagliptin started (arrow) before ischemia did not improve functional recovery after I/R injury in the isolated heart from WT mice. *P < 0.05, numbers in brackets correspond to number of mice per treatment.
Acute administration of metformin or sitagliptin in vivo improved recovery from subsequent I/R injury in normoglycemic mice (Fig. 7B). Recovery of LVDP was also greater in Dpp4−/− hearts versus Dpp4+/+ littermate controls (Fig. 7B). By contrast, sitagliptin administered to the coronary circulation ex vivo (and immediately before I/R injury) exerted no direct cardioprotective actions in isolated mouse hearts (Fig. 7C). Taken together, these data show that the cardioprotective effects of genetic or pharmacological inhibition of DPP-4 activity are not strictly glucose dependent and depend on one or more DPP-4–dependent actions in vivo.
DISCUSSION
Analysis of the cardiovascular profile of antidiabetic agents involves ascertainment of the effects of each drug on the myocardium and endothelium, and on secondary risk factors such as control of blood pressure and cholesterol. Although preclinical studies may be useful in generating hypotheses about the putative cardiovascular actions of different drug classes, the results of subsequent clinical studies have not always been concordant with predictions made from preclinical analyses. For example, although both thiazolidinediones, pioglitazone and rosiglitazone, exert beneficial effects on inflammation and endothelial function (29), pioglitazone, but not rosiglitazone, is associated with reduced cardiovascular events in human studies (3,30). Similarly, although data from both preclinical (31) and clinical studies (32) suggests that metformin therapy may be cardioprotective, the mechanisms through which metformin therapy is associated with cardioprotection remain poorly understood. Moreover, sulfonylureas have been associated with increased rates of death from cardiovascular disease in some (33) but not all studies (32).
We have now investigated the cardiovascular consequences arising from genetic elimination or pharmacological inhibition of DPP-4 activity. DPP-4 has three major functions; adenosine deaminase binding, peptidase activity, and extracellular matrix binding, all of which potentially influence the activity of the immune and/or endocrine systems (34). Although DPP-4 cleaves and inactivates several cardioactive peptides, including neuropeptide Y, brain natriuretic peptide, SDF-1, and GLP-1, there is little information on the cardiovascular consequences of reduced or absent DPP-4 activity. Normoglycemic Dpp4−/− mice exhibit normal cardiac structure and function in the basal state, yet increased survival after experimental MI. Whether the increased survival after LAD ligation is directly due to loss of DPP-4 activity per se in cardiomyocytes or blood vessels, or indirectly due to the subsequent upregulation of cardioprotective molecules such as GLP-1 (18) or SDF-1 (35), cannot be inferred from the present study. Zaruba et al. (35) also observed modest improvements in survival after experimental MI in Dpp4−/− mice or in WT mice treated with a DPP-4 inhibitor, and more robust improvements in survival were observed after administration of G-CSF, findings attributed to SDF-1–dependent mobilization of cardiac stem cells. Our studies extend their observations through examination of the cardiovascular effects of DPP-4 inhibition in diabetic mice and by demonstrating that direct sitagliptin administration into the circulation of the ischemic mouse heart is not directly cardioprotective ex vivo, suggesting that acute reduction of cardiac DPP-4 activity is not sufficient to produce cardioprotection.
Our findings demonstrating that both sitagliptin and the GLP-1R agonist liraglutide upregulated levels of cardioprotective proteins in the nonischemic myocardium suggest a possible role for GLP-1 in the context of enhanced survival after DPP-4 inhibition and experimental MI. Nevertheless, we did not observe a sustained induction of cardioprotective proteins in sitagliptin-treated murine hearts when the same proteins were examined after MI. Moreover, we did not detect significant changes in infarct size or cardiac function after MI that might directly account for the improved survival seen with genetic or chemical reduction of DPP-4 activity. Hence, the precise mechanisms mediating the improvements in survival observed after pharmacological treatment with sitagliptin in diabetic mice or genetic reduction of DPP-4 activity in normoglycemic Dpp4−/− mice require further investigation, ideally through examination of whether DPP-4 inhibitors exert cardioprotective actions in Glp1r−/− mice.
