Abstract
A number of lifestyle factors that reduce cancer risk in the primary prevention setting may be potential new targets for use in combination with cancer vaccines. This review discusses the modulation of energy balance (physical activity, calorie restriction, and obesity prevention), and the supplementation with natural and synthetic analogs of vitamins A and E, as potential interventions for use in combination with cancer vaccines. Additionally, the pharmacologic manipulation of nutrient metabolism in the tumor microenvironment (e.g., arachidonic acid, arginine, tryptophan, and glucose metabolism) is discussed. This review includes a brief overview of the role of each agent in primary cancer prevention; outlines the effects of these agents on immune function, specifically adaptive and/or anti-tumor immune mechanisms, when known; and discusses the potential use of these interventions in combination with therapeutic cancer vaccines. Modulation of energy balance through exercise and strategies targeting nutrient metabolism in the tumor microenvironment represent the most promising interventions to partner with therapeutic cancer vaccines. Additionally, the use of vitamin E succinate and the retinoid X receptor-directed rexinoids in combination with cancer vaccines offer promise. In summary, a number of energy balance- and nutrition-related interventions are viable candidates for further study in combination with cancer vaccines.
Keywords: exercise; calorie restriction; obesity prevention; Vitamin A; Vitamin, E; arachidonic acid; arginine; tryptophan; glucose; immunotherapy; cancer vaccine; review
2. INTRODUCTION
Following completion of primary therapy, a limited number of interventions have demonstrated success in reducing the risk of recurrence of the primary tumor, preventing a secondary malignancy and increasing survival (1). Therapies targeting the immune system offer promise in controlling micro-metastases and increasing survival (2-5), but are likely to yield greater success if used in combination with other strategies that may either slow tumor growth or increase the efficacy of therapeutic cancer vaccines. New targets for use in combination with cancer vaccines need to be identified and explored.
A number of lifestyle factors that reduce cancer risk in the primary prevention setting may be promising candidates such as the modulation of energy balance, as well as supplementation with vitamins and minerals. Changes in energy balance (i.e. physical activity, calorie restriction, and obesity prevention) alter cancer risk. Physical activity significantly reduces the risk of several types of cancer. The data on this are most consistent for colon and breast cancer in humans (6) and animals (7;8). Calorie restriction also consistently and significantly reduces the risk of tumor formation in a variety of animal models (8;9). At the other end of the energy balance spectrum, obesity increases cancer risk and mortality in humans (10;11) and in animal models (8;12-17). With respect to vitamins and minerals, the vitamins A, B6, B12, C, D, E, folate, selenium, and zinc have been shown to reduce the risk of cancer in humans (recently reviewed in (18-22)).
The biological mechanisms underlying the cancer preventive effects of both changes in energy balance and of specific nutrients differ based on the intervention or agent, and are reviewed in detail elsewhere (23-29). A variety of biological mechanisms have been proposed to explain the relationship between changes in energy balance and cancer prevention including: 1) alterations in growth factors; 2) reductions in reproductive and metabolic hormones; 3) enhanced antioxidant defense mechanisms; and 4) enhanced immune function. The chemopreventive activity of many nutrients is achieved through the inhibition of proliferation, promotion of differentiation, and/or induction of apoptosis of tumor cells, as well as increased antioxidant, anti-inflammatory, and immunomodulatory activities. An understanding of the immunoregulatory capabilities of these lifestyle interventions is particularly relevant if these are to be used in combination with cancer vaccines and will be explored in this review.
The target population to receive therapeutic cancer vaccines is likely an older cohort since 60% of all newly diagnosed malignant tumors and 70% of all cancer deaths occur in persons 65 years and older (30). It is well documented that components of both the innate and adaptive immune system decline with advancing age (31-33) and that the T cell population is most affected by the aging process (32). Immunosenescence has been hypothesized to contribute to a greater risk of tumor formation and infection with advancing age, and may contribute to a less effective response to vaccination. In particular, the age-associated decline in T cell function has been associated with thymic involution and atrophy, as well as acquired defects in the bone marrow stroma, hematopoietic stem cell populations, and peripheral lymphoid tissues (34). Therefore, the energy balance- and nutrition-related intervention strategies proposed need to be effective within the context of an aging immune system in order to benefit the patient population most likely to receive cancer vaccines.
The scope of this review was narrowed by only including lifestyle interventions that have shown efficacy in primary prevention coupled with those that regulate immune function. Using these two criteria, several traditional cancer prevention interventions emerge as promising strategies for use in combination with various cancer vaccine platforms. In this review, changes in energy balance are discussed in combination with therapeutic vaccines. The use of systemic supplementation with natural and synthetic analogs of vitamins A and E are also reviewed. Additionally, the pharmacologic manipulation of nutrient metabolism in the tumor microenvironment (e.g., arachidonic acid, arginine, tryptophan, and glucose metabolism), all of which have been shown to contribute to immunosuppression in cancer, are discussed. This review includes a brief overview of the role of each agent in primary cancer prevention; outlines the effects of these agents on immune function, in particular adaptive and/ or anti-tumor immune mechanisms, when known; and discusses the potential use of these interventions in combination with therapeutic cancer vaccines (Tables 1-3). There is a paucity of work in the nexus between nutrition, energy balance, and cancer vaccines. Therefore, the goal of this review is to discuss the rationale for using these prevention strategies in combination with cancer vaccines and to outline key questions that need to be addressed to incorporate these interventions with cancer vaccines in the future.
Table 1.
Summary of the effects of changes1 in energy balance and the impact of nutrients on anti-tumor activity, immune function and use in combination with vaccines in preclinical studies
| Immune Function | Use with | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-Tumor Activity | NK Cell Function | T Cell Function | Cancer Vaccine | ||||||||
| Prevention | Therapy/ Survival |
Ref.2 | Cytotoxicity | Ref.2 | Proliferation | Cytokine Production |
Cytotoxicity | Ref.2 | Ref.2 | ||
| Changes in Energy Balance | |||||||||||
| Physical Activity | + + | + |
7;35-38; 41-46; 48-58 |
+ + |
68-70; 72; 354 |
+/− | + | ND |
72;76-78; 81-83;85; 90;94;95; 102;104; 355 |
ND | |
| Calorie Restriction3 | + + | +/− |
8;9; 115-125 |
( − ) |
139; 140 |
+/− | +/− | ND |
82; 130-138; 168;356 |
ND | |
| Obesity | (−) | (−) |
8;12-14; 16;17; 357;358 |
( − ) |
168- 170 |
(−) | +/− | ND |
169;174; 175;359 |
ND | |
| Impact of Nutrients | |||||||||||
| Retinoids and Carotenoids | |||||||||||
| Retinoid Analogs4 | + + | + |
223;224; 233-236; 268 |
+ |
257; 259; 265 |
+ | + | + |
251-254; 257-259 |
+ |
266; 268 |
| Synthetic Rexinoids5 | + + | + + |
242-248; 250;270 |
ND | ND | ND | ND | ND | |||
| Tocopherols and Tocotrienols | |||||||||||
| Alpha-Tocopherol | ND | ND | ND | + | + | ND | 290-292 | ND | |||
| Vitamin E Succinate (VES) |
+ + | + + |
274-281; 293 |
ND | ND | ND | ND | + + |
294- 296 |
||
Changes in activities outlined in this table are designated with the following symbols: (−) = negative effect, +/− = inconsistent results, + = mild/moderate stimulatory effect, + + = strong stimulatory effect, ND = No data available
Ref. = References
Only studies utilizing adult onset calorie restriction are reviewed in this table
Retinoid Analogs (all-trans-, 9-cis-, and 13-cis-retinoic acid)
Synthetic Rexinoids (Bexarotene (LGD1069) and LG100268).
Table 3.
Summary of the immunosuppressive effects of metabolic factors in the tumor microenvironment and the use small molecule inhibitors in combination with cancer vaccines
| Metabolic Factor | Key Pathway(s) |
Cellular Distribution |
Metabolic Changes |
Mechanisms of Immune Suppression |
Inhibitor | Use with Cancer Vaccine |
References |
|---|---|---|---|---|---|---|---|
| Arachidonic Acid | COX-2 | Tumor TAMs |
↑ PGE2 ↑ Angiogenesis |
↓ B and T cell proliferation ↓ NK cell cytoxicity ↑ Tregs ↓ DC function ↓ TH1 and ↑ TH2 cytokines |
Celecoxib | Yes | 297;300;301;304;308 |
| Arginine | ARG1 iNOS ODC |
Tumor TAMs MDSCs |
↓ Arginine ↑ Polyamines |
↓ TCR CD3 zeta chain expression ↓ IL-2R signaling ↓ IL-2 ↑ Effector T cell apoptosis |
NCX-4016 | Yes | 297;313;316;319;321;322 |
| Tryptophan | IDO | Tumor TAMs MDSCs DCs |
↓ Tryptophan ↑ Kynurenines |
↓ TCR CD3 zeta chain expression ↑ Tregs ↑ TGF-beta ↑ IL-10 |
1-MT | Yes | 297;325;333;334 |
| Glucose | Glut-1 MCT-4 HIF-1-alpha |
Tumor | ↓ Glucose ↓ pH ↑ Hypoxia ↑ Angiogenesis |
↓ Effector T cell function | ND1 | ND1 | 337;342;348;350 |
ND = No data available.
3. CHANGES IN ENERGY BALANCE
3.1. Physical activity
3.1.1. Role of physical activity in cancer prevention
An International Agency for Research on Cancer (IARC) Working Group on the Evaluation of Weight Control and Physical Activity concluded that consistent epidemiologic evidence exists demonstrating that physical activity reduces the risk of some forms of cancer. The evidence is conclusive for a protective effect of physical activity on colon and postmenopausal breast cancer risk, and is mounting for a protective effect of physical activity on endometrial, ovarian, and prostate cancers (35). Some of the reported benefit of physical activity on cancer risk reduction is independent of weight loss. These data suggest that the impact of physical activity on cancer risk reduction may not be solely due to the prevention of obesity (another potential modifiable risk factor that may negatively impact response to vaccine discussed in a later section), but rather due to a cancer preventive effect specifically of exercise.
A variety of animal models have been used to explore the effects of exercise on carcinogenesis including chemically-induced, transplantable, and spontaneous tumor models (7). In these studies, the effect of exercise on intestinal tumor incidence and multiplicity have been the best characterized, with a protective effect of exercise observed in most reports. Early studies showed a significant reduction in the incidence of carcinogen-induced tumors in exercising animals (36-39). Several subsequent studies have examined polyp development following exercise training in the APCMin mouse, a model in which an APC tumor suppressor gene mutation results in multiple intestinal polyps (40). Although some variability has been reported due to the type of exercise and gender of the animals studied, collectively these studies show a decrease in polyp number in exercising male animals (41-44). A protective effect of exercise on mammary tumor incidence, multiplicity, growth rate and/or survival has also been reported (45-52). In additional to colon and breast cancer models, exercise has been shown to be effective in reducing tumor incidence or burden in carcinogen-induced pancreatic (53;54) and liver (55-57) neoplasias. One study has shown that exercise delays tumor growth and enhanced regression of an allogeneic tumor (a murine T cell lymphoma cell line) (58). Thus, exercise has been shown to be effective in reducing the number and size of tumors in a number of models.
Numerous mechanisms have been proposed to explain the relationship between exercise and cancer prevention (29), including an exercise-induced stimulation of anti-tumor immunity. Little work has been done to examine those components of the immune system that are most likely mediating anti-tumor immunity or the effects of exercise on antigen-specific immune responses. However, the work in this area is promising and suggests that an exercise-induced enhancement of immune function may be one of the mechanisms underlying the protective effect of exercise on tumor formation.
3.1.2. Modulation of immune function by physical activity
The current theory to explain the relationship between exercise and immune function is the Inverted J Hypothesis (59). This hypothesis proposes that regular, moderate exercise enhances immune function and in turn, reduces the susceptibility to cancer. In contrast, sedentary behavior, at one end of the curve, and overtraining, at the opposite end of the curve, both lead to suppressed immunity and elevated risk of tumor development. In terms of anti-tumor immunity, NK cell function has been studied in response to exercise to a greater extent than either CD4+ and/or CD8+ T cell function. Little work has been done examining the effect of exercise on antigen-specific T cell functions such as cytokine production, proliferation, and/or cytotoxicity.
Overall, moderate exercise enhances NK cell activity, although there are some inconsistencies in the literature. Several cross-sectional studies have demonstrated higher NK cell function in trained athletes as compared to sedentary individuals (60;61). Longitudinal studies have shown that aerobic training over a period of several months in untrained individuals enhances NK cell activity in humans (59;62-66) and experimental animals (67-72). The beneficial effect of long-term aerobic training on NK cell function (four to seven months) has also been observed in postmenopausal breast cancer survivors (73;74), suggesting that exercise may benefit those patients who may be immunosuppressed following adjuvant therapy. However, another study in breast cancer survivors reported that eight weeks of aerobic training had no effect on NK cell function (61). The lack of a statistically significant effect of exercise on NK cell cytotoxicity in the latter study may be due to a relatively short training period. Recent studies suggest that the duration of the training period influences the effect of exercise on splenic NK cell cytotoxicity in mice. A signficant beneficial effect of running was observed only after 11 weeks of voluntary running (not at six or eight weeks) with the greatest increase in NK cell cytotoxicity observed following 15 weeks of training (72).
The effect of regular moderate exercise on mitogen-induced T cell proliferation has been examined in many studies which have yielded inconsistent results. Some studies have reported an increase (64;75-82), a decrease (83;84), or no effect (59;64;85-93) of regular, moderate exercise on T cell proliferative responses to mitogens in both humans and in experimental animals. Part of the heterogeneity of proliferative responses reported in the literature may be due to variability in the timing of lymphocyte collection with respect to the last exercise bout; to varying intensity and duration of exercise employed in the different studies; and to the origin of lymphoid tissue. With respect to the latter, it has been shown that lymphocytes isolated from different lymphoid tissues are differentially impacted by exercise training. For example, eight weeks of training has been shown to enhance the concanavalin A (Con A)-induced proliferation of T cells collected from the peripheral blood but not the spleen of hamsters (85). Additionally, exercise in rats resulted in an increase in Con A-induced lymphocyte proliferation in the mesenteric lymph nodes, but not the spleen (81). These results are consistent with recent findings in which T cell proliferative responses following six weeks of voluntary exercise differed by tissue type, with exercise significantly enhancing Con A-induced lymphocyte proliferation in intestinal lymphocytes but not splenocytes (82). These data suggest that voluntary exercise enhances T cell proliferation in some but not all lymphoid organs. This is an important distinction since many clinical studies only collect peripheral blood from patients. The assessment of immune status based on the response of one population of lymphoid cells (e.g., PBMC) following an intervention such as exercise may not be representative of the effect on all lymphoid cells and should be taken into consideration.
In tumor-bearing animals, splenic Con A-induced lymphocyte proliferation was increased following exercise training in two studies (94;95). In the first study, increased lymphocyte proliferation was correlated with reduced tumor growth and increased survival (94). However in the second report, final tumor volumes did not differ between the exercising and control animals despite an increase in T cell proliferative responses in the exercise group (95). A study in early-stage breast cancer patients three to six months following chemotherapy found that women who participated in a six-month exercise program showed a greater percentage of CD4+CD69+ cells and a greater level of mitogen-induced proliferation at the end of the intervention (96). The results from these limited studies suggest that exercise may enhance T cell proliferation under conditions where immunosuppressive factors, i.e. the presence of a tumor or following chemotherapy, are exerting an inhibitory effect on the immune system.
There have been considerably fewer studies examining the effect of exercise on antigen-specific T cell function. Most of these have explored the interaction between exercise, aging and T cell function; however, none to date have examined the effect of exercise on the generation of tumor-specific T cells. One line of evidence to suggest that regular, moderate exercise can stimulate antigen-specific immunity is the observation that the incidence and duration of upper-respiratory tract infections is significantly lower in postmenopausal women who are moderately active as compared to sedentary controls (63;64;97). Although no adaptive immune endpoints were measured in any of these studies, adequate cellular immune responses play a critical role in the clearance of viral infections of the respiratory tract (98;99). Two other studies that have examined the effect of exercise on antigen-specific T cell responses have reported a stimulatory effect. Physically fit older men (age 60-79) had significantly greater delayed-type hypersensitivity (DTH) reaction to keyhole limpet hemocyanin (KLH) and higher anti-KLH antibody titers than sedentary controls (100). Older men and women (age 65 or older) who were active or moderately active prior to influenza immunization had greater in vitro antigen-specific T cell proliferation and antibody titers than sedentary subjects (101). Furthermore, several studies in animals have demonstrated a beneficial effect of exercise on T cell function in aged mice. Kohut and colleagues demonstrated that eight weeks of exercise prior to herpes simplex virus-1 (HSV-1) infection enhanced in vitro HSV-1 specific cytokine production (IL-2 and IFN-gamma) in older (16-18 months) but not younger mice (2-4 months) (102;103).
Recent studies conducted to assess the effect of exercise on antigen-specific immunity in young, non-tumor bearing animals have demonstrated that eight weeks of voluntary running prior to vaccination with either a protein or viral based vaccine enhances antigen-specific immune responses. Specifically, antigen-specific proliferation of CD4+ T cells collected from the spleens and inguinal lymph nodes of animals vaccinated subcutaneously with a protein-based vaccine (ovalbumin plus lymphotactin) was significantly higher in exercising animals (104) Additionally, eight weeks of training prior to vaccination enhanced antigen-specific splenic CD4+ T cell proliferation following vaccination with a pox virus based vaccine (recombinant vaccinia/fowlpox NP34 plus recombinant fowlpox GMCSF) (72). In subsequent studies, the minimum length of training time needed to enhance antigen-specific immune responses in C57BL/6 mice was shown to be eight weeks. Importantly, initiating exercise concurrently with the administration of the primary vaccination did not yield significant increases in CD4+ T cell proliferation (72). These data suggest that a training period of eight weeks prior to the primary vaccination is required to achieve the stimulatory effect of exercise on adaptive immune function and that exercise can be used effectively in combination with vaccination.
3.1.3. Physical activity in combination with cancer vaccine
No studies to date have combined moderate physical activity with the administration of a therapeutic cancer vaccine. However, this combinatorial approach seems promising for several reasons. Moderate exercise alone following cancer treatment has been shown to decrease recurrence and increase survival in cancer patients. Compelling findings from the Nurses Health Study, one of the largest prospective investigations examining chronic disease risk factors in women, demonstrated that women who exercised for the equivalent of walking 3-5 hours per week at an average pace had a 50% reduction in breast cancer mortality risk (105). Importantly, women who exercised for the equivalent of walking 1-3 hours per week had a 20% reduction in breast cancer mortality risk, suggesting that modest increases in physical activity can have a profound impact on clinical outcomes. A second report examining physical activity and colorectal cancer outcomes from the Nurses Health Study found similar results. Female nonmetastatic colorectal cancer patients who exercised for the equivalent of walking six or more hours per week at an average pace had approximately a 50% reduction in both colorectal cancer-specific and overall mortality (106). A third study of patients enrolled in an adjuvant chemotherapy trial for stage III colon cancer who exercised for the equivalent of walking six or more hours per week at an average pace had a 47% improvement in disease free survival compared to sedentary patients (107). In addition to the robust effect of exercise on clinical outcomes, exercise interventions in women with breast cancer have been shown to be safe, have had high compliance levels and result in improved fitness and quality of life (108;109). These data suggest that combining an exercise intervention with other therapeutic strategies, such as cancer vaccine treatment, may be relatively easy to implement and confer significant benefit to the patient independent of any enhancement of vaccine efficacy.
Numerous cancer vaccine platforms have already been shown to stimulate tumor-antigen specific immune responses (110-113) and increase disease free survival (4;114) in cancer patients over 65 years old. Moderate exercise has also been shown to enhance the antigen-specific immune responses in aged humans and animal models. Furthermore, moderate exercise can be used effectively in combination with a variety of vaccination protocols to enhance antigen-specific T cell responses in preclinical models. Combined, these data suggest that moderate physical activity is a likely candidate to partner with therapeutic cancer vaccine treatment to enhance the vaccine efficacy. Studies are currently underway to examine this combination in preclinical animal models.
3.2. Calorie restriction
3.2.1. Role of calorie restriction in cancer prevention
The best studied alteration of energy balance in experimental tumor models is on the energy intake side of the equation, specifically involving obesity prevention through calorie restriction (CR). Studies in rodents have most often intiated CR early in life (at the time of weaning) and maintained the CR for the life of the animal. This long term CR inhibits the incidence and growth rate of a variety of spontaneous neoplasias in experimental cancer model systems, including tumors arising in several genetically altered mouse models (e.g., p53-deficient mice, APCmin mice and Wnt-1 transgenic mice); as well as carcinogen and radiation induced cancer (recently reviewed in (8;9)). Additionally, several studies have shown that CR initiated in adult animals in mid to late life also reduces the incidence (115-118) and/or delays the onset (118;119) of spontaneous tumors. CR has also been shown to increase survival in most tumor models (120-125). Thus, the inhibitory action of CR on carcinogenesis is effective in several species for a variety of tumor types and importantly, when intiated in either early and late life.
