Abstract
It has been previously reported that vasopressin 1b receptor knockout mice (Avpr1b−/−) have reduced levels of aggressive behavior compared to wildtype littermates. However, as the background of the mice were always a mixture of 129/SvJ and C57BL/6, we wanted to determine if the phenotype persisted when our laboratory line was crossed with a wild-derived sub-species of house mice. To this end, we crossed our Avpr1b−/− mice with Mus musculus castaneus, one of few sub-species that will breed with laboratory strains. Subsequent F2 offspring, which were approximately 50% Mus musculus castaneus, were tested in a resident-intruder behavioral test to assess aggressive behavior. We found that even on this more “wild” background, Avpr1b−/− continued to demonstrate longer attack latencies and fewer attacks in a resident-intruder test than wildtype littermates. These findings are consistent with previous reports of reduced aggressive behavior in Avpr1b−/− mice and show that the deficit does persist on a different background strain. Further, these findings confirm the importance of the Avpr1b to normal displays of social forms of aggressive behavior.
Keywords: Avpr1b receptor, M.m. castaneus, hippocampus
Introduction
The nonapeptide arginine vasopressin (Avp) has been consistently implicated in the regulation of aggressive behavior across species [1-12]. Currently there are two identified receptor subtypes that are centrally expressed: the vasopressin 1a receptor (Avpr1a) [13, 14] and the vasopressin 1b receptor (Avpr1b) [15, 16]. The Avpr1a has a wide distribution [14, 17, 18] and has been implicated in the regulation of a variety of behaviors, including aggressive behavior [5, 9, 19-26]. The Avpr1b, on the other hand, appears to have a somewhat more restricted distribution, but has also been implicated in the regulation of aggression [1, 10, 11, 15, 27-30]. Unfortunately, compared to work on the Avpr1a, there is a lack of knowledge about how the Avpr1b regulates aggressive behavior.
One of the hurdles to examining the contribution of the Avpr1b in the regulation of aggressive behavior has been the deficiency of commercially available pharmacological tools. To address this, Wersinger and colleagues generated a mouse line with a genetic disruption of the Avpr1b [10]. The Avpr1b knockout (−/−) mice have reduced levels of social forms of aggressive behavior and mildly impaired social recognition. Specifically, Avpr1b−/− male mice have longer attack latencies and fewer attacks toward an intruder compared to wildtype controls in neutral arena and resident-intruder tests [10, 11]. In a reversed resident-intruder test, where the experimental animals are the intruders, Avpr1b−/− mice will display defensive postures but do not initiate many defensive attacks [11]. While male Avpr1b−/− mice demonstrate deficits in offensive and defensive aggression, female Avpr1b−/− mice have deficits in maternal aggression [11]. So, while there are deficits in forms of aggression that have a “social” component, there is not a global deficit in aggressive behavior as Avpr1b−/− mice have normal predatory aggression, as measured by the time to attack a cricket [11].
One ongoing issue when using laboratory strains of mice is the consistency in the phenotype of the null mutant when it is on a different background. To explore this we examined whether the reduced aggressive behavior that is observed in Avpr1b−/− mice would endure on a more “wild” background. However, getting laboratory strains to mate with more “wild” mice can often be challenging. Fortunately we had access to a population of wild-derived Mus musculus castaneus (M.m. castaneus) mice that have been reported to have high levels of aggressive behavior and are one of the few wild-derived subspecies that will breed with laboratory strains [31]. We then crossed our Avpr1b line with M.m castaneus to generate a new line that represented a genetic mixture somewhere between the two and measured aggressive behavior.
Methods
Targeted Disruption of the Vasopressin 1b Receptor Gene and PCR Analysis
The generation of the Avpr1b−/− mouse line has been previously described [10]. The offspring were genotyped at weaning using PCR analysis of DNA isolated from tail clips as previously described [10, 11]. All experimental procedures were approved by the National Institute of Mental Health Animal Care and Use Committee, and followed the NIH guidelines “Using Animals in Intramural Research.”