Western blot analysis of proteins in nonischemic hearts demonstrated that both sitagliptin and metformin therapy induced an overlapping set of cardioprotective proteins. Metformin is thought to exert its cardioprotective actions through distinct mechanisms requiring activation of AMP kinase and endothelial nitric oxide (31). Intriguingly, administration of metformin has also been associated with reduction of DPP-4 activity (36) and increased circulating levels of GLP-1 in both rodent (37) and clinical studies (38), and we detected increased levels of GLP-1 in both metformin- and sitagliptin-treated mice. Accordingly, the extent to which therapy with sitagliptin and metformin produces an overlapping spectrum of actions reflecting similarities in their mechanism(s) of action through enhanced levels of GLP-1 requires further clarification.
Both metformin and sitagliptin significantly increased survival in diabetic mice, possibly due to a comparable reduction in blood glucose achieved with either agent. Hyperglycemia is a risk factor for a poor outcome after MI in humans (39), and there is considerable interest in determining whether intensive glucose control safely and consistently improves outcomes post-MI (40). Similarly, hyperglycemia is known to be associated with reduced survival and impaired LV function in mice after MI (41–43), and it seems likely that reduction in the severity of hyperglycemia contributes to improved survival, perhaps independent of the antidiabetic mechanisms unique to each agent under study.
In contrast, the increased survival observed in normoglycemic Dpp4−/− mice after MI supports the concept that reduction of DPP-4 activity may be cardioprotective in the absence of hyperglycemia (35). Similarly, our observations demonstrating that genetic or chemical inhibition of DPP-4 is associated with enhanced recovery of LVDP in the normoglycemic ischemic murine heart ex vivo suggest that DPP-4 modifies cardiovascular outcomes independent of glucoregulation and provide a useful model for future studies. Given the increasing interest in using strategies based on DPP-4 inhibition for the treatment of diabetes, a more detailed understanding of the role of DPP-4 in the normal and diabetic cardiovascular system is clearly warranted.
Supplementary Material
ACKNOWLEDGMENTS
M.S. was supported by a BBDC–Novo Nordisk studentship award and a Government of Ontario/Heart and Stroke Foundation of Ontario (OGSST) Award. D.J.D. was supported by a Chair in Regulatory Peptides from the Canada Research Chairs Program. D.J.D. has served as an advisor or consultant within the past 12 months to Amylin Pharmaceuticals, Arena Pharmaceuticals, Arisaph Pharmaceuticals, Eli Lilly, GlaxoSmithKline, Glenmark Pharmaceuticals, Hoffman LaRoche, Isis Pharmaceuticals, Merck Research Laboratories, Metabolex, Novartis Pharmaceuticals, Novo Nordisk, Phenomix, and Transition Pharmaceuticals. D.J.D. receives partial operating grant support for DPP-4–related research from Merck Frosst. M.H. has served as a consultant within the past 12 months to sanofi-aventis and Merck. M.H. was supported by a Career Investigator Award (CI-5503) of the Heart & Stroke Foundation of Ontario (HSFO). This work was supported in part by operating grants IRO-80668 from the Canadian Institutes of Health Research (to D.J.D.) and NA-5926 from the HSFO (to M.H. and D.J.D.) in conjunction with grant support from Merck Frosst Inc. No other potential conflicts of interest relevant to this article were reported.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW, Pignone MP, Plutzky J, Porte D, Redberg R, Stitzel KF, Stone NJ: American Heart Association, American Diabetes Association Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30: 162– 172 [DOI] [PubMed] [Google Scholar]
- 2.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT: Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358: 2545– 2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nissen SE, Wolski K: Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356: 2457– 2471 [DOI] [PubMed] [Google Scholar]
- 4.Drucker DJ, Nauck MA: The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006; 368: 1696– 1705 [DOI] [PubMed] [Google Scholar]
- 5.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ: Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 2007; 293: E1289– E1295 [DOI] [PubMed] [Google Scholar]
