Abstract
OBJECTIVE
Newer medications offer more options for glycemic control in type 2 diabetes. However, they come at considerable costs. We undertook a health economic analysis to better understand the value of adding two newer medications (exenatide and sitagliptin) as second-line therapy to glycemic control strategies for patients with new-onset diabetes.
RESEARCH DESIGN AND METHODS
We performed a cost-effectiveness analysis for the U.S. population aged 25–64. A lifetime analytic horizon and health care system perspective were used. Costs and quality-adjusted life years (QALYs) were discounted at 3% annually, and costs are presented in 2008 U.S. dollars. We compared three glycemic control strategies: 1) glyburide as a second-line agent, 2) exenatide as a second-line agent, and 3) sitagliptin as a second-line agent. Outcome measures included QALYs gained, incremental costs, and the incremental cost-effectiveness ratio associated with each strategy.
RESULTS
Exenatide and sitagliptin conferred 0.09 and 0.12 additional QALYs, respectively, relative to glyburide as second-line therapy. In base case analysis, exenatide was dominated (cost more and provided fewer QALYs than the next most expensive option), and sitagliptin was associated with an incremental cost-effectiveness ratio of $169,572 per QALY saved. Results were sensitive to assumptions regarding medication costs, side effect duration, and side effect–associated disutilities.
CONCLUSIONS
Exenatide and sitagliptin may confer substantial costs to health care systems. Demonstrated gains in quality and/or quantity of life are necessary for these agents to provide economic value to patients and health care systems.
Diabetes is increasingly endemic in the U.S. In 2007, 23.5 million Americans aged >20 years had diabetes compared with 18.0 million in 2002 (1). Diabetes was the seventh leading cause of death in 2006 (1). It remains the leading cause of blindness, end-stage renal disease, and nontraumatic amputations. A total of $116 billion in direct health care costs are attributable to diabetes annually (2).
Large clinical trials from the U.S. and Europe have demonstrated that tighter glycemic control can prevent diabetes complications in individuals with recent-onset disease (3,4); in older individuals with longer disease duration, recent studies have found no cardiovascular benefit of tight control (5) and possible harm (6). In the past several years, the U.S. Food and Drug Administration (FDA) approved nine new products for glycemic control (7). Some are new forms or combinations of existing classes, whereas others belong to new therapeutic classes such as amylin analogs, glucagon-like peptide-1 receptor agonists, incretins, and dipeptidyl peptidase-IV inhibitors.
Although these agents increase the management options available, they come at increased costs (8). Previous analyses of the health economics of glycemic control were published before the FDA approval of many new agents (9–11). Recent studies have examined the cost-effectiveness of exenatide or sitagliptin in European populations, reflecting costs and management appropriate for the modeled populations but not necessarily reflective of the U.S. (12–14).
In this analysis, we estimate the costs associated with two of the most prescribed examples of these new medications: exenatide and sitagliptin. We project the gains in health outcomes necessary to have these newer medications pose good economic value for patients with new-onset diabetes, using the incremental cost-effectiveness ratio as our metric.
RESEARCH DESIGN AND METHODS
Our model is an extension of a previously published model (11), using as its platform the published model's analytic algorithm but changing treatment regimens and inputs, in keeping with the newer medications being considered. Adults enter the analysis at diabetes diagnosis and progress through the model until death or age 95. Only patients between the ages of 25 and 64 with newly diagnosed diabetes are included. It is assumed that there is a 10-year lag between diabetes onset and diagnosis.
Patients have an annual risk of diabetes complications, modified by age, race, and sex, time since diabetes onset, time since diagnosis, treatment, A1C achieved, smoking, hypertension, and/or concomitant hypercholesterolemia. It is assumed that hypertensive patients develop complications more rapidly than nonhypertensive patients and that glycemic control has no impact on the progression of coronary heart disease. Costs accrue due to diabetes treatment and treatment of diabetes complications. Costs are averted when complications are averted (15).
The summary metric used to estimate the value of exenatide and sitagliptin is the incremental cost-effectiveness ratio (ICER). In this analysis, ICER = (costs of treatment–averted diabetes complication-related costs)/(quality-adjusted life years [QALYs] gained). Costs were calculated from a health care system perspective, using a lifetime analytic horizon. Key model assumptions are summarized in Tables 1 and 2.