Generation of the “wild” line of Avpr1b mice
The new “wild” line of mice was generated by breeding Avpr1b−/− females, on a mixed background of C57BL/6J and 129X1/SvJ generated by W.S.Y [10], with male M.m. castaneus mice to generate Avpr1b heterozygous (+/−) mice. The Avpr1b+/− mice were then bred with one another to generate F2 Avpr1b+/+ and Avpr1b−/− mice that were on average 50% M.m. castaneus. However, it should be noted that it is likely that the flanking regions of the disrupted Avpr1b gene would be likely to come from the 129X1/SvJ embryonic stem cell background. These animals were then tested in a resident-intruder behavioral test as described below. It was observed that the new “wild” F2 generation were much more “reactive” than were the original line; they tended to jump out of the cage more and were highly aggressive when handled. While the observations were not systematic, this increase in aggression likely reflects heightened defensive aggression rather than offensive aggression (H.K.C., personal observations).
Animals
The M.m castaneus were provided to us by Dr. Christine Kozak in the Viral Biology Section of NIAID. These mice were received in approximately 1998 from a colleague at Roswell Park who had derived them from wild-trapped mice. They have been randomly-bred since in NIH animal facilities [32-34].
Stimulus “intruders” were 10-week old Balb/c males purchased from NCI-Frederick, Different intruders were used to test the “original” and the “wild” lines of mice. Intruders were group-housed over the course of the experiment. Whether the subject “residents” were of the “original” line or the more “wild” line, within each line the Avpr1b+/+ and Avpr1b−/− mice were of comparable ages (“original” line - Avpr1b+/+: 98.13 ± 8.53 versus Avpr1b−/−: 94.88 ± 7.92 days of age; “wild” line- Avpr1b+/+: 104.42 ± 11.55 versus Avpr1b−/−: 118.57 ± 9.58 days of age) at the time of testing. Residents were initially group-housed in single-sex cages. All animals were housed in a 12L:12D light cycle with food and water available ad libitum.
Resident-Intruder Test
Two different resident-intruder tests were completed at two different times on the two lines of mice. The “original” line was tested in March of 2007 while the “wild” line was tested in April 2007. Aside from the dates of testing all other aspects, including housing in the same animal facility, were identical.
Resident males (“original” line: Avpr1b+/+ (n=8) and Avpr1b−/− (n=8) ; “wild” line: Avpr1b+/+ (n=7) and Avpr1b−/− (n=7))) were singly housed for at least 14 days prior to testing.. Testing was conducted during the dark phase of the light:dark cycle approximately 1 hour after lights out. The test was initiated when an intruder was added to the home cage of the resident male. If no aggressive behavior was observed in the first 5 minutes, a latency of 300s was recorded and the test ended. Otherwise, the test lasted 2 minutes after an attack was first observed. For each resident the latencies to attack as well as the attack frequencies were scored by an observer blind to the genotypes. Subjects were given 3 tests with 3 days between each test. Intruders were only used once each day and residents were never tested with the same intruder.
Statistical Analysis
The data collected across days were analyzed using a repeated measures analysis of variance (ANOVA), with genotype as the between-subjects factor and day as the within-subjects factor. The cumulative attack latencies and attack frequencies were also calculated and compared between groups using a one-way ANOVA. Only animals that attacked were included in the statistical analyses, this resulted in one Avpr1b+/+ animal being excluded. Additionally, the latency to attack and attack frequency on day 3 of testing for another Avpr1b+/+ mouse was not videotaped due to experimenter error, so the data for this animal was only included in the cumulative attack latency.
To compare the levels of aggressive behavior of the new “wild” line of Avpr1b−/− mice with the previous “original” line, a repeated measures ANOVA was used with genotype and line as the between-subjects factor and day as the within-subjects factor. For all analyses, a p value of < 0.05 was considered statistically significant.
