Abstract
Purpose: The purpose of the present review was to assess the quality of evidence in the literature regarding the specific benefits of inspiratory muscle training (IMT) with an emphasis on training intensity and the relationships between changes in inspiratory muscle function and other clinical outcome measures. Methods: Articles were found by searching CINAHL, PubMed, Medline via First Search, and ProQuest databases. Articles used in the review were randomized trials of IMT vs. sham IMT or no intervention, published in English in a peer-reviewed journal, included patients with chronic obstructive pulmonary disease (COPD), and specified the intensity of training. The quality of the studies was evaluated by 2 independent reviewers using the methodological rigor scale described by Medlicott and Harris as well as Sackett's levels of evidence. Fifteen articles met the inclusion criteria and were used in this review. Results: Consistent improvements in maximal inspiratory pressures (ranging from −11 to −30 cm H2O) and inspiratory muscle endurance were found. Improvements in dyspnea and health-related quality of life were also observed. Inspiratory muscle training may result in improved exercise tolerance as measured using walking tests. High-intensity IMT resulted in improved training efficiency with respect to inspiratory muscle strength, but evidence of the effect of high-intensity IMT on other clinical outcomes is lacking. Conclusion: Despite research spanning decades, there are numerous limitations in the literature regarding IMT. IMT appears to improve dyspnea, waking test distance, and health-related quality of life in individuals with COPD, but it is not clear whether this improvement is mediated through improved inspiratory muscle strength and endurance. This review discussed several considerations critical to the design of future trials.
Key Words: inspiratory muscle training, COPD
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) affects 4% to 6% of the population.1 It is a major cause of morbidity throughout the world, and the fourth leading cause of death within the United States.2 Chronic bronchitis and emphysema are the disease entities that may be present in varying combinations in individuals with COPD, with dyspnea and exercise intolerance being the most prevalent presenting complaints.3 The mechanisms for dyspnea and exercise intolerance are multifactorial and include increased resistance to airflow (especially during expiration),4 impaired gas exchange resulting in hypoxemia and hypercapnia,4 dynamic hyperinflation,5 and skeletal muscle dysfunction.6 While each of these contributing factors interact with each other, individuals with COPD demonstrate an early onset of lactic acidosis during exercise and an inability to meet the associated ventilatory demand.4 Therefore, interventions that improve ventilation, such as inspiratory muscle training (IMT), may have the potential to reduce dyspnea and improve exercise tolerance.
The contribution of respiratory muscle fatigue to dyspnea and exercise intolerance in individuals with COPD is not clear. Diaphragmatic dysfunction and susceptibility to injury is described in two thorough reviews7,8 which suggest that increased airway resistance and hyperinflation result in the need for a greater inspiratory pressure for producing inspiratory flow compared to that of normal individuals. Bellemere and Grassino9 described inspiratory muscle fatigue as a function of the duration and mean force of diaphragm contraction as a percentage of the respiratory cycle and diaphragmatic peak force, respectively. They were able to demonstrate increased susceptibility of diaphragm fatigue in individuals with COPD compared to those with lung disease using inspiratory muscle loading. However, two studies10,11 were unable to demonstrate diaphragm fatigue during peak exercise testing in comparable individuals with COPD, which suggested that diaphragm fatigue was not a limiting factor to exercise tolerance. A review by Hill et al12 summarizes alternate explanations in the literature for the mechanisms by which respiratory muscle dysfunction might result in dyspnea and exercise intolerance. These include: (1) reductions in the relative proportion of maximal inspiratory pressure to the pressure required for generating inspiratory flow, thereby increasing the perceived effort of breathing; (2) reduced inspiratory flow, thereby lengthening the inspiratory phase and shortening the time available for expiration and subsequently inducing dynamic hyperinflation; and (3) increasing oxygen consumption and lactic acid production related to the work of ventilation.
Despite the lack of a clear explanation for how inspiratory muscle dysfunction contributes to the dyspnea and exercise intolerance experienced by those with COPD, there is a considerable amount of research that has investigated the efficacy of IMT for improving inspiratory muscle strength, inspiratory muscle endurance, dyspnea, and exercise tolerance. To date, 6 systematic reviews13–18 have been completed. The first review with meta-analysis by Smith et al13 in 1991 concluded that no beneficial effects of IMT were seen in inspiratory muscle function or exercise tolerance, but this review was limited by the inclusion of studies that did not control for training intensity. A 2002 update by Lotters et al14 reviewed articles investigating IMT alone or in combination with exercise, and demonstrated improvements in inspiratory muscle strength, inspiratory muscle endurance, and dyspnea. In 2005 a third group of investigators performed further meta-analysis and review of IMT alone15 and IMT in combination with aerobic exercise.18 They examined outcomes for inspiratory muscle strength, endurance, dyspnea, exercise tolerance, and health-related quality of life (HRQL). This group then further updated these reviews in 2008.16,17 These updates affirmed previous results that IMT alone significantly improves inspiratory muscle strength, inspiratory muscle endurance, and dyspnea. They also concluded that IMT alone also improves HRQL and exercise tolerance.15 Further, they demonstrated that IMT in combination with exercise compared to exercise alone may only have additional benefit with respect to improvements in maximal inspiratory pressure (PImax).17
Due to limitations in previous reviews and meta analyses regarding IMT, it remains difficult for the clinician to identify the best indications, training methods, and expected outcomes for this intervention. First, there is considerable variation across reviews as to what studies were included (Table 1). Second, previous reviews have not commented on the possible effects of hyperinflation and training intensity on outcome. Third, previous reviews have not discussed what constitutes a clinically meaningful improvement in inspiratory muscle function. Fourth, there is heterogeneity in the method of IMT examined in previous reviews: normocapnic hyperpnea, threshold loading, and resistive loading. Normocapnic hyperpnea requires specific instrumentation/devices that are not routinely available for clinical use. Resistive loading is accomplished using commonly available mouthpieces with small diameter apertures. However, the resistance is dependent on flow rate and therefore training intensity is poorly controlled. Many of the reviewed studies using resistive loading did not control for actual training intensity.
