Abstract
A growing number of promoters have key components of the transcription machinery, such as TATA-binding protein (TBP) and RNA polymerase II (RNAPII), present at the promoter prior to activation of transcription. Thus, while transcriptional output undergoes a dramatic increase between uninduced and induced conditions, occupancy of a large portion of the transcription machinery does not. As such, activation of these poised promoters depends on rate-limiting steps after recruitment of TBP and RNAPII for regulated expression. Little is known about the transcription components required in these latter steps of transcription in vivo. To identify components with critical roles in transcription after recruitment of TBP in Saccharomyces cerevisiae, we screened for loss of gene expression activity from promoter-tethered TBP in >100 mutant strains deleted for a transcription-related gene. The assay revealed a dramatic enrichment for strains containing deletions in genes encoding subunits of the Spt-Ada-Gcn5-acetyltransferase (SAGA) complex and Mediator. Analysis of an authentic postrecruitment-regulated gene (CYC1) reveals that SAGA occupies the promoter under both uninduced and induced conditions. In contrast, Mediator is recruited only after transfer to inducing conditions and correlates with activation of the preloaded polymerase at CYC1. These studies indicate the critical functions of SAGA and Mediator in the mechanism of activation of genes with rate-limiting steps after recruitment of TBP.
THE regulation of gene expression by RNA polymerase II (RNAPII) is a fundamental and highly complex process. Transcription by RNAPII involves a number of steps including the recruitment of a pre-initiation complex to the promoter, promoter melting, initiation of transcription, promoter clearance, elongation, and termination (for review see Hahn 2004). An assortment of factors is required for these events to take place efficiently and accurately. Initiation of transcription is dependent upon RNAPII and the general transcription factors TFIID [composed of the TATA-binding protein (TBP) and TBP-associated factors (TAFs)], TFIIA, TFIIB, TFIIE, TFIIF, and TFIIH (Lemon and Tjian 2000), which together form the pre-initiation complex (PIC). For a large number of well-characterized promoters, the rate-limiting step in the transcription process is the formation of the PIC at the promoter. For these genes, the recruitment and occupancy of TBP and RNAPII to the promoter correlates strongly with transcriptional output (Kuras and Struhl 1999; Kim et al. 2005; Reppas et al. 2006). Indeed, artificially tethering TBP or RNAPII to a promoter is sufficient for gene activation in many contexts (Kuras and Struhl 1999; Ptashne 2003, 2005). Despite this, an increasing number of promoters are regulated after recruitment of the PIC (for reviews see Lis 2007; Margaritis and Holstege 2008; Price 2008). These preloaded, yet transcriptionally inactive, promoters can be defined as poised for subsequent activation. Poised promoters are found across the evolutionary spectrum, including bacteria, yeast, Drosophila, and humans (Kuras and Struhl 1999; Andrulis et al. 2000; Kim et al. 2005; Reppas et al. 2006; Zhang et al. 2008; Kininis et al. 2009; Venters and Pugh 2009). Indeed, whole-genome studies suggest that transcription of a significant part of the human genome may be regulated at rate-limiting steps after recruitment of the PIC (Kim et al. 2005; Guenther et al. 2007). Importantly, the transcription factors involved in this mechanism of regulation in vivo are currently poorly defined.
To discover transcription factors with roles in rate-limiting steps after formation of the PIC, we took advantage of the fact that tethering TBP to a reporter promoter in a wild-type strain results in robust gene expression (Chatterjee and Struhl 1995; Stargell and Struhl 1996a,b). We used this plasmid-based system to screen mutant strains in search of those that are unable to activate the reporter gene, which would suggest involvement of the gene product in the essential steps of transcription after TBP recruitment. We initially analyzed 10 SPT (Suppressor of Ty) yeast deletion strains in the screen since this family of genes encodes proteins intimately involved in various transcription-related processes. In fact, TBP itself is encoded by the essential SPT15 gene. Products of the yeast SPT gene family are implicated in various processes such as transcription initiation, elongation, and RNA processing and in maintaining chromatin structure (Winston et al. 1984, 1987; Fassler and Winston 1988; Yamaguchi et al. 2001). The Spt1, Spt10, and Spt21 proteins are the regulatory factors that control the expression levels of histone genes (Sherwood et al. 1993; Dollard et al. 1994; Natsoulis et al. 1994; Hess et al. 2004). SPT23 encodes an activator protein involved in transcription of genes involved in lipid biosynthesis (Chellappa et al. 2001). SPT2 and SPT4 encode transcription elongation factors (Wada et al. 1998; Nourani et al. 2006). Finally, several SPT genes are subunits of the Spt-Ada-Gcn5-acetyltransferase (SAGA) coactivator complex, including Spt3, Spt7, Spt8, and Spt20 (Grant et al. 1997; Roberts and Winston 1997).
Using the TBP-tethering approach, we identified several subunits of SAGA and Mediator with potential postrecruitment functions. These results were corroborated with studies of the authentic poised promoter at the CYC1 gene. Timing of SAGA and Mediator occupancy at CYC1, and the lack of interdependency of the two coactivator complexes, indicates distinct functional roles for each complex in activating the poised promoter. Our results underscore the versatility of SAGA and Mediator in mechanisms of gene regulation because both complexes also have well-established roles in the regulation of recruitment-regulated genes.
MATERIALS AND METHODS
Yeast strains, media, and DNA:
Strains are listed in Table 1. The parent strain (BY4741, MATa his3Δ1 ura3Δ0 leu2Δ0 met15Δ0), as well as most of the deletion strains, was purchased from Research Genetics. The med2Δ strain was created using standard techniques (Longtine et al. 1998). The yeast complete and synthetic complete (SC) media were made as described (Hampsey 1997). To assay reporter gene expression, SC-based plates lacking uracil, leucine, and histidine were supplemented with 3-aminotriazol (AT). The reporter plasmid was created by first amplifying the HIS3 gene from the SK1 strain (Fischbeck et al. 2002), which has the Gcn4-binding sites replaced by the LexA operator. The amplified product was then subcloned into the YCp111 plasmid (LEU2, CEN). LexA and LexA-TBP derivatives cloned into pRS316 (URA3, CEN) were obtained from previous studies (Stargell and Struhl 1996b). Both LexA plasmids have an HA epitope in front of the LexA–protein fusion sequence. Strains containing proteins tagged with either the HA or the Myc epitope were generated according to the literature (Longtine et al. 1998). The gcn5E173Q open reading frame (ORF) was a gift from Shelley Berger (Trievel et al. 1999). The ORF was subcloned into the pRS313 plasmid, which was transformed into the BY4741 background. The Med15 (Gal11)-myc, Med15-myc spt20Δ, and Med15-myc gcn5Δ strains were a gift from Alan Hinnebusch (Qiu et al. 2005).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Research Genetics |
| med2Δ | BY4741 med2Δ∷URA3 | This work |
| SPT20-HA | BY4741 SPT20-HA3∷HIS3 | This work |
| GCN5-myc | BY4741 GCN5-myc13∷HIS3 | This work |
| SPT8-HA | BY4741 SPT8-HA3∷HIS3 | This work |
| TAF1-HA | BY4741 TAF1-HA3∷HIS3 | This work |
| MED12-HA | BY4741 MED12-HA3∷HIS3 | This work |
| MED12-HA, spt20Δ | BY4741 MED12-HA3∷HIS3, spt20Δ∷kanMX4 | This work |
| MED15-myc | BY4741 MED15-myc13∷HIS3 | Qiu et al. (2005) |
| MED15-myc, spt20Δ | BY4741 MED15-myc13∷HIS3, spt20Δ∷kanMX4 | Qiu et al. (2005) |
| MED15-myc, gcn5Δ | BY4741 MED15-myc13∷HIS3, gcn5Δ∷kanMX4 | Qiu et al. (2005) |
| gcn5E173Q | BY4741 gcn5Δ∷kanMX4/pRS313-GCN5-E173Q (HIS3) | This work |
Plasmid-based TBP-tethering screen:
Yeast cells were transformed with plasmids using standard procedures (Beggs 1978). Briefly, yeast cells were first transformed with the LexAopHIS3 plasmid. LEU2+ cells were then transformed with the LexA and LexA–TBP fusion constructs. Strains were streaked or spotted in serial dilutions onto SC–UL medium and SC–ULH medium containing 20–40 mm AT on the basis of cell growth. Cell growth was scored as ranging from “±” to “+++” with “±” indicating little or no growth and “+++” indicating robust growth.
Western blot analysis:
Yeast cells (10 ml) were grown in SC–UL to an OD600 of ∼0.8. Cells were harvested, washed with sterile water, and resuspended in 200 μl lysis buffer (0.5 m phosphate buffer, pH 7.5). Whole-cell extracts were prepared by vigorous bead beating. Cellular debris was removed by spinning the extracts at 10,000 × g at 4° for 10 min. Protein concentrations were determined by the Bradford assay (Bio-Rad). An equal amount of whole-cell extracts was separated on 10% SDS–PAGE and transferred to a nitrocellulose membrane. The following antibodies were used at the given dilutions: anti-HA (12CA5 from Covance; 1:1000), anti-myc (Upstate, 1:500), and polyclonal anti-Toa1 or anti-TBP (1:10,000). Horseradish peroxidase-conjugated secondary antibodies were used at a 1:20,000 dilution, and protein bands were detected using reagents from Amersham Biosciences.
Transcript assays:
S1 nuclease assays were conducted as described (Iyer and Struhl 1996). For ethanol induction, cells were grown overnight in medium containing 2% glucose and washed three times in medium lacking glucose prior to dilution into medium containing 3% ethanol. Cells were then cultured at 30° for 6 hr. For galactose induction, cells were grown in rich medium containing 2% glucose and then washed and transferred to YP galactose (2%). For uninduced samples, cells were grown in medium containing 2% glucose at 30° to an optical density of 0.8–1.0. Yeast cells were harvested and total RNA was isolated by hot-acid phenol extraction. A total of 25–30 μg of RNA was hybridized with an excess of 32P-labeled probe overnight at 55°. S1 nuclease digestion was performed for 30–45 min at 37°. The probe was visualized by phosphorimager, and CYC1 or GAL1 loading normalized to the intensity of the tRNAw band. At least three biological replicates (starting from independent cultures) were assayed for each indicated condition.