3.2.2. Modulation of immune function by calorie restriction
Long term CR throughout the life course prevents a variety of age-associated decrements in immune function (117;126-129), and is an intriguing model to explore the effects of aging on the immune system. However, two aspects of long term CR make it difficult to translate into a viable intervention for use in humans: 1) the early age of onset of CR in animal models and 2) the duration of this exposure over the life course. CR started in older animals and/or shorter duration of CR (weeks to months) are both more appropriate model systems to investigate the effects of CR on immune function, particularly if CR is being considered as a potential intervention for use in combination with therapeutic cancer vaccines. Much less is known about the effects of CR initiated in mid-life on immune function. Adult onset CR initiated at 12 months of age (117;130) and at 17 months of age (131) and lasting for up to eight months increased splenic mitogen-induced proliferation and increased the percentage of splenic T cells in mice (116). Additionally, 27 month old mice restricted at 12 months of age had significantly higher allogeneic T cell responses compared to their ad libitum fed, age-matched controls (116;130). Although a limited number of studies have explored the immune sequelae of CR started in mid life, the results to date suggest that a beneficial effect on immune function can be achieved when CR is initiated in older animals.
In contrast, studies exploring the effects of shorter term CR (up to six months) on immune function in animals of varying ages have reported inhibitory effects. A dose dependent inhibition of antigen-specific T cell proliferation following eight weeks of either mild (20%) or severe (50%) CR was observed in normal C57BL6 mice (132). This CR-induced inhibition of T cell proliferation was due to both a deficit in the antigen presenting capabilities of macrophages and proliferative capacity of T cells. In one study, moderate CR significantly reduced mitogen-induced and allogeneic T cell proliferation during the first month of restriction, but after six months on the diet the proliferative responses of CR and ad libitum animals were no longer different (131), suggesting that the time of exposure to CR may influence immune responsiveness. Additionally, in several rodent autoimmunity models, short-term CR in young animals decreases antigen-specific proliferation of T cells, as well as decreases IFN-gamma, IL-12 and autoantibody production (133;134). Futhermore, 40% CR beginning at six weeks of age and lasting for several months, dampens autoreactive lymphocyte proliferation and cytokine production (IL-2 and IFN-gamma) by both CD4+ and CD8+ lymphocytes in the autoimmune prone (NZBxNZW)F1 (B/W) murine model of systemic lupus erythematosis (135-137). Using autoimmune prone (B/W) mice another group has shown decreased mRNA expression of IL-6 and TNF-alpha, and increased expression of TGF-beta in splenocytes harvested from CR animals (138). Finally, four to eight weeks of moderate (30%) to severe (50%) CR significantly lowered splenic NK cell cytotoxicity (139;140). These data suggest that short-term CR in non-aged animals may suppress a number of effector cell functions. Futhermore, these results suggest that a longer duration of exposure to CR (greater than six months) may be necessary to achieve the stimulatory effects on immune function. Additional studies are needed to further characterize the effects of adult onset CR on antigen-specific T cell functions and NK cell cytotoxicity to determine what factors (e.g., age of the animal at the onset of CR, duration of CR exposure, severity of CR, etc…) influence immune responses.
3.2.3. Calorie restriction in combination with cancer vaccine
No studies to date have implemented CR prior to the administration of a therapeutic cancer vaccine. In our view, CR may be an effective strategy to reduce weight in overweight and obese individuals (discussed in the next section). However, additional studies are needed to futher examine the effects of short term CR on immune function in middle-aged normal and overweight animals before any recommendations regarding its use in combination with therapeutic vaccines can be made.
3.3. Obesity: prevention and reversal
3.3.1. Links between obesity and cancer
Obesity continues to be one of the leading health issues facing the nation. The prevalence of obesity has risen significantly over the past several decades and there is no indication that this trend is declining (141;142). In 2004, 66.3% of adults were overweight (body mass index or BMI = 25.0-29.9 kg/m2), 32.2% were obese (BMI ≥ 30.0 kg/m2), and 4.8% were extremely obese (BMI ≥ 40 kg/m2) (142). This trend in the prevalence of obesity is related to an increased incidence of a myriad of chronic diseases, including cancer (143). A body of convincing epidemiologic evidence has accumulated suggesting that overweight or obesity increases risk of several types of cancer including colon, breast (in postmenopausal women), endometrial, ovarian, kidney, esophageal, pancreatic and gallbladder (35;144-147). Furthermore, data from a large cohort study of more than 900,000 adults in the U.S. has demonstrated that in both men and women, increased BMI is associated with increased mortality from tumors of the esophagus, colon, liver, gallbladder, pancreas, and kidney, as well as death due to non-Hodgkin’s lymphoma and multiple myeloma (11). In this same cohort, increased BMI is associated with increased mortality from cancers of the stomach and prostate in men; and mortality from cancers of the breast, uterus, cervix, and ovary in women (11). In addition to providing recommendations on physical activity, the IARC Working Group on the Evaluation of Weight Control and Physical Activity concluded that excess body weight and physical inactivity account for approximately a quarter to one third of cancers of the colon, breast, endometrium, kidney and esophagus (35). Thus, excess adiposity and physical inactivity appear to be the most important avoidable causes of these cancers.
3.3.2. Obesity and immune function
Although obesity is believed to adversely impact immunity, the effect of overweight and obesity on the function of specific immune cell types has not been well studied. However, obesity has been consistently associated with a state of low grade, chronic inflammation (148;149). Cross-sectional analyses show elevated levels of C-reactive protein (CRP) (150;151), tumor necrosis factor alpha (TNF-alpha) (152-154), and IL-6 (155) in the serum of overweight and obese individuals. A variety of pro-inflammatory markers are produced in adipose tissue and increase with increasing adiposity, including: TNF-alpha, IL-6, monocyte chemotatic protein-1 (MCP-1), inducible nitric oxide synthase (iNOS), TGF-beta-1, and plasminogen activator inhibitor type 1 (PAI-1) (149). Macrophages are the main source of pro-inflammatory cytokines in the adipose tissue of obese subjects (156-158) and the number of adipose tissue macrophages increases with increasing body fat (156;157). Increased expression of inflammation-specific genes by adipose tissue macrophages has been shown in obese mice preceding the development of insulin resistance, a well-documented consequence of obesity (155). Both TNF-alpha and IL-6 block insulin action by triggering key steps in the insulin signaling pathway (155;159;160). In the study by Xu and colleagues, obese mice treated with rosiglitazone (an insulin-sensitizing drug) had a decreased expression of inflammation-specific genes in adipose tissue macrophages (157), suggesting an additional feedback loop between insulin signaling and inflammatory cytokines. Currently it is uncertain if the chronic elevation of circulating pro-inflammatory cytokines observed in obese subjects cause and/or contribute to any of the obesity-induced changes in innate and adaptive immune function (discussed below) or if the elevation of inflammatory cytokines and alterations in immunity are both downstream consequences of obesity.
In addition to the elevation of inflammatory markers in obesity, several reports have documented an obesity-induced impairment in innate immunity. Wound healing is significantly delayed and the reported incidence of wound complications is significantly greater in obese patients compared with normal-weight patients (161-163). Additionally, the incidence of nosocomial infections is higher in overweight and obese patients compared with normal-weight patients (161) and obese burn patients have an increased risk of infection and bacteremia during recovery (164). Taken together, these data suggest that several components of innate immunity are adversely impacted by obesity and the surgical resection of a primary tumor may be more complicated in obese patients.
The effect of obesity on NK cell function has been examined in several studies, but the results are inconsistent. Two cross-sectional studies have reported no statistically significant difference in NK cell cytotoxicity between obese subjects and lean controls (165;166). In another report, the influence of obesity on NK cell function differed by age. Obesity had no effect on NK cell cytotoxicity in younger subjects, but obese subjects over 60 years old had lower NK cell function than the lean age-matched controls (167). In animal studies, diet-induced obese animals have lower NK cell function than lean control animals (168;169). Moreover, in a metastasis model, the number of experimental lung colonies was significantly higher in obese mice compared to lean controls (170). Depletion of NK cells with anti-asialo-GM1 antibody led to increased metastases in both control and obese mice, but eliminated the obesity-induced difference in tumor metastasis (170). These results demonstrate that the obesity-induced increase in metastasis in this model is mainly due to impaired NK cell function in obese mice. These data suggest that obesity adversely impacts NK cell function in mice. However, additional studies are needed to further characterize the effects of overweight and obesity on NK cell function in both humans and animal models.
Little work has been done to date to identify the effect of obesity on adaptive immune function. Phenotypic studies have shown increased numbers of CD19+ and CD3+ cells among obese individuals. The increase in CD3+ T cells is due to a selective increase in CD4+ T cells (166;171). There are conflicting results on the effects of obesity on the CD8+ T cell population. One report showed no effect of obesity on the CD8+ T cell population (84) while another showed a decreased frequency of CD8+ T cells (171). Despite increases in the number of CD4+ T cells, their functional capacity is impaired by obesity. Reduced mitogen-induced T cell proliferation has been observed in obese subjects (166;172;173) and in experimental animals (174;175). Additionally, obese children and adolescents have impaired DTH responses compared to normal weight controls (172). Finally, a recent study has demonstrated that obesity impairs antigen-specific CD4+ T cell proliferation and cytokine production following vaccination with a pox virus based vaccine (169). Taken together, these preliminary results suggest that T cell function is impaired by obesity. Therefore, the generation of adequate immunological response to common vaccinations (e.g., flu, tetanus, etc…), and potentially, therapeutic cancer vaccines may be significantly impaired by obesity.
If obesity impairs innate and adaptive immune function, several critical questions need to be addressed. First, can weight loss reverse the adverse effects of obesity on the immune system? Second, does the method of weight loss, i.e. CR (diet), exercise or a combination of CR and exercise, differentially alter the immunological response to weight loss? A few studies have examined immunological endpoints following weight loss in obese subjects using either CR, exercise or a combination of CR and exercise and have reported inconsistent results. Obese subjects maintained on a very low calorie diet for 12 weeks lost a significant amount of body weight and had increased mitogen-induced T cell proliferation after 12 weeks on the diet (173). Similarly, overweight rats that were calorie restricted had a significantly higher number of splenic CD4+ T cells and enhanced mitogen-induced proliferation compared to overweight ad libitum-fed rats (168). However, in another study, obese women completing a 26-week weight loss program involving severe CR followed by a period of incremental refeeding had a significant loss in body weight and percent body fat, but also had a significant decline in DTH responsiveness (176). Importantly, DTH responses remained suppressed for eight months after the end of the diet in those subjects who regained less than 40% of their original weight (176). Additionally, obese women who completed a 12-week weight loss program using a low calorie diet (50% reduction in calories) had a 30-35% reduction in the number of circulating NK cells that persisted following 35 days of normal food intake (177). Finally, one report showed that repeated cycles of weight loss were associated with lower NK cell function in postmenopausal, obese women (178).
In contrast, studies that have incorporated exercise into the weight loss program have reported better immune responses than CR alone. For example, in one report obese women were randomized to one of four groups: control, exercise (walking 45 minutes, 5 days/wk); CR (1200-1300 kcal/day) or a combined CR and exercise group. Women in the CR and CR plus exercise groups lost weight and lowered their percent body fat, however, only the obese exercisers had significantly lower upper respiratory tract infections (84). In another study, obese women were randomized into CR (925 kcal/day) or CR plus exercise (925 kcal/day plus 20 minutes aerobic activity 3 days/wk) groups for eight weeks. Women in the CR only group had significantly lower NK cell function following the diet, but the CR plus exercise group had no decrements in NK cell function (179). In animal studies, exercise but not weight loss enhanced NK cell cytotoxicity and Con A-induced proliferation of splenic lymphocytes in obese Zucker rats (174). These limited results suggest that the incorporation of physical activity into a weight loss regimen may enhance a broader repertoire of immunological responses than dieting alone.
3.3.3. Weight loss strategies in combination with cancer vaccine
No studies to date have implemented weight loss strategies (either via CR, increased physical activity or a combination of both) prior to the administration of a therapeutic cancer vaccine. However, reducing body weight and/or percent body fat may provide a better host environment to generate antigen-specific vaccine responses since obesity had been linked to adverse clinical outcomes, as well as potentially impaired immune function.
In numerous studies, being overweight at the time of diagnosis has been identified as a predictor of adverse clinical outcomes in women with breast cancer (10). To date, 39 studies have examined the relationship between obesity and risk of recurrence or survival in breast cancer patients. A significant association between obesity and recurrence or survival for breast cancer was observed in 31 of these studies (180-185); and the negative effects of obesity on recurrence and survival were observed in both pre and postmenopausal women (185-187). Weight gain after diagnosis is also related to poorer survival in breast cancer patients (183;188;189). Weight gain after diagnosis is commonly reported in breast cancer patients, particularly those receiving adjuvant chemotherapy (190-193). Furthermore, several studies have demonstrated that a return to pre-diagnosis body weight seldom occurs in breast cancer patients (188;194), suggesting that the adverse effects of obesity, at least the risk associated with post-diagnosis weight gain, impact the vast majority of breast cancer patients.
Obesity is also predictive of other less favorable breast cancer clinical outcomes and related co-morbidities. Obesity is associated with more advance stage of disease at diagnosis (195-198), greater lymph node involvement (199-201), and greater risk of contralateral breast cancer in pre (202) and postmenopausal (202-204) women. Obese women also have greater wound complications (e.g., slower healing and greater infections) and lymphedema following breast cancer surgery (205;206).
Although less well studied, similar patterns are emerging between obesity and clinical outcomes related to prostate and colorectal cancer. A number of large cohort studies have demonstrated a clear association between obesity and prostate cancer mortality (11;207-209). In addition, several of these studies have also demonstrated that obesity is strongly correlated with more aggressive disease (207-210). Obese prostate cancer patients have higher grade and/or stage tumors and higher rates of positive surgical margins at the time of radical prostatectomy (210-214), and are at greater risk for biochemical failure following surgery (210;214). Two recent studies have demonstrated that obese men are at an increased risk of developing metastases (215;216). Finally, several recent studies have shown a positive association between obesity at the time of diagnosis and prostate cancer recurrence (210;217-219).
Less data is available regarding the impact of obesity on clinical outcomes related to colorectal cancer. In a randomized adjuvant chemotherapy trial for colorectal cancer, obese women had 34% greater risk of overall mortality and 24% increased risk of recurrence than normal weight women (220). In a second trial investigating clinical outcomes in subjects from the National Surgical Adjuvant Breast and Bowel Project, obesity at the time of diagnosis was associated with increased risk of colon cancer recurrence and death (221). Lastly, in an Australian cohort, incremental increases in percent body fat were positively associated with colorectal-specific mortality (222). Together these data suggest that obesity is associated with adverse clinical outcomes following diagnosis of breast, prostate and colon cancer. Reduction in body weight either through moderate calorie restriction and/or physical activity following diagnosis may reduce the risk of these cancer-related events. However, no studies to date have addressed this issue.
The adverse effects of obesity on recurrence, survival, and other clinical outcomes, and the potential for obesity to impair immunological response to vaccine provide compelling rationale for reducing obesity in cancer patients in general, but also specifically prior to receiving a therapeutic vaccine. However, it may not be feasible to recommend weight loss in overweight and obese patients until after the completion of adjuvant therapy given the potential for adverse side effects associated with treatment (e.g., nausea, fatigue, etc ). Furthermore, the few studies that have explored the effect of weight loss on immune endpoints in obese subjects suggest that weight loss achieved via dieting versus dieting in combination with exercise may have different consequences on immune function, with exercise providing greater benefit. Given the number of potential cancer patients who are likely to be overweight or obese at the time of diagnosis or become obese following treatment, future studies are warranted to first, determine the mechanisms by which obesity impairs immune function; and second, determine if these immunological impairments can be reversed though weight loss or other pharmacological interventions that can be used prior to or in combination with vaccine to enhance vaccine efficacy.
4. IMPACT OF NUTRIENTS
4.1. Retinoids and carotenoids
4.1.1. Role of retinoids and carotenoids in cancer prevention
Vitamin A and its natural analogues, all-trans, 9-cis, and 13-cis-retinoic acids, have been shown to promote the differentiation of normal and neoplastic cells, in vitro and in vivo (223;224). The carotenoids are fat-soluble, antioxidant compounds (xanthophylls, carotenes, and lycopene) that are found in green and yellow leafy vegetables and serve as precursors to vitamin A and its derivatives. Epidemiological studies indicate that diets deficient in the retinoids and carotenoids or individuals with low serum retinol levels are associated with increased relative risk of cancer (225-227). However, large, randomized studies of dietary supplementation with beta-carotene reported significant increases in lung cancer incidence and mortality in heavy smokers and asbestos workers (228-231). These data led the Institute of Medicine (IOM) to conclude that beta-carotene supplementation is not advisable for the general population, but may be appropriate in vitamin A deficient populations (232).
In preclinical studies, a liposomal formulation of all-trans retinoic acid (ATRA) was shown to induce sustained remission of acute promyelocytic leukemia in immunocompetent but not immunodeficient mice, suggesting an immunological mechanism of action (233). The use of 9-cis-retinoic acid (9-cis-RA) has been shown to inhibit the growth of ER+ (234;235) and ER− (236) mammary tumors in the rat. Clinical trials of 13-cis-retinoic acid (13-cis-RA, Isotretinoin, Accutane, Hoffman-La Roche Inc.) show regression of oral leukoplakia (237) and significant reductions in the incidence of secondary squamous cell carcinomas of the head and neck (238;239). A topical formulation of 9-cis-RA (Alitretinoin gel 0.1%, Panretin, Ligand Pharmaceuticals) is FDA approved for the treatment of AIDS-related Kaposis’s sarcoma (KS) where it is thought to inhibit the proliferation and neoangiogenesis of KS lesions (240).
To avoid the toxicities associated with the retinoids (241), synthetic analogues that preferentially bind to the retinoid X receptor (RXR) termed “rexinoids”, LGD1069 (Bexarotene, Targretin, Ligand Pharmaceuticals) and LG100268, were developed. These synthetic analogues show preventive and therapeutic efficacy against animal models of ER+ (242-245) and ER− (244;246-248) mammary carcinogenesis. In a phase II clinical trial of metastatic breast cancer patients, Bexarotene (Targretin, LGD1069) was shown to benefit 20% of the patients without significant toxicity (249). Even greater promise may lie in the new rexinoid, LG100268, which has been shown to synergize with the selective estrogen receptor modulators, Arzoxifene and Acolbifene (244;248), and may be more potent and less toxic than LGD1069 due to its greater specificity of RXR binding (250).
4.1.2. Modulation of immune function by retinoids and carotenoids
There is support in the literature for a diverse immunomodulatory role of the retinoids. Preclinical studies suggest that ATRA therapy promotes Th2-mediated immune responses (251-253). Vertuani and colleagues (254) demonstrated that the retinoids, ATRA and 9-cis-RA, stabilized the class I major histocompatibility complex (MHC) and enhanced both MHC class I restricted peptide-specific lysis by CD8+ cytotoxic T lymphocytes (CTLs) and NK cell cytotoxicity of neuroblastoma cells. Treatment with ATRA works additively with IFN-gamma (255) and high dose gamma irradiation (256) to upregulate human leukocyte antigen (HLA) class I and intercellular adhesion molecule 1 (ICAM-1) on human cervical carcinoma cell lines. Against a panel of human uveal melanoma cells, ATRA treatment was shown to induce G1 arrest, apoptosis, and sensitize cells to MHC class I restricted CTL killing and NK mediated lysis (257). Furthermore, the in vitro proliferation of T cells in response to anti-CD3/anti-CD28 stimulation is significantly improved with ATRA (258). Following chemotherapy in advanced ovarian cancer patients, the combination of low-dose IL-2 with 13-cis-RA was shown to reduce circulating vascular endothelial growth factor (VEGF) levels, increase lymphocyte and NK cell numbers, and significantly improve both progression-free and overall survival (259).
Bexarotene (Targretin) is FDA approved for the treatment of early (260) and advanced (261) cutaneous T cell lymphoma (CTCL). Both Bexarotene (Targretin) and Alitretinoin (Panretin) enhance the sensitivity of T- and B-leukemia cells to denileukin diftitox (ONTAK) due to upregulation of the α/p55/CD25 and β/p75/CD122 chains of the high-affinity IL-2 receptor (262). The combination of Bexarotene (Targretin) and ONTAK (Panretin) is currently being evaluated in clinical trials of patients with relapsed or refractory CTCL (263).