Table 1.
Studies Included in Previous Systematic Reviews
| Smith 1992 | Lotters 2002 | Geddes 2005 | Geddes 2008 | Crowe 2005 | O'Brien 2008 | Present Review | |
|---|---|---|---|---|---|---|---|
| Bjerre-Jepsen 1981∗ | X | X | X | ||||
| Asher1982∗ | X | ||||||
| Reid 1984 ‡ | X | X | X | X | |||
| Kim 1984 | X | X | X | X | |||
| Jones 1985∗ | X | X | |||||
| Falk 1985∗ | X | X | X | ||||
| Chen 1985∗† | X | X | X | ||||
| Mckeon 1986∗ | X | X | X | ||||
| Levine 1986∗ | X | X | X | ||||
| Ries 1986 † | X | X | X | ||||
| Sobush 1986∗ | X | ||||||
| Mcintosh 1987∗ | X | ||||||
| Noseda 1987∗ | X | X | X | ||||
| Bellman 1988∗ | X | X | X | X | |||
| Larson 1988 | X | X | X | X | X | ||
| Richardson 1989∗ | X | X | |||||
| Patessio 1989 | X | X | X | X | |||
| Harver 1989 | X | X | X | X | X | ||
| Goldstein 1989 † | X | X | X | X | |||
| Guyatt 1991∗ | X | ||||||
| DekHuijzen 1991∗† | X | X | X | X | |||
| Weiner 1992 † | X | X | X | ||||
| Guyatt 1992∗ | X | X | |||||
| Nosworthy 1992 ‡ | X | X | |||||
| Kim 1993 | X | X | X | X | |||
| Preusser 1994‡ | X | X | X | ||||
| Wanke 1994† | X | X | X | ||||
| Berry 1996 † | X | X | X | ||||
| Heijdra 1996 | X | X | X | X | |||
| Lisboa 1997 | X | X | X | X | |||
| Villafranca 1998 | X | X | X | X | |||
| Larson 1999 † | X | X | X | ||||
| Scherer 2000∗∗ | X | X | |||||
| Covey 2001 | X | X | X | ||||
| Sanchez Riera 2001 | X | X | X | ||||
| Ramirez-Sarmiento 2002 | X | X | X | ||||
| Miniguchi 2002‡ | X | X | |||||
| Weiner 2003 | X | X | |||||
| Hsiao 2003 | X | X | X | ||||
| Weiner 2004 ‡ | X | ||||||
| Beckerman 2005 | X | X | |||||
| Hill 2006 | X | X | |||||
| Koppers 2006∗∗ | X | ||||||
| Weiner 2006 | X | X | |||||
Reason excluded from the present review:
Flow-dependent/intensity not controlled
Normocapnic hyperpnea
Combo
Other
Therefore, the purpose of the present review was to interpret the literature and assess the quality of evidence regarding the clinical benefits of IMT and the application of this evidence and its limitations to clinical practice by reviewing studies that used training intensity-controlled IMT compared to sham or no intervention. Particular attention was given to training intensity and the relationships between changes in inspiratory muscle function and other clinical outcome measures in each individual study.
METHODS
Literature Search
The literature search was completed using CINAHL, PubMed, Medline via First Search, and ProQuest databases. Key words used in the search were: “inspiratory muscle training,” “respiratory muscle training,” “ventilatory muscle training,” “breathing exercise,” and “resistive breathing.” Reference lists from articles found by these searches were also utilized to discover additional articles. No limit with regard to year of publication was used.
Study Selection Criteria
Inclusion criteria for the present review were as follows: (1) randomized design, (2) published in a peer-reviewed journal, (3) adults with COPD, (4) specified/quantified intensity of training, (5) published in English, and (6) IMT as the sole intervention compared to a sham or no-intervention control group. Because identification of the optimal training intensity was a primary objective of this review, “low-intensity training” was defined at as anything ≤ 30% of PImax. “High-intensity training” was defined as training at ≥ 50% of PImax.
Levels of Evidence
The strength of the evidence was rated using levels of evidence as described by Sackett.15 The levels are ordered 1 to 5, with 1 being the strongest. Articles were evaluated independently by the authors (SD and AL). Differences in scoring were discussed and a consensus was reached by the authors if opinions varied. Levels 1b and 2b in Sackett's schema were included in this review as long as the inclusion criteria required randomization.
Methodological Rigor
The methodological quality of the studies was evaluated using a scale developed by Medlicott and Harris19 (Table 2). The methodological quality was rated as follows: “Strong” 100% to 80%, “Moderate” 60% to 79%, and “Weak” 59% or less. These percentages were calculated by the number of “yes” scores divided by the total number “yes” scores that were applicable for that particular article. Studies with weak methodological rigor were assigned a level of evidence of “2b.”
