Abstract
Background: Our previous study has shown that nuclear factor-kappa B (NF-κB)-signaling pathway was associated with a higher rate of recurrence in head and neck squamous cell carcinoma (HNSCC). The combination of bortezomib, an NF-κB inhibitor by inhibition of proteasomes, plus docetaxel was assessed for efficacy and toxicity.
Materials and methods: Patients with recurrent and/or metastatic HNSCC were enrolled on a phase II bortezomib/docetaxel trial (bortezomib 1.6 mg/m2 and docetaxel 40 mg/m2 on days 1 and 8 of a 21-day cycle). Response was assessed using RECIST. Tissue specimens were evaluated for the presence of human papillomavirus (HPV) and expression of NF-κB-associated genes.
Results: Twenty-one of 25 enrolled patients were assessable for response; one partial response (PR, 5%), 10 stable disease (SD, 48%) and 10 progressive disease (PD, 48%). Patients with PR/SD had significantly longer survival compared with patients with PD and the regimen was well tolerated. Only one of 20 tumors was positive for HPV. Patients with PD had higher expression of NF-κB and epidermal growth factor receptor-associated genes in their tumors by gene expression analysis.
Conclusion: Further understanding of treatment resistance and interactions between bortezomib and docetaxel may provide novel approaches in managing HNSCC.
Keywords: bortezomib, docetaxel, epidermal growth factor receptor, head and neck squamous cell carcinoma, nuclear factor-kappa B
introduction
Head and neck squamous cell carcinoma (HNSCC) remains one of the most devastating cancers in the world. Recent studies have shown that patients with human papillomavirus (HPV) infection have significantly better prognosis compared with HPV-negative (−) patients; however, ∼10% of HPV-positive (+) and 40%–50% of HPV(−) patients have recurrences [1, 2]. Once it recurs, therapeutic options are limited. Therapeutic agents employed in the management of recurrent and/or metastatic (R/M) HNSCC include methotrexate, taxanes, 5-fluorouracil (5-FU), platinum and cetuximab. Single-agent response rates range from 10% to 40% [3–6]. Combination therapy with cetuximab has demonstrated improvement in survival; however, median survival remains brief at 9.2–10.1 months [7, 8]. Thus, identification of new targets with active agents and combination regimens is urgently needed in the management of R/M HNSCC.
Nuclear factor-kappa B (NF-κB) is overexpressed in HNSCC [9]. Furthermore, its expression is associated with poor prognosis [9]. In our previous gene expression study, we also showed that tumors with high risk of recurrence harbored higher expression of genes that are associated with the activation of NF-κB signaling [10]. Therefore, we hypothesized that the patients with recurrence would benefit from NF-κB inhibitors. Bortezomib is a small-molecule proteasome inhibitor [11]. While it affects multiple signaling pathways, one of the mechanisms of antitumor activity is NF-κB inhibition [11, 12]. Bortezomib has also been shown to inhibit tumor growth, induce apoptosis and decrease vessel density in HNSCC in vitro and in vivo [13–15]. Docetaxel is a taxane that promotes tubulin assembly into microtubules and inhibits microtubule depolymerization inducing antitumor activity [16, 17]. In early studies, docetaxel showed overall response rates of 21%–42% and median response duration of 4.7–6.5 months as a monotherapy in R/M HNSCC [5, 18, 19].
A phase I/II study of the combination of bortezomib and docetaxel was conducted in patients with advanced androgen-independent prostate cancer and showed good tolerability and antitumor activity [20]. In HNSCC cell lines, the combination showed significantly increased growth inhibition compared with bortezomib or docetaxel alone [15]. Based on these data, we conducted a phase II trial of combination bortezomib and docetaxel to determine the efficacy and toxicity in R/M HNSCC patients. Also, HPV status of the tumors was determined to assess an interaction with the response rate. To determine the possible mechanism of resistance, the tumors were examined for differential expression of NF-κB pathway-related genes using DNA microarrays.