4.1.3. Use of retinoids and carotenoids in combination with cancer vaccine
Several lines of evidence suggest that the retinoids may be effective adjuncts to antigen-specific immunotherapy. The expansion of Gr-1+/CD11b+ myeloid-derived suppressor cells (MDSCs) in tumor-bearing hosts are reported to be a major mechanism of tumor-associated immunosuppression (264). ATRA therapy has been shown to improve antigen-specific T cell responses through reductions in the Gr-1+/CD11b+ MDSC population in both mice (265;266) and humans (267). In an adoptive transfer model, ATRA therapy reduced the proportions of Gr-1+/CD11b+ MDSCs with concomitant increases in the proportions of CD11c+/I-Ab+ dendritic cells (DCs), F4/80+ macrophages, and Gr-1+/CD11b− granulocytes (266). Moreover, the loss of Gr-1+/CD11b+ MDSCs was associated with improvements in CD4+- and CD8+- mediated immune responses. ATRA therapy was shown to significantly enhance the antitumor efficacy of two vaccine strategies: 1) a peptide-based vaccine against C3 fibrosarcomas, and 2) a DC-based vaccine against MethA sarcomas (266).
In myeloid leukemia, reciprocal DNA translocations result in the formation of unique tumor-associated antigens such as the promyelocytic leukemia-retinoic acid receptor-α (PML-RARA) fusion protein which represents over 95% of the fusion proteins found in acute promyelocytic leukemia patients (268). Padua and colleagues (268) developed a DNA-based vaccine that linked the PML-RARA oncogene with fragment c of the tetanus toxin (PML-RARA-FrC). The DNA vaccine successfully targeted the oncoprotein and combination therapy with ATRA was shown to improve survival, induce antibody production against RARA, and increase serum IFN-gamma levels in vivo (268).
These studies provide mechanistic insights and demonstrate the potential of combination immunotherapy with retinoids such as ATRA; however, significant toxicities associated with the use of the retinoids (269) have led to the development of the synthetic rexinoids with fewer RAR-mediated side effects. The rexinoids show tremendous potential for chemoprevention, but have not been tested in combination with cancer vaccines (personal communication with Dr. Michael Sporn). Interestingly, the rexinoid, Bexarotene (Targretin, LGD1069) has been reported to inhibit cyclooxygenase-2 (COX-2) expression through transrepression of the AP-1 transcription factor in both normal and malignant mammary tissue (270). As pharmacologic inhibition of COX-2 expression has been shown to synergize with a variety of anti-tumor vaccine strategies (see Arachidonic Acid Metabolism), the potential for combination immunotherapy with the rexinoids is intriguing.
4.2. Tocopherols and tocotrienols
4.2.1. Role of tocopherols and tocotrienols in cancer prevention
Vitamin E, a fat-soluble antioxidant vitamin found in plant oils, grains, and seeds, is comprised of a family of four tocopherols and four tocotrienols. The tocopherols have been shown to scavenge free radicals, prevent lipid peroxidation in cell membranes and low-density lipoprotein cholesterol, and inhibit production of the nitrosamines, carcinogens derived from dietary nitrites. Epidemiological studies offer mixed results with some trials suggesting that alpha-tocopherol supplementation may protect against cancers of the prostate (231) and upper gastrointestinal tract (271) in high-risk populations. The Heart Outcomes Prevention Evaluation (HOPE) trial observed a protective effect of vitamin E supplementation (400 IU/day of RRR-alpha-tocopheryl acetate) on incident cases of lung cancer; however, vitamin E supplementation was not shown to be protective against prostate or colorectal cancers (272). In a phase II clinical trial of the Community Clinical Oncology Program (CCOP), alpha-tocopherol supplementation (800 IU/day for 24 weeks) resulted in clinical or histological responses in 61% of patients with premalignant oral leukoplakia (273).
Vitamin E succinate (VES) is a redox-inactive ester analog of vitamin E and a potent chemopreventive agent in many types of cancer with remarkable specificity for malignant tissues (274;275). In prostate cancer cells, VES induces apoptosis by suppressing NF-kB activity, inhibiting the interaction between Bcl-XL and Bcl-2 proteins, and sensitizing androgen-dependent prostate cancer cells to androgen deprivation in vitro (276;277). Malafa and colleagues (278) demonstrated that VES induced caspase 4-dependent apoptosis and inhibited lung metastases in human prostate tumors growing in SCID mice. In malignant mesothelioma cells, VES activates the expression of death receptors 4 and 5 (DR4 and DR5); thereby, rendering malignant mesothelioma cells sensitive to TNF-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in a p53-dependent manner (279). The antitumor activity of VES has also been associated inhibition of angiogenesis through the suppression of VEGF expression in B16F10 melanoma (280) and MDA-MB-231 breast cancer cells (281).
4.2.2. Modulation of immune function by tocopherols and tocotrienols
There is evidence from clinical trials that vitamin E supplementation may boost immune function, particularly in high-risk elderly populations. Meydani and colleagues (282) evaluated the effects of vitamin E supplementation on indices of cell-mediated immunity in a randomized, double-blinded, placebo-controlled trial of healthy older subjects. Supplementation with 30 days of vitamin E (dl-alpha-tocopheryl acetate) at 800 IU/day was shown to increase DTH responses, Con A-stimulated T cell proliferation, and IL-2 release (282). In a subsequent randomized, double-blinded, placebo-controlled trial of elderly subjects, vitamin E supplementation for 4 months at 200 mg/day was shown to enhance DTH responses and antibody responses to hepatitis B and tetanus toxoid vaccination (283). In this study, subjects in the highest tertile of serum alpha-tocopherol concentration (> 2.08 mg/dL) following supplementation had the highest antibody titers to hepatitis B and DTH responses (283). These findings are consistent with a study in a Japanese cohort of nursing home residents in which pre-vaccination vitamin E status was positively associated with response to the influenza vaccine (284). In yet a third supplementation study by Meydani’s group, nursing home residents receiving one year of vitamin E supplementation (200 IU/day) were significantly protected from upper respiratory tract infections such as the common cold (285). However, this effect was not observed in another randomized trial of non-institutionalized elderly individuals receiving vitamin E supplementation (200 mg/day) for up to 15 months (286).
In advanced colorectal cancer patients, short-term, high-dose vitamin E supplementation (750 mg/day for 2 weeks) increased CD4+/CD8+ T cell ratios, increased T cell production of IL-2 and IFN-gamma, and significantly improved NK function through induction of NKG2D expression (287;288). The beneficial effects of vitamin E supplementation on cellular and humoral immunity appear to be dose-dependent as De Waart and colleagues (289) reported that 3 months of supplementation with 100 mg/day of dl-alpha-tocopheryl acetate did not significantly alter mitogen-activated lymphoproliferative responses or antigen-specific IgG or IgA antibody titers.
Several hypotheses have been forwarded to explain the effects of vitamin E supplementation on age-associated decrements in cell-mediated immunity. In a recent study, in vivo vitamin E supplementation of aged mice improved the development of an effective immune synapse between naive CD4+ T cells and APCs resulting in improved T cell signaling and activation (290). Han and colleagues (291) reported that vitamin E supplementation in aged mice resulted in up-regulation of IL-2 and down-regulation of IL-4 expression following anti-CD3/anti-CD28 stimulation suggesting a shift in Th1/Th2 balance in the T cells of older mice receiving vitamin E in vivo. Another hypothesis is based on the observation that macrophages from aging animals produce more prostaglandin E2 (PGE2) which has been shown to inhibit the proliferative capacity and IL-2 secretion of T cells, in vitro. In co-culture experiments, vitamin E supplementation and the cyclooxygenase inhibitor, indomethacin, were shown to improve T cell responsiveness in old mice primarily by reducing macrophage-derived PGE2 production (292). Similarly, VES is hypothesized to inhibit the initiation and progression of lung cancer through its ability to suppress COX-2 activity and PGE2 production in phorbol ester-stimulated human lung epithelial cell lines (293). Thus, the synthetic rexinoid, Bexarotene, and the esterified vitamin E analog, VES, both appear to mediate at least some of their chemopreventive and immunomodulatory effects through the inhibition of COX-2 activity and PGE2 production in target tissues.
4.2.3. Use of tocopherols and tocotrienols in combination with cancer vaccine
To date, three preclinical studies have evaluated VES in combination with DC-based cancer vaccines. Ramanathapuram and colleagues (294) demonstrated that DCs pulsed with tumor cell lysate and injected either intratumorally or subcutaneously in combination with locally or systemically administered VES resulted in significant growth inhibition of established Lewis lung carcinomas. In this study, the adjuvant effect of VES was shown to outperform that of cyclophosphamide. One of the impediments to the clinical applicability of VES is its requirement for ethanol, DMSO, or sesame oil for solubilization. A more soluble formulation of vitamin E succinate, vesiculated alpha-tocopherol succinate (V-alpha-TOS), was shown to be soluble in aqueous solutions and enhance the anti-tumor efficacy of DC-based vaccines against 4T1 breast (295) and Lewis lung (296) carcinomas. In both studies, non-matured DCs incubated with the lysates of V-alpha-TOS-treated tumor cells were shown to mature as evidenced by the upregulation of costimulatory molecules (CD40, CD80, and CD86) and the secretion of IL-12p70 (295;296). Two mechanisms were proposed to explain how V-alpha-TOS improves DC-based immunotherapy: 1) V-alpha-TOS directly lyses tumor cells thereby cross-priming DCs through antigen cascade, and 2) V-alpha-TOS induces the expression of heat shock proteins 60, 70, and 90 in tumor cells which present a “danger” signal to antigen presenting cells (APCs) leading to their activation (295;296). The improved solubility and demonstrated adjuvant characteristics of V-alpha-TOS make this compound a promising candidate for additional research and clinical development.
5. OTHER METABOLIC FACTORS
5.1. Introduction to other metabolic factors
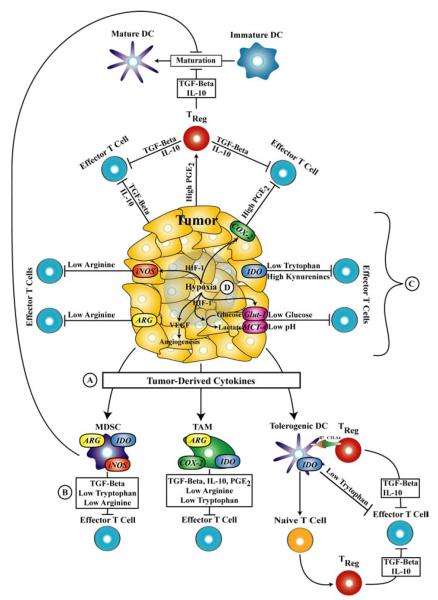
Thus far, this review has focused on systemic modulation of energy balance and nutritional status to optimize innate and adaptive immune function and responses to antigen-directed cancer immunotherapy (Tables 1 and 2). However, there is a growing body of evidence demonstrating that nutrient metabolism at the level of the tumor microenvironment (tumor cells, stroma, and tumor-draining lymph nodes) is employed by the growing tumor to induce peripheral immune tolerance and tumor escape (reviewed in (297)). Below, we have reviewed complex immunosuppressive networks in which arachidonic acid metabolism, the catabolism of the amino acids arginine and tryptophan, and the depletion of glucose from the tumor microenvironment contribute individually, and in combination to inactivate effector T cell function and promote a tolerogenic state (Figure 1). Where available, we have provided examples of small molecule inhibitors that target these pathways and their utility in combination with cancer vaccines (Table 3).
Table 2.
Summary of the effects of changes1 in energy balance and the impact of nutrients on anti-tumor activity, immune function and use in combination with vaccines in human studies
| Immune Function | Use with | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-Tumor Activity | NK Cell Function | T Cell Function | Cancer Vaccine | ||||||||
| Prevention | Therapy/ Survival |
Ref.2 | Cytotoxicity | Ref.2 | Proliferation | Cytokine Production |
Cytotoxicity | Ref.2 | Ref.2 | ||
| Changes in Energy Balance | |||||||||||
| Physical Activity | + + | + |
35; 105-107 |
+ + |
59-66; 73;74 |
+/− | +/− | ND |
59;64;75;79; 80;82;84; 86-89;91- 93;100;101 |
ND | |
| Calorie Restriction2 | ND | ND | (−) |
177- 179 |
+/− | ND | ND | 173;176 | ND | ||
| Obesity | (−) | (−) |
10;11;35; 144-147; 180-193; 195-222 |
+/− |
165- 167 |
(−) | +/− | ND |
149-158; 166;171-173 |
ND | |
| Impact of Nutrients | |||||||||||
| Retinoids and Carotenoids | |||||||||||
| Retinoid Analogs3 | + + | + |
223; 237-240 |
ND | + | ND | ND | 267 | ND | ||
| Synthetic Rexinoids4 | ND | + |
249; 260-263 |
ND | ND | ND | ND | ND | |||
| Tocopherols and Tocotrienols | |||||||||||
| Alpha-Tocopherol | +/− | ND |
231; 271-273 |
+ | 288 | + | + | + |
282;283; 287;288 |
ND | |
| Vitamin E Succinate (VES) |
ND | ND | ND | ND | ND | ND | ND | ||||
Changes in activities outlined in this table are designated with the following symbols: (−) = negative effect, +/− = inconsistent results, + = mild/moderate stimulatory effect, + + = strong stimulatory effect, ND = No data available
Ref. = References
Only studies utilizing adult onset calorie restriction are reviewed in this table
Retinoid Analogs (all-trans-, 9-cis-, and 13-cis-retinoic acid)
Synthetic Rexinoids (Bexarotene (LGD1069) and LG100268).
Figure 1.
Nutrient metabolism in the tumor microenvironment contributes to immunosuppression and tumor escape from immune surveillance. A) Tumor-derived cytokines and others soluble factors such as interleukin-10 (IL-10), transforming growth factor-beta (TGF-beta), vascular endothelial growth factor (VEGF), prostaglandin E2 (PGE2), and colony stimulating factor (CSF) promote the expansion of immunosuppressive cell populations such as myeloid-derived suppressor cells (MDSCs), tumor-associated macrophages (TAMs), and tolerogenic dendritic cells (DCs). B) Both tumor cells and immunosuppressive cell types can inhibit CD4+ and CD8+ effector T cells through the: 1) release of TGF-beta and IL-10, and 2) activation of CD4+/CD25+/FoxP3+ regulatory T (TREG) cells. C) The enzymatic activity of indoleamine 2,3-dioxygenase (IDO), inducible nitric oxide synthase (iNOS), arginase I (ARG), and cyclooxygenase-2 (COX-2) found in tumor cells and tolerogenic cells contributes to effector T cell inactivation by depleting local concentrations of nutrients such as tryptophan, arginine, and producing the immunosuppressive factor, PGE2. D) Hypoxia generated in rapidly growing tumors further induces a tolerogenic state in the tumor microenvironment in a HIF-1-dependent manner by depleting glucose concentrations through increased uptake and metabolism, lowering the pH through lactate production, depleting arginine concentrations through upregulation of iNOS, and increasing the production of PGE2 through COX-2 induction, and increased angiogenesis through transcriptional activation of VEGF expression.
5.2. Arachidonic acid metabolism
Arachidonic acid is a 20-carbon polyunsaturated fatty acid hydrolyzed from membrane phospholipids by the enzyme phospholipase A2 (298). The cyclooxygenase-2 enzyme, COX-2, is the rate-limiting step in the metabolism of arachidonic acid to the prostaglandins (PGE2, PGF2α, PGD2, PGI2) and the thromboxanes (TXA2) (298). Overexpression of COX-2 activity has been reported in preneoplastic lesions as well as gastric cancers and carcinomas of the lung, colon, and breast (299). The synthesis of PGE2 contributes to tumor promotion and progression through its effects on apoptosis, inflammation, angiogenesis, tumor invasiveness, and immunosuppression (298). Tumor or macrophage-derived PGE2 production induces immunosuppression by impairing B and T cell proliferation, inhibiting NK cell cytotoxicity, and inducing FoxP3 expression and regulatory T cell (Treg) function in naïve CD4+CD25− cells (298;300;301). Additionally, PGE2 inhibits the production of TNF-alpha while promoting the expression of the immunosuppressive cytokine, IL-10 (302). Clinically, COX-2 and PGE2 expression were correlated with reduced Th1 and increased Th2 serum cytokine levels and impaired DC and T cell function in breast cancer patients (303). Thus, selective COX-2 inhibition is reported to reverse tumor immunosuppression by reducing intratumoral CD4+CD25+FoxP3+ Treg cells, increasing the production of IFN-gamma by tumor infiltrating lymphocytes (TILs), and normalizing the balance between Th1 (IL-12) and Th2 (IL-10) cytokines (297;298;301;304).
Stolina and colleagues (304) were the first to report that COX-2 inhibition could promote anti-tumor immunity. In this study of Lewis lung carcinoma, the selective COX-2 inhibitor, SC-58236, led to a reduction in IL-10, an increase in IL-12 production by APCs, significant tumor growth inhibition, and prolonged survival (304). Subsequent animal studies demonstrated that selective COX-2 inhibition significantly improves the efficacy of anti-tumor vaccines to inhibit tumor growth and promote survival in murine models of mesothelioma (305), breast (306;307), lung (301;306), and familial adenomatous polyposis (FAP) (308). Due to the safety concerns regarding the selective COX-2 inhibitors (309-311), efforts are now being made to develop small molecule inhibitors that target the prostaglandin receptors (EP1-4) through which PGE2 is known to induce cell signaling such as the induction of FoxP3 expression in Treg cells (297). Thus, the available evidence provides ample rationale for combining strategies for targeted inhibition of arachidonic acid metabolism such as COX-2 inhibition or PGE2 receptor antagonism with antigen-directed cancer immunotherapy.
5.3. Arginine metabolism
L-arginine is a conditionally essential amino acid and its local catabolism by tumor cells, tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs) is reported to modulate tumorigenesis and immune function (297;312;313). The catabolism of arginine is catalyzed by two classes of enzymes: 1) arginase (ARG) encoded by the ARG1 and ARG2 genes, and 2) nitric-oxide synthase (NOS) encoded by the NOS1 (neuronal or nNOS), NOS2 (inducible or iNOS), and NOS3 (endothelial or eNOS) (297;313). As a component of the urea cycle, the ARG1 isoform is highly expressed in the liver where it catalyzes the conversion of L-arginine to L-ornithine and urea (297). The enzyme ornithine decarboxylase (ODC) acts on L-ornithine to initiate the first step in the biosynthesis of the polyamines (putrescine, spermidine, and spermine), a class of organic by-products of L-arginine catabolism known to act as endogenous tumor promoters (314). The intracellular accumulation of polyamines have been reported to be a consequence of elevated ARG and ODC-mediated arginine catabolism in tumor cells (312;315). Consistent with these findings, elevated ARG activity has been reported in patients with non-small cell lung cancer (NSCLC), skin, colorectal, breast, and prostate cancer (313). However, other studies suggest that MDSCs and TAMs are the more significant source of tumor-associated arginine catabolism (316-319).
Myeloid-derived suppressor cells, expressing ARG and/or NOS activity, induce arginine catabolism in the tumor microenvironment and contribute to tumorigenesis and immunosuppression by several mechanisms (Figure 1). First, MDSC- or TAM-derived ARG1 activity has been shown to inhibit the function of adjacent effector T cells by down-regulating the expression of the T cell receptor (TCR) CD3 zeta chain (313;318;320). Second, Chang and colleagues (316) demonstrated that murine macrophages overexpressing ARG1 promote the proliferation of ZR-75-1 breast cancer cells through the conversion of L-arginine to L-ornithine and the subsequent activation of polyamine biosynthesis. This proliferative effect was inhibited by the arginase inhibitor, L-norvaline (316). These observations gain support from findings in patients with metastatic renal cell carcinoma (RCC) in which the arginase activity of peripheral blood mononuclear cells (PBMCs) was significantly higher while TCR CD3 zeta chain expression, IL-2, and IFN-gamma production were significantly lower in RCC patients compared to age- and gender-matched healthy controls (321). Depletion of the CD11b+/CD14− subpopulation from RCC samples restored T cell proliferative capacity, IL-2 and IFN-gamma production, and TCR CD3 zeta chain expression suggesting a role for MDSC-derived ARG1 activity in local immunosuppression (321). Third, the catabolism of L-arginine via the iNOS pathway results in the production of nitric oxide (NO) and L-citrulline. The NO produced by MDSCs abrogates the effector function of adjacent T cells by inhibiting IL-2 receptor signaling and IL-2 production (reviewed by (313)). Finally, activation of both the ARG1 and iNOS enzymes synergize to induce effector T cell apoptosis through the previously described mechanisms and the production of cytotoxic peroxynitrites (313;317;319).
