Abstract
OBJECTIVE
Patients with diabetes suffer high rates of mental health problems, and this combination is associated with poor outcomes. Although effective treatments exist for both diabetes and mental health problems, delivering services for physical and mental health problems separately ignores their interaction and may be inefficient. This systematic review sought to identify psychosocial interventions that could improve both the physical and mental health of patients with diabetes.
RESEARCH DESIGN AND METHODS
Studies were identified from the following databases: CENTRAL, MEDLINE, Excerpta Medica (EMBASE), Psychinfo, and Cumulative Index to Nursing and Allied Health Literature (CINAHL). The review included randomized controlled trials in patients with type 1 and type 2 diabetes who received psychosocial interventions and where both mental health and physical health outcomes were reported. Data were extracted on study quality, the content and process of interventions, and outcomes.
RESULTS
Eighty-five eligible comparisons were identified, of which 49 reported sufficient data for analysis. Psychosocial interventions modestly improved A1C (standardized mean difference −0.29 [95% CI −0.37 to −0.21]) and mental health outcomes (−0.16 [−0.25 to −0.07]). However, there was a limited association between the effects on A1C and mental health, and no intervention characteristics predicted benefit on both outcomes.
CONCLUSIONS
Managing physical and mental health in long-term conditions are increasingly important. The review did not identify types of interventions that consistently provide benefits for both physical and mental health. Developing such interventions remains an important challenge. The findings have implications for understanding the interaction between physical and mental health problems and for the coordination of care.
Diabetes is a major cause of mortality and morbidity (1). Comorbidity (the presence of other conditions) is highly prevalent (2), and patients with diabetes have elevated rates of mental health problems such as depression (3).
Diabetes and mental health interact in harmful ways (4). Patients with diabetes and mental health problems are less adherent to medical care (5) and suffer more complications (6). Comorbid depression and diabetes result in worse health outcomes than other combinations of chronic disease (7).
Although effective treatments exist for both diabetes and mental health problems, these are often delivered separately. This is potentially inefficient and inconvenient for patients, and can lead to problems in care coordination (8). Ignoring comorbidity also causes missed therapeutic opportunities. Reducing depressive symptoms might improve patient self-management with consequent benefits for diabetes outcomes. Improved diabetes control might reduce distress associated with complications and poor physical health.
Identifying interventions that impact both mental health and diabetes outcomes simultaneously would allow practitioners to provide truly biopsychosocial care, to improve efficiency, and could reduce problems in coordinating care. Although there is evidence that psychological interventions improve mental health in some studies and diabetic control in others (9,10), achieving simultaneous improvements in both outcomes is a far greater challenge (11). Effective treatment of depressive symptoms in patients with diabetes often fails to improve glycemic control (12) and vice versa (13).
This systematic review aimed to identify psychosocial interventions that improved both the physical and mental health of patients with diabetes.
RESEARCH DESIGN AND METHODS
The review is reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (14). There was no formal published protocol.
Eligible studies were randomized controlled trials including patients of all ages with both type 1 and type 2 diabetes receiving a psychosocial intervention, which was defined as either 1) lifestyle intervention to manage diabetes (e.g., education, skills training, and exercise interventions) or 2) psychological intervention to manage mental health (e.g., problem solving, cognitive-behavior therapy, and social support).
These interventions were compared with studies using no treatment, waiting list, or usual care comparators. Studies were excluded if the independent effect of a psychosocial intervention could not be determined (e.g., a combined antidepressant medication and counseling intervention compared with usual care).
The studies needed to report both mental and physical health outcomes. To maximize comparability, glycosylated hemoglobin (A1C) was used as the sole physical health outcome. Standardized measures of depression, anxiety, or mental health were used as mental health outcomes. Diabetes-specific outcomes (e.g., diabetes-related quality of life or adjustment to diabetes) were excluded to avoid confounding with physical health outcomes.