materials and methods
patient eligibility and baseline assessment
The protocol was approved by the Institutional Review Board at the Vanderbilt University School of Medicine. All patients signed a written informed consent. The inclusion criteria for the study were the following: (i) patients with R/M squamous cell carcinoma of the oral cavity, oropharynx, hypopharynx, larynx and unknown primary with cervical lymph node metastasis; (ii) patients may not have received prior chemotherapy for recurrent disease but one prior chemotherapy regimen was allowed for distant metastatic disease at diagnosis (initial stage IVC); (iii) patients must not be candidates for curative therapy; (iv) patients must have a measurable disease defined by RECIST [21]; (v) patients with Eastern Cooperative Oncology Group performance status of zero, one or two; (vi) peripheral neuropathy must be grade 1 or lower; (vii) hematologic criteria with an absolute neutrophil count ≥1500/mm3, hemoglobin level >8.0 g/dl and platelet count ≥100 000/mm3 and (viii) hepatic criteria with total bilirubin within normal limits and hepatic transaminases ≤5 times the upper limits of normal.
The exclusion criteria for the study were the following: (i) patients with a platelet count of <100 000/mm3, an absolute neutrophil count of <1500/mm3, a serum creatinine clearance of >2.0 mg/dl and grade 2 or higher peripheral neuropathy within 28 days before enrollment; (ii) prior malignancy within the previous 3 years except early-stage nonmelanomatous skin cancers, carcinoma in situ of the cervix or early-stage prostate cancer; (iii) patients have received other investigational drugs within 28 days before enrollment and (iv) patients have a known hypersensitivity to bortezomib, boron, mannitol, docetaxel or other drugs formulated with polysorbate 80.
chemotherapy regimen
For the first treatment cycle, patients received bortezomib 1.6 mg/m2 infused i.v. as a 3- to 5-s bolus on day 1. On day 8, patients received bortezomib 1.6 mg/m2 followed by docetaxel 40 mg/m2 i.v. for 30 min. On all subsequent cycles, patients received both bortezomib and docetaxel on days 1 and 8 of a 21-day cycle as described by Dreicer et al. [20]. Treatment was continued until progression, undue toxicity or patient withdrawal of consent. Response was assessed every two cycles (6 weeks) using RECIST [21].
The toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria version 3 [22]. Dose modifications were made for grade 3 and 4 toxic effects. Dose reductions of docetaxel and bortezomib were (i) dose level −1 at docetaxel 30 mg/m2 and bortezomib 1.3 mg/m2 and (ii) dose level −2 at docetaxel 20 mg/m2 and bortezomib 1.0 mg/m2. Dose reductions were permanent. If the dose level −2 could not be tolerated by a patient, the patient was removed from the study.
comparison of response assessments by RECIST and World Health Organization criteria
Because the response rates of the early docetaxel monotherapy studies were determined by the 1979 World Health Organization (WHO) criteria, tumor measurements were repeated using the WHO criteria for comparison with RECIST used in the protocol [21, 23].
statistical consideration of the clinical trial
The primary end point of the study was overall response rate of the combination of bortezomib and docetaxel. Simon’s (1989) optimal two-stage design was used to test the null hypothesis that the true response rate was 10% against the alternative that it was at least 25%. Twenty-one patients would be enrolled in the first stage. If two or fewer responses were observed, the trial would be terminated for lack of activity. Otherwise, an additional 29 patients would be entered for a total of 50. This design had an alpha level of 0.10 and 90% power under the alternative hypothesis. The probability of stopping after the first stage was 0.65 if the true response rate was only 10%.
The overall survival (OS) time was defined as the time from the study enrollment to death or to last follow-up date. The progression-free survival (PFS) time was defined as the time from the study enrollment to date of disease progression. Univariate survival analysis was carried out using log-rank test using the SAS/STAT software package (SAS Institute, Research Triangle Park, NC) and plotted as a Kaplan–Meier curve.