De Santo and colleagues (322) demonstrated that T cell proliferation and cytotoxicity were inhibited by CD11b+ MDSCs expressing both ARG and NOS. In this study, an inhibitor of ARG and NOS, nitroaspirin (NCX-4016), was shown to reverse the effects of MDSC-mediated immunosuppression. In tumor-bearing mice, the combination of NCX-4016 and DNA-based vaccination increased the number of tumor-specific CTLs and significantly extended survival (322). These findings suggest a role for combining pharmacological inhibitors of L-arginine catabolism with cancer immunotherapy. As with celebrex, NCX-4016 targets multiple immunosuppressive pathways by inhibiting COX-1, COX-2, ARG1, and iNOS activities in the absence of the gastrointestinal toxicities observed with traditional aspirin.
5.4. Tryptophan metabolism
Local concentrations of the essential amino acid tryptophan can be depleted through the activity of the cytosolic enzyme indoleamine 2,3-dioxygenase (IDO) which catalyzes the conversion of tryptophan to its major metabolites, known collectively as the kynurenines (297). Munn and colleagues were the first to show that IDO-mediated typtophan catabolism induced peripheral immune tolerance in pregnant mice carrying allogeneic concepti (323). A specific, pharmacologic inhibitor of IDO, 1-methyl-tryptophan (1-MT), was shown to reverse maternal tolerance in this model (323). As illustrated in Figure 1, localized immunosuppression leading to tumorigenesis and immune escape is hypothesized to be the result of cumulative tryptophan catabolism by IDO-positive cell types in the tumor microenvironment including tumor cells, stroma, tolerogenic DCs, MDSCs, and TAMs (297).
Mechanistic studies suggest that IDO-mediated tryptophan catabolism regulates adaptive T cell immunity (324). Fallarino and colleagues (325) demonstrated that the absence of tryptophan and the presence of the immunotoxic kynurenines can inhibit the cytotoxic effector function of murine CD8+ T cells through down-regulation of the TCR CD3 zeta chain. Prolonged tryptophan deprivation and exposure to kynurenine metabolites, or co-incubation with IDO-expressing DCs induces a TGF-beta dependent regulatory phenotype in naïve CD4+CD25− T cells (326). Antigen-specific anergy is thought to be mediated by IDO-expressing tolerogenic DCs which have been found to accumulate in the tumor draining lymph nodes of both mice (327) and humans (297). The cytotoxic T lymphocyte antigen-4 (CTLA-4) on Tregs engages B7 receptor molecules on tolerogenic DCs to promote the release of IFN-gamma which acts in an autocrine feedback loop to activate IDO expression in tolerizing DCs (297). Immunosuppression in the tumor microenvironment is maintained when IDO-positive DCs promote Treg expansion, differentiation, and local production of the immunosuppressive cytokines, IL-10 and TGF-beta (297).
A large number of human tumors are reported to constitutively express IDO including but not limited to prostatic, colorectal, pancreatic, cervical, and ovarian carcinomas (327) and the level of IDO expression is negatively correlated with survival in colorectal (328) and ovarian (329) cancer patients. The serum ratio of kynurenine to tryptophan, an indirect marker of IDO activity, has been shown to increase with age (330) and predict all-cause mortality in nonagenarians (331). Murine MC57G fibrosarcoma cells transfected with IDO inhibit CD8+ T cell function in vitro (332) and IDO-transfection of P815 mastocytoma cells prevents the rejection of P815 tumors in mice vaccinated against the P1A tumor associated antigen (327). The IDO inhibitor, 1-MT, inhibits the growth of Lewis lung carcinomas (LLC) by antagonizing the immunosuppressive effects of IDO-positive mononuclear cells in LLC tumors and the tumor-draining lymph nodes (333). Recently, tumor-induced COX-2 activity was shown to positively regulate IDO expression in a murine model of mammary carcinogenesis, PyV MT mice (334). The combination of celecoxib plus a DC-based vaccine improved IFN-gamma production and cytotoxicity of CD8+ T cells from the tumor-draining lymph nodes, slowed the growth of mammary tumors, prevented lung metastases, and improved survival (334). These data suggest a role for the pharmacologic management of immunosuppression with selective inhibitors of inflammation (e.g., COX-2 with celecoxib) and/or tryptophan metabolism (e.g., IDO with 1-MT) in combination cancer immunotherapy.
5.5. Glucose metabolism
In an experiment comparing the gene expression profiles of naïve and primed CD8+ effector CTLs, the effector state was associated with a significant increase in the expression of genes encoding glycolytic enzymes such as α-enolase, pyruvate kinase, triosephosphate isomerase, hexokinase II, and 6-phosphofructokinase type C (335). Consistent with these findings, effector CD8+ T cells display greater glucose uptake, a higher glycolytic rate, and increased lactate production than naïve cells (336). In this study, glucose deprivation was shown to inhibit IFN-gamma gene expression suggesting that CD8+ effector T cell function is uniquely sensitive to glucose availability (337). Despite the greater glucose uptake and utilization by effector versus naïve T cells, tumor cells are reported to take up and metabolize 10 to 100 fold more glucose per unit of time (336).
Enhanced glucose metabolism and angiogenesis are hallmarks of the cellular response to hypoxia in cancer. The central regulator of this response is the transcription factor hypoxia inducible factor 1 (HIF-1) which functions as a heterodimer between HIF-1-alpha and HIF-1-beta (338). Under normoxic conditions, intracellular HIF-1-alpha concentrations are regulated through interaction with the von Hippel-Lindau (VHL) tumor suppressor protein which induces ubiquitination and proteosomal degradation of HIF-1-alpha (339). Under hypoxic conditions (Figure 1), HIF-1-alpha is stabilized, heterodimerizes with HIF-1-beta, and binds to hypoxia response elements in the promoter regions or enhancer elements of genes involved in glucose metabolism (glucose transporters and glycolytic enzymes), angiogenesis (VEGF), inflammation (COX-2), and arginine metabolism (iNOS) (340-345). Similarly, under normoxic conditions in VHL-deficient renal cell carcinomas, HIF-1-alpha is stabilized (339) and glucose uptake is increased (346). Thus, tumors adapt to hypoxia by shunting ATP production away from the tricarboxylic acid (TCA) cycle and oxidative phosphorylation in favor of glycolysis (342). The microenvironments of many solid tumors are reported to be acidic (347) and the greater reliance on glycolysis contributes to this acidification by increasing the export of lactic acid into the tumor microenvironment (348). An acidic microenvironment may also contribute to metastasis by increasing the expression and activity of extracellular matrix-degrading proteases such as the collagenases (349).
Collectively, these findings suggest that hypoxia in the developing tumor acts as a master regulator of glucose metabolism, inflammation, angiogenesis, and metastasis. As such, HIF-1-alpha-directed immunotherapy offers the specificity of targeting hypoxic or VHL-deficient tissues and the opportunity to modulate glucose uptake and metabolism, neovascularization of the tumor, and the immunosuppressive effects of PGE2 production and arginine depletion (Figure 1). This strategy is plausible given that HIF-1-alpha antisense gene transfer was shown to significantly enhance B7-1-mediated immunotherapy of EL-4 tumors in preclinical studies (350). Alternative immunotherapeutic strategies have targeted HIF-1-alpha-regulated gene products such as carbonic anhydrase IX, involved in pH control (351). Another strategy may lie in the use of NSAIDs such as ibuprofen which have been shown to upregulate the VHL protein leading to down-regulation of HIF-1-alpha, the Glut-1 transporter, VEGF, and the VEGF receptor, Flt-1 (352;353).
6. FUTURE RESEARCH DIRECTIONS
Reduction of obesity is an important health issue; however, additional preclinical and clinical work is needed to determine the effects of weight loss either through CR or exercise on immune function before any recommendations can be made about initiating weight loss prior to receiving a cancer vaccine. Modulation of energy balance through exercise is the most promising energy balance related intervention to partner with therapeutic cancer vaccines. Exercise interventions are safe, have high compliance levels and have significant positive effects on clinical outcomes in cancer patients. Exercise training has also been shown to have stimulatory effects on NK cell function, and antigen-specific T cell proliferation and cytokine production. These data suggest that combining exercise interventions with therapeutic cancer vaccine strategies may enhance vaccine efficacy and improve clinical outcomes in cancer patients. Future studies should focus on exploring the effects of combining physical activity with known cancer vaccine platforms to determine the dose, duration and frequency of exercise needed to achieve the maximum stimulatory effects of exercise on anti-tumor immune function.
Synthetic analogs of vitamins A and E are shown to have significant chemopreventive and immunomodulatory activity. Preliminary data suggests that vesiculated vitamin E succinate may synergize with cancer immunotherapy while the RXR-directed rexinoids offer intriguing potential in this regard. A robust and growing body of literature suggests that the pharmacologic manipulation of nutrient metabolism in the tumor microenvironment may offset immunosuppressive mechanisms activated by the growing tumor and tolerogenic cell populations induced by tumor-derived cytokines. Several promising intervention strategies for translational development in combination cancer immunotherapy include COX-2 inhibition with celebrex or PGE2 receptor antagonism, the use of NO-aspirin (NCX-4016), and the IDO inhibitor, 1-MT. Finally, the diverse repertoire of metabolic, immunosuppressive, and angiogenic mechanisms induced by tumor hypoxia make HIF-1α-directed immunotherapy a potential target for future vaccine development.
In summary, changes in energy balance via exercise, supplementation with specific nutrients (e.g., synthetic analogs of vitamins A and E) and modulation of nutrient metabolism in the tumor microenvironment all are viable candidates for further study in combination with cancer vaccines.
7. ACKNOWLEDGMENTS
Kenneth W. Hance and Connie J. Rogers contributed equally in the preparation of this manuscript. The authors gratefully acknowledge the National Cancer Institute Cancer Prevention Fellowship Program.
8. REFERENCES
- 1.Padzur R, Coia LR, Hoskins WJ, Wagman LD. Cancer managment: a multidisciplinary approach. PRR; New York: 2000. [Google Scholar]
- 2.Vermorken JB, Claessen AM, van Tinteren H, Gall HE, Ezinga R, Meijer S, Scheper RJ, Meijer CJ, Bloemena E, Ransom JH, Hanna MG, Jr., Pinedo HM. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 3.Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosenbach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arlen PM, Pazdur M, Skarupa L, Rauckhorst M, Gulley JL. A randomized phase II study of docetaxel alone or in combination with PANVAC-V (vaccinia) and PANVAC-F (fowlpox) in patients with metastatic breast cancer (NCI 05-C-0229) Clin Breast Cancer. 2006;7:176–179. doi: 10.3816/CBC.2006.n.032. [DOI] [PubMed] [Google Scholar]
- 5.McNeel DG. Cellular immunotherapies for prostate cancer. Biomed Pharmacother. 2007 doi: 10.1016/j.biopha.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Friedenreich CM. Physical activity and cancer prevention: from observational to intervention research. Cancer Epidemiol Biomarkers Prev. 2001;10:287–301. [PubMed] [Google Scholar]
- 7.Hoffman-Goetz L. Physical activity and cancer prevention: animal-tumor models. Med Sci Sports Exerc. 2003;35:1828–1833. doi: 10.1249/01.MSS.0000093621.09328.70. [DOI] [PubMed] [Google Scholar]
- 8.Hursting SD, Nunez NP, Patel AC, Perkins SN, Lubet RA, Barrett JC. The utility of genetically altered mouse models for nutrition and cancer chemoprevention research. Mutat Res. 2005;576:80–92. doi: 10.1016/j.mrfmmm.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Hursting SD, Kari FW. The anti-carcinogenic effects of dietary restriction: mechanisms and future directions. Mutat Res. 1999;443:235–249. doi: 10.1016/s1383-5742(99)00021-6. [DOI] [PubMed] [Google Scholar]
- 10.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 11.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 12.Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/- mice: ectopic expression of the agouti gene. Faseb J. 1994;8:479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 13.Hirose Y, Hata K, Kuno T, Yoshida K, Sakata K, Yamada Y, Tanaka T, Reddy BS, Mori H. Enhancement of development of azoxymethane-induced colonic premalignant lesions in C57BL/KsJ-db/db mice. Carcinogenesis. 2004;25:821–825. doi: 10.1093/carcin/bgh059. [DOI] [PubMed] [Google Scholar]
- 14.Pendas AM, Folgueras AR, Llano E, Caterina J, Frerard F, Rodriguez F, Astudillo A, Noel A, Birkedal-Hansen H, Lopez-Otin C. Diet-induced obesity and reduced skin cancer susceptibility in matrix metalloproteinase 19-deficient mice. Mol Cell Biol. 2004;24:5304–5313. doi: 10.1128/MCB.24.12.5304-5313.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cleary MP, Grande JP, Juneja SC, Maihle NJ. Diet-induced obesity and mammary tumor development in MMTV-neu female mice. Nutr Cancer. 2004;50:174–180. doi: 10.1207/s15327914nc5002_7. [DOI] [PubMed] [Google Scholar]
- 16.Hance KW, Rogers CJ, Zaharoff DA, Perkins SN, Schlom J, Hursting SD, Greiner JW. Diet-Induced obesity promotes pancreatic tumor growth in mice; AACR Frontiers in Cancer Prevention Meeting; 2006. [Google Scholar]
- 17.Yakar S, Nunez NP, Pennisi P, Brodt P, Sun H, Fallavollita L, Zhao H, Scavo L, Novosyadlyy R, Kurshan N, Stannard B, East-Palmer J, Smith NC, Perkins SN, Fuchs-Young R, Barrett JC, Hursting SD, LeRoith D. Increased tumor growth in mice with diet-induced obesity: impact of ovarian hormones. Endocrinology. 2006;147:5826–5834. doi: 10.1210/en.2006-0311. [DOI] [PubMed] [Google Scholar]
- 18.Kune G, Watson L. Colorectal Cancer Protective Effects and the Dietary Micronutrients Folate, Methionine, Vitamins B6, B12, C, E, Selenium, and Lycopene. Nutrition and Cancer. 2006;56:11–21. doi: 10.1207/s15327914nc5601_3. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective Study of Predictors of Vitamin D Status and Cancer Incidence and Mortality in Men. J.Natl.Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 20.Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, Newmark HL, Giovannucci E, Wei M, Holick MF. Optimal Vitamin D Status for Colorectal Cancer Prevention: A Quantitative Meta Analysis. American Journal of Preventive Medicine. 2007;32:210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Plummer M, Vivas J, Lopez G, Bravo JC, Peraza S, Carillo E, Cano E, Castro D, Andrade O, Sanchez V, Garcia R, Buiatti E, Aebischer C, Franceschi S, Oliver W, Munoz N. Chemoprevention of precancerous gastric lesions with antioxidant vitamin supplementation: a randomized trial in a high-risk population. J Natl Cancer Inst. 2007;99:137–146. doi: 10.1093/jnci/djk017. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald P, Anderson D, Nelson SA, Taylor PR. Clinical trials of vitamin and mineral supplements for cancer prevention. Am J Clin Nutr. 2007;85:314S–317S. doi: 10.1093/ajcn/85.1.314S. [DOI] [PubMed] [Google Scholar]
- 23.Singh DK, Lippman SM. Cancer chemoprevention. Part 1: Retinoids and carotenoids and other classic antioxidants. Oncology (Williston Park) 1998;12:1643–1648. [PubMed] [Google Scholar]
- 24.Singh DK, Lippman SM. Cancer chemoprevention. Part 2: Hormones, nonclassic antioxidant natural agents, NSAIDs, and other agents. Oncology (Williston Park) 1998;12:1787–1800. [PubMed] [Google Scholar]
- 25.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Rundle A. Molecular epidemiology of physical activity and cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:227–236. [PubMed] [Google Scholar]
- 28.Hursting SD, Rogers CJ, Mahabir S, Nunez NP, Barrett JC, Perkins SN, Forman MR. Energy Balance and Cancer. Academic Press; Amsterdam: 2006. [Google Scholar]
- 29.Rogers CJ, Berrigan D, Colbert LH, Greiner JW, Perkins SD, Hursting SD. Mechanisms Linking Physical Activity and Cancer Prevention. Sports Medicine. 2007 doi: 10.2165/00007256-200838040-00002. (submitted) [DOI] [PubMed] [Google Scholar]
- 30.Ries LAG, Harkins D, Krapcho M, Mariotto A, Miller BA, Feuer EJ, Clegg L, Eisner MP, Horner MJ, Howlader N, Hayat M, Hankey BF, Edwards BK. SEER Cancer Statistics Review, 1975-2003. based on November. National Cancer Institute; Bethesda, MD: 2006. [Google Scholar]
- 31.Hirokawa K. Understanding the mechanism of the age-related decline in immune function. Nutr Rev. 1992;50:361–366. doi: 10.1111/j.1753-4887.1992.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 32.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273:70–74. doi: 10.1126/science.273.5271.70. [DOI] [PubMed] [Google Scholar]
- 33.Miller RA. The aging immune system: subsets, signals, and survival. Aging (Milano) 1997;9:23–24. doi: 10.1007/BF03339690. [DOI] [PubMed] [Google Scholar]
- 34.Gruver AL, Hudson LL, Sempowski GD. Immunosenescence of ageing. J Pathol. 2007;211:144–156. doi: 10.1002/path.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IARC . Weight control and physical activity. International Agency for Research on Cancer; 2002. [Google Scholar]
- 36.Andrianopoulos G, Nelson RL, Bombeck CT, Souza G. The influence of physical activity in 1,2 dimethylhydrazine induced colon carcinogenesis in the rat. Anticancer Res. 1987;7:849–852. [PubMed] [Google Scholar]
- 37.Reddy BS, Sugie S, Lowenfels A. Effect of voluntary exercise on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 1988;48:7079–7081. [PubMed] [Google Scholar]
- 38.Thorling EB, Jacobsen NO, Overvad K. Effect of exercise on intestinal tumour development in the male Fischer rat after exposure to azoxymethane. Eur J Cancer Prev. 1993;2:77–82. doi: 10.1097/00008469-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Thorling EB, Jacobsen NO, Overvad K. The effect of treadmill exercise on azoxymethane-induced intestinal neoplasia in the male Fischer rat on two different high-fat diets. Nutr Cancer. 1994;22:31–41. doi: 10.1080/01635589409514329. [DOI] [PubMed] [Google Scholar]
- 40.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 41.Colbert LH, Davis JM, Essig DA, Ghaffar A, Mayer EP. Exercise and tumor development in a mouse predisposed to multiple intestinal adenomas. Med Sci Sports Exerc. 2000;32:1704–1708. doi: 10.1097/00005768-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Colbert LH, Mai V, Perkins SN, Berrigan D, Lavigne JA, Wimbrow HH, Alvord WG, Haines DC, Srinivas P, Hursting SD. Exercise and intestinal polyp development in APCMin mice. Med Sci Sports Exerc. 2003;35:1662–1669. doi: 10.1249/01.MSS.0000089349.54813.41. [DOI] [PubMed] [Google Scholar]
- 43.Mehl KA, Davis JM, Clements JM, Berger FG, Pena MM, Carson JA. Decreased intestinal polyp multiplicity is related to exercise mode and gender in ApcMin/+ mice. J Appl Physiol. 2005;98:2219–2225. doi: 10.1152/japplphysiol.00975.2004. [DOI] [PubMed] [Google Scholar]
- 44.Colbert LH, Mai V, Tooze JA, Perkins SN, Berrigan D, Hursting SD. Negative energy balance induced by voluntary wheel running inhibits polyp development in APCMin mice. Carcinogenesis. 2006;27:2103–2107. doi: 10.1093/carcin/bgl056. [DOI] [PubMed] [Google Scholar]
- 45.Cohen LA, Choi KW, Wang CX. Influence of dietary fat, caloric restriction, and voluntary exercise on N-nitrosomethylurea-induced mammary tumorigenesis in rats. Cancer Res. 1988;48:4276–4283. [PubMed] [Google Scholar]
- 46.Cohen LA, Choi K, Backlund JY, Harris R, Wang CX. Modulation of N-nitrosomethylurea induced mammary tumorigenesis by dietary fat and voluntary exercise. In vivo. 1991;5:333–344. [PubMed] [Google Scholar]
- 47.Cohen LA, Boylan E, Epstein M, Zang E. Voluntary exercise and experimental mammary cancer. Adv Exp Med Biol. 1992;322:41–59. doi: 10.1007/978-1-4684-7953-9_5. [DOI] [PubMed] [Google Scholar]
- 48.Cohen LA, Kendall ME, Meschter C, Epstein MA, Reinhardt J, Zang E. Inhibition of rat mammary tumorigenesis by voluntary exercise. In vivo. 1993;7:151–158. [PubMed] [Google Scholar]
- 49.Westerlind KC, McCarty HL, Schultheiss PC, Story R, Reed AH, Baier ML, Strange R. Moderate exercise training slows mammary tumour growth in adolescent rats. Eur J Cancer Prev. 2003;12:281–287. doi: 10.1097/00008469-200308000-00007. [DOI] [PubMed] [Google Scholar]
- 50.Thompson HJ, Westerlind KC, Snedden J, Briggs S, Singh M. Exercise intensity dependent inhibition of 1-methyl-1-nitrosourea induced mammary carcinogenesis in female F-344 rats. Carcinogenesis. 1995;16:1783–1786. doi: 10.1093/carcin/16.8.1783. [DOI] [PubMed] [Google Scholar]