Chromatin immunoprecipitation analysis:
Chromatin immunoprecipitations (ChIPs) were performed as described (Zhang et al. 2008) with few modifications. Cells (50 ml) were grown to an OD600 of 0.8–1.0 and were treated with formaldehyde (1%) for 15 min with swirling every 5 min. Glycine (125 mm) was added for 5 min to stop the crosslinking. Cells were collected and washed twice in ice-cold TBS. Cells were then resuspended in FA–lysis buffer [50 mm Hepes, pH 7.5, 140 mm NaCl, 1 mm EDTA, 1% Triton X-100, 0.1% Na–deoxycholate, and a 1× protease inhibitor cocktail of PMSF, benzamidine, pepstatin, leupeptin, and chymostatin]. Chromatin was sheared by sonication using a Branson W-350 model sonifier (10 times at 10 sec each on continuous pulse at a microtip power setting of 6). Ten percent of the chromatin material used for the immunoprecipitation was processed as the input after reversing the crosslinks and purifying the DNA. Chromatin material (500 μl) was incubated with 5–10 μl of anti-TBP, anti-RNAPII (8WG16, Covance), anti-HA (Santa Cruz), or anti-Myc (Upstate) antibodies, rotating overnight at 4°. Fifty microliters of protein-A sepharose beads (Pharmacia-prepared as slurry as per the manufacturer's directions) was incubated with the chromatin material for 3 hr at room temperature. The beads were collected by centrifugation, and the antigen–antibody complexes were recovered and treated with elution buffer (50 mm Tris, 10 mm EDTA, 1% SDS) for 15 min at 65° to elute the complexes. Protein–DNA crosslinks were reversed by incubation overnight at 65°, and the DNA was purified by phenol–chloroform extraction and used for linear PCR analysis or quantitative PCR analysis.
Linear PCR reactions were carried out in a total volume of 25 μl. Each reaction contained 1 μl of 1/100 dilution of 32P-labeled ATP. Different dilutions of each input and immunoprecipitated material were used to determine the linear range of the PCR reaction. Samples were analyzed on a 5% native polyacrylamide gel in 0.5× TBE buffer. The gels were dried and exposed to a phosphorimager screen. The image was scanned on a STORM and quantified using ImageQuant software to detect the signal intensities. Samples with no antibody were used as controls. The ratio between the precipitated sample and the input, minus the background of no antibody control, was used as an indication of the protein occupancy.
Quantitative PCR reactions were carried out in a volume of 50 μl using a BioRad iCycler and SYBR fluorescein mix. Standard curves were generated using 10-fold serial dilutions of Input DNA. PCR efficiencies ranged from 90 to 100%, with a correlation coefficient of 0.95 or greater. Threshold cycle data were quantified relative to the input as described (Frank et al. 2001). For each immunoprecipitation, the highest value was set at 10, and other values were expressed relative to this maximum.
For linear PCR analysis of the occupancy of the LexA derivative, primers were designed to encompass the engineered HIS3 reporter promoter region and amplified a product of 646 bp. Two sets of primers were designed for the promoter region of the CYC1 gene (−230 to +80 for both linear and quantitative PCR and −150 to +40 for quantitative PCR). Occupancy at the GAL promoter was analyzed using quantitative PCR with primers designed for the GAL1,10 promoter region (−1 to −146 relative to the ATG of GAL10).
RESULTS
Classification of mutant strains in a TBP-tethering assay suggests roles in postrecruitment functions:
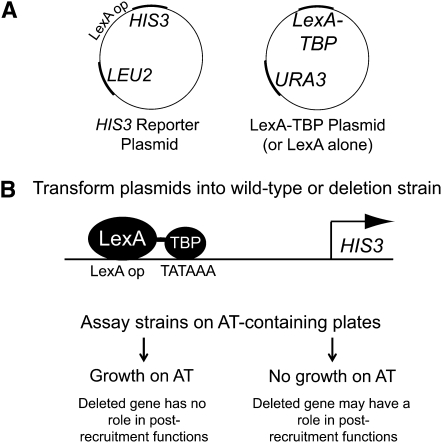
We used a TBP-tethering assay to identify nonessential SPT gene family members with potential functions in rate-limiting steps after TBP recruitment. The assay consists of two plasmids: a HIS3 reporter plasmid with the HIS3 promoter replaced by a LexA operator and a plasmid expressing either the LexA DNA-binding domain or LexA fused to TBP (Figure 1). This fusion results in binding of LexA–TBP to the promoter, which drives HIS3 expression (Chatterjee and Struhl 1995). Reporter gene expression is assayed by cell growth on media containing 3-AT, a competitive inhibitor of the HIS3 gene product. Growth properties on AT correlate very well with quantitative measurements of HIS3 RNA (Chen and Struhl 1988). In wild-type cells expressing LexA–TBP, growth on medium containing AT is robust, whereas LexA alone shows little growth (Figure 2). To assay the postrecruitment functions of the SPT gene family members, the reporter system was transformed into a variety of strains, each with a deletion of one nonessential SPT gene. If the SPT deletion strains are defective for TBP recruitment, artificially recruiting TBP in the tethering assay will correct these defects, and growth on AT will be similar to the wild-type strain. However, if the SPT deletion strains are defective for functions after TBP recruitment, these defects will not be corrected, and growth on AT will be poor. Therefore, the behavior of the deletion strain reflects the involvement of the wild-type protein in regulation of transcription after recruitment of TBP.
Figure 1.—
Schematic of the tethering assay. (A) The two-plasmid system for the tethering assay. The LEU2-marked plasmid contains the LexA operator–HIS3 reporter. The URA3-marked plasmid contains either LexA–TBP or LexA alone. (B) The wild-type strain or a strain with a deletion in one nonessential gene is transformed with the HIS3 reporter plasmid and the LexA–TBP or LexA plasmid. LexA–TBP binds to the LexA operator in the HIS3 reporter plasmid and results in TBP tethering. HIS3 gene expression is assayed by monitoring cell growth on media containing a competitive inhibitor of the HIS3 gene product, 3-AT.
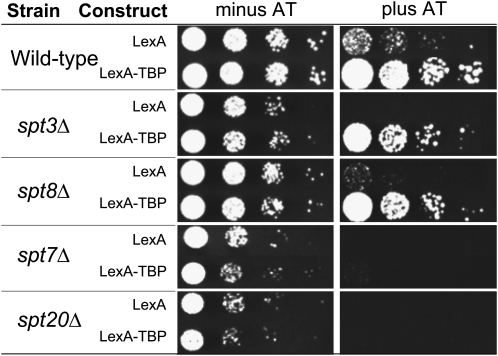
Figure 2.—
The spt7Δ and spt20Δ strains are compromised for function in the tethering system. The wild-type strain and representative SPT gene deletion strains (as indicated) were transformed with the tethering plasmids and monitored for growth. Serial dilutions of each strain were spotted on media with or without AT and incubated for 3 days.
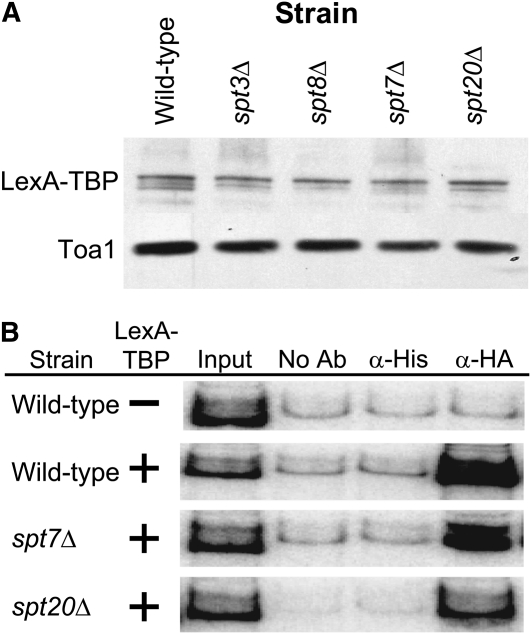
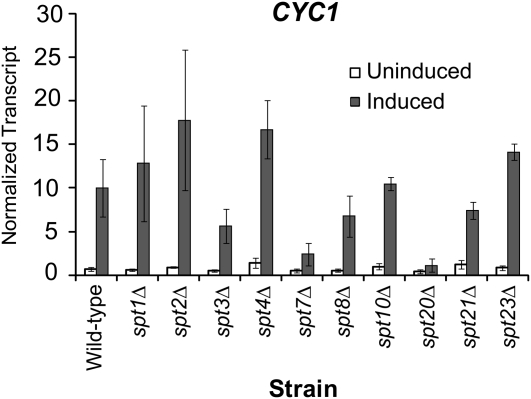
A majority of strains (spt1Δ, spt2Δ, spt3Δ, spt4Δ, spt8Δ, spt10Δ, spt21Δ, and spt23Δ) transformed with the two plasmids grew similar to the wild-type strain on medium containing 20 to 40 mm AT (Table 2 and Figure 2). Thus, the proteins expressed by these SPT genes are unlikely to play critical functions after TBP associates with the promoter. In contrast, strains containing deletions of SPT7 and SPT20 grew poorly on medium containing AT (Table 2 and Figure 2). Loss of reporter gene expression in strains lacking SPT7 and SPT20 suggests that these genes have a post-TBP recruitment role in transcription, but could also be due to less interesting indirect effects. For example, poor reporter expression could be due to low expression of the LexA–TBP fusion protein because low levels of LexA–TBP would prevent the formation of the PIC on the reporter gene and result in no growth on AT. To test this, levels of LexA–TBP protein were assayed via immunoblot analysis. Expression levels of LexA–TBP were comparable in all strains tested (Figure 3A). Another indirect explanation for failure to grow on AT is that SPT7 or SPT20 are required for LexA–TBP protein occupancy at the reporter promoter. To test this, we used a ChIP assay to measure the occupancy of LexA–TBP at the HIS3 reporter promoter. We found LexA–TBP was recruited to the HIS3 reporter gene promoter to comparable levels in the wild-type strain and the spt7Δ and the spt20Δ strains (Figure 3B). These results indicate that LexA–TBP is expressed and recruited to the promoter, but this is not sufficient for reporter gene expression in the absence of the gene products encoded by SPT7 and SPT20. This suggests that these two gene products are involved in regulatory steps after the recruitment of TBP.