Table 2.
Scoring Criteria for Methodological Rigor
| 1 | Randomization |
| 2 | Inclusion and exclusion criteria were listed for subjects |
| 3 | Similarity of groups at baseline |
| 4 | The treatment protocol was sufficiently described to be replicable |
| 5 | Reliability of data obtained with the outcome measures was investigated |
| 6 | Validity data obtained with the outcome measures was addressed |
| 7 | Blinding of patient, and/or treatment provider, and/or assessor was performed (if possible and appropriate) |
| 8 | Dropouts were reported |
| 9 | Long-term (6 month or greater) results were addressed via follow-up |
| 10 | Adherence to home programs was investigated (if included in the intervention) |
RESULTS
Six-hundred ninety-one articles were found with the search parameters previously outlined. The most frequent reasons for exclusion were diagnoses other than COPD, using IMT in combination with other interventions, using flow-dependent resistive training without specification of training intensity, not investigating IMT, and not using a control group (Table 3). Ultimately 15 studies20–34 met the inclusion criteria for this review. These studies were then categorized according to training intensity: low (5),20–24 progression from low to high (5),25–29 and high intensity (5).30–34 Tables 4 and 5 display the methodological rigor and summaries of the included articles. The primary outcomes reported were as follows: 15 examined inspiratory muscle strength as by measured by PImax. Ten examined inspiratory muscle endurance, with 3 using incremental threshold loading pressure (PITL), 5 using inspiratory muscle endurance time (IMET) at a specified percentage of PImax, and 2 using maximal sustained inspiratory pressure (SIPmax). Six studies measured HRQL, 6 measured dyspnea, and 9 measured exercise tolerance.
Table 3.
Search Methods and Results
| Databases: | CINAHL, PubMed, Medline via First Search, ProQuest Health |
| Search Terms: | “inspiratory muscle training,” “respiratory muscle training,” “ventilatory muscle training,” “breathing exercise,” “resistive breathing” |
| Number of Articles Found: | 691 |
| Number of Articles Meeting Inclusion Criteria: | 15 |
| Excluded Articles: | |
| Reason | Number of Articles |
| Diagnosis other than COPD | 562 |
| IMT performed in combination with other interventions | 18 |
| Intensity Not Specified or Not Controlled | 15 |
| Non-English Language | 5 |
| Other: expiratory muscle training, no IMT, other review articles, no control group | 69 |
COPD = chronic obstructive pulmonary disease, IMT = inspiratory muscle training
Methodological Rigor
Medlicott and Harris scores of methodological rigor ranged from 40% to 90%. Six articles20,21,24,26,28,34 were rated ‘strong,’ 822,23,25,27,29,31–33 as ‘moderate,’ and 130 as ‘weak.’
-
Randomization
Subjects were randomly assigned in all 15 studies, but only 2 described the randomization process.28,34
-
Subject inclusion and exclusion criteria
All but 130 of the studies provided specific inclusion and exclusion criteria. Exclusion criteria varied between the studies more than the inclusion criteria. All but 4 studies21,23,25,30 used spirometry to specifically define the presence of COPD. The most typical exclusion criteria were cardiac disease,20,24,27,28,32,33 any disease other than COPD interfering with the ability to exercise or participate,20,23,24,26,32,34 presence asthma,20,26,32,33 of presence of restrictive lung disease,20,21,33 use of supplemental oxygen,27,28,34 use of corticosteroids,21,26,32,34 and poor adherence.20,27,28,33
-
Similarity of groups at baseline
All studies reported that there were no statistically significant differences between subject groups at baseline based on age, pulmonary function, or outcome measures.
-
Repeatability of the treatment protocol
All studies reported the training intensities for the training and control groups. The 3 most replicable studies26,32,34 used interval training and provided the relevant detail about IMT prescription. The remaining 12 studies described the training session by duration (eg, IMT for 15 minutes). It is assumed that subjects used IMT continuously for the specified duration, but no studies provided this information.
-
Outcome measure reliability
Only 1 study21 conducted reliability testing or reported any values of intra- or inter-rater reliability for the measurement instruments used.
-
Outcome measure validity
All studies clearly outlined the procedures for inspiratory muscle testing and referenced appropriate sources. All studies used valid, standard measures to assess the effects of IMT on dyspnea,22,24–28 HRQL,20,21,24,26,28,34 and exercise tolerance.20,21,22,24,27,28,32–34 Dyspnea measures included the Transition Dyspnea Index,22,24,25 the Borg Scale,22,27 the Baseline Dyspnea Index,27 and the Perception of Dypsnea scale.28 The HQRL measures included St. George's Respiratory Questionaire (SGRQ),28 the Chronic Respiratory Disease Questionnaire (CRQ),24,26,34 Bronchitis Emphysema Symptoms Checklist (BESC),21 Profile of Mood States,20 Sickness Impact Profile,20 and the Health Perception Questionaire.20 Exercise tolerance was measured by the 12-minute walk test (12MWT),20,21 the 6-minute walk test (6MWT),22,27,28,32–34 cycle ergometry,22,24,32,34 and the shuttle walk test (SWT).24
-
Blinding
All but 5 studies25,29,30,32,33 used blinding of subjects and/ or investigators in their studies. Seven were double-blinded studies.20,22,23,24,27,28,34 Three studies21,26,31 used blinding of the investigator only, of which 126 blinded only the investigators performing measurements.