- 51.Thompson HJ, Westerlind KC, Snedden JR, Briggs S, Singh M. Inhibition of mammary carcinogenesis by treadmill exercise. J Natl Cancer Inst. 1995;87:453–455. doi: 10.1093/jnci/87.6.453. [DOI] [PubMed] [Google Scholar]
- 52.Whittal KS, Parkhouse WS. Exercise during adolescence and its effects on mammary gland development, proliferation, and nitrosomethylurea (NMU) induced tumorigenesis in rats. Breast Cancer Res Treat. 1996;37:21–27. doi: 10.1007/BF01806628. [DOI] [PubMed] [Google Scholar]
- 53.Roebuck BD, McCaffrey J, Baumgartner KJ. Protective effects of voluntary exercise during the postinitiation phase of pancreatic carcinogenesis in the rat. Cancer Res. 1990;50:6811–6816. [PubMed] [Google Scholar]
- 54.Giles TC, Roebuck BD. Effects of voluntary exercise and/or food restriction on pancreatic tumorigenesis in male rats. Adv Exp Med Biol. 1992;322:17–27. doi: 10.1007/978-1-4684-7953-9_3. [DOI] [PubMed] [Google Scholar]
- 55.Baracos VE. Exercise inhibits progressive growth of the Morris hepatoma 7777 in male and female rats. Can J Physiol Pharmacol. 1989;67:864–870. doi: 10.1139/y89-135. [DOI] [PubMed] [Google Scholar]
- 56.Sugie S, Reddy BS, Lowenfels A, Tanaka T, Mori H. Effect of voluntary exercise on azoxymethane-induced hepatocarcinogenesis in male F344 rats. Cancer Lett. 1992;63:67–72. doi: 10.1016/0304-3835(92)90091-9. [DOI] [PubMed] [Google Scholar]
- 57.Ikuyama T, Watanabe T, Minegishi Y, Osanai H. Effect of voluntary exercise on 3′-methyl-4-dimethylaminoazobenzene-induced hepatomas in male Jc1:Wistar rats. Proc Soc Exp Biol Med. 1993;204:211–215. doi: 10.3181/00379727-204-43655. [DOI] [PubMed] [Google Scholar]
- 58.Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol. 2004;96:2249–2256. doi: 10.1152/japplphysiol.01210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woods JA, Davis JM, Smith JA, Nieman DC. Exercise and cellular innate immune function. Med Sci Sports Exerc. 1999;31:57–66. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen BK, Tvede N, Christensen LD, Klarlund K, Kragbak S, Halkjr-Kristensen J. Natural killer cell activity in peripheral blood of highly trained and untrained persons. Int J Sports Med. 1989;10:129–131. doi: 10.1055/s-2007-1024888. [DOI] [PubMed] [Google Scholar]
- 61.Nieman DC, Cook VD, Henson DA, Suttles J, Rejeski WJ, Ribisl PM, Fagoaga OR, Nehlsen-Cannarella SL. Moderate exercise training and natural killer cell cytotoxic activity in breast cancer patients. Int J Sports Med. 1995;16:334–337. doi: 10.1055/s-2007-973015. [DOI] [PubMed] [Google Scholar]
- 62.Crist DM, Mackinnon LT, Thompson RF, Atterbom HA, Egan PA, Peake GT, Sibbitt WL, Jr., Kraner JC. Physical exercise increases natural cellular-mediated tumor cytotoxicity in elderly women. Gerontology. 1989;35:66–71. doi: 10.1159/000213001. [DOI] [PubMed] [Google Scholar]
- 63.Nieman DC, Nehlsen-Cannarella SL, Markoff PA, Balk-Lamberton AJ, Yang H, Chritton DB, Lee JW, Arabatzis K. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int J Sports Med. 1990;11:467–473. doi: 10.1055/s-2007-1024839. [DOI] [PubMed] [Google Scholar]
- 64.Nieman DC, Henson DA, Gusewitch G, Warren BJ, Dotson RC, Butterworth DE, Nehlsen-Cannarella SL. Physical activity and immune function in elderly women. Med Sci Sports Exerc. 1993;25:823–831. doi: 10.1249/00005768-199307000-00011. [DOI] [PubMed] [Google Scholar]
- 65.Yan H, Kuroiwa A, Tanaka H, Shindo M, Kiyonaga A, Nagayama A. Effect of moderate exercise on immune senescence in men. Eur J Appl Physiol. 2001;86:105–111. doi: 10.1007/s004210100521. [DOI] [PubMed] [Google Scholar]
- 66.DiPenta JM, Johnson JG, Murphy RJ. Natural killer cells and exercise training in the elderly: a review. Can J Appl Physiol. 2004;29:419–443. doi: 10.1139/h04-027. [DOI] [PubMed] [Google Scholar]
- 67.MacNeil B, Hoffman-Goetz L. Effect of exercise on natural cytotoxicity and pulmonary tumor metastases in mice. Med Sci Sports Exerc. 1993;25:922–928. [PubMed] [Google Scholar]
- 68.Hoffman-Goetz L. Exercise, natural immunity, and tumor metastasis. Med Sci Sports Exerc. 1994;26:157–163. doi: 10.1249/00005768-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 69.Hoffman-Goetz L, May KM, Arumugam Y. Exercise training and mouse mammary tumour metastasis. Anticancer Res. 1994;14:2627–2631. [PubMed] [Google Scholar]
- 70.Jonsdottir IH, Johansson C, Asea A, Johansson P, Hellstrand K, Thoren P, Hoffmann P. Duration and mechanisms of the increased natural cytotoxicity seen after chronic voluntary exercise in rats. Acta Physiol Scand. 1997;160:333–339. doi: 10.1046/j.1365-201X.1997.00185.x. [DOI] [PubMed] [Google Scholar]
- 71.Jonsdottir IH, Hellstrand K, Thoren P, Hoffmann P. Enhancement of natural immunity seen after voluntary exercise in rats. Role of central opioid receptors. Life Sci. 2000;66:1231–1239. doi: 10.1016/s0024-3205(00)00428-8. [DOI] [PubMed] [Google Scholar]
- 72.Rogers CJ, Hance KW, Zaharoff DA, Perkins SN, Hursting SD, Schlom J, Greiner JW. Minimal duration of voluntary exercise training necessary to enhance innate and antigen-specific immune responses. AACR Annual Meeting 2582.2007. [Google Scholar]
- 73.Peters C, Lotzerich H, Niemeier B, Schule K, Uhlenbruck G. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer Res. 1994;14:1033–1036. [PubMed] [Google Scholar]
- 74.Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol. 2005;98:1534–1540. doi: 10.1152/japplphysiol.00566.2004. [DOI] [PubMed] [Google Scholar]
- 75.Watson RR, Moriguchi S, Jackson JC, Werner L, Wilmore JH, Freund BJ. Modification of cellular immune functions in humans by endurance exercise training during beta-adrenergic blockade with atenolol or propranolol. Med Sci Sports Exerc. 1986;18:95–100. [PubMed] [Google Scholar]
- 76.Hoffman-Goetz L, Thorne RJ, Houston ME. Splenic immune responses following treadmill exercise in mice. Can J Physiol Pharmacol. 1988;66:1415–1419. doi: 10.1139/y88-230. [DOI] [PubMed] [Google Scholar]
- 77.Tharp GD, Preuss TL. Mitogenic response of T-lymphocytes to exercise training and stress. J Appl Physiol. 1991;70:2535–2538. doi: 10.1152/jappl.1991.70.6.2535. [DOI] [PubMed] [Google Scholar]
- 78.Coleman KJ, Rager DR. Effects of voluntary exercise on immune function in rats. Physiol Behav. 1993;54:771–774. doi: 10.1016/0031-9384(93)90090-3. [DOI] [PubMed] [Google Scholar]
- 79.Shinkai S, Kohno H, Kimura K, Komura T, Asai H, Inai R, Oka K, Kurokawa Y, Shephard R. Physical activity and immune senescence in men. Med Sci Sports Exerc. 1995;27:1516–1526. [PubMed] [Google Scholar]
- 80.Gueldner SH, Poon LW, La Via M, Virella G, Michel Y, Bramlett MH, Noble CA, Paulling E. Long-term exercise patterns and immune function in healthy older women. A report of preliminary findings. Mech Ageing Dev. 1997;93:215–222. doi: 10.1016/s0047-6374(96)01820-9. [DOI] [PubMed] [Google Scholar]
- 81.Dos Santos Cunha WD, Giampietro MV, De Souza DF, Vaisberg M, Seelaender MC, Rosa LF. Exercise restores immune cell function in energy-restricted rats. Med Sci Sports Exerc. 2004;36:2059–2064. doi: 10.1249/01.mss.0000147626.32295.38. [DOI] [PubMed] [Google Scholar]
- 82.Rogers CJ, Berrigan D, Patel AC, Zaharoff DA, Hance KW, Greiner JW, Hursting SD. Effects of exercise and calorie restriction on systemic and mucosal immune function in C57BL/6 mice. Cancer Epidemiol Biomarkers Prev. 2004;13:1926S–1927S. [Google Scholar]
- 83.Lin YS, Jan MS, Chen HI. The effect of chronic and acute exercise on immunity in rats. Int J Sports Med. 1993;14:86–92. doi: 10.1055/s-2007-1021151. [DOI] [PubMed] [Google Scholar]
- 84.Nieman DC, Nehlsen-Cannarella SL, Henson DA, Koch AJ, Butterworth DE, Fagoaga OR, Utter A. Immune response to exercise training and/or energy restriction in obese women. Med Sci Sports Exerc. 1998;30:679–686. doi: 10.1097/00005768-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 85.Peters BA, Sothmann M, Wehrenberg WB. Blood leukocyte and spleen lymphocyte immune responses in chronically physically active and sedentary hamsters. Life Sci. 1989;45:2239–2245. doi: 10.1016/0024-3205(89)90065-9. [DOI] [PubMed] [Google Scholar]
- 86.Nehlsen-Cannarella SL, Nieman DC, Balk-Lamberton AJ, Markoff PA, Chritton DB, Gusewitch G, Lee JW. The effects of moderate exercise training on immune response. Med Sci Sports Exerc. 1991;23:64–70. [PubMed] [Google Scholar]
- 87.Baslund B, Lyngberg K, Andersen V, Kristensen J. Halkjaer, Hansen M, Klokker M, Pedersen BK. Effect of 8 wk of bicycle training on the immune system of patients with rheumatoid arthritis. J Appl Physiol. 1993;75:1691–1695. doi: 10.1152/jappl.1993.75.4.1691. [DOI] [PubMed] [Google Scholar]
- 88.Mitchell JB, Paquet AJ, Pizza FX, Starling RD, Holtz RW, Grandjean PW. The effect of moderate aerobic training on lymphocyte proliferation. Int J Sports Med. 1996;17:384–389. doi: 10.1055/s-2007-972865. [DOI] [PubMed] [Google Scholar]
- 89.Rhind SG, Shek PN, Shinkai S, Shephard RJ. Effects of moderate endurance exercise and training on in vitro lymphocyte proliferation, interleukin-2 (IL-2) production, and IL-2 receptor expression. Eur J Appl Physiol Occup Physiol. 1996;74:348–360. doi: 10.1007/BF02226932. [DOI] [PubMed] [Google Scholar]
- 90.Jonsdottir IH, Asea A, Hoffmann P, Dahlgren UI, Andersson B, Hellstrand K, Thoren P. Voluntary chronic exercise augments in vivo natural immunity in rats. J Appl Physiol. 1996;80:1799–1803. doi: 10.1152/jappl.1996.80.5.1799. [DOI] [PubMed] [Google Scholar]
- 91.Shore S, Shinkai S, Rhind S, Shephard RJ. Immune responses to training: how critical is training volume? J Sports Med Phys Fitness. 1999;39:1–11. [PubMed] [Google Scholar]
- 92.Fahlman M, Boardley D, Flynn MG, Braun WA, Lambert CP, Bouillon LE. Effects of endurance training on selected parameters of immune function in elderly women. Gerontology. 2000;46:97–104. doi: 10.1159/000022142. [DOI] [PubMed] [Google Scholar]
- 93.Hayes SC, Rowbottom D, Davies PS, Parker TW, Bashford J. Immunological changes after cancer treatment and participation in an exercise program. Med Sci Sports Exerc. 2003;35:2–9. doi: 10.1097/00005768-200301000-00002. [DOI] [PubMed] [Google Scholar]
- 94.Bacuau RF, Belmonte MA, Seelaender MC, Rosa L. F. Costa. Effect of a moderate intensity exercise training protocol on the metabolism of macrophages and lymphocytes of tumour-bearing rats. Cell Biochem Funct. 2000;18:249–258. doi: 10.1002/1099-0844(200012)18:4<249::AID-CBF879>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 95.Shewchuk LD, Baracos VE, Field CJ. Dietary L-glutamine supplementation reduces the growth of the Morris Hepatoma 7777 in exercise-trained and sedentary rats. J Nutr. 1997;127:158–166. doi: 10.1093/jn/127.1.158. [DOI] [PubMed] [Google Scholar]
- 96.Hutnick NA, Williams NI, Kraemer WJ, Orsega-Smith E, Dixon RH, Bleznak AD, Mastro AM. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005;37:1827–1835. doi: 10.1249/01.mss.0000175857.84936.1a. [DOI] [PubMed] [Google Scholar]
- 97.Chubak J, McTiernan A, Sorensen B, Wener MH, Yasui Y, Velasquez M, Wood B, Rajan KB, Wetmore CM, Potter JD, Ulrich CM. Moderate-intensity exercise reduces the incidence of colds among postmenopausal women. Am J Med. 2006;119:937–942. doi: 10.1016/j.amjmed.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 98.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193:981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Woodland DL, Hogan RJ, Zhong W. Cellular immunity and memory to respiratory virus infections. Immunol Res. 2001;24:53–67. doi: 10.1385/IR:24:1:53. [DOI] [PubMed] [Google Scholar]
- 100.Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004;97:491–498. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- 101.Kohut ML, Cooper MM, Nickolaus MS, Russell DR, Cunnick JE. Exercise and psychosocial factors modulate immunity to influenza vaccine in elderly individuals. J Gerontol A Biol Sci Med Sci. 2002;57:M557–M562. doi: 10.1093/gerona/57.9.m557. [DOI] [PubMed] [Google Scholar]
- 102.Kohut ML, Boehm GW, Moynihan JA. Moderate exercise is associated with enhanced antigen-specific cytokine, but not IgM antibody production in aged mice. Mech Ageing Dev. 2001;122:1135–1150. doi: 10.1016/s0047-6374(01)00255-x. [DOI] [PubMed] [Google Scholar]
- 103.Kohut ML, Senchina DS. Reversing age-associated immunosenescence via exercise. Exerc Immunol Rev. 2004;10:6–41. [PubMed] [Google Scholar]
- 104.Rogers CJ, Hance KW, Zaharoff DA, Perkins SN, Schlom J, Hursting SD, Greiner JW. Exercise enhances antigen specific immune responses in both systemic and mucosal compartments. Cancer Epidemiol Biomarkers Prev. 2005;14(Part):2747S. [Google Scholar]
- 105.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–2486. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 106.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, Fuchs CS. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–3534. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 107.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, Thomas J, Nelson H, Whittom R, Hantel A, Schilsky RL, Fuchs CS. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–3541. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 108.McTiernan A, Ulrich C, Kumai C, Bean D, Schwartz R, Mahloch J, Hastings R, Gralow J, Potter JD. Anthropometric and hormone effects of an eight-week exercise-diet intervention in breast cancer patients: results of a pilot study. Cancer Epidemiol Biomarkers Prev. 1998;7:477–481. [PubMed] [Google Scholar]
- 109.Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. J Clin Oncol. 2003;21:1660–1668. doi: 10.1200/JCO.2003.04.093. [DOI] [PubMed] [Google Scholar]
- 110.Marshall JL, Hoyer RJ, Toomey MA, Faraguna K, Chang P, Richmond E, Pedicano JE, Gehan E, Peck RA, Arlen P, Tsang KY, Schlom J. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J Clin Oncol. 2000;18:3964–3973. doi: 10.1200/JCO.2000.18.23.3964. [DOI] [PubMed] [Google Scholar]
- 111.Zhu MZ, Marshall J, Cole D, Schlom J, Tsang KY. Specific cytolytic T-cell responses to human CEA from patients immunized with recombinant avipox-CEA vaccine. Clin Cancer Res. 2000;6:24–33. [PubMed] [Google Scholar]
- 112.von Mehren M, Arlen P, Gulley J, Rogatko A, Cooper HS, Meropol NJ, Alpaugh RK, Davey M, McLaughlin S, Beard MT, Tsang KY, Schlom J, Weiner LM. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–1191. [PubMed] [Google Scholar]
- 113.Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, Tsang K, Panicali D, Poole D, Schlom J, Hamilton J. Michael. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 114.Marshall JL, Gulley JL, Arlen PM, Beetham PK, Tsang KY, Slack R, Hodge JW, Doren S, Grosenbach DW, Hwang J, Fox E, Odogwu L, Park S, Panicali D, Schlom J. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 115.Tannenbaum A. The genesis and growth of tumors. II. Effects of caloric restriction per se. Cancer Res. 1942;2:460–464. [Google Scholar]
- 116.Weindruch R, Gottesman SR, Walford RL. Modification of age-related immune decline in mice dietarily restricted from or after midadulthood. Proc Natl Acad Sci U S A. 1982;79:898–902. doi: 10.1073/pnas.79.3.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weindruch R, Walford RL. Dietary restriction in mice beginning at 1 year of age: effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 118.Yoshida K, Inoue T, Hirabayashi Y, Nojima K, Sado T. Calorie restriction and spontaneous hepatic tumors in C3H/He mice. J Nutr Health Aging. 1999;3:121–126. [PubMed] [Google Scholar]
- 119.Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis. 2002;23:817–822. doi: 10.1093/carcin/23.5.817. [DOI] [PubMed] [Google Scholar]
- 120.Giovanella BC, Shepard RC, Stehlin JS, Venditti JM, Abbott BJ. Calorie restriction: effect on growth of human tumors heterotransplanted in nude mice. J Natl Cancer Inst. 1982;68:249–257. [PubMed] [Google Scholar]
- 121.Ershler WB, Berman E, Moore AL. Slower B16 melanoma growth but greater pulmonary colonization in calorie-restricted mice. J Natl Cancer Inst. 1986;76:81–85. [PubMed] [Google Scholar]
- 122.Shields BA, Engelman RW, Fukaura Y, Good RA, Day NK. Calorie restriction suppresses subgenomic mink cytopathic focus-forming murine leukemia virus transcription and frequency of genomic expression while impairing lymphoma formation. Proc Natl Acad Sci U S A. 1991;88:11138–11142. doi: 10.1073/pnas.88.24.11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci U S A. 1994;91:7036–7040. doi: 10.1073/pnas.91.15.7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fernandes G, Chandrasekar B, Troyer DA, Venkatraman JT, Good RA. Dietary lipids and calorie restriction affect mammary tumor incidence and gene expression in mouse mammary tumor virus/v-Ha-ras transgenic mice. Proc.Natl Acad.Sci.U.S.A. 1995;92:6494–6498. doi: 10.1073/pnas.92.14.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Program, National Toxicology Effect of Dietary Restriction on Toxicology and Carcinogenesis Studies in F344/N Rats and B6C3F1 Mice. 1997. [PubMed]
- 126.Walford RL, Lui RK, Gerbase-Lima M, Mathies M, Smith GS. Longterm dietray restriction and immune function in aged mice: Response to sheep red blood cells and mitogenic agents. Mech Ageing Dev. 1974;2:447–454. doi: 10.1016/0047-6374(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 127.Miller RA, Harrison DE. Delayed reduction in T cell precursor frequencies accompanies diet-induced lifespan extension. J Immunol. 1985;134:1426–1429. [PubMed] [Google Scholar]
- 128.Nikolich-Zugich J, Messaoudi I. Mice and flies and monkeys too: caloric restriction rejuvenates the aging immune system of non-human primates. Exp Gerontol. 2005;40:884–893. doi: 10.1016/j.exger.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 129.Messaoudi I, Warner J, Fischer M, Park B, Hill B, Mattison J, Lane MA, Roth GS, Ingram DK, Picker LJ, Douek DC, Mori M, Nikolich-Zugich J. Delay of T cell senescence by caloric restriction in aged long-lived nonhuman primates. Proc Natl Acad Sci U S A. 2006;103:19448–19453. doi: 10.1073/pnas.0606661103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Weindruch RH, Kristie JA, Cheney KE, Walford RL. Influence of controlled dietary restriction on immunologic function and aging. Fed Proc. 1979;38:2007–2016. [PubMed] [Google Scholar]
- 131.Mann PL. The effect of various dietary restricted regimes on some immunological parameters of mice. Growth. 1978;42:87–103. [PubMed] [Google Scholar]
- 132.Christadoss P, Talal N, Lindstrom J, Fernandes G. Suppression of cellular and humoral immunity to T-dependent antigens by calorie restriction. Cell Immunol. 1984;88:1–8. doi: 10.1016/0008-8749(84)90046-7. [DOI] [PubMed] [Google Scholar]
- 133.Abe T, Nakajima A, Satoh N, Ohkoshi M, Sakuragi S, Koizumi A. Suppression of experimental autoimmune uveoretinitis by dietary calorie restriction. Jpn J Ophthalmol. 2001;45:46–52. doi: 10.1016/s0021-5155(00)00303-8. [DOI] [PubMed] [Google Scholar]
- 134.Shibolet O, Alper R, Avraham Y, Berry EM, Ilan Y. Immunomodulation of experimental colitis via caloric restriction: role of Nk1.1+ T cells. Clin Immunol. 2002;105:48–56. doi: 10.1006/clim.2002.5260. [DOI] [PubMed] [Google Scholar]
- 135.Jolly CA, Fernandez R, Muthukumar AR, Fernandes G. Calorie restriction modulates Th-1 and Th-2 cytokine-induced immunoglobulin secretion in young and old C57BL/6 cultured submandibular glands. Aging (Milano) 1999;11:383–389. doi: 10.1007/BF03339817. [DOI] [PubMed] [Google Scholar]
- 136.Jolly CA, Muthukumar A, Avula CP, Troyer D, Fernandes G. Life span is prolonged in food-restricted autoimmune-prone (NZB × NZW)F(1) mice fed a diet enriched with (n-3) fatty acids. J Nutr. 2001;131:2753–2760. doi: 10.1093/jn/131.10.2753. [DOI] [PubMed] [Google Scholar]
- 137.Jolly CA, Muthukumar A, Avula C. P. Reddy, Fernandes G. Maintenance of NF-kappaB activation in T-lymphocytes and a naive T-cell population in autoimmune-prone (NZB/NZW)F(1) mice by feeding a food-restricted diet enriched with n-3 fatty acids. Cell Immunol. 2001;213:122–133. doi: 10.1006/cimm.2001.1866. [DOI] [PubMed] [Google Scholar]
- 138.Chandrasekar B, McGuff HS, Aufdermorte TB, Troyer DA, Talal N, Fernandes G. Effects of calorie restriction on transforming growth factor beta 1 and proinflammatory cytokines in murine Sjogren’s syndrome. Clin Immunol Immunopathol. 1995;76:291–296. doi: 10.1006/clin.1995.1128. [DOI] [PubMed] [Google Scholar]
- 139.Blank SE, Duncan DA, Meadows GG. Suppression of natural killer cell activity by ethanol consumption and food restriction. Alcohol Clin Exp Res. 1991;15:16–22. doi: 10.1111/j.1530-0277.1991.tb00514.x. [DOI] [PubMed] [Google Scholar]
- 140.Weindruch R, Devens BH, Raff HV, Walford RL. Influence of dietary restriction and aging on natural killer cell activity in mice. J Immunol. 1983;130:993–996. [PubMed] [Google Scholar]
- 141.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 142.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 143.C. f. D. C. a. Prevention The Burden of Chronic Diseases and Their Risk Factors: National and State Perspectives 2004. 2004 Available at: http://www.cdc.gov/nccdphp/burdenbook2004.