TABLE 2.
Growth phenotypes in the tethering assay of the indicated strains
| Straina | LexA | LexA–TBP | Strain | LexA | LexA–TBP | Strain | LexA | LexA–TBP |
|---|---|---|---|---|---|---|---|---|
| BY4741 | + | +++ | Activators | Elongation factors | ||||
| SPTs | asc1Δ | ± | +++ | dst1Δ | ± | +++ | ||
| spt1Δ | + | +++ | bas1Δ | — | +++ | ela1Δ | ± | +++ |
| spt2Δ | + | +++ | gal4Δ | + | +++ | elc1Δ | + | +++ |
| spt3Δ | + | +++ | gal80Δ | + | +++ | elp2Δ | ++ | ++ |
| spt4Δ | + | +++ | hpc2Δ | + | +++ | elp3Δ | + | +++ |
| spt7Δ | ± | — | tbs1Δ | ± | +++ | elp4Δ | ++ | +++ |
| spt8Δ | + | +++ | mbf1Δ | — | +++ | elp6Δ | + | ++ |
| spt10Δ | + | +++ | met18Δ | — | +++ | iki3Δ | + | +++ |
| spt20Δ | ± | + | mot3Δ | — | +++ | nhp6aΔ | — | +++ |
| spt21Δ | + | +++ | swi5Δ | + | +++ | rtf1Δ | — | +++ |
| spt23Δ | + | +++ | swi6Δ | + | ++ | thp1Δ | ± | +++ |
| SAGA/ADA | Repressors | thp2Δ | — | +++ | ||||
| ada1Δ | — | ± | caf4Δ | + | +++ | Swi/Snf | ||
| ada2Δ | — | ++ | caf16Δ | — | +++ | snf2Δ | — | +++ |
| ada3Δ | — | ++ | caf17Δ | — | +++ | snf5Δ | — | +++ |
| ahc1Δ | + | +++ | ccr4Δ | ± | +++ | snf6Δ | — | ++ |
| chd1Δ | — | +++ | not3Δ | — | +++ | snf11Δ | + | +++ |
| gcn5Δ | ± | + | not5Δ | — | +++ | swi3Δ | + | +++ |
| sgf11Δ | ± | ++ | nrg2Δ | + | +++ | Protein kinase subunits | ||
| Mediator | pop2Δ | — | +++ | cka1Δ | + | +++ | ||
| med1Δ | + | ++ | sig1Δ | — | ++ | cka2Δ | ± | +++ |
| med3Δ | ± | +++ | ssn6Δ | — | +++ | ckb1Δ | + | +++ |
| med5Δ | + | +++ | sut1Δ | ± | +++ | ckb2Δ | — | +++ |
| med9Δ | — | ++ | tup1Δ | — | +++ | ctk1Δ | ± | +++ |
| med12Δ | + | +++ | HDACs | ctk2Δ | — | +++ | ||
| med13Δ | + | +++ | hda1Δ | ± | +++ | ctk3Δ | ± | ++ |
| med15Δ | + | +++ | hos1Δ | — | +++ | H4/H2A HAT complexes | ||
| med16Δ | + | +++ | hos2Δ | — | +++ | eaf3Δ | ++ | +++ |
| med18Δ | ± | + | hos3Δ | + | +++ | eaf6Δ | ± | +++ |
| med19Δ | ± | + | hst1Δ | + | +++ | hat1Δ | — | ++ |
| med20Δ | ± | ++ | pho23Δ | ± | +++ | hat2Δ | + | +++ |
| med31Δ | ± | ++ | rpd3Δ | — | +++ | taf14Δ | + | ++ |
| cdk8Δ | + | +++ | sap30Δ | ± | +++ | yng1Δ | — | +++ |
| cyccΔ | + | +++ | sin3Δ | — | +++ | Paf1 complex | ||
| RNA Pol II subunits | Subunits of ISW1/2 complex | cdc73Δ | + | +++ | ||||
| rpb4Δ | — | +++ | isw1Δ | + | +++ | paf1Δ | — | +++ |
| rpb9Δ | — | +++ | isw2Δ | — | +++ | Other | ||
| itc1Δ | + | ++ | mcm22Δ | — | ++ | |||
| mhr1Δ | + | +++ | ||||||
| rad26Δ | + | +++ | ||||||
Relative growth rate on 20–40 mm AT of strains harboring the LexA- or LexA–TBP-expressing plasmid. Robust growth is scored as “+++” and is the result of HIS3 gene activation. Intermediate-to-weak growth is indicated by “++,” “+,” or “±.”
Strains assayed were wild type (BY4741) or contained a deletion in the gene indicated. All strains were purchased from Research Genetics.
Figure 3.—
LexA–TBP is expressed in various SPT gene deletion strains and is recruited to the promoter of the HIS3 reporter plasmid. (A) Expression levels of LexA–TBP protein are similar in SPT deletion strains to the wild-type parent strain. Protein extracts from the indicated strains expressing LexA–TBP were separated on an SDS–PAGE gel and subjected to Western blot analysis. Levels of LexA–TBP were detected via anti-HA antibody against the HA tag on the N terminus of the fusion protein. Anti-Toa1 antibody was used to detect Toa1 levels for a loading control. (B) LexA–TBP is recruited to the reporter HIS3 gene in the deletion strains. ChIP assays using anti-HA antibody from strains expressing LexA–TBP were performed to determine the occupancy of LexA–TBP on the HIS3 promoter. Antibody to an irrelevant His-tag was used as a control. ChIP assays were repeated a minimum of three times using independent cultures of cells.
Proper regulation of the poised CYC1 promoter requires the function of SPT7 and SPT20:
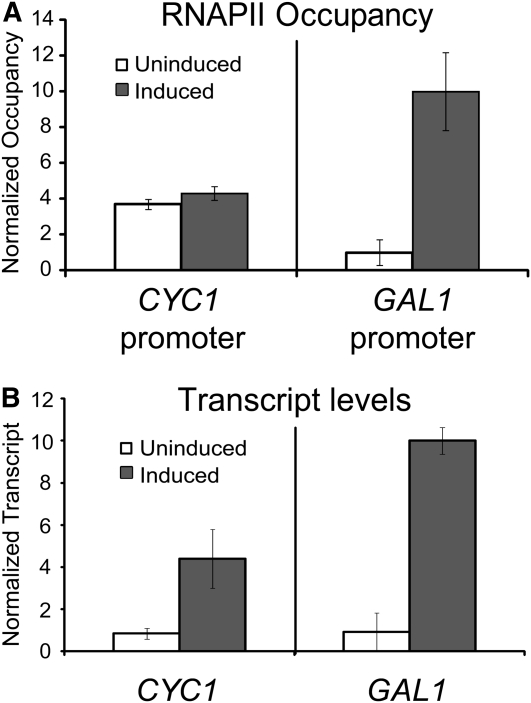
We next compared the results from the tethering assay to transcription of an authentic postrecruitment-regulated promoter. CYC1 is regulated after the recruitment of TBP and RNAPII (Lue et al. 1989; Chen et al. 1994; Kuras and Struhl 1999; Martens et al. 2001; Zhang et al. 2008). Therefore, RNAPII occupies the promoter to a similar degree under both uninduced and induced conditions (Figure 4A). This is despite a dramatic change in transcript levels during induction (Figure 4B). This preloading of key members of the transcription machinery at the promoter of CYC1 is fundamentally different from recruitment-regulated genes such as GAL1. Occupancy of RNAPII at the GAL1 promoter undergoes a large change (10-fold) upon transcriptional activation (Figure 4, A and B, respectively). CYC1 is therefore regulated in a postrecruitment fashion. We refer to CYC1 as having a poised promoter because preloaded TBP and RNAPII mark the promoter for future activation.
Figure 4.—
CYC1 and GAL1 represent two different classes of gene regulation. (A) Chromatin immunoprecipitation for RNAPII during uninduced (glucose: open bars) and induced (galactose: solid bars) conditions at the CYC1 and GAL1 promoter regions. RNAPII occupies the CYC1 promoter in both conditions but is recruited to the GAL1 promoter during the same conditions. (B) CYC1 and GAL1 transcript levels are induced during growth in medium containing galactose as a carbon source. Total RNA from the wild-type strain grown in glucose (uninduced: open bars) or in galactose (induced: solid bars) was analyzed via S1 nuclease assay using 32P-labeled CYC1, GAL1, and tryptophan tRNA probes. The tRNAw signal was used as a loading control and to normalize transcript levels. In A and B, the mean ± SD of three separate biological samples is shown.
To examine the correlation between the tethering assay and regulation of the poised CYC1 promoter, we tested whether SPT genes were required for CYC1 expression. Transcript levels of CYC1 were measured using RNA harvested from wild-type and SPT deletion strains and S1 nuclease protection assays. CYC1 transcript levels in the uninduced condition were not significantly changed upon deletion of any of the SPT genes (Figure 5). However, during induction, activated transcription from CYC1 was dramatically abolished in strains deleted for SPT7 and SPT20. Thus, SPT7 and SPT20 are specifically required for activation of the poised CYC1 promoter. Significantly, these are the two SPT strains that were also identified in the tethering assay. Both SPT7 and SPT20 encode subunits of the yeast SAGA complex (Grant et al. 1997; Roberts and Winston 1997). Because both subunits are required for the structural integrity of the complex (Grant et al. 1997; Sterner et al. 1999), we next focused on SAGA.
Figure 5.—
The spt7Δ and spt20Δ strains are defective for CYC1 expression. Total RNA from indicated strains grown in glucose (uninduced: open bars) and in ethanol (induced: solid bars) were analyzed via S1 nuclease assay using 32P-labeled CYC1 and tryptophan tRNA probes. The tRNAw signal was used as a loading control and to normalize transcript levels. The mean ± SD of three separate biological samples is shown.