HPV detection in patients’ tumors
Using sterile surgical blades, slides containing sections cut from formalin-fixed paraffin-embedded (FFPE) tumor blocks were scraped, and DNA was isolated using Ambion RecoverAll™ Total Nucleic Acid Isolation Kit (Ambion Inc., Austin, TX) according to the manufacturer's protocol. The tumor DNA was tested for the presence of HPV DNA using a previously established PCR-based method [24]. This method employs degenerate PCR primers (MY09 and MY11, WD72/76 and WD66/67/154) that are designed to represent highly conserved HPV L1 and E6 sequences present in all major types of HPV. As a positive internal control for amplification, primers for β-globin were included with each sample. As positive controls for HPV specificity of PCR, DNA samples from HPV(+) cell lines, HeLa and SiHa, were used as previously described [25].
DNA microarray analyses
Gene expression analyses were carried out using 25 samples (five frozen and 18 FFPE tumors and two normal adjacent mucosal epithelia) from 16 patients from the trial. The hematoxylin–eosin-stained slides for each tumor were examined and areas with >70% tumor cellularity were chosen for macrodissection. Total RNA isolation was carried out using Ambion RecoverAll™ Total Nucleic Acid Isolation Kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Demethylation step was carried out by incubating the RNA in 50 mM Tris–HCl (pH 8) at 70°C for 10 min. The RNA was amplified using Nugen WT-Ovation FFPE amplification and labeling kits (Nugen Technologies, Inc., San Carlos, CA) according to the manufacturer's protocol. The labeled and fragmented RNA was loaded on to the Affymetrix Human Genome U133 plus 2.0 GeneChip (Affymetrix, Santa Clara, CA) and processed according to the manufacturer's recommendations. The raw microarray data were uploaded to the Vanderbilt Microarray Shared Resource database.
From a published study, 277 unique genes associated with activation of NF-κB on murine expression arrays were obtained [26]. By Unigene mapping, 111 of the 277 unique genes were present on the Affymetrix Human Genome U133 plus 2.0 GeneChip and the expression levels of these genes were examined in the 25-sample dataset. Furthermore, to determine the differentially expressed genes between the tumors from patients with partial response (PR)/stable disease (SD) and with progressive disease (PD), a supervised analysis of 25 samples was carried out using Significance Analysis of Microarray [27]. The genes with false discovery rate (FDR) <5% and greater than twofold difference in the expression levels were selected to be statistically significant.
results
patient characteristics
Twenty-five patients were enrolled on the study from August 2005 to April 2008 at Vanderbilt University Medical Center and Vanderbilt-Ingram Cancer Center Affiliate Network; 21 patients were assessable for response. Three patients withdrew their consent and one patient was not assessable for response. Detailed patient characteristics are summarized in Table 1. Patient demographics were representative of the R/M HNSCC patient population in previously published studies [6, 28].
Table 1.
Patient characteristics
| Characteristics | N = 25 |
| Age (median) | 56 |
| Sex | |
| Male | 18 |
| Female | 7 |
| Race | |
| White | 22 |
| Black | 2 |
| Other | 1 |
| Performance status | |
| 0 | 3 |
| 1 | 20 |
| 2 | 2 |
| Tumor sites | |
| Oral cavity | 8 |
| Oropharynx | 11 |
| Hypopharynx | 1 |
| Larynx | 4 |
| Unknown primary | 1 |
| Stage at diagnosis | |
| I–II | 2 |
| III–IV | 22 |
| Unknown | 1 |
| Tumor grade | |
| Well | 1 |
| Moderate | 12 |
| Poor | 9 |
| Unknown | 3 |
| Pattern of failure | |
| LC | 9 |
| DM | 4 |
| Both LC and DM | 12 |
| Primary treatments at diagnosis | |
| Surgery alone | 3 |
| Surgery + chemo + XRT | 6 |
| Surgery + XRT | 3 |
| Chemo alone | 5 |
| Chemo + XRT | 8 |
| Response | |
| Partial response | 1 |
| Stable disease | 10 |
| Progressive disease | 10 |
| Not evaluable | 4 |
LC, locoregional; DM, distant metastasis; XRT, radiation.