- 144.Carroll KK. Obesity as a risk factor for certain types of cancer. Lipids. 1998;33:1055–1059. doi: 10.1007/s11745-998-0305-8. [DOI] [PubMed] [Google Scholar]
- 145.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 146.Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control. 2007;18:165–175. doi: 10.1007/s10552-006-0100-0. [DOI] [PubMed] [Google Scholar]
- 147.Olsen CM, Green AC, Whiteman DC, Sadeghi S, Kolahdooz F, Webb PM. Obesity and the risk of epithelial ovarian cancer: A systematic review and meta-analysis. Eur J Cancer. 2007 doi: 10.1016/j.ejca.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 148.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 150.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 151.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 152.Mousa SA. Elevation of plasma von Willebrand factor and tumor necrosis factor-a in obese subjects and their reduction by the low molecular weight heparin tinzaparin. Int Angiol. 2005;24:278–281. [PubMed] [Google Scholar]
- 153.Aldhahi W, Hamdy O. Adipokines, inflammation, and the endothelium in diabetes. Curr Diab Rep. 2003;3:293–298. doi: 10.1007/s11892-003-0020-2. [DOI] [PubMed] [Google Scholar]
- 154.Halle M, Berg A, Northoff H, Keul J. Importance of TNF-alpha and leptin in obesity and insulin resistance: a hypothesis on the impact of physical exercise. Exerc Immunol Rev. 1998;4:77–94. [PubMed] [Google Scholar]
- 155.Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 156.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- 159.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 161.Canturk Z, Canturk NZ, Cetinarslan B, Utkan NZ, Tarkun I. Nosocomial infections and obesity in surgical patients. Obes Res. 2003;11:769–775. doi: 10.1038/oby.2003.107. [DOI] [PubMed] [Google Scholar]
- 162.Espejo B, Torres A, Valentin M, Bueno B, Andres A, Praga M, Morales JM. Obesity favors surgical and infectious complications after renal transplantation. Transplant Proc. 2003;35:1762–1763. doi: 10.1016/s0041-1345(03)00718-8. [DOI] [PubMed] [Google Scholar]
- 163.Gore JL, Pham PT, Danovitch GM, Wilkinson AH, Rosenthal JT, Lipshutz GS, Singer JS. Obesity and outcome following renal transplantation. Am J Transplant. 2006;6:357–363. doi: 10.1111/j.1600-6143.2005.01198.x. [DOI] [PubMed] [Google Scholar]
- 164.Gottschlich MM, Mayes T, Khoury JC, Warden GD. Significance of obesity on nutritional, immunologic, hormonal, and clinical outcome parameters in burns. J Am Diet Assoc. 1993;93:1261–1268. doi: 10.1016/0002-8223(93)91952-m. [DOI] [PubMed] [Google Scholar]
- 165.Dovio A, Caramello V, Masera RG, Sartori ML, Saba L, Tinivella M, Prolo P, Termine A, Avagnina P, Angeli A. Natural killer cell activity and sensitivity to positive and negative modulation in uncomplicated obese subjects: relationships to leptin and diet composition. Int J Obes Relat Metab Disord. 2004;28:894–901. doi: 10.1038/sj.ijo.0802639. [DOI] [PubMed] [Google Scholar]
- 166.Nieman DC, Henson DA, Nehlsen-Cannarella SL, Ekkens M, Utter AC, Butterworth DE, Fagoaga OR. Influence of obesity on immune function. J Am Diet Assoc. 1999;99:294–299. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 167.Moriguchi S, Oonishi K, Kato M, Kishino Y. Obesity is a risk factor for deteriorating cellular immune function with aging. Nutr Res. 1995;15:151–160. [Google Scholar]
- 168.Lamas O, Martinez JA, Marti A. Energy restriction restores the impaired immune response in overweight (cafeteria) rats. J Nutr Biochem. 2004;15:418–425. doi: 10.1016/j.jnutbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 169.Rogers CJ, Hance KW, Zaharoff DA, Perkins SN, Schlom J, Hursting SD, Greiner JW. Diet-induced obesity impairs both innate and adaptive immune responses. AACR Frontiers in Cancer Prevention Meeting.2006. [Google Scholar]
- 170.Mori A, Sakurai H, Choo MK, Obi R, Koizumi K, Yoshida C, Shimada Y, Saiki I. Severe pulmonary metastasis in obese and diabetic mice. Int J Cancer. 2006;119:2760–2767. doi: 10.1002/ijc.22248. [DOI] [PubMed] [Google Scholar]
- 171.O’Rourke RW, Kay T, Scholz MH, Diggs B, Jobe BA, Lewinsohn DM, Bakke AC. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes Surg. 2005;15:1463–1468. doi: 10.1381/096089205774859308. [DOI] [PubMed] [Google Scholar]
- 172.Chandra RK, Kutty KM. Immunocompetence in obesity. Acta Paediatr Scand. 1980;69:25–30. doi: 10.1111/j.1651-2227.1980.tb07024.x. [DOI] [PubMed] [Google Scholar]
- 173.Tanaka S, Inoue S, Isoda F, Waseda M, Ishihara M, Yamakawa T, Sugiyama A, Takamura Y, Okuda K. Impaired immunity in obesity: suppressed but reversible lymphocyte responsiveness. Int J Obes Relat Metab Disord. 1993;17:631–636. [PubMed] [Google Scholar]
- 174.Moriguchi S, Kato M, Sakai K, Yamamoto S, Shimizu E. Exercise training restores decreased cellular immune functions in obese Zucker rats. J Appl Physiol. 1998;84:311–317. doi: 10.1152/jappl.1998.84.1.311. [DOI] [PubMed] [Google Scholar]
- 175.Tanaka S, Isoda F, Yamakawa T, Ishihara M, Sekihara H. T lymphopenia in genetically obese rats. Clin Immunol Immunopathol. 1998;86:219–225. doi: 10.1006/clin.1997.4467. [DOI] [PubMed] [Google Scholar]
- 176.Stallone DD, Stunkard AJ, Zweiman B, Wadden TA, Foster GD. Decline in delayed-type hypersensitivity response in obese women following weight reduction. Clin Diagn Lab Immunol. 1994;1:202–205. doi: 10.1128/cdli.1.2.202-205.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kelley DS, Daudu PA, Branch LB, Johnson HL, Taylor PC, Mackey B. Energy restriction decreases number of circulating natural killer cells and serum levels of immunoglobulins in overweight women. Eur J Clin Nutr. 1994;48:9–18. [PubMed] [Google Scholar]
- 178.Shade ED, Ulrich CM, Wener MH, Wood B, Yasui Y, Lacroix K, Potter JD, McTiernan A. Frequent intentional weight loss is associated with lower natural killer cell cytotoxicity in postmenopausal women: possible long-term immune effects. J Am Diet Assoc. 2004;104:903–912. doi: 10.1016/j.jada.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 179.Scanga CB, Verde TJ, Paolone AM, Andersen RE, Wadden TA. Effects of weight loss and exercise training on natural killer cell activity in obese women. Med Sci Sports Exerc. 1998;30:1666–1671. doi: 10.1097/00005768-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 180.Senie RT. Breast cancer prognosis: role of obesity? J Surg Oncol. 1994;57:30. doi: 10.1002/jso.2930570109. [DOI] [PubMed] [Google Scholar]
- 181.Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol. 2002;20:1128–1143. doi: 10.1200/JCO.2002.20.4.1128. [DOI] [PubMed] [Google Scholar]
- 182.Berclaz G, Li S, Price KN, Coates AS, Castiglione-Gertsch M, Rudenstam CM, Holmberg SB, Lindtner J, Erien D, Collins J, Snyder R, Thurlimann B, Fey MF, Mendiola C, Werner ID, Simoncini E, Crivellari D, Gelber RD, Goldhirsch A. Body mass index as a prognostic feature in operable breast cancer: the International Breast Cancer Study Group experience. Ann Oncol. 2004;15:875–884. doi: 10.1093/annonc/mdh222. [DOI] [PubMed] [Google Scholar]
- 183.Kroenke CH, Chen WY, Rosner B, Holmes MD. Weight, weight gain, and survival after breast cancer diagnosis. J Clin Oncol. 2005;23:1370–1378. doi: 10.1200/JCO.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 184.Whiteman MK, Hillis SD, Curtis KM, McDonald JA, Wingo PA, Marchbanks PA. Body mass and mortality after breast cancer diagnosis. Cancer Epidemiol Biomarkers Prev. 2005;14:2009–2014. doi: 10.1158/1055-9965.EPI-05-0106. [DOI] [PubMed] [Google Scholar]
- 185.Loi S, Milne RL, Friedlander ML, McCredie MR, Giles GG, Hopper JL, Phillips KA. Obesity and outcomes in premenopausal and postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1686–1691. doi: 10.1158/1055-9965.EPI-05-0042. [DOI] [PubMed] [Google Scholar]
- 186.Holmberg L, Ohlander EM, Byers T, Zack M, Wolk A, Bergstrom R, Bergkvist L, Thurfjell E, Bruce A, Adami HO. Diet and breast cancer risk. Results from a population-based, case-control study in Sweden. Arch Intern Med. 1994;154:1805–1811. doi: 10.1001/archinte.154.16.1805. [DOI] [PubMed] [Google Scholar]
- 187.Lethaby AE, O’Neill MA, Mason BH, Holdaway IM, Harvey VJ. Overall survival from breast cancer in women pregnant or lactating at or after diagnosis. Auckland Breast Cancer Study Group. Int J Cancer. 1996;67:751–755. doi: 10.1002/(SICI)1097-0215(19960917)67:6<751::AID-IJC1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 188.Camoriano JK, Loprinzi CL, Ingle JN, Therneau TM, Krook JE, Veeder MH. Weight change in women treated with adjuvant therapy or observed following mastectomy for node-positive breast cancer. J Clin Oncol. 1990;8:1327–1334. doi: 10.1200/JCO.1990.8.8.1327. [DOI] [PubMed] [Google Scholar]
- 189.Chlebowski RT, Weiner JM, Reynolds R, Luce J, Bulcavage L, Bateman JR. Long-term survival following relapse after 5-FU but not CMF adjuvant breast cancer therapy. Breast Cancer Res Treat. 1986;7:23–30. doi: 10.1007/BF01886732. [DOI] [PubMed] [Google Scholar]
- 190.Demark-Wahnefried W, Winer EP, Rimer BK. Why women gain weight with adjuvant chemotherapy for breast cancer. J Clin Oncol. 1993;11:1418–1429. doi: 10.1200/JCO.1993.11.7.1418. [DOI] [PubMed] [Google Scholar]
- 191.Demark-Wahnefried W, Rimer BK, Winer EP. Weight gain in women diagnosed with breast cancer. J Am Diet Assoc. 1997;97:519–26. 529. doi: 10.1016/s0002-8223(97)00133-8. [DOI] [PubMed] [Google Scholar]
- 192.Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, Trudeau M, Hood N, Redwood S. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17:120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 193.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19:2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 194.Faber-Langendoen K. Weight gain in women receiving adjuvant chemotherapy for breast cancer. JAMA. 1996;276:855–856. [PubMed] [Google Scholar]
- 195.Jones BA, Kasi SV, Curnen MG, Owens PH, Dubrow R. Severe obesity as an explanatory factor for the black/white difference in stage at diagnosis of breast cancer. Am J Epidemiol. 1997;146:394–404. doi: 10.1093/oxfordjournals.aje.a009292. [DOI] [PubMed] [Google Scholar]
- 196.Jain M, Miller AB. Tumor characteristics and survival of breast cancer patients in relation to premorbid diet and body size. Breast Cancer Res Treat. 1997;42:43–55. doi: 10.1023/a:1005798124538. [DOI] [PubMed] [Google Scholar]
- 197.Moorman PG, Jones BA, Millikan RC, Hall IJ, Newman B. Race, anthropometric factors, and stage at diagnosis of breast cancer. Am J Epidemiol. 2001;153:284–291. doi: 10.1093/aje/153.3.284. [DOI] [PubMed] [Google Scholar]
- 198.Cui Y, Whiteman MK, Langenberg P, Sexton M, Tkaczuk KH, Flaws JA, Bush TL. Can obesity explain the racial difference in stage of breast cancer at diagnosis between black and white women? J Womens Health Gend Based Med. 2002;11:527–536. doi: 10.1089/152460902760277886. [DOI] [PubMed] [Google Scholar]
- 199.Zumoff B, Dasgupta I. Relationship between body weight and the incidence of positive axillary nodes at mastectomy for breast cancer. J Surg Oncol. 1983;22:217–220. doi: 10.1002/jso.2930220402. [DOI] [PubMed] [Google Scholar]
- 200.Verreault R, Brisson J, Deschenes L, Naud F. Body weight and prognostic indicators in breast cancer. Modifying effect of estrogen receptors. Am J Epidemiol. 1989;129:260–268. doi: 10.1093/oxfordjournals.aje.a115131. [DOI] [PubMed] [Google Scholar]
- 201.Maehle BO, Tretli S, Thorsen T. The associations of obesity, lymph node status and prognosis in breast cancer patients: dependence on estrogen and progesterone receptor status. Apmis. 2004;112:349–357. doi: 10.1111/j.1600-0463.2004.apm1120605.x. [DOI] [PubMed] [Google Scholar]
- 202.Dignam JJ, Wieand K, Johnson KA, Fisher B, Xu L, Mamounas EP. Obesity, tamoxifen use, and outcomes in women with estrogen receptor-positive early-stage breast cancer. J Natl Cancer Inst. 2003;95:1467–1476. doi: 10.1093/jnci/djg060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Huang Z, Hankinson SE, Colditz GA, Stampfer MJ, Hunter DJ, Manson JE, Hennekens CH, Rosner B, Speizer FE, Willett WC. Dual effects of weight and weight gain on breast cancer risk. JAMA. 1997;278:1407–1411. [PubMed] [Google Scholar]
- 204.van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152:514–527. doi: 10.1093/aje/152.6.514. [DOI] [PubMed] [Google Scholar]
- 205.Barber J. Breast pain. Bmj. 1995;310:669–670. doi: 10.1136/bmj.310.6980.669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Forouhi P, Dixon JM, Leonard RC, Chetty U. Prospective randomized study of surgical morbidity following primary systemic therapy for breast cancer. Br J Surg. 1995;82:79–82. doi: 10.1002/bjs.1800820127. [DOI] [PubMed] [Google Scholar]
- 207.Snowdon DA, Phillips RL, Choi W. Diet, obesity, and risk of fatal prostate cancer. Am J Epidemiol. 1984;120:244–250. doi: 10.1093/oxfordjournals.aje.a113886. [DOI] [PubMed] [Google Scholar]
- 208.Andersson SO, Wolk A, Bergstrom R, Adami HO, Engholm G, Englund A, Nyren O. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J Natl Cancer Inst. 1997;89:385–389. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 209.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–353. [PubMed] [Google Scholar]
- 210.Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP, Chung AK, McLeod DG. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 2004;22:439–445. doi: 10.1200/JCO.2004.03.132. [DOI] [PubMed] [Google Scholar]
- 211.Amling CL, Kane CJ, Riffenburgh RH, Ward JF, Roberts JL, Lance RS, Friedrichs PA, Moul JW. Relationship between obesity and race in predicting adverse pathologic variables in patients undergoing radical prostatectomy. Urology. 2001;58:723–728. doi: 10.1016/s0090-4295(01)01373-5. [DOI] [PubMed] [Google Scholar]
- 212.Mydlo JH, Tieng NL, Volpe MA, Chaiken R, Kral JG. A pilot study analyzing PSA, serum testosterone, lipid profile, body mass index and race in a small sample of patients with and without carcinoma of the prostate. Prostate Cancer Prostatic Dis. 2001;4:101–105. doi: 10.1038/sj.pcan.4500514. [DOI] [PubMed] [Google Scholar]
- 213.Rohrmann S, Roberts WW, Walsh PC, Platz EA. Family history of prostate cancer and obesity in relation to high-grade disease and extraprostatic extension in young men with prostate cancer. Prostate. 2003;55:140–146. doi: 10.1002/pros.10211. [DOI] [PubMed] [Google Scholar]
- 214.Freedland SJ, Aronson WJ, Kane CJ, Presti JC, Jr., Amling CL, Elashoff D, Terris MK. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 215.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007 doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 216.Loeb S, Yu X, Nadler RB, Roehl KA, Han M, Hawkins SA, Catalona WJ. Does body mass index affect preoperative prostate specific antigen velocity or pathological outcomes after radical prostatectomy? J Urol. 2007;177:102–106. doi: 10.1016/j.juro.2006.08.097. [DOI] [PubMed] [Google Scholar]
- 217.Amling CL. Relationship between obesity and prostate cancer. Curr Opin Urol. 2005;15:167–171. doi: 10.1097/01.mou.0000165550.94663.fb. [DOI] [PubMed] [Google Scholar]
- 218.Bassett WW, Cooperberg MR, Sadetsky N, Silva S, DuChane J, Pasta DJ, Chan JM, Anast JW, Carroll PR, Kane CJ. Impact of obesity on prostate cancer recurrence after radical prostatectomy: data from CaPSURE. Urology. 2005;66:1060–1065. doi: 10.1016/j.urology.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 219.Strom SS, Kamat AM, Gruschkus SK, Gu Y, Wen S, Cheung MR, Pisters LL, Lee AK, Rosser CJ, Kuban DA. Influence of obesity on biochemical and clinical failure after external-beam radiotherapy for localized prostate cancer. Cancer. 2006;107:631–639. doi: 10.1002/cncr.22025. [DOI] [PubMed] [Google Scholar]
- 220.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Benson AB, III, Macdonald JS, Fuchs CS. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 221.Dignam JJ, Polite BN, Yothers G, Raich P, Colangelo L, O’Connell MJ, Wolmark N. Body mass index and outcomes in patients who receive adjuvant chemotherapy for colon cancer. J Natl Cancer Inst. 2006;98:1647–1654. doi: 10.1093/jnci/djj442. [DOI] [PubMed] [Google Scholar]
- 222.Haydon AM, Macinnis RJ, English DR, Giles GG. Effect of physical activity and body size on survival after diagnosis with colorectal cancer. Gut. 2006;55:62–67. doi: 10.1136/gut.2005.068189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10:1031–1039. doi: 10.1096/fasebj.10.9.8801164. [DOI] [PubMed] [Google Scholar]
- 224.Wang J. g., Barsky LW, Davicioni E, Weinberg KI, Triche TJ, Zhang X. k., Wu L. Retinoic acid induces leukemia cell G1 arrest and transition into differentiation by inhibiting cyclin-dependent kinase-activating kinase binding and phosphorylation of PML/RAR{alpha} FASEB J. 2006;20:2142–2144. doi: 10.1096/fj.06-5900fje. [DOI] [PubMed] [Google Scholar]
- 225.Bjelke E. Dietary vitamin A and human lung cancer. Int J Cancer. 1975;15:561–565. doi: 10.1002/ijc.2910150405. [DOI] [PubMed] [Google Scholar]
- 226.Shekelle RB, Lepper M, Liu S, Maliza C, Raynor WJ, Jr., Rossof AH, Paul O, Shryock AM, Stamler J. Dietary vitamin A and risk of cancer in the Western Electric study. Lancet. 1981;2:1185–1190. doi: 10.1016/s0140-6736(81)91435-5. [DOI] [PubMed] [Google Scholar]