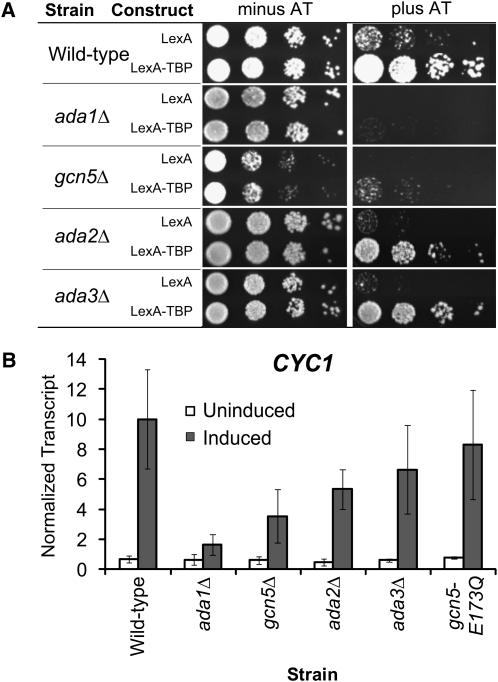
ADA1 and GCN5 are also critical for postrecruitment regulation:
SAGA is a highly conserved, multiple-subunit coactivator complex composed of Spt proteins, TAFs, Ada proteins, and the histone acetyltransferase enzyme Gcn5 (for reviews see Daniel and Grant 2007; Rodriguez-Navarro 2009). SAGA also links other histone modifications with transcriptional processes: histone H3 methylation via Chd1 (Pray-Grant et al. 2005) and H2B deubiquination via Sgf11 (Ingvarsdottir et al. 2005). Thus, we expanded our screen to include additional SAGA subunits (Table 2). A majority of strains grew similarly to the wild-type strain on medium containing AT. In contrast, the ada1Δ and the gcn5Δ deletion strains showed poor growth on AT (Figure 6A). We next tested the consequence of these deletions on CYC1 expression levels. Transcript levels in the ada1Δ and the gcn5Δ strains were similar to wild-type levels in the uninduced condition but were compromised during induction. Deletion of ADA2 or ADA3 had little influence on CYC1 transcript levels in the uninduced condition; during activation there was a slight decrease in the ada2Δ strain, and no significant effect in the ada3Δ strain (Figure 6B). Additionally, we found no significant effect upon deletion of UBP8 and SGF11 (data not shown), which provide the deubiquination activity of the SAGA complex (Ingvarsdottir et al. 2005; Lee et al. 2005; Shukla et al. 2006). Thus, four subunits of SAGA are critical for the postrecruitment regulation of CYC1, and three of those (Spt7, Spt20, and Ada1) are involved in the integrity of the complex (Grant et al. 1997; Sterner et al. 1999).
Figure 6.—
Additional SAGA subunits have postrecruitment functions. (A) The wild-type strain and strains deleted for individual genes encoding representative subunits of the SAGA complex were assayed using the tethering system. Serial dilutions were spotted on media with and without AT and incubated for 3 days. (B) CYC1 expression levels in the indicated strains during growth in glucose (uninduced: open bars) and ethanol (induced: solid bars) were measured by S1 nuclease protection. Mean ± SD of three separate biological replicates (independent cultures) is shown.
The histone acetyltransferase activity of SAGA is not required for proper regulation of CYC1:
Gcn5 is a histone acetyltransferase (HAT), which is an enzyme that transfers acetyl groups to histones (Brownell et al. 1996; Grant et al. 1997, 1998; Utley et al. 1998). To determine if the HAT activity of SAGA is important in the postrecruitment regulation of CYC1, we utilized a strain containing a Gcn5 derivative defective for histone acetylation, gcn5E173Q (Trievel et al. 1999). We found no change in CYC1 expression in a strain with this mutant protein as compared to wild-type GCN5 (Figure 6B). Thus, the HAT activity of the SAGA complex is not important for the activation of the preloaded complex on CYC1. Consistent with this finding, the deletion of the HAT-related SAGA subunits, Ada2 and Ada3, which together with Gcn5 comprise the catalytic core (Balasubramanian et al. 2002), has little effect on CYC1 expression (Figure 6B).
CYC1 is SAGA dependent and TFIID independent:
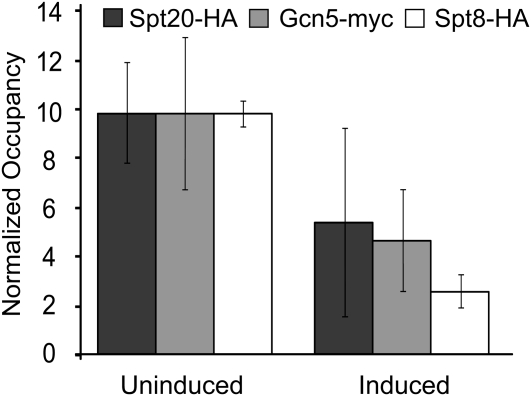
To test for a direct role of SAGA in CYC1 transcription, we performed chromatin immunoprecipitation assays to determine the occupancy of tagged derivatives of the Spt20, Gcn5, and Spt8 proteins both before and after activation of transcription. Importantly, the Spt8 subunit is present only in the SAGA complex, and not in the related SLIK complex, unlike Spt20 and Gcn5 (Pray-Grant et al. 2002). Interestingly, Spt20, Gcn5, and Spt8 occupy the CYC1 promoter in both the uninduced and induced state. Changes in occupancy do occur, however, with a drop in occupancy observed for each of the three subunits after activation of transcription (Figure 7). SAGA-dependent genes are largely TFIID independent (Huisinga and Pugh 2004). To determine if CYC1 is TFIID independent, we examined the occupancy of Taf1, a TFIID-specific TAF (Grant et al. 1998). We found occupancy of Taf1 was not greater than background either before or after activation (data not shown). These results are also consistent with the fact that CYC1 is a TATA-containing gene, which is typically SAGA dependent (Basehoar et al. 2004; Huisinga and Pugh 2004).
Figure 7.—
SAGA occupies the CYC1 promoter. The indicated strains were grown under uninduced (glucose) or induced (ethanol) conditions. ChIP analyses was performed to measure the occupancy of Spt20-HA, Gcn5-myc, or Spt8-HA at the CYC1 promoter region (−230 to +80 relative to the ATG). Normalized occupancy was calculated by determining the tagged derivative occupancy and dividing it by the occupancy observed in an untagged strain and setting the highest value for each strain at 10. The mean ± SD of three separate biological samples is shown. While the normalized occupancy value of Spt8-HA appears low in induced conditions, the immunoprecipitation value was higher than what is observed at an induced (galactose-grown) SAGA-dependent GAL promoter, suggesting that this protein still occupies the CYC1 promoter in induced conditions (data not shown).
TBP recruitment function of SAGA is not required for CYC1 regulation:
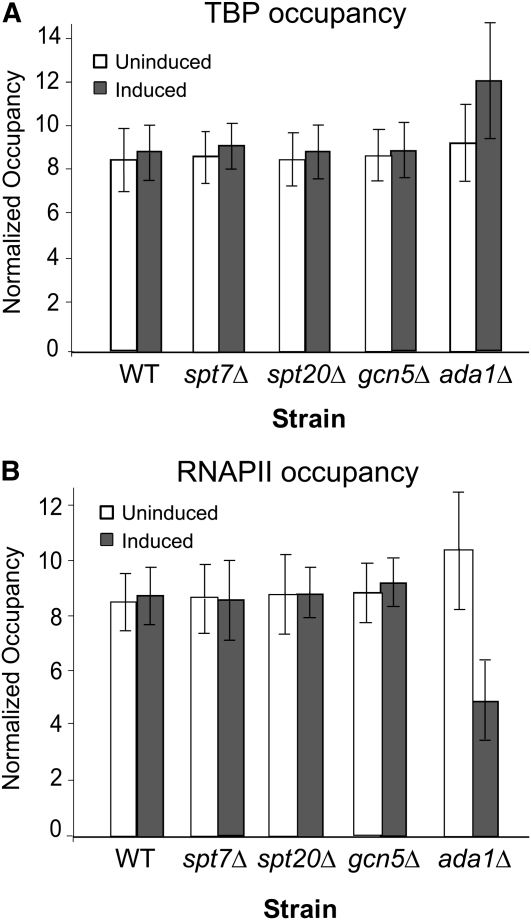
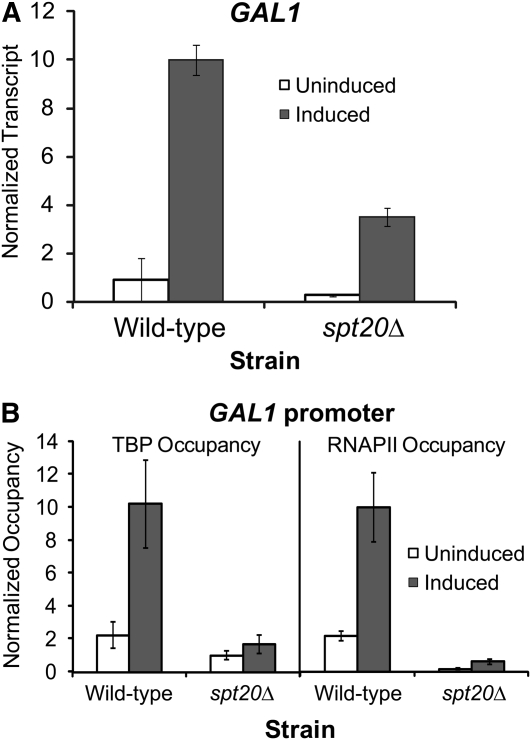
In addition to histone acetyltransferase activity, SAGA also has a well-characterized role in TBP delivery at recruitment-regulated promoters (Dudley et al. 1999; Belotserkovskaya et al. 2000; Bhaumik and Green 2001, 2002; Larschan and Winston 2001; Qiu et al. 2005). SAGA binds TBP (Sterner et al. 1999) and transfers it to the TATA box (Sermwittayawong and Tan 2006). A defining feature of the poised CYC1 promoter is that it has TBP bound at the promoter in the uninduced state prior to activated levels of transcription (Lue et al. 1989; Chen et al. 1994; Kuras and Struhl 1999; Martens et al. 2001). The above results indicate that SAGA also occupies this poised promoter. To test whether loss of SAGA results in loss of TBP occupancy at the CYC1 promoter, we performed chromatin immunoprecipitation assays for TBP occupancy in several deletion backgrounds (spt7Δ, spt20Δ, gcn5Δ, and ada1Δ) of SAGA. In all four deletion strains, TBP occupied the CYC1 promoter similar to that of a wild-type strain in both the uninduced and induced condition (Figure 8A). Thus, the TBP recruitment function of SAGA is not involved in the regulation of CYC1. This is consistent with the observation that the spt3Δ and spt8Δ strains were not defective in any of the assays. We also found that RNAPII occupies the CYC1 promoter in the spt7Δ, spt20Δ, gcn5Δ, and ada1Δ strains during the uninduced condition to levels comparable to the wild-type strain (Figure 8B). Taken together, these results indicate that loss of SAGA does not impact preloading of TBP and RNAPII at the CYC1 promoter; therefore, the CYC1 promoter is still poised in the absence of this coactivator complex. Furthermore, SAGA plays a rate-limiting role downstream of the recruitment of both TBP and RNAPII at CYC1. This is in striking contrast to the recruitment-regulated GAL1 gene. Like CYC1, GAL1 is dependent upon SAGA for normal expression (Figure 9A). However, at GAL1, SPT20 functions in TBP and RNAPII recruitment, as occupancy of these members of the transcription machinery are compromised in the spt20Δ strain (Figure 9B).