assessment of clinical response and toxicity
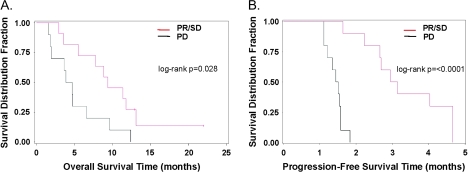
The median follow-up time across all patients was 5.1 months. Clinical response was assessed by RECIST according to the protocol. Among the 21 assessable patients, there was one (5%) PR, 10 (48%) SD and 10 (48%) PD. [SD was defined as less than a 50% reduction and less than a 25% increase in the sum of the products of two perpendicular diameters of all measured lesions and the appearance of no new lesions and the appearance of no new lesions.] The median duration of clinical benefit was 3.0 months in patients with PR or SD. The median OS was 6.6 months. Compared with patients with PD, patients with PR or SD had longer median OS (9.4 versus 4.3 months; log-rank test P = 0.028; Figure 1) and PFS (3.0 versus 1.5 months; log-rank test P < 0.0001). The trial was terminated because the required number of responses was not seen to allow progression to the second stage.
Figure 1.
Kaplan–Meier plots comparing patients with partial response (PR) and stable diseases (SD) versus progressive disease (PD). (A) Overall survival; median survival time 9.4 versus 4.3 months; log-rank test P = 0.028. (B) Progression-free survival; median survival time 3.0 versus 1.5 months; log-rank test P <0.0001. Red line: PR/SD; black line: PD.
Because the response rate was significantly lower than the historical controls of docetaxel monotherapy, the tumors from 12 patients with available imaging studies were measured again retrospectively using the WHO criteria [5, 18, 19, 23]. Responses by the WHO criteria were six SD (50%) and six PD (50%). One patient with PR by RECIST could not be reevaluated because one of the measured lesions was a clinical measurement and a bidimensional measurement could not be obtained retrospectively. The two response criteria were similar except that two patients with SD by RECIST were determined to be PD by WHO criteria.
Overall, the regimen was well tolerated. The detailed toxicity assessments were summarized in Table 2. Two deaths were reported while on study: one patient died of progression of disease and one patient died suddenly of unknown cause. Two grade 4 toxic effects were reported: one patient developed hypercalcemia secondary to disease and one patient developed severe bleeding also from disease. Grade 3 toxic effects were confined to gastrointestinal complaints including nausea, vomiting and diarrhea.
Table 2.
Common toxic effects: serious adverse events
| Grade | Category | Toxicity | Total no. of patients (N = 25) |
| Grade 1 | Allergy/immunology | Allergy/immunology—other | 1 |
| Dermatology/skin | Dermatology/skin—other | 1 | |
| Metabolic/laboratory | Sodium, serum—low (hyponatremia) | 1 | |
| Grade 2 | Neurology | Mental status | 1 |
| Cardiac general | Hypotension | 1 | |
| Dermatology/skin | Flushing | 1 | |
| Gastrointestinal | Dehydration | 1 | |
| Nausea | 1 | ||
| Musculoskeletal/soft tissue | Fracture | 1 | |
| Pain | Pain—other | 1 | |
| Pulmonary/upper respiratory | Dyspnea | 1 | |
| Pulmonary/upper respiratory—other | 1 | ||
| Grade 3 | Gastrointestinal | Nausea | 1 |
| Vomiting | 3 | ||
| Diarrhea | 3 | ||
| Mucositis/stomatitis—oral cavity | 1 | ||
| Neurology | Seizure | 1 | |
| Grade 4 | Metabolic/laboratory | Calcium, serum high (hypercalcemia) | 1 |
| Hemorrhage/bleeding | Hemorrhage, gastrointestinal—oral cavity | 1 | |
| Grade 5 | Death | Death not associated with CTCAE term—disease progression NOS | 1 |
| Death not associated with CTCAE term—sudden death | 1 |
CTCAE, Common Toxicity Criteria Adverse Event; NOS, not otherwise specified.
determination of HPV status in patients’ tumors
Twenty tumors from 21 assessable patients were available for HPV testing and 10 of 20 tumors were from oropharynx. Only one tumor from oropharynx (5% of all tumors or 10% of oropharyngeal tumors) was positive for HPV. The patient with the HPV(+) tumor had PD. This is consistent with the findings that patients with HPV(+) tumors have less recurrence and better survival compared with HPV(−) patients [1].