Results
The attack latencies of the “wild” line showed a main effect of day (F(2,20)=7.71, p=0.003) and of genotype (F(1,10)=12.79, p=0.005) but no interaction (Figure 1A). Attack latencies shortened from day to day with repeated testing and Avpr1b−/− mice had longer attack latencies compared to Avpr1b+/+ mice. In the analysis of attack frequencies across days there was a main effect of day (F(2,20)=8.28, p=0.002) and of genotype (F(1,10)=1268.01, p=0.036) but no interaction (Figure 2A). There was the expected increase in attack frequency from day to day with repeated testing and Avpr1b−/− mice displayed fewer attacks than Avpr1b+/+ mice. These genotypic differences in attack latency and attack frequency were reflected in statistically significant differences between the groups in cumulative attack latency (F(1,10)=5.87, p=0.038) (Figure 1B) and in cumulative attack frequency (F(1,12)=5.12, p=0.045) (Figure 2B).
Figure 1.
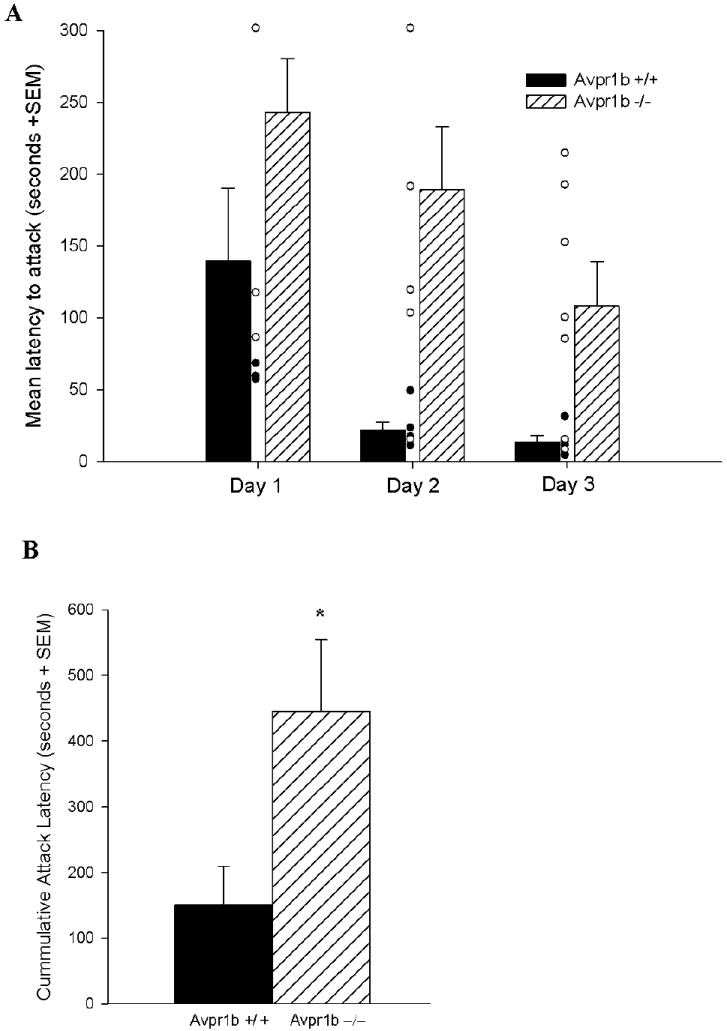
Attack latencies (A) across test days (including individual data points) and (B) cumulatively for Avpr1b wildtype (+/+) and knockout (−/−) mice on a more “wild” background. In (A) there were main effects of day and of genotype, but no interaction. Avpr1b−/− mice had longer attack latencies compared to Avpr1b+/+ mice. In (B) Avpr1b−/− mice had longer cumulative attack latencies than Avpr1b+/+ mice. (Mean ± SEM)
Figure 2.