Information sources and search strategy
The comprehensive coverage of the CENTRAL database makes exhaustive searching of individual bibliographic databases unnecessary (15). We therefore carried out a search of the CENTRAL database of controlled trials on the Cochrane Library (Issue 2, 2007). To identify recently published trials, additional searches were conducted in MEDLINE and EMBASE. We also carried out a full search of Psychinfo (1985–2007) and CINAHL (1980–2007) using the first two stages only of the Cochrane Highly Sensitive Search Strategy for randomized controlled trials to identify any further trials not identified by the other searches. All searches were carried out between April and August 2007 and updated in April 2009. Electronic searches were augmented by checking 24 reviews of diabetes care. The full search strategy is provided in the online appendix (available at http://care.diabetesjournals.org/cgi/content/full/dc09-1519/DC1). Non-English publications were translated. Potentially eligible studies were identified by title and abstract and the full text was obtained to check eligibility.
Analytic methods
The different components in each intervention were categorized, and data were extracted on intervention intensity (number of sessions, duration), setting (e.g., primary care, hospital), the professionals involved, delivery method (e.g., individual or group, face-to-face or remote delivery), and quality control (training, supervision, written manuals, and assessments of adherence or competence). Patient characteristics included age, type of diabetes, and whether patients were recruited on the basis of either poor diabetic control or identified depression.
Methodological quality at the level of the study was assessed by two independent reviewers who rated sequence generation, allocation concealment, blinding, intention-to-treat analyses, and attrition. For analysis, a binary measure of allocation concealment served as the primary quality measure (16).
Continuous outcome measures were translated to a standardized effect size (mean of intervention group minus mean of control group, divided by the pooled standard deviation). Negative effect sizes corresponded to positive outcomes for the intervention compared with the control (i.e., reductions in A1C or depressive symptoms). Dichotomous outcomes, missing SDs, and other nonstandard data formats were dealt with using conventional methods (17,18). The outcome data closest to 6-months follow-up was used to maximize consistency across the studies. Where the studies reported two interventions versus a control group, sample sizes were halved to avoid double counting. All coding was done by groups of two raters working independently with disagreements resolved by discussion.
Initial meta-analyses using random effects modeling were conducted to estimate the pooled effect of psychosocial interventions, to allow calculation of the I2 statistic to quantify heterogeneity (19), and to explore publication bias. Publication bias is where published research is systematically unrepresentative of the population of completed studies (20). A funnel plot (21) plots effect size against the SE of the effect size. Asymmetry in the plot is often related to an absence of small studies with small effect sizes, which is suggestive of publication bias. Asymmetry can be formally tested through the Egger regression method (21).
The main aim was to identify the characteristics of psychosocial interventions that impacted on both mental and physical health outcomes. The association between the effect of psychosocial interventions on physical and mental health outcomes was examined using scatterplots and the Pearson correlation coefficient. The primary analysis examined the association between intervention characteristics and outcomes using random effects meta-regression (22). Associations may be confounded by study characteristics other than the intervention, particularly methodological quality and patient characteristics. Initial analyses examined the univariate relationship between allocation concealment and patient characteristics, and each outcome in turn. If allocation concealment or patient characteristics were significant (using the liberal criteria of P < 0.10) in these univariate analyses, they were then entered into the meta-regression models, together with each intervention characteristic. This approach examined whether intervention characteristics were significant predictors of variation in outcomes over and above differences in study quality and patient populations.
Separate meta-regressions were conducted on physical and mental health outcomes, to identify intervention characteristics that predicted each individual outcome, and those that were significant predictors across both outcomes. The regression coefficient represents the difference in effect size between studies with different characteristics, either different levels of categorical variables (e.g., presence of exercise in the intervention) or the change in effect size associated with a one-unit increase in a continuous variable (e.g., each hour of additional treatment). Analyses were conducted in Stata version 9, using the metan and metareg macros.
RESULTS
We identified 652 potentially relevant abstracts, and included 73 studies reporting 85 relevant comparisons (supplemental Fig. 1). Of these, 49 comparisons included data for meta-analysis. Supplemental Table 1 provides descriptive data on the studies, interventions, and study quality.