Table 1.
Assumptions regarding model parameters
| Value | Reference | |
|---|---|---|
| Diabetes-related parameters | ||
| Interval between onset of diabetes and diagnosis (years) | 10 | 15 |
| Average hemoglobin A1C at time of diagnosis (%) | 6.80 | 15 |
| Treatment impact on A1C (%) | −2.90 | 15 |
| Rate of change in A1C, on treatment (%) | 0.20 | 15 |
| Hazard rates | ||
| Normal to microalbuminuria | 0.02371 | |
| Microalbuminuria to nephropathy | 0.06561 | |
| Normal to peripheral neuropathy | 0.0294 | |
| Normal to photocoagulation | 0.0079 | |
| Side effect–related parameters | ||
| Probability of weight gain while on glyburide (first 2 years) (%) | 100 | 33 |
| Probability of weight loss while on exenatide (first 2 years) (%) | 100 | 33 |
| Probability of hypoglycemia (%) | ||
| Glyburide | 36.10 | 12 |
| Sitagliptin | 6.20 | 12 |
| Exenatide | 16.00 | 34 |
| Probability of nausea/other gastrointestinal side effects while taking glyburide (%) | 4.20 | 19 |
| Probability of nausea/other gastrointestinal side effects while taking exenatide (%) | 57.00 | 13 |
| Probability of upper respiratory infection while on sitagliptin (%) | 3.50 | 19 |
| Costs per day ($) | ||
| Metformin, 2,000 mg | 1.42 | WSP, 35 |
| Glyburide, 7.5 mg | 0.42 | WSP, 35 |
| Sitagliptin, 100 mg | 6.06 | WSP, 35 |
| Exenatide, 20 μg | 8.37 | WSP, 35 |
| Rosiglitazone, 8 mg | 5.59 | WSP, 35 |
| NPH insulin, 10 units | 0.90 | WSP, 35 |
| Injection-related supplies | 0.52 | WSP, 35 |
| Annual utilities following | ||
| Blindness | 0.69 | 15 |
| Stroke | 0.5 | 15 |
| End-stage renal disease | 0.61 | 15 |
| Lower extremity amputation | 0.8 | 15 |
WSP, wholesale price.
Table 2.
Side effect–related quality-of-life assumptions
| Side effect | Glyburide | Exenatide | Sitagliptin | Reference |
|---|---|---|---|---|
| Weight gain/loss | −0.0031 | 0.0013 | 0 | 33 |
| Hypoglycemia | −0.0064 | −0.0005 | −0.0002 | 12,34,36 |
| Nausea/gastrointestinal side effects | 0 | −0.0005 | 0 | Authors' assumption; 13,33 |
| Upper respiratory infections | 0 | 0 | −0.0001 | 13 |
| Injectable | 0 | −0.0032 | 0 | Authors' assumption |
| Overall disutility associated with | ||||
| side effects, after weighting* | −0.0095 | −0.0029 | −0.0003 |
A positive number (utility) indicates a gain in quality of life, and a negative number (disutility) indicates a loss in quality of life.
*The overall disutility was calculated as the weighted sum of the side effect utilities/disutilities, where the weights were 1) the probability a patient was on a given medication at a point in time and 2) the probability the side effect occurred.
Treatment strategies
We assumed that intensive glycemic control is now the standard of care in the U.S. Three intensive glycemic control strategies were modeled: 1) glyburide as second-line treatment strategy, 2) exenatide as second-line treatment strategy, and 3) sitagliptin as second-line treatment strategy. In each strategy, patients were treated with combinations of metformin, the second-line agent specific to the strategy, rosiglitazone, and NPH insulin.
In all three strategies, patients requiring medication were given metformin initially. If glycemic control was not achieved with metformin alone, other medications were added, based on modeled rates of treatment failure (see supplementary Table, available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc09-1488/DC1). All three strategies incorporated rosiglitazone as third-line therapy.