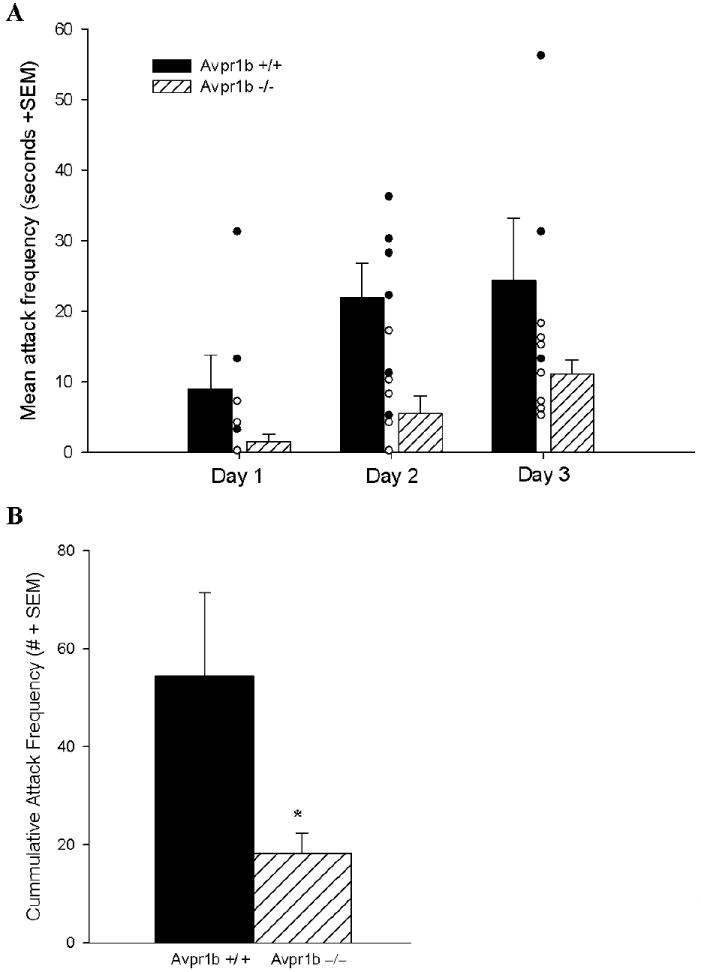
Attack frequencies (A) across test days (including individual data points) and (B) cumulatively for Avpr1b wildtype (+/+) and knockout (−/−) mice on a more “wild” background. In (A) there were main effects of day and of genotype, but no interaction. Avpr1b−/− mice had fewer attacks compared to Avpr1b+/+ mice. In (B) Avpr1b−/− mice displayed fewer cumulative attacks than Avpr1b+/+ mice. (Mean ± SEM)
When the “wild” line was statistically compared to the “original” line there were no differences between the two strains in either the latency to attack or the attack frequency. There were, however, the expected main effects of day and of genotype on the latency to attack (day: F(2,46)=11.21, p=0.001; genotype: F(1,23)=20.77, p=0.001) and attack frequency (day: F(2,46)=16.04, p=0.001; genotype: F(2,23)=9.03, p=0.006) (Table 1).
Table 1.
| Attack Latency (Mean ±SEM) | Attack Frequency (Mean ±SEM) | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | ||
| Avpr1b+/+ | “wild” line | 107.40 ± 48.19 | 16.80 ± 2.33 | 13.80 ± 4.44 | 10.80 ± 5.50 | 19.20 ± 4.85 | 24.40 ± 8.84 |
| “original line” | 119.50 ± 30.34 | 50.88 ± 18.36 | 56.13 ± 35.56 | 9.13 ± 2.15 | 9.38 ± 2.27 | 16.38 ± 3.09 | |
| Avpr1b−/− | “wild” line | 243.00 ± 36.95 | 189.14 ± 43.70 | 108.43 ± 30.65 | 1.57 ± 1.07 | 5.57 ± 2.45 | 11.14 ± 1.99 |
| “original” line | 249.00 ± 24.95 | 182.25 ± 43.45 | 192.13 ± 45.65 | 2.13 ± 1.04 | 5.63 ± 1.94 | 10.88 ± 4.41 | |
Discussion
In the current study we have demonstrated that, on a different background strain, absence of a functional Avpr1b gene results in significant reductions in aggressive behavior compared to wildtype controls. These results provide compelling evidence that the role of the Avpr1b is conserved within sub-species and likely across species as these results are consistent with studies in Syrian hamsters and mice that found that oral administration of an Avpr1b antagonist results in reduced aggression [1, 35].