The characteristics of and references to individual studies are shown in supplemental Tables 2 and 3. The majority of studies were in specialist settings, using patients aged over 50 years with type 2 diabetes. Only a minority of patients were specifically recruited on the basis of poor diabetic control (22%) or baseline depression (10%). Interventions were delivered by a range of professionals and peers, and the bulk were delivered face to face with an equal split between group and individual interventions. Fifty-three percent of interventions focused on lifestyle alone, 29% focused on mental health, and 18% targeted both. The most common lifestyle interventions were education and theory-based psychological treatments such as cognitive-behavior therapy. Psychological interventions were more varied, including cognitive-behavior therapy, social support, and relaxation. Approximately half the studies used a specific depression outcome, and the rest used a combination of mental health scales (e.g., the SF36 mental component scale) or anxiety scales. The mean length of follow-up was 6.8 months (SD 5.9).
Meta-analysis of the effect of interventions on physical and mental health outcomes
Psychosocial interventions were associated with modest improvements in A1C (standardized mean difference −0.29 [95% CI −0.37 to −0.21], I2 = 45%) (supplemental Fig. 2) and smaller improvements in mental health (−0.16, −0.25 to −0.07, I2 = 56%) (supplemental Fig. 3). These results were consistent across studies using different mental health outcomes (e.g., depression vs. mental health quality of life or anxiety). Interventions focused on lifestyle alone were not significantly more effective in controlling A1C than those focused on mental health or combined interventions. Interventions including both a lifestyle and a mental health component were significantly more effective than lifestyle interventions alone in improving mental health.
There was evidence of funnel plot asymmetry in both meta-analyses (supplemental Fig. 4 and 5).
Association between effects of interventions on physical and mental health outcomes
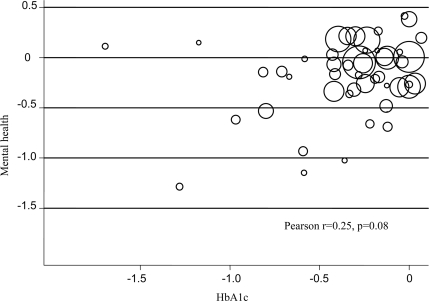
The scatterplot of mental health and physical health outcomes is shown in Fig. 1. The relationship between the effect of interventions on mental health and A1C was modest and not statistically significant (Pearson r = 0.25, P = 0.08). One study was an outlier on both outcomes and post hoc removal of that study reduced the correlation markedly (Pearson r = 0.11, P = 0.45). Using conventional criteria for the magnitude of effect sizes, only five (10%) studies reported at least “medium” effects on both outcomes (standardized mean difference of −0.5 or greater) and only 11 (23%) reported at least “small” effects on both (standardized mean difference of −0.2 or greater).
Figure 1.
Scatterplot of the standardized effect on A1C and mental health outcomes.
Meta-regression to identify study characteristics associated with physical and mental health outcomes
Allocation concealment did not predict A1C. The benefits of psychosocial interventions on A1C were less in elderly (mean age >50 years) patients (coefficient 0.16 [95% CI −0.02 to 0.34]) and greater in those recruited on the basis of poor baseline diabetes control (−0.17 [−0.37 to 0.03]).
In the multivariate model including age and poor baseline diabetes control, the benefits of psychosocial interventions on A1C were greater only in interventions that included education and skills training (−0.17 [−0.33 to −0.01]).
Allocation concealment did not predict benefits of psychosocial interventions on mental health, but benefits were greater in those studies recruited specifically on the basis of poor mental health (−0.36 [−0.67 to −0.06]).
In the multivariate model including recruitment on the basis of mental health, the benefits of psychosocial interventions on mental health were greater in interventions including other psychological therapies (−0.29 [−0.57 to −0.01]).
CONCLUSIONS
The study aimed to identify psychosocial interventions that improved both physical and mental health in patients with diabetes. This could arise through the effective integration of physical and mental health interventions into a single package or through positive interactions (e.g., where improvements in lifestyle and physical outcomes impacted on mental health or vice versa).