-
Reporting Drop-outs
All but 2 studies23,30 reported drop-outs or made clear in their methods and results how many subjects were recruited in comparison to the number included in data analysis.
-
Long-term results
Only 2 studies reported long-term results: 1 for 6 months24 and 1 for 12 months.28
-
Adherence to Program
Eight studies recorded either adherence to home-based or attendance to clinic-based training programs.20,25–28,32–34 Of these, all reported good adherence to the intervention. One study22 not reporting adherence stated that despite not using an adherence log, the weekly assessment and progression of the training protocol by investigators provided no indication of poor adherence.
Inspiratory Muscle Function
All 15 studies demonstrated statistically significant improvements in PImax ranging from −11 to −30 cmH2O and all 10 studies measuring endurance20,21,24,26,27,30–34 demonstratedstatisticallysignificantimprovementsineither PITL, IMET, or SIPmax. Further, improvements in inspiratory strength were always accompanied by improvements in inspiratory muscle endurance.20,21,24,26,27,30–34
Dyspnea
All 3 studies22,24,25 measuring dyspnea during functional activities and all 3 studies26–28 measuring dyspnea during inspiratory muscle training demonstrated improvements. Of the 3 studies measuring dyspnea during exercise tolerance,22,24,34 only Lisboa et al22 found reduced dyspnea following the intervention.
Health-Related Quality of Life
Five of 6 studies measuring HRQL demonstrated statistically and clinically significant improvements in HRQL using the CRQ,24,26,34 SGRQ,28 or the BESC.21
Exercise Tolerance
All 4 studies22,24,32,34 measuring maximal aerobic capacity with cycle ergometry failed to demonstrate statistically significant improvements in either maximal oxygen consumption or peak work. Eight of 9 studies demonstrated improvementinsubmaximalwalkingtestmeasuresofexercise tolerance: 12MWT,20,21 6MWT,22,27,28,33,34 and SWT.24
DISCUSSION
The results of this systematic review, which are in agreement with previous meta-analyses,16,17 found statistically significant improvements in PImax ranging from −11 to −30 cm H2O as well as improvements in either PITL, IMET, or SIPmax. However, previous reviews have not provided an interpretation of the clinical benefit of these improvements. Because there are no established thresholds for what constitutes a clinically meaningful change in inspiratory muscle strength or endurance, other methods must be utilized to infer clinical benefit. For example, restoration of PImax to “normal” could be considered a clinically significant change. The mean changes in PImax in the studies included in the present review appeared to cross the threshold of “normal” based on measured PImax, but only 3 of these studies31,32,34 reported PImax based on percent of predicted, which all showed improvement to near 100% of predicted. Because there are considerable gender differences in PImax,35,36 reporting means and mean changes in this measure would allow for stronger conclusions about the significance of observed changes. The inference that improvement in PImax results in clinically meaningful benefit would also be enhanced if concurrent changes in other clinical measures such as dyspnea, HRQL, and exercise tolerance were observed. However, only 3 of the 6 studies25,27,30 measuring dyspnea, 1 of 626 measuring HRQL, and 2 of 927,33 measuring exercise tolerance examined and subsequently demonstrated weak to moderate correlations between improvement in PImax and these other clinically meaningful measures.
Regarding the effect on dyspnea, only 3 studies22,24,25 support using IMT for reducing dyspnea during activities of daily living. This is in agreement with the review by Geddes et al.16 Three other studies26–28 in the present review measured dyspnea only during inspiratory muscle endurance testing. The clinical significance of reduced dyspnea during inspiratory muscle endurance testing is not clear, but perhaps simulates the perceived effort during high inspiratory muscle demand similar to that during exercise.