Figure 8.—
Occupancy of TBP and RNAPII at CYC1 are unchanged in SAGA deletion strains compromised for activated transcription. (A) The indicated strains were grown under uninduced (glucose: open bars) or induced (ethanol: solid filled bars) conditions for the CYC1 gene. ChIP analyses were performed to determine the occupancy of TBP at the CYC1 promoter. The occupancy of TBP during uninduced and induced conditions at CYC1 does not change in the deletion strains (mean ± SD). (B) RNAPII occupancy at the CYC1 promoter in the wild-type (BY4741) strain and strains with deletions in genes encoding SAGA subunits during uninduced (open bars) and induced (solid bars) conditions. RNAPII occupancy does not change in the deletion strains (mean ± SD). The ratio between the immunoprecipitated sample and the input—minus the background of no antibody control—was used to calculate the protein occupancy.
Figure 9.—
SAGA is required for TBP and RNAPII recruitment at the recruitment-regulated GAL1 gene. (A) GAL1 is dependent on the SPT20 gene for normal levels of expression. GAL1 expression levels in the indicated strains during growth in glucose (uninduced: open bars) and galactose (induced: solid bars) were measured by S1 nuclease protection. Mean ± SD of three separate biological replicates (independent cultures) is shown. (B) SPT20 is required for TBP and RNAPII occupancy at the GAL1 promoter. Occupancy of TBP and RNAPII in the indicated strains at the GAL1 promoter is shown.
Expansion of the tethering assay reveals an enrichment in Mediator subunits:
As the tethering assay accurately revealed a postrecruitment function of the SAGA complex at the CYC1 promoter, we expanded the screen to include other transcription-related factors. Specifically, we examined strains containing deletions of subunits of the Mediator complex, RNA polymerase II, activators, repressors, histone deacetylases (HDACs), ISW1/2 complexes, elongation factors, Swi/Snf, protein kinases, HAT components, the PAF complex, and others for a role in postrecruitment regulation. The assay was highly selective, identifying only a few additional genes with putative postrecruitment functions (Table 2). Strikingly, of 85 additional strains, only two strains (med18Δ and med19Δ) showed severe phenotypes in the tethering assay. MED18 and MED19 encode subunits of Mediator, an important multisubunit coactivator complex with both positive and negative roles in transcription (Li et al. 1995; Holstege et al. 1998; Myers and Kornberg 2000; Casamassimi and Napoli 2007).
Mediator is required for proper expression of CYC1:
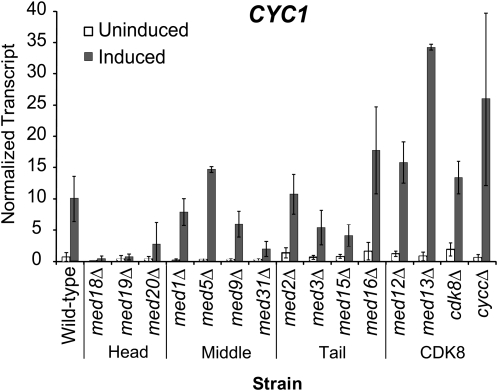
We next examined the role of the Mediator complex at the poised CYC1 promoter. Mediator subunits can be classified into the head, middle, tail, and CDK8 module of the complex (Davis et al. 2002). Under uninduced conditions, CYC1 transcript levels were mildly diminished in strains containing deletions of the head and middle module subunits, and increases in expression were observed in strains containing deletions of the tail and CDK8 module subunits (Figure 10). Activation of CYC1 transcription under inducing conditions was compromised in a number of strains, with the most significant effects associated with deletions of head module subunits (Figure 10). In all three strains tested (med18Δ, med19Δ, and med20Δ), transcription was drastically compromised compared to wild type. Other modules exhibited more complex patterns with deletions of particular subunits resulting in disparate effects. For example, deletion of the middle module subunit MED31 abolished CYC1 activation, whereas deletion of other members of this module was well tolerated. Enhanced transcriptional activation was observed in the CDK8 module deletions, which is entirely consistent with a role for this module in the negative regulation of gene expression (Carlson 1997; Hampsey 1998; van de Peppel et al. 2005). Taken together, our results demonstrate that a functional Mediator complex is required for proper expression of CYC1, with a clear requirement for the head module and the Med31 subunit of the middle module in activation of the poised CYC1 promoter.
Figure 10.—
A functional Mediator complex is required for proper CYC1 expression. CYC1 transcript levels during growth in uninduced (glucose: open bars) and induced (ethanol: solid bars) conditions in each strain harboring a deletion in a Mediator subunit. Strains are grouped according to the corresponding Mediator module. Total RNA from the indicated strains was analyzed via S1 nuclease assay using 32P-labeled CYC1 and tryptophan tRNA probes as in Figure 5.
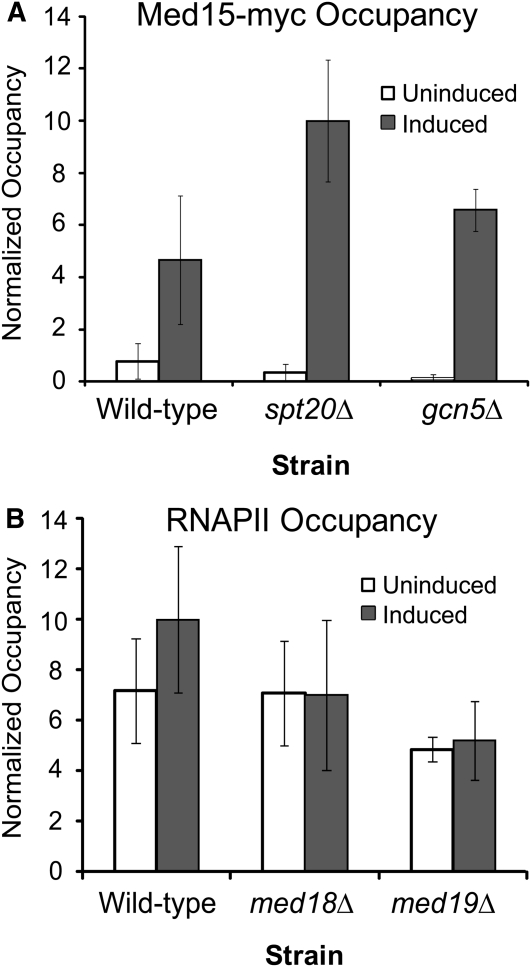
We next determined the occupancy of Mediator at CYC1 using chromatin immunoprecipitation. The head, middle, and tail modules of the complex are distributed in virtually identical patterns across the yeast genome (Venters and Pugh 2009), so we chose Med15 to represent this grouping because it has been successfully used in ChIP assays (Qiu et al. 2005; Fan and Struhl 2009). The CDK8 module is proposed to transiently associate with certain genomic locations (Andrau et al. 2006); thus we also determined the occupancy of Med12 to represent this module. We found that both Med15 (Figure 11A) and Med12 (data not shown) exhibited low occupancy under noninducing conditions and high occupancy during growth in inducing conditions. Thus, whereas TBP, RNAPII, and SAGA occupy the poised CYC1 promoter in the uninduced condition, Mediator has high occupancy only after transfer to inducing conditions. As such, Mediator recruitment correlates with the activation of the preloaded polymerase at the CYC1 promoter.
Figure 11.—
Mediator is recruited to the CYC1 promoter upon induction in a SAGA-independent manner, and Mediator is not required for RNAPII occupancy. (A) Chromatin immunoprecipitation of Med15 (Med15-myc) shows increased CYC1 promoter occupancy after transfer to inducing conditions. An increase in occupancy is maintained in the spt20Δ strain or gcn5Δ strain, indicating that core Mediator is recruited to this promoter independently of the SAGA subunits. Normalized occupancy was determined by subtracting occupancy at the GAL1 promoter from occupancy at the CYC1 promoter. (B) Chromatin immunoprecipitation of RNAPII at the CYC1 promoter in the wild-type, med18Δ, and med19Δ strains. Occupancy of RNAPII is independent of these two Mediator proteins. Occupancy is shown as fold over the occupancy at a telomere-proximal region on the right arm of chromosome VI. In A and B, the highest value is set to 10. Bars represent the average occupancy at CYC1 (−150 to +40 relative to the ATG) of three biological replicates ± SD.
SAGA is dispensable for recruitment of Mediator to CYC1:
Because SAGA and Mediator subunits were both overrepresented in the tethering screen, and these complexes are mutually dependent at several promoters (Roberts and Winston 1997; Larschan and Winston 2005; Qiu et al. 2005), we investigated their connection at CYC1. We examined Mediator recruitment to the CYC1 promoter in a strain containing a deletion of the SAGA complex genes GCN5 or SPT20. We found that Med15-myc is recruited to the CYC1 promoter in both the gcn5Δ and the spt20Δ strains (Figure 11A). Importantly, SPT20 is absolutely required for the structural integrity of the SAGA complex (Grant et al. 1997; Sterner et al. 1999) and also for function in the tethering assay and for CYC1 transcription.