However, the analysis suggested that very few such interventions have been identified, and that there are no characteristics of interventions that predict substantial benefits in both outcomes. Despite the potential interactions between mental health and diabetes, a common therapeutic pathway has proven elusive.
Limitations of the study
There was significant variation in interventions, patient populations, study quality, and follow-up times, raising concerns about pooling outcomes with clinical and methodological heterogeneity. We maximized homogeneity through the use of A1C as a common outcome and by standardizing the follow-up period as much as possible. We also note that estimates of statistical heterogeneity were moderate by current convention (19). More importantly, the analytic focus was on the relationships between outcomes (Fig. 1) and the meta-regression. Meta-regression does not assume homogeneity but specifically seeks to explore variation in outcomes.
Patients recruited on the basis of poor diabetic control showed greater impact from psychosocial interventions. This may reflect their greater capacity to benefit, as poor control may reflect deficits in knowledge and skills that are amenable to change. Similarly, only 10% of studies specifically recruited patients based on mental health problems at baseline, and again, this was a significant predictor of benefit. The results may represent a “floor effect” among patients who were not significantly distressed and therefore had little room for improvement in symptoms. However, even though most patients were not recruited on the basis of a mental health problem, they were not excluded on that basis either. Epidemiological work has shown that any unselected sample of patients with diabetes will include significant numbers of depressed patients (3) and others with significant symptoms, where an intervention may have a preventive effect. There is evidence that the effects of depression on diabetes self-care are present across the whole range of symptoms, and that even mild levels of depression are associated with important levels of nonadherence (23).
Unlike previous analyses (9), the current study excluded psychological outcomes such as diabetes-specific quality of life and self-efficacy. These are likely to be confounded with physical health, and there is evidence that depression is a more effective predictor of problems in diabetes self-care (24). We did not code other aspects of the intervention (e.g., pharmacological interventions), partly because such data were often not presented in detail.
Meta-regression has a number of weaknesses (22). The analysis only represents an observational association and is limited by the small sample size, which also restricted the scope for the modeling of interactions.
Implications for policy and practice
Although previous reviews have suggested that psychological therapy can improve both diabetes and mental health outcomes (9,10), the current review has been unable to identify the types of interventions that can impact on both.
There are a number of reasons for this failure. Providing such interventions requires significant expertise, and professionals may lack the necessary expertise across mental and physical health. The results could also reflect difficulties for patients: modifying behavior to meet guidelines for physical health in diabetes requires significant effort, and adding mental health interventions may tax time, attention, and motivation (25). Although there may be useful therapeutic synergies between conditions (e.g., exercise may improve both A1C outcomes and depression), conditions may also be antagonistic (2). For example, improvements in depressive symptoms may cause an increase in appetite and unhealthy eating, while active monitoring of physical symptoms may itself be distressing (11).
Managing mental and physical health issues is an important part of care for long-term conditions, and the current study suggests that achieving integrated, biopsychosocial care remains a significant challenge. Interesting avenues for future research might include the development of interventions designed around the management of comorbidity as a specific task (11) or the identification of particular groups of patients that are amenable to an integrated approach (e.g., where depression is causally related to the diabetes, as opposed to those cases where they simply co-occur).
Supplementary Material
Acknowledgments
This article presents independent research commissioned by the National Institute for Health Research (NIHR). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, or the Department of Health. The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; and the preparation, review, or approval of the manuscript.
P.B. is a paid scientific consultant to the British Association of Counselling and Psychotherapy. L.G. has received funding from Servier for research and from a number of different pharmaceutical companies for lecturing at meetings. No other potential conflicts of interest relevant to this article were reported.