correlation between gene expression of NF-κB-associated genes and clinical response to bortezomib and docetaxel
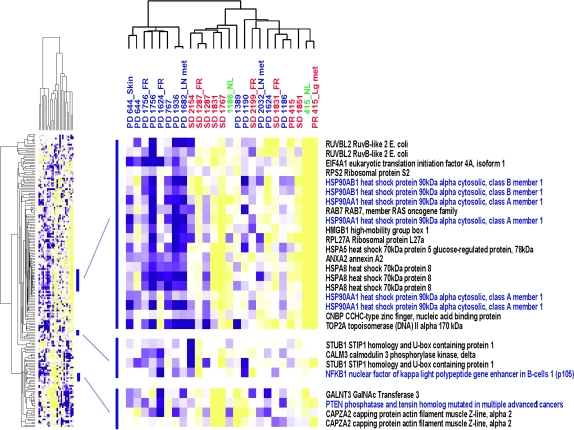
The 111 genes that associate with activation of the NF-κB-signaling pathway were examined in a microarray dataset of 25 samples obtained from 16 patients in the trial (Figure 2). When the expression signature was compared between tumors from patients with PR/SD or PD, the tumors from patients with PD had higher expression of activated NF-κB-associated genes including NF-κB1 (p105), heat shock proteins (HSP) 70 and 90, topoisomerase II and phosphatase and tensin homolog. This indicates that the NF-κB pathway activity was relatively greater in tumors that progressed and that higher NF-κB activity may correspond with bortezomib resistance.
Figure 2.
Hierarchical clustering of 25 samples using the 111-gene nuclear factor-kappa B (NF-κB) signature known to be modulated by NF-κB as previously published [10, 26]. Red: PR, partial response and SD, stable disease; blue: PD, progressive disease; green: normal mucosa adjacent to the tumors; purple squares: higher gene expression; yellow squares: lower gene expression.
differentially expressed genes between tumors with PR/SD and PD
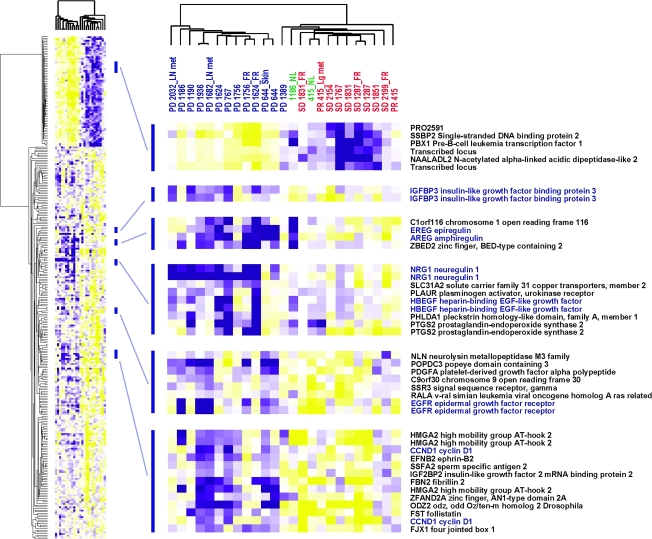
To identify a set of genes that associate with bortezomib and docetaxel treatment resistance, supervised analysis of the array data was carried out using PR/SD and PD as supervising parameters. There were 239 unique genes that passed the filtering criteria of greater than twofold difference and FDR <5% (supplemental Table 1, available at Annals of Oncology online and Figure 3). The most predominant pathway among the differentially expressed genes was the epidermal growth factor receptor (EGFR) pathway, including higher expression of EGFR itself and its ligands epiregulin, amphiregulin, neuregulin 1 and heparin-binding epidermal growth factor-like growth factor. Also, insulin-like growth factor-binding protein 3 as well as cell cycle regulators such as cyclin D1 had higher expression in the tumors with PD.
Figure 3.