Although we found that Mediator occupies the CYC1 promoter only after activation, the Mediator complex has a well-established role in RNAPII recruitment (Biddick et al. 2008; Esnault et al. 2008). To rule out the possibility that Mediator influences recruitment of RNAPII to the CYC1 promoter, chromatin immunoprecipitation of RNAPII was performed in the med18Δ and med19Δ strains. We found that RNAPII occupancy at the CYC1 promoter is unaffected by deletion of either MED18 or MED19 (Figure 11B). Importantly, these two subunits of the Mediator complex came out of the TBP-tethering assay and are crucial for CYC1 gene expression. These results indicate that Mediator is not required for RNAPII preloading at the CYC1 promoter. Thus, Mediator recruitment is independent of SAGA, and recruitment of Mediator is not sufficient for transcriptional activation of the poised promoter.
DISCUSSION
Transcription by RNAPII is a regulated and complex process that depends upon the coordinate activities of a large number of factors. Coactivators represent an important and highly conserved class of factors that mediate and integrate signals to arrive at the appropriate level of gene expression for a particular condition. Coactivators, like the multiprotein complexes SAGA, Mediator, and TFIID, associate with the relevant promoter via protein–protein interactions with sequence-specific DNA-binding proteins (Brown et al. 2001; Zhang et al. 2004; Govind et al. 2005; Thakur et al. 2008; Liu et al. 2009). Coactivators facilitate transcription by recruiting RNAPII and/or enhancing the formation of the preinitiation complex by a variety of mechanisms (Torchia et al. 1998; Naar et al. 2001; Kornberg 2005; Baker and Grant 2007). Here we report that the SAGA and Mediator coactivator complexes also play essential and distinct roles in the regulation of gene expression after the recruitment of TBP and RNAPII.
It is now clear that a growing number of genes are regulated at a step (or steps) after the recruitment of the general transcription machinery (for recent reviews see Core and Lis 2008; Margaritis and Holstege 2008; Price 2008). Such genes include the yeast CYC1 gene, the Drosophila heat-shock genes, and mammalian c-myc and HIV-1 genes (Krumm et al. 1992; Kuras and Struhl 1999; Andrulis et al. 2000; Martens et al. 2001; Stevens et al. 2006; Zhang et al. 2008). Genomewide studies also indicate that a large number of developmental and stress-inducible genes have RNAPII preloaded at promoter-proximal regions (Guenther et al. 2007; Muse et al. 2007; Zeitlinger et al. 2007). Our mechanistic understanding of these poised yet inactive promoters is woefully incomplete, and there is very little information on the regulatory factors that are required to activate these genes. As such, we set out to identify gene products with important roles in transcription after the recruitment of TBP and RNAPII.
We initially used a TBP-tethering screen with a large number of haploid deletion strains to search for gene products with required functions after TBP associates with the promoter. We observed a significant dependence on gene products in the SAGA and Mediator complexes for activity of the tethered TBP derivative (Table 2). Importantly, we also found that deletion of these gene products leads to diminished transcription of CYC1, an authentic postrecruitment-regulated promoter.
Four genes encoding subunits of the SAGA complex were identified with potential roles in postrecruitment functions: ADA1, SPT7, SPT20, and GCN5. Three of these (ADA1, SPT7, and SPT20) are required for the structural integrity of the SAGA complex (Grant et al. 1997; Sterner et al. 1999; Qiu et al. 2005), indicating that an intact complex is required for the activation functions. We therefore investigated the three well-established functions of SAGA for a role at CYC1: HAT activity (Brownell et al. 1996; Grant et al. 1997, 1998; Utley et al. 1998), TBP recruitment (Dudley et al. 1999; Belotserkovskaya et al. 2000; Bhaumik and Green 2001, 2002; Larschan and Winston 2001; Qiu et al. 2005), and histone H2B deubiquitination (Ingvarsdottir et al. 2005; Lee et al. 2005; Shukla et al. 2006). Our data indicate that, despite the SAGA dependency that we observe at CYC1, these traditional functions are not involved in the activation of the preloaded complex. Therefore, this promoter provides a useful tool for probing additional functions of this large complex.
The dependency on SAGA at CYC1 may reflect a functional interaction with Mediator, since these two coactivators collaborate in regulating the expression of a wide array of recruitment-regulated promoters (Larschan and Winston 2005; Leroy et al. 2006; Liu et al. 2008; Qiu et al. 2005). The connection between SAGA and Mediator functions is fairly well established. Multiple genetic interactions between SAGA and Mediator subunits were shown over a decade ago (Roberts and Winston 1997). Deletion of the SAGA subunit SPT20 is lethal in combination with deletions in several nonessential genes encoding the Mediator subunits Med16 (Sin4), Med15 (Gal11), Med20 (Srb2), and Med18 (Srb5). Synthetic lethality was also observed with spt20Δ using a truncation mutation in the essential MED14 gene (Roberts and Winston 1997). Further, using synthetic genetic array and diploid-based synthetic lethality analysis on microarrays, negative genetic interactions are observed between SPT3 and SPT8 subunits of SAGA and MED16 (SIN4), MED15 (GAL11), MED2, MED3 (PGD1), MED31 (SOH1), MED20 (SRB2), and MED5 (NUT1), genes which encompass subunits within the head, middle, and tail modules of Mediator (Collins et al. 2007). These genetic interactions give strong support for SAGA and Mediator acting in concert in the process of transcription. Indeed, we find that both complexes play important postrecruitment functions in the TBP-tethering assay and the native CYC1 gene. However, there are significant differences in the functional roles for the two coactivators at CYC1. In contrast to SAGA (and TBP and RNAPII), we find that Mediator is recruited to the CYC1 promoter only upon activation of the preloaded complex. Thus, Mediator occupancy correlates with transcriptional output of the poised CYC1 promoter. This occupancy is not just fortuitous, but is functional, since Mediator subunits (Med18, Med19, Med20, and Med31) are essential for transcriptional activation of the poised promoter.
Importantly, Mediator occupancy at CYC1 is not dependent upon SAGA because disruption of SAGA (in the spt20Δ strain) or alteration of SAGA (in the gcn5Δ strain) does not alter Mediator occupancy. This suggests that Mediator and SAGA are required for independent steps in the activation process of this poised promoter. It should be noted that particular recruitment-regulated genes require SAGA and Mediator for independent functions as well (Bryant and Ptashne 2003; Leroy et al. 2006). The mechanistic requirement of SAGA and Mediator at recruitment-regulated promoters is based on PIC formation, which is distinct from their requirement at the poised promoter.
In addition to recruiting RNAPII and enhancing PIC formation, Mediator can also stimulate phosphorylation of the C-terminal domain (CTD) of RNAPII by the general transcription factor TFIIH (Kim et al. 1994; Esnault et al. 2008). Phosphorylation of the CTD at serine 5 is a prerequisite for the transition from initiation to elongation (reviewed in Orphanides and Reinberg 2002; Meinhart et al. 2005; Phatnani and Greenleaf 2006). However, we have previously shown that serine 5 is phosphorylated at the poised promoter prior to activation (Zhang et al. 2008). In keeping with this, TFIIH is also already present at the poised promoter before transcriptional activation (Zhang et al. 2008). Thus, Mediator does not function to stimulate this step in the process at the poised CYC1 promoter.
What, then, is the functional activity provided by Mediator at the poised promoter? An involvement of Mediator in postrecruitment functions has been described at the mouse Egr1 gene (Wang et al. 2005). The authors suggest that an isomerization of the transcription complex may be the functional role of Mediator, and others concur (Kornberg 2005; Struhl 2005). It is interesting to note that the head module, which interfaces with RNAPII (Cai et al. 2009), plays a critical role in activation of the CYC1 poised promoter (Figure 10), whereas it is not as uniformly required for a recruitment-regulated promoter (Leroy et al. 2006). Furthermore, the Med31 subunit is also important for CYC1 activation. Med31 belongs to the middle module, which also makes direct contact with RNAPII (Davis et al. 2002). This subunit is well conserved across evolution (Linder and Gustafsson 2004), suggesting a possible role in the activation of poised promoters in higher eukaryotes. Taken together, it is interesting to speculate that recruitment of Mediator results in a reorganization of the poised promoter into a transcriptionally active conformation. Consistent with this hypothesis, we see changes in the crosslinking pattern of occupancy of SAGA after activation of the poised promoter. Further work will be required to reveal the nature and extent of these changes and how they lead to a productive RNAPII machinery.