- 6.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP: Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation 2004; 109: 962– 965 [DOI] [PubMed] [Google Scholar]
- 7.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP: Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail 2006; 12: 694– 699 [DOI] [PubMed] [Google Scholar]
- 8.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM: Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes 2005; 54: 146– 151 [DOI] [PubMed] [Google Scholar]
- 9.Bose AK, Mocanu MM, Carr RD, Yellon DM: Myocardial ischaemia-reperfusion injury is attenuated by intact glucagon like peptide-1 (GLP-1) in the in vitro rat heart and may involve the p70s6K pathway. Cardiovasc Drugs Ther 2007; 21: 253– 256 [DOI] [PubMed] [Google Scholar]
- 10.Noyan-Ashraf MH, Momen MA, Ban K, Sadi AM, Zhou YQ, Riazi AM, Baggio LL, Henkelman RM, Husain M, Drucker DJ: GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes 2009; 58: 975– 983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garber A, Henry R, Ratner R, Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM, Zdravkovic M, Bode B: LEAD-3 (Mono) Study Group. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 2009; 373: 473– 481 [DOI] [PubMed] [Google Scholar]
- 12.Moretto TJ, Milton DR, Ridge TD, Macconell LA, Okerson T, Wolka AM, Brodows RG: Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug-naive patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther 2008; 30: 1448– 1460 [DOI] [PubMed] [Google Scholar]
- 13.Lovshin JA, Drucker DJ: Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol 2009; 5: 262– 269 [DOI] [PubMed] [Google Scholar]
- 14.Drucker DJ: Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care 2007; 30: 1335– 1343 [DOI] [PubMed] [Google Scholar]
- 15.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M: Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008; 117: 2340– 2350 [DOI] [PubMed] [Google Scholar]
- 16.Sonne DP, Engstrøm T, Treiman M: Protective effects of GLP-1 analogues exendin-4 and GLP-1(9–36) amide against ischemia-reperfusion injury in rat heart. Regul Pept 2008; 146: 243– 249 [DOI] [PubMed] [Google Scholar]
- 17.Nikolaidis LA, Elahi D, Shen YT, Shannon RP: Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol 2005; 289: H2401– H2408 [DOI] [PubMed] [Google Scholar]
- 18.Marguet D, Baggio L, Kobayashi T, Bernard AM, Pierres M, Nielsen PF, Ribel U, Watanabe T, Drucker DJ, Wagtmann N: Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci U S A 2000; 97: 6874– 6879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamont BJ, Drucker DJ: Differential antidiabetic efficacy of incretin agonists versus DPP-4 inhibition in high fat fed mice. Diabetes 2008; 57: 190– 198 [DOI] [PubMed] [Google Scholar]
- 20.Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE: Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes 2005; 54: 2235– 2244 [DOI] [PubMed] [Google Scholar]
- 21.Maida A, Hansotia T, Longuet C, Seino Y, Drucker DJ: Differential importance of glucose-dependent insulinotropic polypeptide vs glucagon-like peptide 1 receptor signaling for beta cell survival in mice. Gastroenterology 2009; 137: 2146– 2157 [DOI] [PubMed] [Google Scholar]
- 22.Ohta K, Nakajima T, Cheah AY, Zaidi SH, Kaviani N, Dawood F, You XM, Liu P, Husain M, Rabinovitch M: Elafin-overexpressing mice have improved cardiac function after myocardial infarction. Am J Physiol Heart Circ Physiol 2004; 287: H286– H292 [DOI] [PubMed] [Google Scholar]
- 23.Koehler JA, Drucker DJ: Activation of glucagon-like peptide-1 receptor signaling does not modify the growth or apoptosis of human pancreatic cancer cells. Diabetes 2006; 55: 1369– 1379 [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi I, Melendez J, Ahn Y, Skavdahl M, Murphy E, Welch S, Schaefer E, Walsh K, Rosenzweig A, Torella D, Nurzynska D, Kajstura J, Leri A, Anversa P, Sussman MA: Nuclear targeting of Akt enhances kinase activity and survival of cardiomyocytes. Circ Res 2004; 94: 884– 891 [DOI] [PubMed] [Google Scholar]
- 25.Yet SF, Tian R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee ME, Perrella MA: Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res 2001; 89: 168– 173 [DOI] [PubMed] [Google Scholar]
- 26.Heineke J, Molkentin JD: Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol 2006; 7: 589– 600 [DOI] [PubMed] [Google Scholar]