Regarding the effect of IMT on HRQL, definitive conclusions through meta-analyses have eluded previous reviews due to the multiplicity of HRQL instruments utilized. Qualitatively, however, 5 of 6 studies measuring HRQL demonstrated statistically and clinically significant improvements in HRQL using the CRQ,24,26,34 SGRQ,28 and the BESC.21
Regarding the effect of IMT on exercise tolerance, all 4 studies22,24,32,34 measuring maximal aerobic capacity with cycle ergometry failed to demonstrate statistically significant improvements in either maximal oxygen consumption or peak work. Eight of 9 studies demonstrated improvement in submaximal walking test measures of exercise tolerance: 12MWT,20,21 6MWT,22,27,28,33,34 and SWT.24 Three of these demonstrated improvements that exceeded what is considered to be clinically significant improvement of 54 meters37 for the 6MWT22,27,28 and 47.5 meters38 for the SWT.24 There are no established thresholds for interpreting changes in the 12MWT; however, the changes of approximately 60 meters demonstrated by Larson et al20 and Kim et al21 likely do not reflect meaningful change in the 12MWT. Therefore, improvement in maximal exercise tolerance would likely not be an expected outcome of IMT, but improvements in walking test distance may be expected. Previous meta analyses14–16 support this observation, including a statistically but not clinically significant weighted mean difference for improvement in 6MWT found in the update by Geddes et al.16 However, this conclusion must be considered in the context of the present review where only 2 studies27,33 found significant correlations between improvement in inspiratory muscle function and improvement in 6MWT distance, and only 4 studies reported between-group differences in walking test outcomes.20,24,28,34
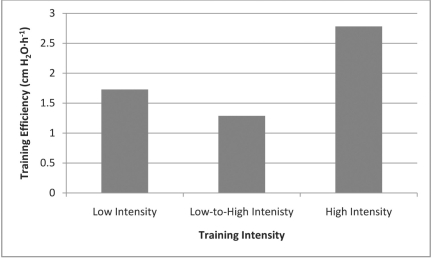
Regarding recommendations to clinicians about IMT training parameters, Hill et al34 found that high intensity training can result in a higher training efficiency, defined as mean change in PImax per hour spent on loaded training (cm H2O·h−1). The results of the present systematic review clearly support this suggestion. In comparing the results of their single trial of high-intensity IMT, Hill et al34 provided graphic comparison of their training efficiency to all but one of the studies included in the present review. A study by Preusser et al39 not included in this review (no control group was used) compared high-intensity to low-intensity IMT in a head-to-head trial, and demonstrated greater improvements in inspiratory muscle strength and endurance in the high-intensity IMT group. It should be noted that the training programs utilized by Hill et al,34 Ramirez-Sarmiento et al,32 and Preusser et al39 were interval-based and resulted in the greatest training efficiency, although the other 3 studies30,31,33 using continuous high-intensity training also resulted in better training efficiency compared to other training intensities. An overall graphic summary of training efficiency based on training intensity is provided in Figure 1.
Figure 1.
Average Training Efficency Based on IMT Training Intensity
Unfortunately, only 1 of the 5 studies34 investigating high-intensity IMT included in the present review used a measure of HRQL (which did show statistically and clinically significant improvements in CRQ scores), and none included any measures of dyspnea. Thus, it is difficult to conclude definitively that high-intensity training would result in better clinical outcomes beyond improved inspiratory muscle function. Training intensity did not appear to result in greater changes in PImax, and it is likely that similar dyspnea and HRQL outcomes would be found compared to lower training intensities. However, it is our experience that patients are much more willing to initiate and adhere to interval-based IMT than training programs of up to 30 minutes of continuous IMT. If similar benefits can be obtained through increased training efficiency, then high-intensity IMT might be an optimal method for training.
Other potential benefits of high-intensity IMT might include reduced outpatient utilization and hospital length of stay. Beckerman et al28 used IMT progressing from 15% to 60% of PImax over a 12-month period compared to a control group, and found that the treatment group spent 2.5 less days in the hospital and had 3.2 less primary care consultations. However, no other study examined this as an outcome of IMT and therefore these results need to be corroborated in future studies to determine if this is a repeatable, expected outcome of IMT, as well as whether this outcome is associated with training intensity.
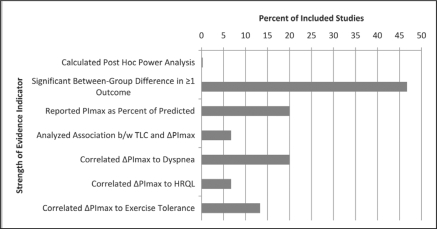
Despite the extensive study of IMT spanning decades, there continue to be limitations to this body of knowledge. Figure 2 presents a summary of these limitations of the strength of evidence regarding IMT. First, most studies have included small sample sizes which have likely resulted in low statistical power (no study reported statistical power post hoc) as evidenced by a limited number of studies finding between-group differences (most conclusions were based on within-group differences qualitatively compared to the control group). Approximately one third of studies found between-group differences for inspiratory muscle strength, inspiratory muscle endurance, and exercise tolerance. Additionally, low statistical power may also account for the apparent lack of effect of IMT on exercise tolerance. Second, there was a lack of uniformity in inclusion and exclusion criteria, resulting in non-uniformity of disease severity, particularly with regard to the presence and degree of hyperinflation. In comparing high versus low intensity, Preusser et al39 observed that the subjects with the greatest amount of hyperinflation made the greatest amount of improvement in PImax when training at high intensity. However, only 4 studies12,20,26,32 in the present review reported total lung capacity (TLC), and only Larson et al20 commented on the relationship between TLC and response to training. They were unable to provide any definitive conclusions. The derth of data about the influence of hyperinflation on IMT is ironic since hyperinflation and its subsequent effects on diaphragm position and function are frequently cited as being a primary contributing factor to the dyspnea experienced by individuals with COPD. A third limitation to the literature regarding IMT is the high number of drop-outs. Of the studies reviewed in this paper, 821,22,25,26,28,32–34 of the 15 reported a combined total of 112 drop-outs compared to 394 subjects who completed these protocols. At least 37 of the 112 drop-outs were due to pulmonary exacerbations. These drop-outs were essentially equal among the treatment and control groups and therefore did not reflect an adverse outcome resulting from IMT.
Figure 2.
Key Strength of Evidence Indicators
TLC = Total Lung Capacity, ΔPImax = change in maximal inspiratory pressure, HRQL = Health-Related Quality of Life
Implications for Future Research
Future research should include larger samples, control for degree of hyperinflation in their analyses, report changes in PImax based on percentage of predicted, and determine correlations between changes in inspiratory muscle function with other clinically important outcomes.