Acknowledgments
We thank Shelley Berger for providing the GCN5 histone acetyltransferase mutant derivative, gcn5E173Q; Alan Hinnebusch for providing the Med15-myc-tagged strain in the spt20Δ and gcn5Δ backgrounds; and Carlos Herrera for his participation in the tethering screen. This project was supported by grants to L.A.S. from the National Science Foundation (MCB-0843073) and the National Institutes of Health (GM056884). It was also supported by a predoctoral grant to S.K.L (F31AG031641) from the National Institute On Aging; the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
References
- Andrau, J. C., L. van de Pasch, P. Lijnzaad, T. Bijma, M. G. Koerkamp et al., 2006. Genome-wide location of the coactivator mediator: binding without activation and transient Cdk8 interaction on DNA. Mol. Cell 22 179–192. [DOI] [PubMed] [Google Scholar]
- Andrulis, E. D., E. Guzman, P. Doring, J. Werner and J. T. Lis, 2000. High-resolution localization of Drosophila Spt5 and Spt6 at heat shock genes in vivo: roles in promoter proximal pausing and transcription elongation. Genes Dev. 14 2635–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker, S. P., and P. A. Grant, 2007. The SAGA continues: expanding the cellular role of a transcriptional co-activator complex. Oncogene 26 5329–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian, R., M. G. Pray-Grant, W. Selleck, P. A. Grant and S. Tan, 2002. Roles of the Ada2 and Ada3 transcriptional coactivators in histone acetylation. J. Biol. Chem. 277 7989–7995. [DOI] [PubMed] [Google Scholar]
- Basehoar, A. D., S. J. Zanton and B. F. Pugh, 2004. Identification and distinct regulation of yeast TATA box-containing genes. Cell 116 699–709. [DOI] [PubMed] [Google Scholar]
- Beggs, J., 1978. Transformation of yeast by a replicating hybrid plasmid. Nature 275 104–109. [DOI] [PubMed] [Google Scholar]
- Belotserkovskaya, R., D. E. Sterner, M. Deng, M. H. Sayre, P. M. Lieberman et al., 2000. Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol. Cell. Biol. 20 634–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik, S. R., and M. R. Green, 2001. SAGA is an essential in vivo target of the yeast acidic activator Gal4p. Genes Dev. 15 1946–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaumik, S. R., and M. R. Green, 2002. Differential requirement of SAGA components for recruitment of TATA-box-binding protein to promoters in vivo. Mol. Cell. Biol. 22 7365–7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddick, R. K., G. L. Law, K. K. Chin and E. T. Young, 2008. The transcriptional coactivators SAGA, SWI/SNF, and mediator make distinct contributions to activation of glucose-repressed genes. J. Biol. Chem. 283 33101–33109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, C. E., L. Howe, K. Sousa, S. C. Alley, M. J. Carrozza et al., 2001. Recruitment of HAT complexes by direct activator interactions with the ATM-related Tra1 subunit. Science 292 2333–2337. [DOI] [PubMed] [Google Scholar]
- Brownell, J. E., J. Zhou, T. Ranalli, R. Kobayashi, D. G. Edmondson et al., 1996. Tetrahymena histone acetyltransferase A: a homolog to yeast Gcn5p linking histone acetylation to gene activation. Cell 84 843–851. [DOI] [PubMed] [Google Scholar]
- Bryant, G. O., and M. Ptashne, 2003. Independent recruitment in vivo by Gal4 of two complexes required for transcription. Mol. Cell 11 1301–1309. [DOI] [PubMed] [Google Scholar]
- Cai, G., T. Imasaki, Y. Takagi and F. J. Asturias, 2009. Mediator structural conservation and implications for the regulation mechanism. Structure 17 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M., 1997. Genetics of transcriptional regulation in yeast: connections to the RNA polymerase II CTD. Annu. Rev. Cell Dev. Biol. 13 1–23. [DOI] [PubMed] [Google Scholar]
- Casamassimi, A., and C. Napoli, 2007. Mediator complexes and eukaryotic transcription regulation: an overview. Biochimie 89 1439–1446. [DOI] [PubMed] [Google Scholar]
- Chatterjee, S., and K. Struhl, 1995. Connecting a promoter-bound protein to the TATA-binding protein overrides the need for a transcriptional activation region. Nature 374 820–822. [DOI] [PubMed] [Google Scholar]
- Chellappa, R., P. Kandasamy, C. S. Oh, Y. Jiang, M. Vemula et al., 2001. The membrane proteins, Spt23p and Mga2p, play distinct roles in the activation of Saccharomyces cerevisiae OLE1 gene expression. Fatty acid-mediated regulation of Mga2p activity is independent of its proteolytic processing into a soluble transcription activator. J. Biol. Chem. 276 43548–43556. [DOI] [PubMed] [Google Scholar]
- Chen, J., M. Ding and D. S. Pederson, 1994. Binding of TFIID to the CYC1 TATA boxes in yeast occurs independently of upstream activating sequences. Proc. Natl. Acad. Sci. USA 91 11909–11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W., and K. Struhl, 1988. Saturation mutagenesis of a yeast his3 TATA element: genetic evidence for a specific TATA-binding protein. Proc. Natl. Acad. Sci. USA 85 2691–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins, S. R., K. M. Miller, N. L. Maas, A. Roguev, J. Fillingham et al., 2007. Functional dissection of protein complexes involved in yeast chromosome biology using a genetic interaction map. Nature 446 806–810. [DOI] [PubMed] [Google Scholar]
- Core, L. J., and J. T. Lis, 2008. Transcription regulation through promoter-proximal pausing of RNA polymerase II. Science 319 1791–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel, J. A., and P. A. Grant, 2007. Multi-tasking on chromatin with the SAGA coactivator complexes. Mutat. Res. 618 135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, J. A., Y. Takagi, R. D. Kornberg and F. A. Asturias, 2002. Structure of the yeast RNA polymerase II holoenzyme: Mediator conformation and polymerase interaction. Mol. Cell 10 409–415. [DOI] [PubMed] [Google Scholar]
- Dollard, C., S. L. Ricupero-Hovasse, G. Natsoulis, J. D. Boeke and F. Winston, 1994. SPT10 and SPT21 are required for transcription of particular histone genes in Saccharomyces cerevisiae. Mol. Cell. Biol. 14 5223–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, A. M., C. Rougeulle and F. Winston, 1999. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 13 2940–2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esnault, C., Y. Ghavi-Helm, S. Brun, J. Soutourina, N. Van Berkum et al., 2008. Mediator-dependent recruitment of TFIIH modules in preinitiation complex. Mol. Cell 31 337–346. [DOI] [PubMed] [Google Scholar]
- Fan, X., and K. Struhl, 2009. Where does mediator bind in vivo? PLoS One 4 e5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassler, J. S., and F. Winston, 1988. Isolation and analysis of a novel class of suppressor of Ty insertion mutations in Saccharomyces cerevisiae. Genetics 118 203–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbeck, J. A., S. M. Kraemer and L. A. Stargell, 2002. SPN1, a conserved yeast gene identified by suppression of a post-recruitment defective yeast TATA-binding protein mutant. Genetics 162 1605–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, S. R., M. Schroeder, P. Fernandez, S. Taubert and B. Amati, 2001. Binding of c-Myc to chromatin mediates mitogen-induced acetylation of histone H4 and gene activation. Genes Dev. 15 2069–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govind, C. K., S. Yoon, H. Qiu, S. Govind and A. G. Hinnebusch, 2005. Simultaneous recruitment of coactivators by Gcn4p stimulates multiple steps of transcription in vivo. Mol. Cell. Biol. 25 5626–5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant, P. A., L. Duggan, J. Cote, S. M. Roberts, J. E. Brownell et al., 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histons: charaterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 11 1640–1650. [DOI] [PubMed] [Google Scholar]
- Grant, P. A., D. Schieltz, M. G. Pray-Grant, D. Steger, J. C. Reese et al., 1998. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcriptional stimulation. Cell 94 45–53. [DOI] [PubMed] [Google Scholar]
- Guenther, M. G., S. S. Levine, L. A. Boyer, R. Jaenisch and R. A. Young, 2007. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn, S., 2004. Structure and mechanism of the RNA polymerase II transcription machinery. Nat. Struct. Biol. 11 394–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampsey, M., 1997. A review of phenotypes in Saccharomyces cerevisiae. Yeast 13 1099–1133. [DOI] [PubMed] [Google Scholar]
- Hampsey, M., 1998. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol. Mol. Biol. Rev. 62 465–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, D., B. Liu, N. R. Roan, R. Sternglanz and F. Winston, 2004. Spt10-dependent transcriptional activation in Saccharomyces cerevisiae requires both the Spt10 acetyltransferase domain and Spt21. Mol. Cell. Biol. 24 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holstege, F. C. P., E. G. Jennings, C. J. Wyrick, T. I. Lee, C. J. Hengartner et al., 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95 717–728. [DOI] [PubMed] [Google Scholar]
- Huisinga, K. L., and B. F. Pugh, 2004. A genome-wide housekeeping role for TFIID and a highly regulated stress-related role for SAGA in Saccharomyces cerevisiae. Mol. Cell 13 573–585. [DOI] [PubMed] [Google Scholar]
- Ingvarsdottir, K., N. J. Krogan, N. C. Emre, A. Wyce, N. J. Thompson et al., 2005. H2B ubiquitin protease Ubp8 and Sgf11 constitute a discrete functional module within the Saccharomyces cerevisiae SAGA complex. Mol. Cell. Biol. 25 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer, V., and K. Struhl, 1996. Absolute mRNA levels and transcriptional initiation rates in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93 5208–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T. H., L. O. Barrera, M. Zheng, C. Qu, M. A. Singer et al., 2005. A high-resolution map of active promoters in the human genome. Nature 436 876–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.-J., S. Bjorklund, Y. Li, M. H. Sayre and R. D. Kornberg, 1994. A multiprotein mediator of transcriptional activation and its interaction with the C-terminal repeat domain of RNA polymerase II. Cell 77 599–608. [DOI] [PubMed] [Google Scholar]
- Kininis, M., G. D. Isaacs, L. J. Core, N. Hah and W. L. Kraus, 2009. Postrecruitment regulation of RNA polymerase II directs rapid signaling responses at the promoters of estrogen target genes. Mol. Cell. Biol. 29 1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg, R. D., 2005. Mediator and the mechanism of transcriptional activation. Trends Biochem. Sci. 30 235–239. [DOI] [PubMed] [Google Scholar]
- Krumm, A., T. Meulia, M. Brunvand and M. Groudine, 1992. The block to transcriptional elongation within the human c-myc gene is determined in the promoter-proximal region. Genes Dev. 6 2201–2213. [DOI] [PubMed] [Google Scholar]