- 227.Kummet T, Moon TE, Meyskens FL., Jr. Vitamin A: evidence for its preventive role in human cancer. Nutr Cancer. 1983;5:96–106. doi: 10.1080/01635588309513785. [DOI] [PubMed] [Google Scholar]
- 228.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Jr., Valanis B, Williams JH, Jr., Barnhart S, Cherniack MG, Brodkin CA, Hammar S. Risk Factors for Lung Cancer and for Intervention Effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J.Natl.Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 229.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL, Valanis B, Williams JH, Barnhart S, Hammar S. Effects of a Combination of Beta Carotene and Vitamin A on Lung Cancer and Cardiovascular Disease. N Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 230.Albanes D, Heinonen OP, Taylor PR, Virtamo J, Edwards BK, Rautalahti M, Hartman AM, Palmgren J, Freedman LS, Haapakoski J, Barrett MJ, Pietinen P, Malila N, Tala E, Liippo K, Salomaa ER, Tangrea JA, Teppo L, Askin FB, Taskinen E, Erozan Y, Greenwald P, Huttunen JK. {alpha}-Tocopherol and beta-Carotene Supplements and Lung Cancer Incidence in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study: Effects of Base-line Characteristics and Study Compliance. J.Natl.Cancer Inst. 1996;88:1560–1570. doi: 10.1093/jnci/88.21.1560. [DOI] [PubMed] [Google Scholar]
- 231.ATBC Study Group The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 232.Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- 233.Westervelt P, Pollock JL, Oldfather KM, Walter MJ, Ma MK, Williams A, DiPersio JF, Ley TJ. Adaptive immunity cooperates with liposomal all-trans-retinoic acid (ATRA) to facilitate long-term molecular remissions in mice with acute promyelocytic leukemia. PNAS. 2002;99:9468–9473. doi: 10.1073/pnas.132657799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Anzano MA, Byers SW, Smith JM, Peer CW, Mullen LT, Brown CC, Roberts AB, Sporn MB. Prevention of Breast Cancer in the Rat with 9-cis-Retinoic Acid as a Single Agent and in Combination with Tamoxifen. Cancer Res. 1994;54:4614–4617. [PubMed] [Google Scholar]
- 235.Anzano MA, Peer CW, Smith JM, Mullen LT, Shrader MW, Logsdon DL, Driver CL, Brown CC, Roberts AB, Sporn MB. Chemoprevention of Mammary Carcinogenesis in the Rat: Combined Use of Raloxifene and 9-cis-Retinoic Acid. J.Natl.Cancer Inst. 1996;88:123–125. doi: 10.1093/jnci/88.2.123. [DOI] [PubMed] [Google Scholar]
- 236.Wu K, Kim HT, Rodriquez JL, Munoz-Medellin D, Mohsin SK, Hilsenbeck SG, Lamph WW, Gottardis MM, Shirley MA, Kuhn JG, Green JE, Brown PH. 9-cis-Retinoic Acid Suppresses Mammary Tumorigenesis in C3(1)-Simian Virus 40 T Antigen-transgenic Mice. Clin Cancer Res. 2000;6:3696–3704. [PubMed] [Google Scholar]
- 237.Hong WK, Endicott J, Itri LM, Doos W, Batsakis JG, Bell R, Fofonoff S, Byers R, Atkinson EN, Vaughan C. 13-cis-retinoic acid in the treatment of oral leukoplakia. N.Engl.J Med. 1986;315:1501–1505. doi: 10.1056/NEJM198612113152401. [DOI] [PubMed] [Google Scholar]
- 238.Hong WK, Lippman SM, Itri LM, Karp DD, Lee JS, Byers RM, Schantz SP, Kramer AM, Lotan R, Peters LJ. Prevention of second primary tumors with isotretinoin in squamous-cell carcinoma of the head and neck. N.Engl.J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 239.Benner SE, Pajak TF, Lippman SM, Earley C, Hong WK. Prevention of Second Primary Tumors With Isotretinoin in Patients With Squamous Cell Carcinoma of the Head and Neck: Long-term Follow-up. J.Natl.Cancer Inst. 1994;86:140–141. doi: 10.1093/jnci/86.2.140. [DOI] [PubMed] [Google Scholar]
- 240.Cattelan AM, Trevenzoli M, Aversa SM. Recent advances in the treatment of AIDS-related Kaposi’s sarcoma. Am J Clin Dermatol. 2002;3:451–462. doi: 10.2165/00128071-200203070-00002. [DOI] [PubMed] [Google Scholar]
- 241.Bendich A, Langseth L. Safety of vitamin A. Am J Clin Nutr. 1989;49:358–371. doi: 10.1093/ajcn/49.2.358. [DOI] [PubMed] [Google Scholar]
- 242.Gottardis MM, Bischoff ED, Shirley MA, Wagoner MA, Lamph WW, Heyman RA. Chemoprevention of Mammary Carcinoma by LGD1069 (Targretin): An RXR-selective Ligand. Cancer Res. 1996;56:5566–5570. [PubMed] [Google Scholar]
- 243.Bischoff ED, Gottardis MM, Moon TE, Heyman RA, Lamph WW. Beyond Tamoxifen: The Retinoid X Receptor-selective Ligand LGD1069 (TARGRETIN) Causes Complete Regression of Mammary Carcinoma. Cancer Res. 1998;58:479–484. [PubMed] [Google Scholar]
- 244.Suh N, Lamph WW, Glasebrook AL, Grese TA, Palkowitz AD, Williams CR, Risingsong R, Farris MR, Heyman RA, Sporn MB. Prevention and Treatment of Experimental Breast Cancer with the Combination of a New Selective Estrogen Receptor Modulator, Arzoxifene, and a New Rexinoid, LG 100268. Clin Cancer Res. 2002;8:3270–3275. [PubMed] [Google Scholar]
- 245.Rendi MH, Suh N, Lamph WW, Krajewski S, Reed JC, Heyman RA, Berchuck A, Liby K, Risingsong R, Royce DB, Williams CR, Sporn MB. The Selective Estrogen Receptor Modulator Arzoxifene and the Rexinoid LG100268 Cooperate to Promote Transforming Growth Factor {beta}-Dependent Apoptosis in Breast Cancer. Cancer Res. 2004;64:3566–3571. doi: 10.1158/0008-5472.CAN-04-0234. [DOI] [PubMed] [Google Scholar]
- 246.Wu K, Kim HT, Rodriquez JL, Hilsenbeck SG, Mohsin SK, Xu XC, Lamph WW, Kuhn JG, Green JE, Brown PH. Suppression of Mammary Tumorigenesis in Transgenic Mice by the RXR-selective Retinoid, LGD1069. Cancer Epidemiol Biomarkers Prev. 2002;11:467–474. [PubMed] [Google Scholar]
- 247.Wu K, Zhang Y, Xu XC, Hill J, Celestino J, Kim HT, Mohsin SK, Hilsenbeck SG, Lamph WW, Bissonette R, Brown PH. The Retinoid X Receptor-Selective Retinoid, LGD1069, Prevents the Development of Estrogen Receptor-Negative Mammary Tumors in Transgenic Mice. Cancer Res. 2002;62:6376–6380. [PubMed] [Google Scholar]
- 248.Liby K, Rendi M, Suh N, Royce DB, Risingsong R, Williams CR, Lamph W, Labrie F, Krajewski S, Xu X, Kim H, Brown P, Sporn MB. The Combination of the Rexinoid, LG100268, and a Selective Estrogen Receptor Modulator, Either Arzoxifene or Acolbifene, Synergizes in the Prevention and Treatment of Mammary Tumors in an Estrogen Receptor-Negative Model of Breast Cancer. Clin Cancer Res. 2006;12:5902–5909. doi: 10.1158/1078-0432.CCR-06-1119. [DOI] [PubMed] [Google Scholar]
- 249.Esteva FJ, Glaspy J, Baidas S, Laufman L, Hutchins L, Dickler M, Tripathy D, Cohen R, DeMichele A, Yocum RC, Osborne CK, Hayes DF, Hortobagyi GN, Winer E, Demetri GD. Multicenter Phase II Study of Oral Bexarotene for Patients With Metastatic Breast Cancer. J Clin Oncol. 2003;21:999–1006. doi: 10.1200/JCO.2003.05.068. [DOI] [PubMed] [Google Scholar]
- 250.Boehm MF, Zhang L, Zhi L, McClurg MR, Berger E, Wagoner M, Mais DE, Suto CM, Davies JA, Heyman RA. Design and synthesis of potent retinoid X receptor selective ligands that induce apoptosis in leukemia cells. J Med Chem. 1995;38:3146–3155. doi: 10.1021/jm00016a018. [DOI] [PubMed] [Google Scholar]
- 251.Cui D, Moldoveanu Z, Stephensen CB. High-Level Dietary Vitamin A Enhances T-Helper Type 2 Cytokine Production and Secretory Immunoglobulin A Response to Influenza A Virus Infection in BALB/c Mice. J.Nutr. 2000;130:1132–1139. doi: 10.1093/jn/130.5.1132. [DOI] [PubMed] [Google Scholar]
- 252.Ma Y, Ross AC. The anti-tetanus immune response of neonatal mice is augmented by retinoic acid combined with polyriboinosinic:polyribocytidylic acid. PNAS. 2005;102:13556–13561. doi: 10.1073/pnas.0506438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Yu S, Xia M, Xu W, Chu Y, Wang Y, Xiong S. All-trans retinoic acid biases immune response induced by DNA vaccine in a Th2 direction. Vaccine. 2005;23:5160–5167. doi: 10.1016/j.vaccine.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 254.Vertuani S, De Geer A, Levitsky V, Kogner P, Kiessling R, Levitskaya J. Retinoids act as multistep modulators of the major histocompatibility class I presentation pathway and sensitize neuroblastomas to cytotoxic lymphocytes. Cancer Res. 2003;63:8006–8013. [PubMed] [Google Scholar]
- 255.Santin AD, Hermonat P, Ravaggi A, Chiriva-Internati M, Hiserodt JC, Tian E, Carter CA, Pecorelli S, Parham GP. Effects of retinoic acid combined with interferon-gamma on the expression of major-histocompatibility-complex molecules and intercellular adhesion molecule-1 in human cervical cancer. Int J Cancer. 1998;75:254–258. doi: 10.1002/(sici)1097-0215(19980119)75:2<254::aid-ijc14>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 256.Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Hiserodt JC, Pecorelli S, Parham GP. Effects of retinoic acid combined with irradiation on the expression of major histocompatibility complex molecules and adhesion/costimulation molecules ICAM-1 in human cervical cancer. Gynecol.Oncol. 1998;70:195–201. doi: 10.1006/gyno.1998.5060. [DOI] [PubMed] [Google Scholar]
- 257.Vertuani S, Dubrovska E, Levitsky V, Jager M, Kiessling R, Levitskaya J. Retinoic acid elicits cytostatic, cytotoxic and immunomodulatory effects on uveal melanoma cells. Cancer Immunology, Immunotherapy. 2007;56:193–204. doi: 10.1007/s00262-006-0185-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ertesvag A, Engedal N, Naderi S, Blomhoff HK. Retinoic acid stimulates the cell cycle machinery in normal T cells: involvement of retinoic acid receptor-mediated IL-2 secretion. J Immunol. 2002;169:5555–5563. doi: 10.4049/jimmunol.169.10.5555. [DOI] [PubMed] [Google Scholar]
- 259.Recchia F, Saggio G, Cesta A, Candeloro G, Nuzzo A, Lombardo M, Carta G, Rea S. Interleukin-2 and 13-cis retinoic acid as maintenance therapy in advanced ovarian cancer. Int J Oncol. 2005;27:1039–1046. [PubMed] [Google Scholar]
- 260.Duvic M, Martin AG, Kim Y, Olsen E, Wood GS, Crowley CA, Yocum RC, for the Worldwide Bexarotene Study Group Phase 2 and 3 Clinical Trial of Oral Bexarotene (Targretin Capsules) for the Treatment of Refractory or Persistent Early-Stage Cutaneous T-Cell Lymphoma. Arch Dermatol. 2001;137:581–593. [PubMed] [Google Scholar]
- 261.Duvic M, Hymes K, Heald P, Breneman D, Martin AG, Myskowski P, Crowley C, Yocum RC. Bexarotene Is Effective and Safe for Treatment of Refractory Advanced-Stage Cutaneous T-Cell Lymphoma: Multinational Phase II-III Trial Results. J Clin Oncol. 2001;19:2456–2471. doi: 10.1200/JCO.2001.19.9.2456. [DOI] [PubMed] [Google Scholar]
- 262.Gorgun G, Foss F. Immunomodulatory effects of RXR rexinoids: modulation of high-affinity IL-2R expression enhances susceptibility to denileukin diftitox. Blood. 2002;100:1399–1403. doi: 10.1182/blood-2002-01-0300. [DOI] [PubMed] [Google Scholar]
- 263.Foss F, Demierre MF, DiVenuti G. A phase-1 trial of bexarotene and denileukin diftitox in patients with relapsed or refractory cutaneous T-cell lymphoma. Blood. 2005;106:454–457. doi: 10.1182/blood-2004-11-4570. [DOI] [PubMed] [Google Scholar]
- 264.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The Terminology Issue for Myeloid-Derived Suppressor Cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yanagisawa K, Exley MA, Jiang X, Ohkochi N, Taniguchi M, Seino K. i. Hyporesponsiveness to Natural Killer T-Cell Ligand {alpha}-Galactosylceramide in Cancer-Bearing State Mediated by CD11b+ Gr-1+ Cells Producing Nitric Oxide. Cancer Res. 2006;66:11441–11446. doi: 10.1158/0008-5472.CAN-06-0944. [DOI] [PubMed] [Google Scholar]
- 266.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 267.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Padua RA, Larghero J, Robin M, le Pogam C, Schlageter MH, Muszlak S, Fric J, West R, Rousselot P, Phan TH, Mudde L, Teisserenc H, Carpentier AF, Kogan S, Degos L, Pla M, Bishop JM, Stevenson F, Charron D, Chomienne C. PML-RARA-targeted DNA vaccine induces protective immunity in a mouse model of leukemia. Nat Med. 2003;9:1413–1417. doi: 10.1038/nm949. [DOI] [PubMed] [Google Scholar]
- 269.Miller VA, Rigas JR, Benedetti FM, Verret AL, Tong WP, Kris MG, Gill GM, Loewen GR, Truglia JA, Ulm EH, Warrell RP., Jr. Initial clinical trial of the retinoid receptor pan agonist 9-cis retinoic acid. Clin Cancer Res. 1996;2:471–475. [PubMed] [Google Scholar]
- 270.Kong G, Kim HT, Wu K, DeNardo D, Hilsenbeck SG, Xu XC, Lamph WW, Bissonnette R, Dannenberg AJ, Brown PH. The retinoid X receptor-selective retinoid, LGD1069, down-regulates cyclooxygenase-2 expression in human breast cells through transcription factor crosstalk: implications for molecular-based chemoprevention. Cancer Res. 2005;65:3462–3469. doi: 10.1158/0008-5472.CAN-03-2912. [DOI] [PubMed] [Google Scholar]
- 271.Taylor PR, Qiao YL, Abnet CC, Dawsey SM, Yang CS, Gunter EW, Wang W, Blot WJ, Dong ZW, Mark SD. Prospective Study of Serum Vitamin E Levels and Esophageal and Gastric Cancers. J.Natl.Cancer Inst. 2003;95:1414–1416. doi: 10.1093/jnci/djg044. [DOI] [PubMed] [Google Scholar]
- 272.The HOPE and HOPE-TOO Trial Investigators Effects of Long-term Vitamin E Supplementation on Cardiovascular Events and Cancer: A Randomized Controlled Trial. JAMA. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 273.Benner SE, Winn RJ, Lippman SM, Poland J, Hansen KS, Luna MA, Hong WK. Regression of oral leukoplakia with alpha-tocopherol: a community clinical oncology program chemoprevention study. J Natl Cancer Inst. 1993;85:44–47. doi: 10.1093/jnci/85.1.44. [DOI] [PubMed] [Google Scholar]
- 274.Fariss MW, Fortuna MB, Everett CK, Smith JD, Trent DF, Djuric Z. The Selective Antiproliferative Effects of {alpha}-Tocopheryl Hemisuccinate and Cholesteryl Hemisuccinate on Murine Leukemia Cells Result from the Action of the Intact Compounds. Cancer Res. 1994;54:3346–3351. [PubMed] [Google Scholar]
- 275.Weber T, Lu M, Andera L, Lahm H, Gellert N, Fariss MW, Korinek V, Sattler W, Ucker DS, Terman A, Schroder A, Erl W, Brunk UT, Coffey RJ, Weber C, Neuzil J. Vitamin E Succinate Is a Potent Novel Antineoplastic Agent with High Selectivity and Cooperativity with Tumor Necrosis Factor-related Apoptosis-inducing Ligand (Apo2 Ligand) in vivo. Clin Cancer Res. 2002;8:863–869. [PubMed] [Google Scholar]
- 276.Crispen PL, Uzzo RG, Golovine K, Makhov P, Pollack A, Horwitz EM, Greenberg RE, Kolenko VM. Vitamin E succinate inhibits NF-kappaB and prevents the development of a metastatic phenotype in prostate cancer cells: Implications for chemoprevention. Prostate. 2007 doi: 10.1002/pros.20468. [DOI] [PubMed] [Google Scholar]
- 277.Shiau CW, Huang JW, Wang DS, Weng JR, Yang CC, Lin CH, Li C, Chen CS. {alpha}-Tocopheryl Succinate Induces Apoptosis in Prostate Cancer Cells in Part through Inhibition of Bcl-xL/Bcl-2 Function. J.Biol.Chem. 2006;281:11819–11825. doi: 10.1074/jbc.M511015200. [DOI] [PubMed] [Google Scholar]
- 278.Malafa MP, Fokum FD, Andoh J, Neitzel LT, Bandyopadhyay S, Zhan R, Iiizumi M, Furuta E, Horvath E, Watabe K. Vitamin E succinate suppresses prostate tumor growth by inducing apoptosis. Int J Cancer. 2006;118:2441–2447. doi: 10.1002/ijc.21689. [DOI] [PubMed] [Google Scholar]
- 279.Tomasetti M, Andera L, Alleva R, Borghi B, Neuzil J, Procopio A. (alpha)-Tocopheryl succinate induces DR4 and DR5 expression by a p53-dependent route: Implication for sensitisation of resistant cancer cells to TRAIL apoptosis. FEBS Letters. 2006;580:1925–1931. doi: 10.1016/j.febslet.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 280.Malafa MP, Fokum FD, Smith L, Louis A. Inhibition of Angiogenesis and Promotion of Melanoma Dormancy by Vitamin E Succinate. Ann Surg Oncol. 2002;9:1023–1032. doi: 10.1007/BF02574523. [DOI] [PubMed] [Google Scholar]
- 281.Malafa MP, Neitzel LT. Vitamin E succinate promotes breast cancer tumor dormancy. J Surg Res. 2000;93:163–170. doi: 10.1006/jsre.2000.5948. [DOI] [PubMed] [Google Scholar]
- 282.Meydani SN, Barklund MP, Liu S, Meydani M, Miller RA, Cannon JG, Morrow FD, Rocklin R, Blumberg JB. Vitamin E supplementation enhances cell-mediated immunity in healthy elderly subjects. Am J Clin Nutr. 1990;52:557–563. doi: 10.1093/ajcn/52.3.557. [DOI] [PubMed] [Google Scholar]
- 283.Meydani SN, Meydani M, Blumberg JB, Leka LS, Siber G, Loszewski R, Thompson C, Pedrosa MC, Diamond RD, Stollar BD. Vitamin E supplementation and in vivo immune response in healthy elderly subjects. A randomized controlled trial. JAMA. 1997;277:1380–1386. doi: 10.1001/jama.1997.03540410058031. [DOI] [PubMed] [Google Scholar]
- 284.Hara M, Tanaka K, Hirota Y. Immune response to influenza vaccine in healthy adults and the elderly: association with nutritional status. Vaccine. 2005;23:1457–1463. doi: 10.1016/j.vaccine.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 285.Meydani SN, Leka LS, Fine BC, Dallal GE, Keusch GT, Singh MF, Hamer DH. Vitamin E and Respiratory Tract Infections in Elderly Nursing Home Residents: A Randomized Controlled Trial. JAMA. 2004;292:828–836. doi: 10.1001/jama.292.7.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Graat JM, Schouten EG, Kok FJ. Effect of Daily Vitamin E and Multivitamin-Mineral Supplementation on Acute Respiratory Tract Infections in Elderly Persons: A Randomized Controlled Trial. JAMA. 2002;288:715–721. doi: 10.1001/jama.288.6.715. [DOI] [PubMed] [Google Scholar]
- 287.Malmberg KJ, Lenkei R, Petersson M, Ohlum T, Ichihara F, Glimelius B, Frodin JE, Masucci G, Kiessling R. A Short-Term Dietary Supplementation of High Doses of Vitamin E Increases T Helper 1 Cytokine Production in Patients with Advanced Colorectal Cancer. Clin Cancer Res. 2002;8:1772–1778. [PubMed] [Google Scholar]