Hierarchical clustering of 25 samples using the 239 genes that were differentially expressed between tumors with partial response (PR)/stable disease (SD) and progressive disease (PD). Red: PR/SD; blue: PD; green: normal mucosa adjacent to the tumors; purple squares: higher gene expression; yellow squares: lower gene expression.
discussion
Our previous data indicated that patients with high risk of recurrence have activation of the NF-κB-signaling pathway [10]. Bortezomib has been shown to inhibit NF-κB activation and to enhance the cytotoxicity of docetaxel with known efficacy in HNSCC [5, 13–15, 18, 19]. Therefore, we conducted a phase II study with a combination of bortezomib and docetaxel. The response rate for single-agent docetaxel as reported for published phase II trials ranged from 21% to 42% [5, 18, 19]. In our study, the response rate was lower than anticipated. We considered the differences in response criteria because the early studies assessed response using the WHO criteria rather than with RECIST; however, the response assessments of the two criteria were similar. The lower response rate could be explained by several other factors: (i) the dose of docetaxel and/or bortezomib chosen for this trial may have been suboptimal, (ii) our patients’ tumors may have been resistant to therapy as a result of extensive pretreatment while many earlier studies were carried out before patients were treated with upfront intensive multimodality therapies including high-dose induction chemotherapy, concurrent chemoradiation therapy or reirradiation and (iii) bortezomib had an anticytotoxic effect when combined with docetaxel.
There are several reasons why the dose and schedule may have been suboptimal in our study. In earlier phase II docetaxel monotherapy studies by Catimel, Dreyfuss and Couteau, patients were treated with docetaxel 100 mg/m2 every 21 days [5, 18, 19]. This is above the currently recommended dose of 75 mg/m2 every 21 days for HNSCC when it is given as a combination regimen with cisplatin and 5-FU [29]. The combination regimen with bortezomib rather than cisplatin and 5-FU may require higher doses of docetaxel than the current standard in order to achieve the expected response rates. There are data to indicate that a low-dose weekly schedule was well tolerated in combination with bortezomib in prostate cancer [20]. Based on these data, our study dosed docetaxel weekly at 40 mg/m2 on days 1 and 8 (total dose of 80 mg/m2 in a 21-day cycle), but the weekly dosing schedule may have been inferior to the every 3-week high-dose regimen. Although the dose and schedule for bortezomib used in this trial was established via a phase I/II study and may represent the maximum tolerated doses of each agent, the weekly dose of bortezomib was significantly lower than the dose used in multiple myeloma which is twice a week dosing. Also, most of the patients reported in this trial were heavily pretreated with intensive concurrent chemoradiation therapy before enrollment. In comparison, the previously reported single-agent docetaxel studies contained more patients who were either chemotherapy or radiation therapy naive.
The NF-κB-signaling pathway, one of the major pathways that is regulated by proteasomes (the target of bortezomib), is active in HNSCC; thus, it is unclear why bortezomib did not improve patient outcome when added to single-agent docetaxel. One possibility is that bortezomib could not completely inhibit the NF-κB-signaling pathway in R/M HNSCC. There are five members in the NF-κB family (NF-κB1, NF-κB2, REL A, cREL and REL B) and two pathways to activate NF-κB signaling including (i) the canonical (or classic) pathway triggered mainly by tumor necrosis factor (TNF) among others and activate NF-κB1/REL A (p50/p65) or p50/cREL and (ii) the noncanonical (or alternative) pathway activated by other TNF family members signaling through NF-κB2/REL B (p52/REL B) [30, 31]. A recent study by Allen et al. [32] indicated that low-dose bortezomib could inhibit only the canonical pathway and proposed inability to inhibit activation of the noncanonical pathway as a mechanism of bortezomib resistance. To support these data, the supervised analysis of DNA microarray data comparing PR/SD and PD showed increased EGFR-associated genes. One of the downstream effectors of EGFR is AKT, which activates the noncanonical pathway of NF-κB (reviewed in Van Waes [31]). Therefore, the combination of bortezomib with either EGFR inhibitors or HSP90 inhibitors that decrease AKT would be a novel therapeutic option. It also should be considered that NF-κB may not be a main target in HNSCC. Despite the data on overexpression and activation of the pathway in a subset of HNSCC, it remains unclear if this is a passenger rather than an oncogenic driver. Furthermore, in a study using lung cancer cells as a model, it was indicated that the sequence of administering bortezomib and docetaxel could affect the induction of apoptosis [33]. The findings were that bortezomib given first before docetaxel or the two drugs given together would arrest the cell cycle in sub-G1 and antagonize the effect of docetaxel which induces cytotoxicity in cells undergoing mitosis. Although it was a limited study using cell lines, these are intriguing data because docetaxel and bortezomib were given together in our trial rather than using the presumed effective sequence of docetaxel first followed by bortezomib after a certain time interval.