Risks of diabetes complications
The methods used to estimate the probabilities of diabetes complications have been described elsewhere (15). In brief, probabilities depended on time since diagnosis, time between onset and diagnosis, age, sex, race, glycemic levels, smoking, cholesterol levels, and hypertension (Table 1). Time since diagnosis, glycemic level, and hypertension affected all transition probabilities. Time between onset and diagnosis affected the glycemic level at the time of diagnosis. Age, sex, smoking, and cholesterol level affected transition probabilities associated with coronary heart disease (CHD) and stroke. Race affected glycemic levels and mortality. Alternative treatment strategies affected transition probabilities by altering a patient's modeled trajectory of A1C levels over time.
Glycemic control
All three strategies were assumed to provide the same degree of glycemic control and, hence, the same effects on risks of diabetes complications. This assumption was based on results from clinical trials of sitagliptin and exenatide (16–21).
Medication side effects
Health outcomes differed on the basis of side effect profiles. Side effect profiles were developed for each of the second-line medications (glyburide, exenatide, and sitagliptin) based on literature review. Utilities (positive gains in quality of life) or disutilities (losses in quality of life) were applied to reflect these profiles. We grouped these effects into five categories: weight gain/loss, hypoglycemia, nausea/other gastrointestinal effects, upper respiratory infections, and the disutility associated with an injectable formulation. Each side effect could be experienced by the proportion of the population receiving a given drug at a given time. The effects of weight gain/loss, nausea and upper respiratory infection were assumed to last for 2 years (13); all others were assumed to last for the duration that the medication was taken.
Glyburide was associated with a weight gain of 3% experienced by all, nausea experienced by 4.2%, and hypoglycemia experienced by 36.1%. Exenatide was associated with a weight loss of 5% (experienced by all), hypoglycemia (experienced by 16%), nausea and other gastrointestinal effects (experienced by 57%), and a disutility because it was an injectable medication. Sitagliptin was associated with weight neutrality, hypoglycemia (experienced by 6.2%), and an increased risk of minor upper respiratory infections (experienced by 3.5%). All three side effect profiles resulted in a net disutility for each year the respective medication was taken (Table 2).
Management of hypertension and hypercholesterolemia
It was assumed that patients with hypertension would receive antihypertensive medications and that patients with hypercholesterolemia would be given statins. The methods used were analogous to those published previously (11).
Costs
All costs are presented in 2008 U.S. dollars (Table 1). Both costs and health benefits were discounted at 3% annually and estimated from the health care system perspective. Costs of glycemic control included the costs of the drugs themselves, the costs of equipment needed for self-injection of insulin, the costs of glucose monitoring, and the costs of outpatient care associated with routine follow-up for diabetic patients. Costs of diabetes complications were drawn from the same literature sources and used the same methods of calculation as in the model published previously (11). These costs included costs of procedures, inpatient and outpatient care, specialist visits, and medications required for the management of diabetic nephropathy, neuropathy, retinopathy, CHD, and stroke.
Health benefits
Prevention of diabetes complications results in a reduced risk of mortality and improved quality of life. In the model, strategies associated with improved glycemic control reduced the transition probabilities leading to diabetes complications at all stages, thereby reducing the risks of death due to CHD, stroke, nephropathy, or neuropathy. Retinopathy was assumed to lead to blindness but not to alter the risk of death.
Quality of life was captured by incorporating health utilities (Table 1) into the model, where a utility of 1 describes a period of time lived in perfect health and a utility of 0 is assigned to death. Utility values between 0 and 1 describe life lived in less than perfect health and are used in the calculation of QALYs (22). All analyses were performed with custom software built by the original study team (15).
RESULTS
All three strategies were assumed to confer the same benefits in terms of reductions in major health outcomes as a result of diabetes-related complications. They were assumed to differ in their side effect profiles only, and these side effects were not assumed to alter risks of complications. The impacts of these side effects were incorporated into the model as quality-of-life gains or losses.