- 288.Hanson MG, Ozenci V, Carlsten MC, Glimelius BL, Frodin JE, Masucci G, Malmberg KJ, Kiessling RV. A short-term dietary supplementation with high doses of vitamin E increases NK cell cytolytic activity in advanced colorectal cancer patients. Cancer Immunol.Immunother. 2006 doi: 10.1007/s00262-006-0261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.De Waart FG, Portengen L, Doekes G, Verwaal CJ, Kok FJ. Effect of 3 months vitamin E supplementation on indices of the cellular and humoral immune response in elderly subjects. Br.J Nutr. 1997;78:761–774. doi: 10.1079/bjn19970193. [DOI] [PubMed] [Google Scholar]
- 290.Marko MG, Ahmed T, Bunnell SC, Wu D, Chung H, Huber BT, Meydani SN. Age-Associated Decline in Effective Immune Synapse Formation of CD4+ T Cells Is Reversed by Vitamin E Supplementation. J Immunol. 2007;178:1443–1449. doi: 10.4049/jimmunol.178.3.1443. [DOI] [PubMed] [Google Scholar]
- 291.Han SN, Adolfsson O, Lee CK, Prolla TA, Ordovas J, Meydani SN. Age and Vitamin E-Induced Changes in Gene Expression Profiles of T Cells. J Immunol. 2006;177:6052–6061. doi: 10.4049/jimmunol.177.9.6052. [DOI] [PubMed] [Google Scholar]
- 292.Beharka AA, Wu D, Han SN, Meydani SN. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mechanisms of Ageing and Development. 1997;93:59–77. doi: 10.1016/s0047-6374(96)01819-2. [DOI] [PubMed] [Google Scholar]
- 293.Lee E, Choi MK, Lee YJ, Ku JL, Kim KH, Choi JS, Lim SJ. Alpha-tocopheryl succinate, in contrast to alpha-tocopherol and alpha-tocopheryl acetate, inhibits prostaglandin E2 production in human lung epithelial cells. Carcinogenesis. 2006;27:2308–2315. doi: 10.1093/carcin/bgl073. [DOI] [PubMed] [Google Scholar]
- 294.Ramanathapuram LV, Kobie JJ, Bearss D, Payne CM, Trevor KT, Akporiaye ET. alpha-Tocopheryl succinate sensitizes established tumors to vaccination with nonmatured dendritic cells. Cancer Immunol Immunother. 2004;53:580–588. doi: 10.1007/s00262-004-0499-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ramanathapuram LV, Hahn T, Dial SM, Akporiaye ET. Chemo-immunotherapy of breast cancer using vesiculated alpha-tocopheryl succinate in combination with dendritic cell vaccination. Nutr Cancer. 2005;53:177–193. doi: 10.1207/s15327914nc5302_7. [DOI] [PubMed] [Google Scholar]
- 296.Ramanathapuram LV, Hahn T, Graner MW, Katsanis E, Akporiaye ET. Vesiculated alpha-tocopheryl succinate enhances the anti-tumor effect of dendritic cell vaccines. Cancer Immunol Immunother. 2006;55:166–177. doi: 10.1007/s00262-005-0016-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat.Rev.Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 298.Dannenberg AJ, Altorki NK, Boyle JO, Dang C, Howe LR, Weksler BB, Subbaramaiah K. Cyclo-oxygenase 2: a pharmacological target for the prevention of cancer. The Lancet Oncology. 2001;2:544–551. doi: 10.1016/S1470-2045(01)00488-0. [DOI] [PubMed] [Google Scholar]
- 299.PEREG D, LISHNER M. Non-steroidal anti-inflammatory drugs for the prevention and treatment of cancer. Journal of Internal Medicine. 2005;258:115–123. doi: 10.1111/j.1365-2796.2005.01519.x. [DOI] [PubMed] [Google Scholar]
- 300.Baratelli F, Lin Y, Zhu L, Yang SC, Heuze-Vourc’h N, Zeng G, Reckamp K, Dohadwala M, Sharma S, Dubinett SM. Prostaglandin E2 Induces FOXP3 Gene Expression and T Regulatory Cell Function in Human CD4+ T Cells. J Immunol. 2005;175:1483–1490. doi: 10.4049/jimmunol.175.3.1483. [DOI] [PubMed] [Google Scholar]
- 301.Sharma S, Yang SC, Zhu L, Reckamp K, Gardner B, Baratelli F, Huang M, Batra RK, Dubinett SM. Tumor Cyclooxygenase-2/Prostaglandin E2-Dependent Promotion of FOXP3 Expression and CD4+CD25+ T Regulatory Cell Activities in Lung Cancer. Cancer Res. 2005;65:5211–5220. doi: 10.1158/0008-5472.CAN-05-0141. [DOI] [PubMed] [Google Scholar]
- 302.Kambayashi T, Alexander HR, Fong M, Strassmann G. Potential involvement of IL-10 in suppressing tumor-associated macrophages. Colon-26-derived prostaglandin E2 inhibits TNF-alpha release via a mechanism involving IL-10. J Immunol. 1995;154:3383–3390. [PubMed] [Google Scholar]
- 303.Pockaj BA, Basu GD, Pathangey LB, Gray RJ, Hernandez JL, Gendler SJ, Mukherjee P. Reduced T-Cell and Dendritic Cell Function Is Related to Cyclooxygenase-2 Overexpression and Prostaglandin E2 Secretion in Patients With Breast Cancer. Ann Surg Oncol. 2004;11:328–339. doi: 10.1245/aso.2004.05.027. [DOI] [PubMed] [Google Scholar]
- 304.Stolina M, Sharma S, Lin Y, Dohadwala M, Gardner B, Luo J, Zhu L, Kronenberg M, Miller PW, Portanova J, Lee JC, Dubinett SM. Specific Inhibition of Cyclooxygenase 2 Restores Antitumor Reactivity by Altering the Balance of IL-10 and IL-12 Synthesis. J Immunol. 2000;164:361–370. doi: 10.4049/jimmunol.164.1.361. [DOI] [PubMed] [Google Scholar]
- 305.DeLong P, Tanaka T, Kruklitis R, Henry AC, Kapoor V, Kaiser LR, Sterman DH, Albelda SM. Use of cyclooxygenase-2 inhibition to enhance the efficacy of immunotherapy. Cancer Res. 2003;63:7845–7852. [PubMed] [Google Scholar]
- 306.Morecki S, Yacovlev E, Gelfand Y, Trembovler V, Shohami E, Slavin S. Induction of antitumor immunity by indomethacin. Cancer Immunol Immunother. 2000;48:613–620. doi: 10.1007/s002620050009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kundu N, Walser TC, Ma X, Fulton AM. Cyclooxygenase inhibitors modulate NK activities that control metastatic disease. Cancer Immunol Immunother. 2005;54:981–987. doi: 10.1007/s00262-005-0669-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Zeytin HE, Patel AC, Rogers CJ, Canter D, Hursting SD, Schlom J, Greiner JW. Combination of a poxvirus-based vaccine with a cyclooxygenase-2 inhibitor (celecoxib) elicits antitumor immunity and long-term survival in CEA.Tg/MIN mice. Cancer Res. 2004;64:3668–3678. doi: 10.1158/0008-5472.CAN-03-3878. [DOI] [PubMed] [Google Scholar]
- 309.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N.Engl.J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 310.Nussmeier NA, Whelton AA, Brown MT, Langford RM, Hoeft A, Parlow JL, Boyce SW, Verburg KM. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N.Engl.J Med. 2005;352:1081–1091. doi: 10.1056/NEJMoa050330. [DOI] [PubMed] [Google Scholar]
- 311.Solomon SD, McMurray JJ, Pfeffer MA, Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E, Bertagnolli M. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N.Engl.J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 312.Gerner EW, Meyskens FL., Jr. Polyamines and cancer: old molecules, new understanding. Nat Rev Cancer. 2004;4:781–792. doi: 10.1038/nrc1454. [DOI] [PubMed] [Google Scholar]
- 313.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 314.O’Brien TG, Megosh LC, Gilliard G, Soler AP. Ornithine Decarboxylase Overexpression Is a Sufficient Condition for Tumor Promotion in Mouse Skin. Cancer Res. 1997;57:2630–2637. [PubMed] [Google Scholar]
- 315.Singh R, Pervin S, Karimi A, Cederbaum S, Chaudhuri G. Arginase Activity in Human Breast Cancer Cell Lines: N{{omega}}-Hydroxy-L-arginine Selectively Inhibits Cell Proliferation and Induces Apoptosis in MDA-MB-468 Cells. Cancer Res. 2000;60:3305–3312. [PubMed] [Google Scholar]
- 316.Chang CI, Liao JC, Kuo L. Macrophage Arginase Promotes Tumor Cell Growth and Suppresses Nitric Oxide-mediated Tumor Cytotoxicity. Cancer Res. 2001;61:1100–1106. [PubMed] [Google Scholar]
- 317.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid Suppressor Lines Inhibit T Cell Responses by an NO-Dependent Mechanism. J Immunol. 2002;168:689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 318.Rodriguez PC, Quiceno DG, Zabaleta J, Ortiz B, Zea AH, Piazuelo MB, Delgado A, Correa P, Brayer J, Sotomayor EM, Antonia S, Ochoa JB, Ochoa AC. Arginase I Production in the Tumor Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression and Antigen-Specific T-Cell Responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- 319.Kusmartsev S, Gabrilovich DI. STAT1 Signaling Regulates Tumor-Associated Macrophage-Mediated T Cell Deletion. J Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 320.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T Cell Receptor CD3zeta Chain Expression by L-Arginine. J.Biol.Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 321.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-Producing Myeloid Suppressor Cells in Renal Cell Carcinoma Patients: A Mechanism of Tumor Evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 322.De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, Musiani P, Zanovello P, Bronte V. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc.Natl Acad.Sci.U.S.A. 2005;102:4185–4190. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 324.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 325.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Santamaria P, Fioretti MC, Puccetti P. The Combined Effects of Tryptophan Starvation and Tryptophan Catabolites Down-Regulate T Cell Receptor {zeta}-Chain and Induce a Regulatory Phenotype in Naive T Cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 326.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, Orabona C, Bianchi R, Belladonna ML, Volpi C, Fioretti MC, Puccetti P. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transplant Immunology. 2006;17:58–60. doi: 10.1016/j.trim.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 327.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, Boon T, Van den Eynde BJ. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat.Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 328.Brandacher G, Perathoner A, Ladurner R, Schneeberger S, Obrist P, Winkler C, Werner ER, Werner-Felmayer G, Weiss HG, Gobel G, Margreiter R, Konigsrainer A, Fuchs D, Amberger A. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res. 2006;12:1144–1151. doi: 10.1158/1078-0432.CCR-05-1966. [DOI] [PubMed] [Google Scholar]
- 329.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T, Urashima M. Indoleamine 2,3-Dioxygenase Serves as a Marker of Poor Prognosis in Gene Expression Profiles of Serous Ovarian Cancer Cells. Clin Cancer Res. 2005;11:6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 330.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clinical Biochemistry. 2004;37:684–687. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 331.Pertovaara M, Raitala A, Lehtimaki T, Karhunen PJ, Oja SS, Jylha M, Hervonen A, Hurme M. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mechanisms of Ageing and Development. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 332.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells Expressing Indoleamine 2,3-Dioxygenase Inhibit T Cell Responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 333.Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A, Extermann M, Mellor AL, Munn DH, Antonia SJ. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int.J Cancer. 2002;101:151–155. doi: 10.1002/ijc.10645. [DOI] [PubMed] [Google Scholar]
- 334.Basu GD, Tinder TL, Bradley JM, Tu T, Hattrup CL, Pockaj BA, Mukherjee P. Cyclooxygenase-2 inhibitor enhances the efficacy of a breast cancer vaccine: role of IDO. J Immunol. 2006;177:2391–2402. doi: 10.4049/jimmunol.177.4.2391. [DOI] [PubMed] [Google Scholar]
- 335.Cham CM, Xu H, O’Keefe JP, Rivas FV, Zagouras P, Gajewski TF. Gene Array and Protein Expression Profiles Suggest Post-transcriptional Regulation during CD8+ T Cell Differentiation. J.Biol.Chem. 2003;278:17044–17052. doi: 10.1074/jbc.M212741200. [DOI] [PubMed] [Google Scholar]
- 336.Cham CM, Gajewski TF. Metabolic mechanisms of tumor resistance to T cell effector function. Immunol Res. 2005;31:107–118. doi: 10.1385/IR:31:2:107. [DOI] [PubMed] [Google Scholar]
- 337.Cham CM, Gajewski TF. Glucose Availability Regulates IFN-{gamma} Production and p70S6 Kinase Activation in CD8+ Effector T Cells. J Immunol. 2005;174:4670–4677. doi: 10.4049/jimmunol.174.8.4670. [DOI] [PubMed] [Google Scholar]
- 338.Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, Prognosis, and Targeted Therapy for Renal Cell Carcinoma: Exploiting the Hypoxia-Induced Pathway. Clin Cancer Res. 2003;9:4641–4652. [PubMed] [Google Scholar]
- 339.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–275. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 340.Pedersen MW, Holm S, Lund EL, Hojgaard L, Kristjansen PE. Coregulation of glucose uptake and vascular endothelial growth factor (VEGF) in two small-cell lung cancer (SCLC) sublines in vivo and in vitro. Neoplasia. 2001;3:80–87. doi: 10.1038/sj.neo.7900133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Hellwig-Burgel T, Stiehl DP, Wagner AE, Metzen E, Jelkmann W. Review: hypoxia-inducible factor-1 (HIF-1): a novel transcription factor in immune reactions. J Interferon Cytokine Res. 2005;25:297–310. doi: 10.1089/jir.2005.25.297. [DOI] [PubMed] [Google Scholar]
- 342.Kim J. w., Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 343.Kaidi A, Qualtrough D, Williams AC, Paraskeva C. Direct Transcriptional Up-regulation of Cyclooxygenase-2 by Hypoxia-Inducible Factor (HIF)-1 Promotes Colorectal Tumor Cell Survival and Enhances HIF-1 Transcriptional Activity during Hypoxia. Cancer Res. 2006;66:6683–6691. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 344.Csiki I, Yanagisawa K, Haruki N, Nadaf S, Morrow JD, Johnson DH, Carbone DP. Thioredoxin-1 Modulates Transcription of Cyclooxygenase-2 via Hypoxia-Inducible Factor-1{alpha} in Non-Small Cell Lung Cancer. Cancer Res. 2006;66:143–150. doi: 10.1158/0008-5472.CAN-05-1357. [DOI] [PubMed] [Google Scholar]
- 345.Liu XH, Kirschenbaum A, Lu M, Yao S, Dosoretz A, Holland JF, Levine AC. Prostaglandin E2 Induces Hypoxia-inducible Factor-1alpha Stabilization and Nuclear Localization in a Human Prostate Cancer Cell Line. J.Biol.Chem. 2002;277:50081–50086. doi: 10.1074/jbc.M201095200. [DOI] [PubMed] [Google Scholar]
- 346.Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, Czernin J, Sawyers CL. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 347.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother.Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 348.Stubbs M, McSheehy PM, Griffiths JR. Causes and consequences of acidic pH in tumors: a magnetic resonance study. Adv.Enzyme Regul. 1999;39:13–30. doi: 10.1016/s0065-2571(98)00018-1. [DOI] [PubMed] [Google Scholar]
- 349.Schlappack OK, Zimmermann A, Hill RP. Glucose starvation and acidosis: effect on experimental metastatic potential, DNA content and MTX resistance of murine tumour cells. Br J Cancer. 1991;64:663–670. doi: 10.1038/bjc.1991.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Sun X, Kanwar JR, Leung E, Lehnert K, Wang D, Krissansen GW. Gene transfer of antisense hypoxia inducible factor-1 alpha enhances the therapeutic efficacy of cancer immunotherapy. Gene Ther. 2001;8:638–645. doi: 10.1038/sj.gt.3301388. [DOI] [PubMed] [Google Scholar]
- 351.Tso CL, Zisman A, Pantuck A, Calilliw R, Hernandez JM, Paik S, Nguyen D, Gitlitz B, Shintaku PI, de Kernion J, Figlin R, Belldegrun A. Induction of G250-targeted and T-cell-mediated antitumor activity against renal cell carcinoma using a chimeric fusion protein consisting of G250 and granulocyte/monocyte-colony stimulating factor. Cancer Res. 2001;61:7925–7933. [PubMed] [Google Scholar]
- 352.Jones MK, Szabo IL, Kawanaka H, Husain SS, Tarnawski AS. von Hippel Lindau tumor suppressor and HIF-1alpha: new targets of NSAIDs inhibition of hypoxia-induced angiogenesis. Faseb J. 2002;16:264–266. doi: 10.1096/fj.01-0589fje. [DOI] [PubMed] [Google Scholar]
- 353.Palayoor ST, Tofilon PJ, Coleman CN. Ibuprofen-mediated Reduction of Hypoxia-inducible Factors HIF-1{alpha} and HIF-2{alpha} in Prostate Cancer Cells. Clin Cancer Res. 2003;9:3150–3157. [PubMed] [Google Scholar]
- 354.MacNeil B, Hoffman-Goetz L. Chronic exercise enhances in vivo and in vitro cytotoxic mechanisms of natural immunity in mice. J Appl Physiol. 1993;74:388–395. doi: 10.1152/jappl.1993.74.1.388. [DOI] [PubMed] [Google Scholar]
- 355.Kohut ML, Thompson JR, Lee W, Cunnick JE. Exercise training-induced adaptations of immune response are mediated by beta-adrenergic receptors in aged but not young mice. J Appl Physiol. 2004;96:1312–1322. doi: 10.1152/japplphysiol.00792.2003. [DOI] [PubMed] [Google Scholar]
- 356.Weindruch RH, Kristie JA, Naeim F, Mullen BG, Walford RL. Influence of weaning-initiated dietary restriction on responses to T cell mitogens and on splenic T cell levels in a long-lived F1-hybrid mouse strain. Exp Gerontol. 1982;17:49–64. doi: 10.1016/0531-5565(82)90008-0. [DOI] [PubMed] [Google Scholar]
- 357.Weber RV, Stein DE, Scholes J, Kral JG. Obesity potentiates AOM-induced colon cancer. Dig Dis Sci. 2000;45:890–895. doi: 10.1023/a:1005560621722. [DOI] [PubMed] [Google Scholar]
- 358.Hakkak R, Holley AW, Macleod SL, Simpson PM, Fuchs GJ, Jo CH, Kieber-Emmons T, Korourian S. Obesity promotes 7,12-dimethylbenz(a)anthracene-induced mammary tumor development in female zucker rats. Breast Cancer Res. 2005;7:R627–R633. doi: 10.1186/bcr1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Mito N, Hosoda T, Kato C, Sato K. Change of cytokine balance in diet-induced obese mice. Metabolism. 2000;49:1295–1300. doi: 10.1053/meta.2000.9523. [DOI] [PubMed] [Google Scholar]