In summary, our study showed that the addition of bortezomib to docetaxel did not improve efficacy. Because bortezomib affects multiple pathways, our study does not exclude NF-κB as a valid target of novel therapeutic approaches. Further studies are needed to determine NF-κB as an effective target and identify other pathways that are involved in resistance to bortezomib and docetaxel therapy as well as novel therapeutic targets in R/M HNSCC.
funding
Damon Runyon Clinical Investigator Award (CI-28-05); Robert J. and Helen C. Kleberg Foundation; National Institutes of Health (R01-DE-017982) to C.H.C.; Millennium Pharmaceuticals, Inc. and Sanofi-Aventis, Inc.
disclosure
CHC and AJC received honoraria from Bristol-Myers Squibb and Amgen for educational lectures and served on an advisory board. Other authors declared no conflict of interest.
Supplementary Material
References
- 1.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 2.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 3.Urba SG, Forastiere AA. Systemic therapy of head and neck cancer: most effective agents, areas of promise. Oncology (Huntingt) 1989;3:79–88. discussion 88. [PubMed] [Google Scholar]
- 4.Kies MS, Levitan N, Hong WK. Chemotherapy of head and neck cancer. Otolaryngol Clin North Am. 1985;18:533–541. [PubMed] [Google Scholar]
- 5.Couteau C, Chouaki N, Leyvraz S, et al. A phase II study of docetaxel in patients with metastatic squamous cell carcinoma of the head and neck. Br J Cancer. 1999;81:457–462. doi: 10.1038/sj.bjc.6690715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibson MK, Li Y, Murphy B, et al. Randomized phase III evaluation of cisplatin plus fluorouracil versus cisplatin plus paclitaxel in advanced head and neck cancer (E1395): an intergroup trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2005;23:3562–3567. doi: 10.1200/JCO.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 7.Burtness B, Goldwasser MA, Flood W, et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol. 2005;23:8646–8654. doi: 10.1200/JCO.2005.02.4646. [DOI] [PubMed] [Google Scholar]
- 8.Vermorken JB, Mesia R, Rivera F, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359:1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 9.Zhang PL, Pellitteri PK, Law A, et al. Overexpression of phosphorylated nuclear factor-kappa B in tonsillar squamous cell carcinoma and high-grade dysplasia is associated with poor prognosis. Mod Pathol. 2005;18:924–932. doi: 10.1038/modpathol.3800372. [DOI] [PubMed] [Google Scholar]
- 10.Chung CH, Parker JS, Ely K, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-{kappa}B signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006;66:8210–8218. doi: 10.1158/0008-5472.CAN-06-1213. [DOI] [PubMed] [Google Scholar]
- 11.Millennium Pharmaceuticals, Inc. Investigator Brochure, PS-341 (Bortezomib) for Injection. Cambridge, MA: Millennium Pharmaceuticals, Inc; 2004. [Google Scholar]
- 12.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 13.Lun M, Zhang PL, Pellitteri PK, et al. Nuclear factor-kappaB pathway as a therapeutic target in head and neck squamous cell carcinoma: pharmaceutical and molecular validation in human cell lines using Velcade and siRNA/NF-kappaB. Ann Clin Lab Sci. 2005;35:251–258. [PubMed] [Google Scholar]
- 14.Sunwoo JB, Chen Z, Dong G, et al. Novel proteasome inhibitor PS-341 inhibits activation of nuclear factor-kappa B, cell survival, tumor growth, and angiogenesis in squamous cell carcinoma. Clin Cancer Res. 2001;7:1419–1428. [PubMed] [Google Scholar]