CONCLUSION
In summary, the clinical benefits of improved inspiratory muscle strength and endurance resulting from IMT appear to include improvements in dyspnea, walking test distance, and HRQL. However, the strength of these conclusions must be considered in the context of several limitations to the body of evidence regarding the use of IMT in individuals with COPD. Additionally, it is not clear who would benefit most from IMT and what training regimen is optimal.
Table 4.
Results of 10-Point Criteria: Methodological Rigor
| Author | Randomized | Inclusion/Exclusion Criteria | Group Similarity at Baseline | Replicable | Reliable | Valid | Blinding | Dropouts Reported | Long-Term Results | Adherence | Score as Percentage | Level of Evidence |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Larson et al20 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | 80% | 1b |
| Kim et al21 | Y | Y | Y | Y | Y | Y | Y | Y | N | N | 90% | 1b |
| Lisboa et al22 | Y | Y | Y | Y | N | Y | Y | Y | N | N∗ | 70% | 1b |
| Villafranca et al23 | Y | Y | Y | Y | N | Y | Y | N | N | N | 60% | 1b |
| Sanchez-Riera et al24 | Y | Y | Y | Y | N | Y | Y | Y | Y | N | 80% | 1b |
| Harver et al25 | Y | Y | Y | Y | N | Y | N | Y | N | Y | 70% | 1b |
| Covey et al26 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | 80% | 1b |
| Weiner et al27 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | 70% | 1b |
| Beckerman et al28 | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 90% | 1b |
| Weiner & Weiner29 | Y | Y | Y | Y | N | Y | N | Y | N | N | 70% | 1b |
| Patessio et al30 | Y | N | Y | Y | N | Y | N | N | N | N | 40% | 2b |
| Heijdra et al31 | Y | Y | Y | N | N | Y | Y | Y | N | N | 60% | 1b |
| Ramirez-Sarmiento et al32 | Y | Y | Y | Y | N | Y | N | Y | N | Y | 70% | 1b |
| Hsiao et al33 | Y | Y | Y | Y | N | Y | N | Y | N | Y | 70% | 1b |
| Hill et al34 | Y | Y | Y | Y | N | Y | Y | Y | N | Y | 80% | 1b |
=Adherence not measured but the authors stated there was no indication of non-adherence among their subjects
Table 5.
Study Characteristics
| OUTCOMES | ||||||
| Study, Level of Evidence, and Rigor | Subjects | Intervention | Inspiratory Muscle Strength and Endurance | HRQL and Dyspnea | Exercise Tolerance | |
|---|---|---|---|---|---|---|
|
Low Intensity IMT | ||||||
|
|
30%PImax, RV progressing from 15 to 30 min daily for 8 wks vs control of 15% PImax, RV | No significant changes in either group for the Profile of Mood States, Sickness Impact Profile, or Health Perceptions Questionnaire | |||
|
|
30%PImax, RV for 30 min daily for 8 wks vs. control with no load | The treatment group made significant improvements in the Bronchitis Emphysema Symptoms Checklist | |||
|
|
30%PImax, FRC for 30 min daily, 6 days/week for 10 wks vs. control of 10% PImax, FRC |
|
|||
|
|
30%PImax, FRC for 15 min twice daily, 6 days/week for 10 wks vs. control of 10% PImax, FRC | Not Measured | Not Measured | ||
|
|
30%PImax, FRC for 15 min twice daily, 6 days/week for 24 wks vs. control with no load |
|
|||
| Progressing from Low to High Intensity IMT | ||||||
|
|
17%progressing to 45% PImax, RV for 15 min twice daily for 8 wks vs. control at 14% PImax, RV |
|
Transition Dyspnea Index improved 3.17 vs. no change in the control† | Not Measured | |
|
|
30%progressing to 60% PImax, RV, 6 sets of 5 min each, 5 days/week for 16 weeks vs. no IMT for control | Not Measured | |||
|
|
15%progressing to 60% PImax, RV, for 30 min daily 6 days/week for 12 weeks vs. 7cmH2O for control |
|
|||
|
|
15%progressing to 60% PImax, RV for 15 min twice daily, 6 days per week, for 52 wks vs. control at 7% PImax, RV | ||||
|
|
15%progressing to 60% PImax, RV for 60 min daily, 6 days per week, for 8 wks vs. control at 7% PImax, RV | Not Measured | Not Measured | ||
| High Intensity IMT | ||||||
|
|
50% PImax, FRC for 15 min daily for 8 wks vs control with no load | Not Measured | Not Measured | ||
|
|
60% PImax, RV for 10 wks vs control at 10% PImax (Frequency and session duration NR) | Mean nocturnal saturation improved by 2 (2)%, and nocturnal desaturation decreased by 13 (14)% vs. no change in the control‡ | Not Measured | ||
|
|
40–50% PImax, RV progressed as tolerated for 30 min, 5 days/wk, for 5 wks, 3 min:2 min work:rest vs no IMT for control | Proportion of type I and size of type II muscle fibers of the external intercostals improved in the treatment group vs. no change in the control |
|
||
|
|
15%progressing to 60% PImax, RV for 15 min twice daily, 6 days per week, for 52 wks vs. control at 7% PImax, RV | ||||
|
|
50%PImax, RV using threshold (A) or using target flow (B) 15 min twice daily for 8 wks vs control no load |
|
Not Measured | ||
|
|
45%progressing to 100%PImax, FRC 3 days/wk for 8 wks, 7 repetitions of 2 min each vs. control of 10% PImax |
|
|
||
Statistically significant (p<.05) within-group difference,
statistically significant (p<.05) within-group difference and between-group difference compared to control, M&HR=Medlicott and Harris Rating of methodological rigor, SEM=standard error of the mean, NR=not reported, FEV1 =forced expiratory volume in 1 second as percentage of predicted, PImaxFRC=maximal inspiratory pressure measured at functional residual capacity, PImaxRV=maximal inspiratory pressure measured at residual volume, IMET=inspiratory muscle endurance time, SIPmax=maximal sustained inspiratory pressure, PITL=maximal pressure obtained during incremental threshold loading, CRQ=Chronic Respiratory Disease Questionnaire, RPBD=Rating of Perceived Breathing Difficulty, BDI=Baseline Dyspnea Index, POD=Perception of Dyspnea Scale.