The authors would like to thank Ros McNally for expert advice on the searches; Bonnie Sibbald, Christine Bundy, and Chris Dickens for helpful comments on the study; Annette Barber for her hard work on retrieving studies for the review; and Ricci Chang for assistance with the translation of studies. All authors developed the protocol, assessed studies for inclusion, and extracted data. E.H. managed the review process. E.H. and P.B. analyzed the data. All authors had full access to the data and were involved in the interpretation of the findings and the preparation of the manuscript. The authors would like to thank the reviewers for their useful comments on the initial submission.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
References
- 1. World Health Organization. The Global Burden of Disease: 2004 Update. Geneva, Switzerland, World Health Organization Press, 2009. [Google Scholar]
- 2. Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity: implications for understanding and provision of health services and health. Ann Fam Med 2009;7:357–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 2001;24:1069–1078 [DOI] [PubMed] [Google Scholar]
- 4. Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, Lee HB, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. JAMA 2008;299:2751–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piette JD, Richardson C, Valenstein M. Addressing the needs of patients with multiple chronic illnesses: the case of diabetes and depression. Am J Manag Care 2004;10:152–162 [PubMed] [Google Scholar]
- 6. Anderson RJ, Grigsby AB, Freedland KE, de Groot M, McGill JB, Clouse RE, Lustman PJ. Anxiety and poor glycemic control: a meta-analytic review of the literature. Int J Psychiatry Med 2002;32:235–247 [DOI] [PubMed] [Google Scholar]
- 7. Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851–858 [DOI] [PubMed] [Google Scholar]
- 8. Haggerty JL, Reid RJ, Freeman GK, Starfield BH, Adair CE, McKendry R. Continuity of care: a multidisciplinary review. BMJ 2003;327:1219–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Winkley K, Landau S, Eisler I, Ismail K. Psychological interventions to improve glycemic control in patients with type 1 diabetes: systematic review and meta-analysis of randomized controlled trials. BMJ 2006;333:65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ismail K, Winkley K, Rabe-Hesketh S. Systematic review and meta-analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363:1589–1597 [DOI] [PubMed] [Google Scholar]
- 11. Detweiler-Bedell JB, Friedman MA, Leventhal H, Miller IW, Leventhal EA. Integrating co-morbid depression and chronic physical disease management: identifying and resolving failures in self-regulation. Clin Psychol Rev 2008;28:1426–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Katon WJ, Von Korff M, Lin EH, Simon G, Ludman E, Russo J, Ciechanowski P, Walker E, Bush T. The Pathways Study: a randomized trial of collaborative care in patients with diabetes and depression. Arch Gen Psychiatry 2004;61:1042–1049 [DOI] [PubMed] [Google Scholar]
- 13. Ismail K, Thomas SM, Maissi E, Chalder T, Schmidt U, Bartlett J, Patel A, Dickens CM, Creed F, Treasure J. Motivational enhancement therapy with and without cognitive behavior therapy to treat type 1 diabetes: a randomized trial. Ann Intern Med 2008;149:708–719 [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Royle P, Waugh N. A simplified search strategy for identifying randomised controlled trials for systematic reviews of health care interventions: a comparison with more exhaustive strategies. BMC Med Res Methodol 2005;5:23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hewitt C, Hahn S, Torgerson DJ, Watson J, Bland JM. Adequacy and reporting of allocation concealment: review of recent trials published in four general medical journals. BMJ 2005;330:1057–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lipsey MW, Wilson D. Practical Meta-Analysis. Thousand Oaks, CA, Sage Publications, 2001. [Google Scholar]
- 18. Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J Clin Epidemiol 2006;59:7–10 [DOI] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull 1979;86:638–641 [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–1573 [DOI] [PubMed] [Google Scholar]
- 23. Gonzalez JS, Safren SA, Cagliero E, Wexler DJ, Delahanty L, Wittenberg E, Blais MA, Meigs JB, Grant RW. Depression, self-care, and medication adherence in type 2 diabetes: relationships across the full range of symptom severity. Diabetes Care 2007;30:2222–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gonzalez JS, Delahanty LM, Safren SA, Meigs JB, Grant RW. Differentiating symptoms of depression from diabetes-specific distress: relationship with self-care in type 2 diabetes. Diabetologia 2008;51:1822–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lin EH, Katon W, Rutter C, Simon GE, Ludman EJ, Von Korff M, Young B, Oliver M, Ciechanowski PC, Kinder L, Walker E. Effects of enhanced depression treatment on diabetes self-care. Ann Fam Med 2006;4:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.