- 27.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ: Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest 2004; 113: 1535– 1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE, Thornberry NA, Zhang BB: Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci U S A 2003; 100: 6825– 6830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parulkar AA, Pendergrass ML, Granda-Ayala R, Lee TR, Fonseca VA: Nonhypoglycemic effects of thiazolidinediones. Ann Intern Med 2001; 134: 61– 71 [DOI] [PubMed] [Google Scholar]
- 30.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE: Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. JAMA 2007; 298: 1180– 1188 [DOI] [PubMed] [Google Scholar]
- 31.Calvert JW, Gundewar S, Jha S, Greer JJ, Bestermann WH, Tian R, Lefer DJ: Acute metformin therapy confers cardioprotection against myocardial infarction via AMPK-eNOS-mediated signaling. Diabetes 2008; 57: 696– 705 [DOI] [PubMed] [Google Scholar]
- 32.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA: 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577– 1589 [DOI] [PubMed] [Google Scholar]
- 33.Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA: Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ 2006; 174: 169– 174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aytac U, Dang NH: CD26/dipeptidyl peptidase IV: a regulator of immune function and a potential molecular target for therapy. Curr Drug Targets Immune Endocr Metabol Disord 2004; 4: 11– 18 [DOI] [PubMed] [Google Scholar]
- 35.Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, Nathan P, Israel L, Imhof A, Herbach N, Assmann G, Wanke R, Mueller-Hoecker J, Steinbeck G, Franz WM: Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell 2009; 4: 313– 323 [DOI] [PubMed] [Google Scholar]
- 36.Green BD, Irwin N, Duffy NA, Gault VA, O'harte FP, Flatt PR: Inhibition of dipeptidyl peptidase-IV activity by metformin enhances the antidiabetic effects of glucagon-like peptide-1. Eur J Pharmacol 2006; 547: 192– 199 [DOI] [PubMed] [Google Scholar]
- 37.Yasuda N, Inoue T, Nagakura T, Yamazaki K, Kira K, Saeki T, Tanaka I: Enhanced secretion of glucagon-like peptide 1 by biguanide compounds. Biochem Biophys Res Commun 2002; 298: 779– 784 [DOI] [PubMed] [Google Scholar]
- 38.Mannucci E, Tesi F, Bardini G, Ognibene A, Petracca MG, Ciani S, Pezzatini A, Brogi M, Dicembrini I, Cremasco F, Messeri G, Rotella CM: Effects of metformin on glucagon-like peptide-1 levels in obese patients with and without type 2 diabetes. Diabetes Nutr Metab 2004; 17: 336– 342 [PubMed] [Google Scholar]
- 39.Vergès B, Zeller M, Dentan G, Beer JC, Laurent Y, Janin-Manificat L, Makki H, Wolf JE, Cottin Y: Impact of fasting glycemia on short-term prognosis after acute myocardial infarction. J Clin Endocrinol Metab 2007; 92: 2136– 2140 [DOI] [PubMed] [Google Scholar]
- 40.Weston C, Walker L, Birkhead J: National Audit of Myocardial Infarction Project, National Institute for Clinical Outcomes Research. Early impact of insulin treatment on mortality for hyperglycaemic patients without known diabetes who present with an acute coronary syndrome. Heart 2007; 93: 1542– 1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shiomi T, Tsutsui H, Ikeuchi M, Matsusaka H, Hayashidani S, Suematsu N, Wen J, Kubota T, Takeshita A: Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J Am Coll Cardiol 2003; 42: 165– 172 [DOI] [PubMed] [Google Scholar]
- 42.Greer JJ, Ware DP, Lefer DJ: Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol 2006; 290: H146– H153 [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Wei J, Peng DH, Layne MD, Yet SF: Absence of heme oxygenase-1 exacerbates myocardial ischemia/reperfusion injury in diabetic mice. Diabetes 2005; 54: 778– 784 [DOI] [PubMed] [Google Scholar]
- 44.Zhou YQ, Foster FS, Nieman BJ, Davidson L, Chen XJ, Henkelman RM: Comprehensive transthoracic cardiac imaging in mice using ultrasound biomicroscopy with anatomical confirmation by magnetic resonance imaging. Physiol Genomics 2004; 18: 232– 244 [DOI] [PubMed] [Google Scholar]
- 45.Zhou YQ, Zhu Y, Bishop J, Davidson L, Henkelman RM, Bruneau BG, Foster FS: Abnormal cardiac inflow patterns during postnatal development in a mouse model of Holt-Oram syndrome. Am J Physiol Heart Circ Physiol 2005; 289: H992– H1001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.