REFERENCES
- 1.Mannino DM. COPD: Epidemiology, prevalence, morbidity, and mortality, and disease heterogeneity. Chest. 2002;121:121–126. doi: 10.1378/chest.121.5_suppl.121s. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 3.Casaburi R. Exercise training in chronic obstructive lung disease. In: Casaburi R, Petty TL, editors. Principles and Practice of Pulmonary Rehabilitation. Philadelphia, PA: Saunders; 1993. pp. 204–224. [Google Scholar]
- 4.Wasserman K, Hansen JE, Sue DY, Stringer WW, Whipp BJ. Principles of Exercise Testing and Interpretation. Philedelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 5.Casaburi R. Combination therapy for exercise tolerance in COPD. Thorax. 2006;61:551–552. doi: 10.1136/thx.2006.058511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Thoracic Society, European Respiratory Society. Skeletal muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:S1–S40. doi: 10.1164/ajrccm.159.supplement_1.99titlepage. [DOI] [PubMed] [Google Scholar]
- 7.Laghi F, Tobin MJ. Disorders of the respiratory muscles. Am J Respir Crit Care Med. 2003;168:10–48. doi: 10.1164/rccm.2206020. [DOI] [PubMed] [Google Scholar]
- 8.Scott A. The diaphragm in COPD – evidence of overuse injury and considerations for treatment. Cardiopulm Phys Ther J. 2004;15:3–8. [Google Scholar]
- 9.Bellemere F, Grassino A. Force reserve of the diaphragm in patients with chronic obstructive pulmonary disease. J Appl Physiol. 1983;55:8–15. doi: 10.1152/jappl.1983.55.1.8. [DOI] [PubMed] [Google Scholar]
- 10.Sinderby C, Spahija J, Beck J, et al. Diaphragm activation during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:1637–1641. doi: 10.1164/ajrccm.163.7.2007033. [DOI] [PubMed] [Google Scholar]
- 11.Mador MJ, Kufel TJ, Pineda LA, Sharma GK. Diaphragmatic fatigue and high-intensity exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:118–123. doi: 10.1164/ajrccm.161.1.9903010. [DOI] [PubMed] [Google Scholar]
- 12.Hill K, Jenkins SC, Hillman DR, Eastwood PR. Dyspnoea in COPD: can inspiratory muscle training help? Australian J Physiotherapy. 2004;50:169–180. doi: 10.1016/s0004-9514(14)60155-0. [DOI] [PubMed] [Google Scholar]
- 13.Smith K, Cook D, Gordon HG, Madhavan J, Oxman AD. Respiratory muscle training in chronic airflow limitation: a meta-analysis. Am Rev Respir Dis. 1992;145:533–539. doi: 10.1164/ajrccm/145.3.533. [DOI] [PubMed] [Google Scholar]
- 14.Lötters F, van Tol B, Kwakkel G, Gosselink R. Effects of controlled inspiratory muscle training in patients with COPD: a meta-analysis. Eur Respir J. 2002;20:570–578. doi: 10.1183/09031936.02.00237402. [DOI] [PubMed] [Google Scholar]
- 15.Geddes L, Reid WD, Crowe J, O'Brien K, Brooks D. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: A systematic review. Respir Med. 2005;99:1440–1458. doi: 10.1016/j.rmed.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Geddes L, O'Brien K, Reid WD, Brooks D, Crowe J. Inspiratory muscle training in adults with chronic obstructive pulmonary disease: an update of a systematic review. Respir Med. 2008;102:1715–1729. doi: 10.1016/j.rmed.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 17.O'Brien K, Geddes EL, Reid WD, Brooks D, Crowe J. Inspiratory muscle training compared with other rehabilitation interventions in chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prevent. 2008;28:128–141. doi: 10.1097/01.HCR.0000314208.40170.00. [DOI] [PubMed] [Google Scholar]
- 18.Crowe J, Reid WD, Geddes EL, O'Brien K, Brooks D. Inspiratory muscle training compared with other rehabilitation interventions in adults with chronic obstructive pulmonary disease: a systematic literature review and meta-analysis. J Chronic Obstructive Pulm Dis. 2005;3:319–329. doi: 10.1080/15412550500218072. [DOI] [PubMed] [Google Scholar]
- 19.Medlicott MS, Harris SR. A systematic review of the effectiveness of exercise, manual therapy, electrotherapy, relaxation training, and biofeedback in the management of temporomandibular disorder. Phys Ther. 2006;86:955–973. [PubMed] [Google Scholar]
- 20.Larson JL, Kim MJ, Sharp JT, Larson DA. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1988;138:689–696. doi: 10.1164/ajrccm/138.3.689. [DOI] [PubMed] [Google Scholar]
- 21.Kim MJ, Larson JL, Covey MK, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Nurs Res. 1993;42:356–362. [PubMed] [Google Scholar]