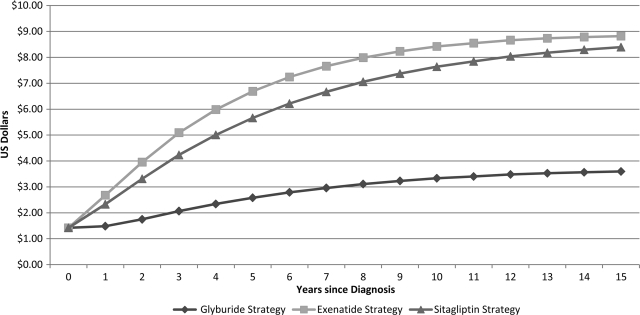
Use of sitagliptin as a second-line treatment for type 2 diabetes in adults <65 years of age is associated with additional intervention costs of $20,213 per person over a lifetime compared with a baseline strategy using glyburide as second-line therapy. Use of exenatide as a second-line treatment is associated with an additional cost of $23,849 over a lifetime compared with glyburide as second-line therapy. The differences in intervention costs among the three strategies were due to differences in medication costs, summarized in Fig. 1.
Figure 1.
Daily treatment costs. Over 15 years, the average daily treatment costs for the glyburide, sitagliptin, and exenatide strategies were $2.98, $6.51, and $7.26, respectively.
Incremental cost-effectiveness results are summarized in Table 3. Changes in costs and QALYs were calculated using comparisons to the next most expensive strategy, as well as to the common baseline of the strategy incorporating glyburide as second-line therapy. The strategy incorporating sitagliptin as second-line therapy was associated with an incremental cost-effectiveness ratio of $169,572 per QALY saved, relative to glyburide as second-line therapy. Because exenatide was 1) associated with an injectable formulation with an accompanying disutility and 2) had higher medication-associated costs, the strategy incorporating exenatide as second-line therapy was dominated by that incorporating sitagliptin, meaning that it was both more expensive and less effective in terms of QALYs saved.
Table 3.
Results of cost-effectiveness analysis
| Intensive control treatment strategies | Cost of medications | Total costs | Incremental costs | QALYs | Incremental QALYs | Incremental cost-effectiveness ratio* | Cost-effectiveness ratio, relative to glyburide strategy |
|---|---|---|---|---|---|---|---|
| Glyburide as second-line therapy | $65,205 | $146,950 | — | 15.2143 | — | — | — |
| Sitagliptin as second-line therapy | $85,418 | $4,167,163 | $20,213 | 15.3335 | 0.1192 | $169,572 | $169,572 |
| Exenatide as second-line therapy | $89,054 | $170,799 | $3,636 | 15.2998 | −0.0337 | Dominated | $278,935 |
*Changes in costs in $ and QALYs were calculated relative to the next most expensive treatment strategy.
In one-way sensitivity analysis, when the disutility associated with an injectable medication was set to 0, exenatide ceased to be dominated and was associated with an incremental cost-effectiveness ratio of $932,308 per QALY saved. In two-way sensitivity analysis, when the disutility associated with an injectable medication was set to zero and the medication cost of exenatide was decreased by 25%, exenatide exerted weak, also termed extended, dominance over sitagliptin and was associated with a cost-effectiveness ratio of $167,002 per QALY saved.
When utility gains and losses associated with weight changes were assumed to last a lifetime, the incremental cost-effectiveness ratio associated with sitagliptin was $141,833 per QALY saved. In this analysis, exenatide ceased to be dominated and was associated with an incremental cost-effectiveness ratio of $932,308 per QALY saved. In both the analysis for which the disutility associated with injectable medication was negated and in this lifetime weight change analysis, the net disutility associated with exenatide was 0, leading to similar results. When incremental cost-effectiveness analysis was performed using metformin and glyburide with insulin as third-line therapy (as opposed to rosiglitazone), exenatide remained dominated and the cost-effectiveness ratio associated with sitagliptin changed minimally to $173,300 per QALY saved. Finally, when discount rates for costs and QALYs were assumed to be 5%, the exenatide strategy remained dominated, and the incremental cost-effectiveness ratio associated with sitagliptin was $154,389 per QALY saved.
CONCLUSIONS
Our results suggest that widespread use of sitagliptin and exenatide as second-line agents in the glycemic control of patients with diabetes could be associated with $731 to $862 million additional direct health care costs in the U.S. Additional quality-adjusted life would be gained because of improved side-effect profiles associated with these drugs. These gains would cost roughly $170 thousand per QALY for sitagliptin. In the base case analysis, exenatide is dominated, being both more costly and generating fewer QALYs than sitagliptin.