A study that described M.m. castaneus as being more aggressive than C57BL6 mice was one of the precipitants of the current study [31]. However, the line generated here that were approximately 50% M.m. castaneus do not appear to have higher levels of aggression compared to the “original” line; though these mice were highly reactive compared to the “original” line (described above). Even with the lack of heightened aggression in this “wild” line compared to the “original” line, the persistence of the phenotype is the most critical observation. It is likely that if these mice were further back-crossed into the M.m. castaneus sub-species they would show increases in their baseline aggressive behavior.
As the evidence supporting a critical role of the Avpr1b in the regulation of aggressive behavior mounts, one issue that still remains is where in the brain Avp is acting via the Avpr1b. While highly expressed in the pituitary, the Avpr1b mRNA is also prominently expressed within the CA2 pyramidal neurons of the hippocampus [28, 30]. A recent publication examining changes in blood oxygen levels (BOLD), although focused on the role of the Avpr1a, showed that the male rat hippocampus has increased activity in the presence of a female mate and intruder male [Imaging the Neural Circuitry and Chemical Control of Aggressive Motivation. Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. BMC Neurosci. 2008 Nov 13;9(1):111. [Epub ahead of print]]. It should be noted that in situ hybridization histochemistry for the Avpr1b, reveals only two other areas that express Avpr1b mRNA, the paraventricular nucleus and the anterior amydala. Within these two nuclei there were relatively few labeled neurons [28]. Detection of Avpr1b protein has remained elusive but the abundance of message within CA2 hippocampus is intriguing. We have hypothesized that the role of the Avpr1b within the CA2 field may be to help in the formation of memories that are accessory olfactory-based [28, 36]. If this is the case, then the Avpr1b may be helping to encode the social context and perhaps even stimulate the retrieval of a previous social memory. Therefore, the reduced aggressive behavior caused by a null mutation of the Avpr1b gene is a part of a larger, more global deficit in response to social stimuli. The presence of the Avpr1b within the CA2 field of hippocampus across mouse, rat, and human suggests that whatever its role may be, its location appears to be evolutionarily conserved [28]. Future work will focus on the contribution of the CA2 field of hippocampus to the behavioral phenotype observed in Avpr1b knockout mice.
Acknowledgments
We thank Dr. Christine Kozak (NIAID) for providing us with the M.m. castaneus mice and the NIMH Animal Facility in Building 14 for their exceptional animal care. This research was supported, in part, by NIMH (Z01-MH-002498-20) from the Division of Intramural Research, National Institute of Mental Health, National Institutes of Health, DHHS.