- 22.Lisboa C, Villafranca C, Levia A, et al. Inspiratory muscle training in chronic airflow limitation: effect on exercise performance. Eur Respir J. 1997;10:537–542. [PubMed] [Google Scholar]
- 23.Villafranca C, Borzone G, Leiva A, Lisboa C. Effects of inspiratory muscle training with an intermediate load on inspiratory power output in COPD. Eur Respir J. 1998;11:28–33. doi: 10.1183/09031936.98.11010028. [DOI] [PubMed] [Google Scholar]
- 24.Riera HS, Rubio TM, Ruiz FO, et al. Inspiratory muscle training in patients with COPD. Chest. 2001;120:748–756. doi: 10.1378/chest.120.3.748. [DOI] [PubMed] [Google Scholar]
- 25.Harver A, Mahler DA, Daubenspeck JA. Targeted inspiratory muscle training improves respiratory muscle function and reduces dyspnea in patients with chronic obstructive pulmonary disease. Ann Intern Med. 1989;111:117–124. doi: 10.7326/0003-4819-111-2-117. [DOI] [PubMed] [Google Scholar]
- 26.Covey MK, Larson JL, Wirtz SE, et al. High-intensity inspiratory muscle training in patients with chronic obstructive pulmonary disease and severely reduced function. J Cardiopulm Rehabil. 2001;21:231–240. doi: 10.1097/00008483-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Weiner P, Magadle R, Beckerman M, Weiner M, BerarYanay N. Comparison of specific expiratory, inspiratory, and combined muscle training programs in COPD. Chest. 2003;124:1357–1364. doi: 10.1378/chest.124.4.1357. [DOI] [PubMed] [Google Scholar]
- 28.Beckerman M, Magadle R, Weiner M, Weiner P. The effects of 1 year of specific inspiratory muscle training in patients with COPD. Chest. 2005;128:3177–3183. doi: 10.1378/chest.128.5.3177. [DOI] [PubMed] [Google Scholar]
- 29.Weiner P, Weiner M. Inspiratory muscle training may increase peak inspiratory flow in chronic pulmonary disease. Respiration. 2006;73:151–156. doi: 10.1159/000088095. [DOI] [PubMed] [Google Scholar]
- 30.Patessio A, Rampulla C, Fracchia C, et al. Relationship between the perception of breathlessness and inspiratory resistive loading: report on a clinical trial. Eur Respir J. 1989;2:587–591. [PubMed] [Google Scholar]
- 31.Heijdra YF, Dekhuijzen PNR, van Herwaarden CLA, Folering HTM. Nocturnal saturation improves by target flow inspiratory muscle training in patients with COPD. Am J Respir Crit Care Med. 1996;153:260–265. doi: 10.1164/ajrccm.153.1.8542126. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez-Sarmiento A, Orozco-Levi M, Güella R, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:1491–1497. doi: 10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 33.Hsiao SF, Wu YT, Wu HD, Wang TG. Comparison of effectiveness of pressure threshold and targeted resistance devices for inspiratory muscle training in patients with chronic obstructive pulmonary disease. J Formos Med Assoc. 2003;102:240–245. [PubMed] [Google Scholar]
- 34.Hill K, Jenkins SC, Philippe DL, et al. High-intensity inspiratory muscle training in COPD. Eur Respir J. 2006;27:1119–1128. doi: 10.1183/09031936.06.00105205. [DOI] [PubMed] [Google Scholar]
- 35.Harik-Kahn RI, Wise RA, Fozard JL. Determinants of maximal inspiratory pressure: the Baltimore Longitudianl Study of Aging. Am J Respir Crit Care Med. 1998;158:1459–1464. doi: 10.1164/ajrccm.158.5.9712006. [DOI] [PubMed] [Google Scholar]
- 36.Hautmann H, Hefele S, Schottem K, Huber RM. Maximal inspiratory mouth pressures (PIMAX) in healthy subjects–what is the lower limit of normal? Respir Med. 2000;94:689–693. doi: 10.1053/rmed.2000.0802. [DOI] [PubMed] [Google Scholar]
- 37.Redelmeier DA, Bayoumi AM, Goldstein RS, Guyatt GH. Interpreting small differences in functional status: The six minute walk test in chronic lung disease patients. Am J Respir Crit Care Med. 1997;155:1278–1282. doi: 10.1164/ajrccm.155.4.9105067. [DOI] [PubMed] [Google Scholar]
- 38.Singh SJ, Jones PW, Evans R, Morgan MDL. Minimum clinically important improvement for the incremental shuttle walking test. Thorax. 2008;63:775–777. doi: 10.1136/thx.2007.081208. [DOI] [PubMed] [Google Scholar]
- 39.Preusser BA, Winningham ML, Clanton TL. High– vs. low-intensity inspiratory muscle interval training in patients with COPD. Chest. 1994;106:110–117. doi: 10.1378/chest.106.1.110. [DOI] [PubMed] [Google Scholar]