- Kuras, L., and K. Struhl, 1999. Binding of TBP to promoters in vivo is stimulated by activators and requires Pol II holoenzyme. Nature 399 609–613. [DOI] [PubMed] [Google Scholar]
- Larschan, E., and F. Winston, 2001. The S. cerevisiae SAGA complex functions in vivo as a coactivator for transcriptional activation by Gal4. Genes Dev. 15 1946–1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larschan, E., and F. Winston, 2005. The Saccharomyces cerevisiae Srb8-Srb11 complex functions with the SAGA complex during Gal4-activated transcription. Mol. Cell. Biol. 25 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, K. K., L. Florens, S. K. Swanson, M. P. Washburn and J. L. Workman, 2005. The deubiquitylation activity of Ubp8 is dependent upon Sgf11 and its association with the SAGA complex. Mol. Cell. Biol. 25 1173–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon, B., and R. Tjian, 2000. Orchestrated response: a symphony of transcription factors for gene control. Genes Dev. 14 2551–2569. [DOI] [PubMed] [Google Scholar]
- Leroy, C., L. Cormier and L. Kuras, 2006. Independent recruitment of mediator and SAGA by the activator Met4. Mol. Cell. Biol. 26 3149–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., S. Bjorklund, Y. W. Jiang, Y. J. Kim, W. S. Lane et al., 1995. Yeast global transcriptional regulators SIN4 and RGR1 are components of mediator complex RNA polymerase II holoenzyme. Proc. Natl. Acad. Sci. USA 92 10864–10868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder, T., and C. M. Gustafsson, 2004. The Soh1/MED31 protein is an ancient component of Schizosaccharomyces pombe and Saccharomyces cerevisiae Mediator. J. Biol. Chem. 279 49455–49459. [DOI] [PubMed] [Google Scholar]
- Lis, J. T., 2007. Imaging Drosophila gene activation and polymerase pausing in vivo. Nature 450 198–202. [DOI] [PubMed] [Google Scholar]
- Liu, W. L., R. A. Coleman, E. Ma, P. Grob, J. L. Yang et al., 2009. Structures of three distinct activator-TFIID complexes. Genes Dev. 23 1510–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X., M. Vorontchikhina, Y. L. Wang, F. Faiola and E. Martinez, 2008. STAGA recruits Mediator to the MYC oncoprotein to stimulate transcription and cell proliferation. Mol. Cell. Biol. 28 108–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine, M., A. McKenzie, III, D.J. Demarini, N.G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 953–961. [DOI] [PubMed] [Google Scholar]
- Lue, N. F., A. F. Buchman and R. D. Kornberg, 1989. Activation of RNA polymerase II transcription by a thymidine rich upstream element in vitro. Proc. Natl. Acad. Sci. USA 86 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaritis, T., and F. C. Holstege, 2008. Poised RNA polymerase II gives pause for thought. Cell 133 581–584. [DOI] [PubMed] [Google Scholar]
- Martens, C., B. Krett and P. Laybourn, 2001. RNA polymerase II and TBP occupy the repressed CYC1 promoter. Mol. Microbiol. 40 1009–1019. [DOI] [PubMed] [Google Scholar]
- Meinhart, A., T. Kamenski, S. Hoeppner, S. Baumli and P. Cramer, 2005. A structural perspective of CTD function. Genes Dev. 19 1401–1415. [DOI] [PubMed] [Google Scholar]
- Muse, G. W., D. A. Gilchrist, S. Nechaev, R. Shah, J. S. Parker et al., 2007. RNA polymerase is poised for activation across the genome. Nat. Genet. 39 1507–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers, L. C., and R. D. Kornberg, 2000. Mediator of transcriptional regulation. Annu. Rev. Biochem. 69 729–749. [DOI] [PubMed] [Google Scholar]
- Naar, A. M., B. D. Lemon and R. Tjian, 2001. Transcriptional coactivator complexes. Annu. Rev. Biochem. 70 475–501. [DOI] [PubMed] [Google Scholar]
- Natsoulis, G., F. Winston and J. D. Boeke, 1994. The SPT10 and SPT21 genes of Saccharomyces cerevisiae. Genetics 136 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nourani, A., F. Robert and F. Winston, 2006. Evidence that Spt2/Sin1, an HMG-like factor, plays roles in transcription elongation, chromatin structure, and genome stability in Saccharomyces cerevisiae. Mol. Cell. Biol. 26 1496–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides, G., and D. Reinberg, 2002. A unified theory of gene expression. Cell 108 439–451. [DOI] [PubMed] [Google Scholar]
- Phatnani, H. P., and A. L. Greenleaf, 2006. Phosphorylation and functions of the RNA polymerase II CTD. Genes Dev. 20 2922–2936. [DOI] [PubMed] [Google Scholar]
- Pray-Grant, M. G., D. Schieltz, S. J. McMahon, J. M. Wood, E. L. Kennedy et al., 2002. The novel SLIK histone acetyltransferase complex functions in the yeast retrograde response pathway. Mol. Cell. Biol. 22 8774–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pray-Grant, M. G., J. A. Daniel, D. Schieltz, J. R. Yates, III and P. A. Grant, 2005. Chd1 chromodomain links histone H3 methylation with SAGA- and SLIK-dependent acetylation. Nature 433 434–438. [DOI] [PubMed] [Google Scholar]
- Price, D. H., 2008. Poised polymerases: on your mark…get set…go! Mol. Cell 30 7–10. [DOI] [PubMed] [Google Scholar]
- Ptashne, M., 2003. Regulated recruitment and cooperativity in the design of biological regulatory systems. Philos. Transact. A Math. Phys. Eng. Sci. 361 1223–1234. [DOI] [PubMed] [Google Scholar]
- Ptashne, M., 2005. Regulation of transcription: from lambda to eukaryotes. Trends Biochem. Sci. 30 275–279. [DOI] [PubMed] [Google Scholar]
- Qiu, H., C. Hu, F. Zhang, G. J. Hwang, M. J. Swanson et al., 2005. Interdependent recruitment of SAGA and Srb mediator by transcriptional activator Gcn4p. Mol. Cell. Biol. 25 3461–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppas, N. B., J. T. Wade, G. M. Church and K. Struhl, 2006. The transition between transcriptional initiation and elongation in E. coli is highly variable and often rate limiting. Mol. Cell 24 747–757. [DOI] [PubMed] [Google Scholar]
- Roberts, S. M., and F. Winston, 1997. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics 147 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Navarro, S., 2009. Insights into SAGA function during gene expression. EMBO Rep. 10 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sermwittayawong, D., and S. Tan, 2006. SAGA binds TBP via its Spt8 subunits in competition with DNA: implications for TBP recruitment. EMBO J. 25 3791–3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood, P. W., S. V. Tsang and M. A. Osley, 1993. Characterization of HIR1 and HIR2, two genes required for regulation of histone gene transcription in Saccharomyces cerevisiae. Mol. Cell. Biol. 13 28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla, A., N. Stanojevic, Z. Duan, P. Sen and S. R. Bhaumik, 2006. Ubp8p, a histone deubiquitinase whose association with SAGA is mediated by Sgf11p, differentially regulates lysine 4 methylation of histone H3 in vivo. Mol. Cell. Biol. 26 3339–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stargell, L. A., and K. Struhl, 1996. a Mechanisms of transcriptional activation in vivo: two steps forward. Trends Genet. 12 311–315. [DOI] [PubMed] [Google Scholar]
- Stargell, L. A., and K. Struhl, 1996. b A new class of activation-defective TATA-binding protein mutants: evidence for two steps of transcriptional activation in vivo. Mol. Cell. Biol. 16 4456–4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterner, D. E., P. A. Grant, S. M. Roberts, L. J. Duggan, R. Belotserkovskaya et al., 1999. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol. Cell. Biol. 19 86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens, M., E. De Clercq and J. Balzarini, 2006. The regulation of HIV-1 transcription: molecular targets for chemotherapeutic intervention. Med. Res. Rev. 26 595–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl, K., 2005. Transcriptional activation: mediator can act after preinitiation complex formation. Mol. Cell 17 752–754. [DOI] [PubMed] [Google Scholar]
- Thakur, J. K., H. Arthanari, F. Yang, S. J. Pan, X. Fan et al., 2008. A nuclear receptor-like pathway regulating multidrug resistance in fungi. Nature 452 604–609. [DOI] [PubMed] [Google Scholar]
- Torchia, J., C. Glass and M. G. Rosenfeld, 1998. Co-activators and co-repressors in the integration of transcriptional responses. Curr. Opin. Cell Biol. 10 373–383. [DOI] [PubMed] [Google Scholar]
- Trievel, R. C., J. R. Rojas, D. E. Sterner, R. N. Venkataramani, L. Wang et al., 1999. Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc. Natl. Acad. Sci. USA 96 8931–8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utley, R. T., K. Ikeda, P. A. Grant, J. Cote, D. J. Steger et al., 1998. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature 394 498–502. [DOI] [PubMed] [Google Scholar]
- van de Peppel, J., N. Kettelarij, H. van Bakel, T. T. Kockelkorn, D. van Leenen et al., 2005. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol. Cell 19 511–522. [DOI] [PubMed] [Google Scholar]
- Venters, B. J., and B. F. Pugh, 2009. A canonical promoter organization of the transcription machinery and its regulators in the Saccharomyces genome. Genome Res. 19 360–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, T., T. Takagi, Y. Yamaguchi, A. Ferdous, T. Imai et al., 1998. DSIF, a novel transcription elongation factor that regulates RNA polymerase II processivity, is composed of human Spt4 and Spt5 homologs. Genes Dev. 12 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, G., M. A. Balamotis, J. L. Stevens, Y. Yamaguchi, H. Handa et al., 2005. Mediator requirement for both recruitment and postrecruitment steps in transcription initiation. Mol. Cell 17 683–694. [DOI] [PubMed] [Google Scholar]
- Winston, F., D. Chaleff, B. Valent and G. Fink, 1984. Mutations affecting Ty-mediated transcription of HIS4 gene of Saccharomyces cerevisiae. Genetics 107 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston, F., C. Dollard, E. A. Malone, J. Clare, J. Kapakos et al., 1987. Three genes are required for trans-activation of Ty transcription in yeast. Genetics 115 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, Y., T. Narita, N. Inukai, T. Wada and H. Handa, 2001. SPT genes: key players in the regulation of transcription, chromatin structure and other cellular processes. J. Biochem. 129 185–191. [DOI] [PubMed] [Google Scholar]
- Zeitlinger, J., A. Stark, M. Kellis, J. W. Hong, S. Nechaev et al., 2007. RNA polymerase stalling at developmental control genes in the Drosophila melanogaster embryo. Nat. Genet. 39 1512–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F., L. Sumibcay, A. G. Hinnebusch and M. J. Swanson, 2004. A triad of subunits from the Gal11/tail domain of Srb mediator is an in vivo target of transcriptional activator Gcn4p. Mol. Cell. Biol. 24 6871–6886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L., A. Fletcher, V. Cheung, F. Winston and L. A. Stargell, 2008. Spn1 regulates the recruitment of Spt6 and the Swi/Snf complex during transcriptional activation by RNA polymerase II. Mol. Cell. Biol. 28 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]