References
- 1.Blanchard RJ, Griebel G, Farrokhi C, Markham C, Yang M, Blanchard DC. AVP V(1b) selective antagonist SSR149415 blocks aggressive behaviors in hamsters. Pharmacol Biochem Behav. 2005;80:189–194. doi: 10.1016/j.pbb.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 2.Ferris CF, Potegal M. Vasopressin receptor blockade in the anterior hypothalamus suppresses aggression in hamsters. Physiol Behav. 1988;44:235–239. doi: 10.1016/0031-9384(88)90144-8. [DOI] [PubMed] [Google Scholar]
- 3.Potegal M, Ferris CF. Intraspecific aggression in male hamsters is inhibited by intrahypothalamic vasopressin-receptor antagonist. Aggressive Behav. 1990;15:311–320. [Google Scholar]
- 4.Compaan JC, Buijs RM, Pool CW, de Ruiter AJ, Koolhaas JM. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res Bull. 1993;30:1–6. doi: 10.1016/0361-9230(93)90032-7. [DOI] [PubMed] [Google Scholar]
- 5.Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17:4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bester-Meredith JK, Young LJ, Marler CA. Species differences in paternal behavior and aggression in Peromyscus and their associations with vasopressin immunoreactivity and receptors. Horm Behav. 1999;36:25–38. doi: 10.1006/hbeh.1999.1522. [DOI] [PubMed] [Google Scholar]
- 7.Stribley JM, Carter CS. Developmental exposure to vasopressin increases aggression in adult prairie voles. Proc Natl Acad Sci USA. 1999;96:12601–12604. doi: 10.1073/pnas.96.22.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bester-Meredith JK, Marler CA. Vasopressin and aggression in cross-fostered California mice (Peromyscus californicus) and white-footed mice (Peromyscus leucopus) Horm Behav. 2001;40:51–64. doi: 10.1006/hbeh.2001.1666. [DOI] [PubMed] [Google Scholar]
- 9.Caldwell HK, Albers HE. Effect of photoperiod on vasopressin-induced aggression in Syrian hamsters. Horm Behav. 2004;46:444–449. doi: 10.1016/j.yhbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Wersinger SR, Ginns EI, O’Carroll AM, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 11.Wersinger SR, Caldwell HK, Christiansen M, Young WS., 3rd Disruption of the vasopressin 1b receptor gene impairs the attack component of social behavior in mice. Genes Brain Behav. 2006;6:653–660. doi: 10.1111/j.1601-183X.2006.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everts HGJ, De Ruiter AJH, Koolhaas JM. Differential lateral septal vasopressin in wild-type rats: correlation with aggression. Horm Behav. 1997;31:136–144. doi: 10.1006/hbeh.1997.1375. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski NL, Lolait SJ, Bradley DJ, O’Carroll A, Brownstein MJ, Young WS., Iii Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology. 1992;131:533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- 14.Johnson AE, Audigier S, Rossi F, Jard S, Tribollet E, Barberis C. Localization and characterization of vasopressin binding sites in the rat brain using an iodinated linear AVP antagonist. Brain Research. 1993;622:9–16. doi: 10.1016/0006-8993(93)90795-o. [DOI] [PubMed] [Google Scholar]
- 15.Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS, III, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci U S A. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Antoni FA. Novel ligand specificity of pituitary vasopressin receptors in the rat. Neuroendocrinology. 1984;39:186–188. doi: 10.1159/000123976. [DOI] [PubMed] [Google Scholar]
- 17.Ostrowski NL, Lolait SJ, Young WS., Iii Cellular localization of vasopressin V1a receptor messenger ribonucleic acid in adult male rat brain, pineal, and brain vasculature. Endocrinology. 1994;135:1511–1528. doi: 10.1210/endo.135.4.7925112. [DOI] [PubMed] [Google Scholar]
- 18.Szot P, Bale TL, Dorsa DM. Distribution of messenger RNA for the vasopressin V1a receptor in the CNS of male and female rats. Brain Res Mol Brain Res. 1994;24:1–10. doi: 10.1016/0169-328x(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 19.Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- 20.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 21.Hammock EAD, Young LJ. Variation in the vasopressin V1a receptor promoter and expression: implications for inter- and intraspecific variation in social behaviour. Eur J Neurosci. 2002;16:399–402. doi: 10.1046/j.1460-9568.2002.02083.x. [DOI] [PubMed] [Google Scholar]