- 15.Wagenblast J, Hambek M, Baghi M, et al. Antiproliferative activity of bortezomib alone and in combination with cisplatin or docetaxel in head and neck squamous cell carcinoma cell lines. J Cancer Res Clin Oncol. 2008;134:323–330. doi: 10.1007/s00432-007-0287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83:288–291. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz SB, Lothstein L, Manfredi JJ, et al. Taxol: mechanisms of action and resistance. Ann N Y Acad Sci. 1986;466:733–744. doi: 10.1111/j.1749-6632.1986.tb38455.x. [DOI] [PubMed] [Google Scholar]
- 18.Dreyfuss AI, Clark JR, Norris CM, et al. Docetaxel: an active drug for squamous cell carcinoma of the head and neck. J Clin Oncol. 1996;14:1672–1678. doi: 10.1200/JCO.1996.14.5.1672. [DOI] [PubMed] [Google Scholar]
- 19.Catimel G, Verweij J, Mattijssen V, et al. Docetaxel (Taxotere): an active drug for the treatment of patients with advanced squamous cell carcinoma of the head and neck. EORTC Early Clinical Trials Group. Ann Oncol. 1994;5:533–537. doi: 10.1093/oxfordjournals.annonc.a058908. [DOI] [PubMed] [Google Scholar]
- 20.Dreicer R, Petrylak D, Agus D, et al. Phase I/II study of bortezomib plus docetaxel in patients with advanced androgen-independent prostate cancer. Clin Cancer Res. 2007;13:1208–1215. doi: 10.1158/1078-0432.CCR-06-2046. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. 2006. http://ctep.cancer.gov 31 March 2003, (accessed 20 October 2009) [Google Scholar]
- 23.Miller AB, Hoogstraten B, Staquet M, et al. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Resnick RM, Cornelissen MT, Wright DK, et al. Detection and typing of human papillomavirus in archival cervical cancer specimens by DNA amplification with consensus primers. J Natl Cancer Inst. 1990;82:1477–1484. doi: 10.1093/jnci/82.18.1477. [DOI] [PubMed] [Google Scholar]
- 25.Slebos RJ, Yi Y, Ely K, et al. Gene expression differences associated with human papillomavirus status in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:701–709. doi: 10.1158/1078-0432.CCR-05-2017. [DOI] [PubMed] [Google Scholar]
- 26.Loercher A, Lee TL, Ricker JL, et al. Nuclear factor-kappaB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res. 2004;64:6511–6523. doi: 10.1158/0008-5472.CAN-04-0852. [DOI] [PubMed] [Google Scholar]
- 27.Tusher V, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gilbert J, Cmelak A, Shyr Y, et al. Phase II trial of irinotecan plus cisplatin in patients with recurrent or metastatic squamous carcinoma of the head and neck. Cancer. 2008;113:186–192. doi: 10.1002/cncr.23545. [DOI] [PubMed] [Google Scholar]
- 29.Taxotere® (Docetaxel) Prescribing Information. Sanofi-Aventis U.S. LLC; 2008. [Google Scholar]
- 30.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 31.Van Waes C. Nuclear factor-kappaB in development, prevention, and therapy of cancer. Clin Cancer Res. 2007;13:1076–1082. doi: 10.1158/1078-0432.CCR-06-2221. [DOI] [PubMed] [Google Scholar]
- 32.Allen C, Saigal K, Nottingham L, et al. Bortezomib-induced apoptosis with limited clinical response is accompanied by inhibition of canonical but not alternative nuclear factor-{kappa}B subunits in head and neck cancer. Clin Cancer Res. 2008;14:4175–4185. doi: 10.1158/1078-0432.CCR-07-4470. [DOI] [PubMed] [Google Scholar]
- 33.Jung CS, Zhou Z, Khuri FR, Sun SY. Assessment of apoptosis-inducing effects of docetaxel combined with the proteasome inhibitor PS-341 in human lung cancer cells. Cancer Biol Ther. 2007;6:749–754. doi: 10.4161/cbt.6.5.3977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.