A prior analysis of the cost-effectiveness of glycemic control, based on the UK Prospective Diabetes Study, was published before FDA approval of many new medications used in diabetes management (11). Our model represents an extension of this previously published model, which has now been used to address multiple diabetes-related policy questions (11,23,24).
Previous analyses compared sitagliptin and exenatide individually with generic drugs. Because the treatment strategies used were different, countries under analysis were different, and comparator strategies were different, direct comparison of these analyses with the current analysis are difficult (12–14). Importantly, this is the first cost-effectiveness analysis to compare exenatide and sitagliptin to one another and to glyburide for second-line therapy in a single model. Because intensive glycemic control has become the accepted standard of care for healthier individuals <65 years of age, our analysis focuses on alternative strategies for achieving this goal (25). Direct comparison of this analysis to the previous analyses is complicated by the fact that the current analysis is a comparison of intensive control strategies alone. In addition, unlike previous analyses, all three strategies made use of a metformin-first approach, with rosiglitazone as a third-line option and second-line therapy varying by strategy. Finally, our analysis incorporated hypertensive control and statin therapy, as would be current standard practice.
Nonetheless, review of previous studies makes clear that the extent to which nonglycemic control effects are attributed to newer glycemic control agents influences cost-effectiveness (12–14). For example, exenatide use in the U.K., relative to that of insulin glargine, was found to be dominated by insulin glargine in one study, whereas its use was found to be cost-effective (with a cost-effectiveness ratio of £22,420) in another. These different results are explained by the former model not attributing to exenatide improvements in blood pressure and in lipid profile (leading to improved cardiovascular outcomes), whereas the latter study did make these attributions. Because clinical trials of exenatide and sitagliptin have not shown significant differences in diabetes complication rates, including cardiovascular events, we chose not to ascribe such benefits to either medication.
Our findings suggest that the potential scale of health benefits gained by use of exenatide and/or sitagliptin, as a result of improved side-effect profiles, may be substantial. Relative to glyburide as second-line therapy, we found the use of exenatide and sitagliptin to be associated with an additional 0.09 and 0.12 QALYs per patient. This result is comparable to the scale of health benefits provided by a number of highly effective preventive and treatment strategies. For example, the use of aspirin for secondary prevention of myocardial infarction in 45-year-old men has been estimated to provide a QALY gain of 0.04 per patient (26). The use of statins in the secondary prevention of coronary artery disease has been associated with a QALY gain of 0.25 per patient (27). The use of 23-valent pneumococcal vaccine to prevent disease in elderly patients has been associated with a QALY gain of 0.003 per patient (28).
We found results to be sensitive to assumptions regarding medication cost, incidence of medication effects, and disutilities due to medication effects. Given the impact that such effects have on patients' daily lives and the importance of these quality-of-life effects on cost-effectiveness, further empirical study is necessary to understand the preference-weighted quality of life impact of these effects, their costs, and their consequences.
The American Diabetes Association and European Diabetes have recently published consensus guidelines for sequencing existing and new classes of medications as initial therapies in diabetes (29). These recommendations are consistent with meta-analyses indicating that antiglycemic oral agents and insulin used to treat diabetes have comparable efficacy, (30,31), although they differ in other effects and significantly in costs. Spending on antidiabetic agents nearly doubled from 6.3% of all prescription drug spending in 2004 to 12.3% in 2006, and costs of treatment increased sharply (9.5%) because of higher prices for nongeneric drugs and a shift in treatment mix toward newer, more expensive products (32).
Although side effects associated with older medications may justify the use of newer ones in individual cases, our study suggests that the additional costs of newer classes of drugs, when widely used in the large U.S. diabetic patient population, require that the value of these drugs be supported by substantial gains in health outcomes to be recommended on a population basis. Better understanding of the quality-of-life impacts of these drugs is necessary to make such a case strongly. For example, understanding the duration of weight loss effects of some of these medications and the potential downstream effects on macrovascular events (coronary heart disease and stroke) could contribute substantially to the value of some of these medications.
