Abstract
A portion of the Mycobacterium tuberculosis gene encoding the beta subunit of RNA polymerase (rpoB) was amplified by PCR using degenerate oligonucleotides and used as a hybridization probe to isolate plasmid clones carrying the entire rpoB gene of M. tuberculosis H37Rv, a virulent, rifampin-susceptible strain. Sequence analysis of a 5,084-bp SacI genomic DNA fragment revealed a 3,534-bp open reading frame encoding an 1,178-amino-acid protein with 57% identity with the Escherichia coli beta subunit. This SacI fragment also carried a portion of the rpoC gene located 43 bp downstream from the 3' end of the rpoB open reading frame; this organization is similar to that of the rpoBC operon of E. coli. The M. tuberculosis rpoB gene was cloned into the shuttle plasmid pMV261 and electroporated into the LR223 strain of Mycobacterium smegmatis, which is highly resistant to rifampin (MIC > 200 micrograms/ml). The resulting transformants were relatively rifampin susceptible (MIC = 50 micrograms/ml). Using PCR mutagenesis techniques, we introduced a specific rpoB point mutation (associated with clinical strains of rifampin-resistant M. tuberculosis) into the cloned M. tuberculosis rpoB gene and expressed this altered gene in the LR222 strain of M. smegmatis, which is susceptible to rifampin (MIC = 25 micrograms/ml). The resulting transformants were rifampin resistant (MIC = 200 micrograms/ml). The mutagenesis and expression strategy of the cloned M. tuberculosis rpoB gene that we have employed in this study will allow us to determine the rpoB mutations that are responsible for rifampin resistance in M. tuberculosis.
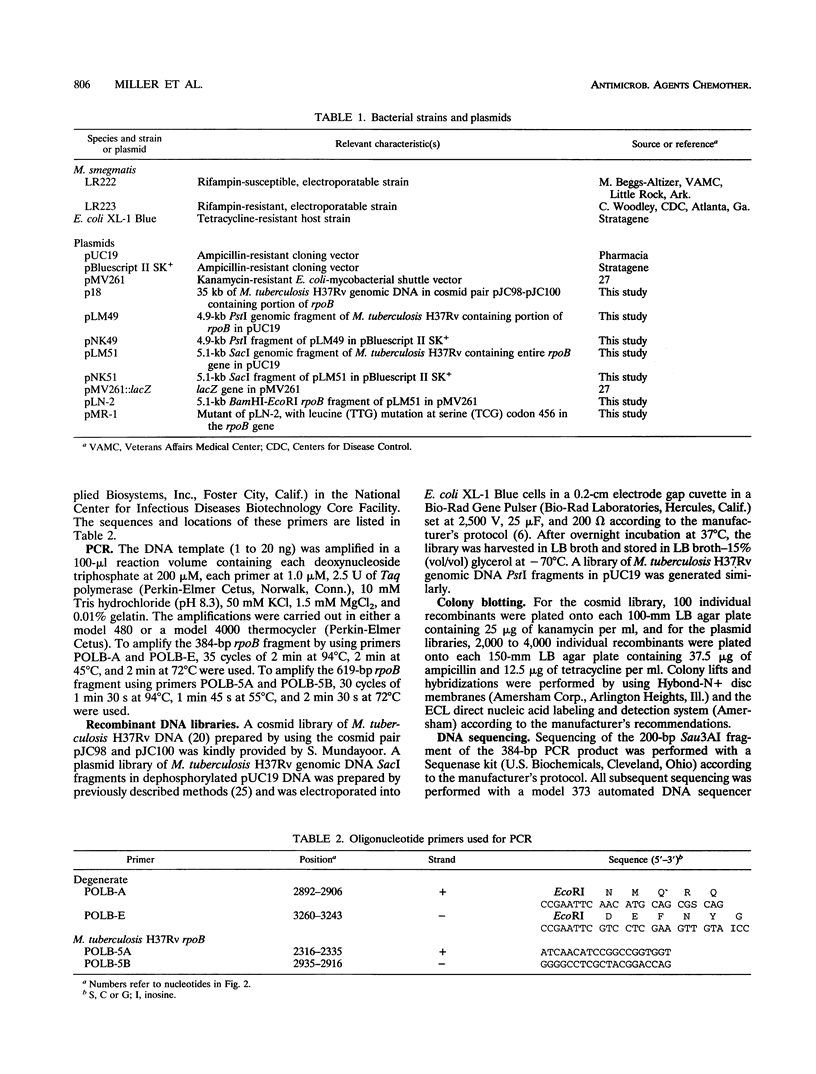
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry G., Squires C. L., Squires C. Control features within the rplJL-rpoBC transcription unit of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4922–4926. doi: 10.1073/pnas.76.10.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. N., Pollack J., Malik F., Ganem D. Cloning and characterization of RNA polymerase core subunits of Chlamydia trachomatis by using the polymerase chain reaction. J Bacteriol. 1990 Oct;172(10):5732–5741. doi: 10.1128/jb.172.10.5732-5741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Honore N., Cole S. T. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother. 1993 Mar;37(3):414–418. doi: 10.1128/aac.37.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honoré N., Bergh S., Chanteau S., Doucet-Populaire F., Eiglmeier K., Garnier T., Georges C., Launois P., Limpaiboon T., Newton S. Nucleotide sequence of the first cosmid from the Mycobacterium leprae genome project: structure and function of the Rif-Str regions. Mol Microbiol. 1993 Jan;7(2):207–214. doi: 10.1111/j.1365-2958.1993.tb01112.x. [DOI] [PubMed] [Google Scholar]
- Ishihama A. Promoter selectivity of prokaryotic RNA polymerases. Trends Genet. 1988 Oct;4(10):282–286. doi: 10.1016/0168-9525(88)90170-9. [DOI] [PubMed] [Google Scholar]