- 22.Bielsky IF, Hu SB, Ren X, Terwilliger EF, Young LJ. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: a gene replacement study. Neuron. 2005;47:503–513. doi: 10.1016/j.neuron.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Caldwell HK, Albers HE. Short-photoperiod exposure reduces vasopressin (V1a) receptor binding but not arginine-vasopressin-induced flank marking in male Syrian hamsters. J Neuroendocrinol. 2003;15:971–977. doi: 10.1046/j.1365-2826.2003.01086.x. [DOI] [PubMed] [Google Scholar]
- 24.Landgraf R, Frank E, Aldag JM, Neumann ID, Sharer CA, Ren X, Terwilliger EF, Niwa M, Wigger A, Young LJ. Viral vector-mediated gene transfer of the vole V1a vasopressin receptor in the rat septum: improved social discrimination and active social behaviour. Eur J Neurosci. 2003;18:403–411. doi: 10.1046/j.1460-9568.2003.02750.x. [DOI] [PubMed] [Google Scholar]
- 25.Ferris CF, Lu SF, Messenger T, Guillon CD, Heindel N, Miller M, Koppel G, Robert BF, Simon NG. Orally active vasopressin V1a receptor antagonist, SRX251, selectively blocks aggressive behavior. Pharmacol Biochem Behav. 2006;83:169–174. doi: 10.1016/j.pbb.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Caldwell HK, Smith DA, Albers HE. Photoperiodic mechanisms controlling scent marking: interactions of vasopressin and gonadal steroids. Eur J Neurosci. 2008;27:1189–96. doi: 10.1111/j.1460-9568.2008.06071.x. [DOI] [PubMed] [Google Scholar]
- 27.Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- 28.Young WS, Li J, Wersinger SR, Palkovits M. The vasopressin 1b receptor is prominent in the hippocampal area CA2 where it is unaffected by restraint stress or adrenalectomy. Neuroscience. 2006;143:1031–1039. doi: 10.1016/j.neuroscience.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saito M, Sugimoto T, Tahara A, Kawashima H. Molecular cloning and characterization of rat V1b vasopressin receptor: evidence for its expression in extra-pituitary tissues. Biochem Biophys Res Commun. 1995;212:751–757. doi: 10.1006/bbrc.1995.2033. [DOI] [PubMed] [Google Scholar]
- 30.Hernando F, Schoots O, Lolait SJ, Burbach JP. Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology. 2001;142:1659–1668. doi: 10.1210/endo.142.4.8067. [DOI] [PubMed] [Google Scholar]
- 31.Le Roy I, Roubertoux PL, Jamot L, Maarouf F, Tordjman S, Mortaud S, Blanchard C, Martin B, Guillot PV, Duquenne V. Neuronal and behavioral differences between Mus musculus domesticus (C57BL/6JBy) and Mus musculus castaneus (CAST/Ei) Behav Brain Res. 1998;95:135–42. doi: 10.1016/s0166-4328(97)00218-0. [DOI] [PubMed] [Google Scholar]
- 32.Lyu MS, Kozak CA. Genetic basis for resistance to polytropic murine leukemia viruses in the wild mouse species Mus castaneus. J Virol. 1996;70:830–3. doi: 10.1128/jvi.70.2.830-833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lyu MS, Nihrane A, Kozak CA. Receptor-mediated interference mechanism responsible for resistance to polytropic leukemia viruses in Mus castaneus. J Virol. 1999;73:3733–6. doi: 10.1128/jvi.73.5.3733-3736.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu T, Yan Y, Kozak CA. Rmcf2, a xenotropic provirus in the Asian mouse species Mus castaneus, blocks infection by polytropic mouse gammaretroviruses. J Virol. 2005;79:9677–84. doi: 10.1128/JVI.79.15.9677-9684.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Serradeil-Le Gal C, Wagnon J, III, Tonnerre B, Roux R, Garcia G, Griebel G, Aulombard A. An overview of SSR149415, a selective nonpeptide vasopressin V(1b) receptor antagonist for the treatment of stress-related disorders. CNS Drug Rev. 2005;11:53–68. doi: 10.1111/j.1527-3458.2005.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caldwell HK, Wersinger SR, Young WS., 3rd The role of the vasopressin 1b receptor in aggression and other social behaviours. Prog Brain Res. 2008;170:65–72. doi: 10.1016/S0079-6123(08)00406-8. [DOI] [PubMed] [Google Scholar]