Our study has several limitations. Recently published long-term follow-up studies of intensive glucose control have demonstrated extended and improved treatment benefits, despite post-trial loss of between-group glycemic differences (4). Our model did not integrate such legacy effects, relying instead on a direct relationship between glycemic control and diabetes complications. If such legacy effects differ by medication class, then significant adjustment of the economic model would be necessary. The model incorporates disutilities due to medication effects, but does not yet account for costs due to management of side effects or medication switches that may occur due to side effects.
Diabetes is an epidemic disease that imposes substantial morbidity, premature mortality, and costs on the U.S. population. Appropriate treatment choices are necessary to minimize the economic burden associated with this prevalent disease. Our study suggests that to provide good economic value, newer medications, such as sitagliptin and exenatide, need to confer health benefits in scale with the additional costs they bring to the health care system.
Supplementary Material
Acknowledgments
This work was funded by Veterans Health Administration (VHA) Grant IIR 06-091 and VHA Research Enhancement Award Program Award 03-021 (to L.P.).
No potential conflicts of interest relevant to this article were reported.
Footnotes
The funding source had no role in the study's design, conduct, and reporting. The views expressed are solely those of the authors and do not necessarily represent the opinion of the Department of Veterans Affairs.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. Centers for Disease Control and Prevention. National Diabetes Fact Sheet, 2007 [article online], 2007. Available from http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2007.pdf. Accessed 13 November 2008
- 2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 4. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 5. ADVANCE Collaborative Group. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 6. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nathan DM. Finding new treatments for diabetes—how many, how fast … how good? N Engl J Med 2007;356:437–440 [DOI] [PubMed] [Google Scholar]
- 8. Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med 2008;168:2088–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Clarke P, Gray A, Adler A, Stevens R, Raikou M, Cull C, Stratton I, Holman R. UK PDS Group. Cost-effectiveness analysis of intensive blood-glucose control with metformin in overweight patients with type II diabetes (UKPDS No. 51). Diabetologia 2001;44:298–304 [DOI] [PubMed] [Google Scholar]
- 10. Clarke PM, Gray AM, Briggs A, Farmer AJ, Fenn P, Stevens RJ, Matthews DR, Stratton IM, Holman RR. UK PDS Group. A model to estimate the lifetime health outcomes of patients with type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS) Outcomes Model (UKPDS no. 68). Diabetologia 2004;47:1747–1759 [DOI] [PubMed] [Google Scholar]
- 11. CDC Diabetes Cost-effectiveness Group. Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA 2002;287:2542–2551 [DOI] [PubMed] [Google Scholar]
- 12. Schwarz B, Gouveia M, Chen J, Nocea G, Jameson K, Cook J, Krishnarajah G, Alemao E, Yin D, Sintonen H. Cost-effectiveness of sitagliptin-based treatment regimens in European patients with type 2 diabetes and haemoglobin A1c above target on metformin monotherapy. Diabetes Obes Metab 2008;10(Suppl. 1):43–55 [DOI] [PubMed] [Google Scholar]
- 13. Ray JA, Boye KS, Yurgin N, Valentine WJ, Roze S, McKendrick J, Tucker DM, Foos V, Palmer AJ. Exenatide versus insulin glargine in patients with type 2 diabetes in the UK: a model of long-term clinical and cost outcomes. Curr Med Res Opin 2007;23:609–622 [DOI] [PubMed] [Google Scholar]
- 14. Woehl A, Evans M, Tetlow A, McEwan P. Evaluation of the cost effectiveness of exenatide versus insulin glargine in patients with sub-optimally controlled type 2 diabetes in the United Kingdom. Cardiovasc Diabetol 2008;7:24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoerger TJ, Richter A, Bethke AD, Gibbons CB. A Markov Model of Disease Progression and Cost-effectiveness for Type II Diabetes (Technical Report). Research Triangle Park, NC, Research Triangle Institute, 2002. [Google Scholar]