- Jacobs W. R., Jr, Kalpana G. V., Cirillo J. D., Pascopella L., Snapper S. B., Udani R. A., Jones W., Barletta R. G., Bloom B. R. Genetic systems for mycobacteria. Methods Enzymol. 1991;204:537–555. doi: 10.1016/0076-6879(91)04027-l. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Levin M. E., Hatfull G. F. Mycobacterium smegmatis RNA polymerase: DNA supercoiling, action of rifampicin and mechanism of rifampicin resistance. Mol Microbiol. 1993 Apr;8(2):277–285. doi: 10.1111/j.1365-2958.1993.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Lisitsyn N. A., Sverdlov E. D., Moiseyeva E. P., Danilevskaya O. N., Nikiforov V. G. Mutation to rifampicin resistance at the beginning of the RNA polymerase beta subunit gene in Escherichia coli. Mol Gen Genet. 1984;196(1):173–174. doi: 10.1007/BF00334112. [DOI] [PubMed] [Google Scholar]
- Mitchison D. A., Nunn A. J. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986 Mar;133(3):423–430. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Ohme M., Tanaka M., Chunwongse J., Shinozaki K., Sugiura M. A tobacco chloroplast DNA sequence possibly coding for a polypeptide similar to E. coli RNA polymerase beta-subunit. FEBS Lett. 1986 May 5;200(1):87–90. doi: 10.1016/0014-5793(86)80516-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Post L. E., Strycharz G. D., Nomura M., Lewis H., Dennis P. P. Nucleotide sequence of the ribosomal protein gene cluster adjacent to the gene for RNA polymerase subunit beta in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1697–1701. doi: 10.1073/pnas.76.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Telenti A., Imboden P., Marchesi F., Lowrie D., Cole S., Colston M. J., Matter L., Schopfer K., Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993 Mar 13;341(8846):647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- Tsukamura M. The pattern of resistance development to rifampicin in Mycobacterium tuberculosis. Tubercle. 1972 Jun;53(2):111–117. doi: 10.1016/0041-3879(72)90027-x. [DOI] [PubMed] [Google Scholar]
- Vall-Spinosa A., Lester W., Moulding T., Davidson P. T., McClatchy J. K. Rifampin in the treatment of drug-resistant mycobacterium tuberculosis infections. N Engl J Med. 1970 Sep 17;283(12):616–621. doi: 10.1056/NEJM197009172831202. [DOI] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Base composition of deoxyribonucleic acid isolated from mycobacteria. J Bacteriol. 1968 Dec;96(6):1915–1919. doi: 10.1128/jb.96.6.1915-1919.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne L. G., Gross W. M. Isolation of deoxyribonucleic acid from mycobacteria. J Bacteriol. 1968 Apr;95(4):1481–1482. doi: 10.1128/jb.95.4.1481-1482.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W. Rifampin: mechanisms of action and resistance. Rev Infect Dis. 1983 Jul-Aug;5 (Suppl 3):S407–S411. doi: 10.1093/clinids/5.supplement_3.s407. [DOI] [PubMed] [Google Scholar]
- Yepiz-Plascencia G. M., Radebaugh C. A., Hallick R. B. The Euglena gracilis chloroplast rpoB gene. Novel gene organization and transcription of the RNA polymerase subunit operon. Nucleic Acids Res. 1990 Apr 11;18(7):1869–1878. doi: 10.1093/nar/18.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]