- 16. Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care 2004;27:2628–2635 [DOI] [PubMed] [Google Scholar]
- 17. Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care 2003;26:2370–2377 [DOI] [PubMed] [Google Scholar]
- 18. Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE. Sitagliptin 036 Study Group. Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care 2007;30:1979–1987 [DOI] [PubMed] [Google Scholar]
- 19. Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP. Sitagliptin Study 024 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab 2007;9:194–205 [DOI] [PubMed] [Google Scholar]
- 20. Scott R, Loeys T, Davies MJ, Engel SS. Sitagliptin Study 801 Group. Efficacy and safety of sitagliptin when added to ongoing metformin therapy in patients with type 2 diabetes. Diabetes Obes Metab 2008;10:959–969 [DOI] [PubMed] [Google Scholar]
- 21. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med 2005;143:559–569 [DOI] [PubMed] [Google Scholar]
- 22. Drummond MF, O'Brien B, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. New York, Oxford University Press, 1997. [Google Scholar]
- 23. Herman WH, Hoerger TJ, Brandle M, Hicks K, Sorensen S, Zhang P, Hamman RF, Ackermann RT, Engelgau MM, Ratner RE. Diabetes Prevention Program Research Group. The cost-effectiveness of lifestyle modification or metformin in preventing type 2 diabetes in adults with impaired glucose tolerance. Ann Intern Med 2005;142:323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoerger TJ, Harris R, Hicks KA, Donahue K, Sorensen S, Engelgau M. Screening for type 2 diabetes mellitus: a cost-effectiveness analysis. Ann Intern Med 2004;140:689–699 [DOI] [PubMed] [Google Scholar]
- 25. National Committee for Quality Assurance. HEDIS 2008 Summary Table of Measures [article online], 2005. Available from http://www.ncqa.org/Portals/0/HEDISQM/HEDIS2008/2008_Measures.pdf. Accessed 2 November 2009
- 26. Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: a cost-utility analysis. Ann Intern Med 2006;144:326–336 [DOI] [PubMed] [Google Scholar]
- 27. Ganz DA, Kuntz KM, Jacobson GA, Avorn J. Cost-effectiveness of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor therapy in older patients with myocardial infarction. Ann Intern Med 2000;132:780–787 [DOI] [PubMed] [Google Scholar]
- 28. Sisk JE, Moskowitz AJ, Whang W, Lin JD, Fedson DS, McBean AM, Plouffe JF, Cetron MS, Butler JC. Cost-effectiveness of vaccination against pneumococcal bacteremia among elderly people. JAMA 1997;278:1333–1339 [PubMed] [Google Scholar]
- 29. Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Medical management of hyperglycaemia in type 2 diabetes mellitus: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia 2008;52:17–30 [DOI] [PubMed] [Google Scholar]
- 30. Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, Wiley C, Selvin E, Wilson R, Bass EB, Brancati FL. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med 2007;147:386–399 [DOI] [PubMed] [Google Scholar]
- 31. Horvath K, Jeitler K, Berghold A, Ebrahim SH, Gratzer TW, Plank J, Kaiser T, Pieber TR, Siebenhofer A. Long-acting insulin analogues versus NPH insulin (human isophane insulin) for type 2 diabetes mellitus. Cochrane Database Syst Rev 2007:CD005613 [DOI] [PubMed] [Google Scholar]
- 32. Castle L, Verbrugge RR. 2008 drug trend report: predictions. Five insights that will shape healthcare [article online], 2008. Available from http://www.medcohealth.com/art/drug_trend/pdf/DT_Report_2008.pdf. Accessed 13 August 2009
- 33. Matza LS, Boye KS, Yurgin N, Brewster-Jordan J, Mannix S, Shorr JM, Barber BL. Utilities and disutilities for type 2 diabetes treatment-related attributes. Qual Life Res 2007;16:1251–1265 [DOI] [PubMed] [Google Scholar]
- 34. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA 2007;298:194–206 [DOI] [PubMed] [Google Scholar]
- 35. Red Book: Pharmacy's Fundamental Reference. Washington, DC, Thompson Healthcare, 2008. [Google Scholar]
- 36. Lundkvist J, Berne C, Bolinder B, Jönsson L. The economic and quality of life impact of hypoglycemia. Eur J Health Econ 2005;6:197–202 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.