Abstract
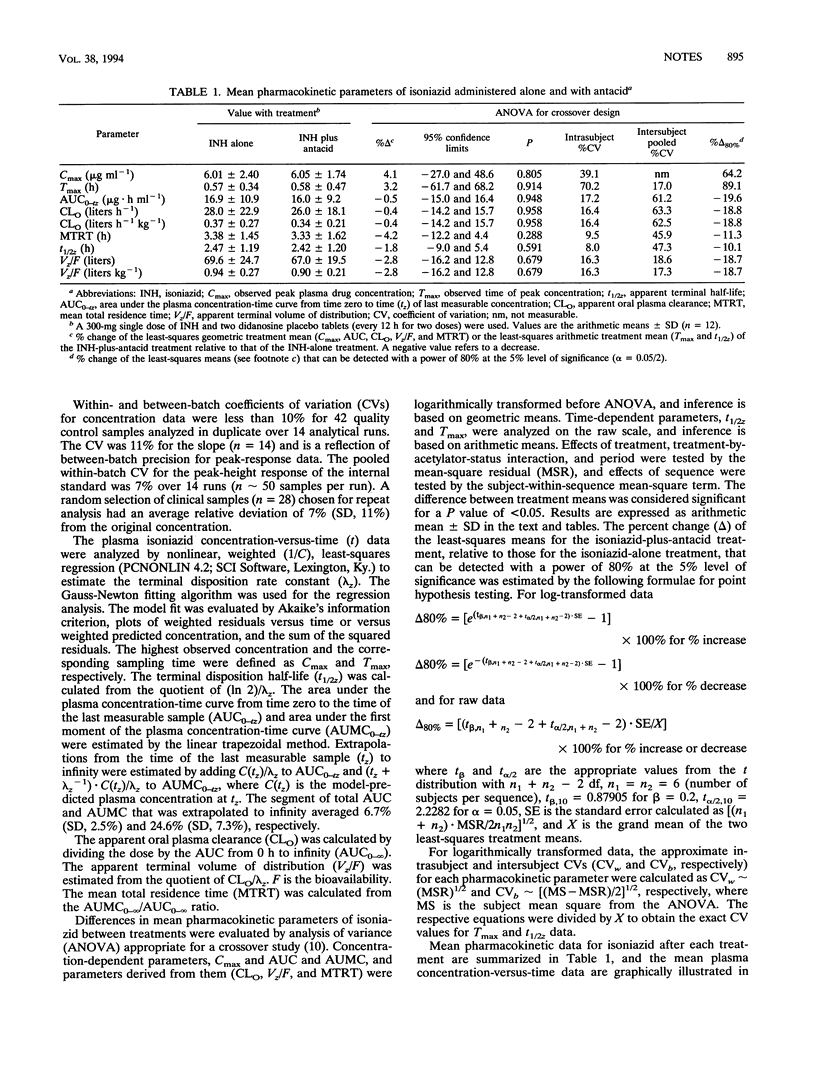
The antacids in two didanosine placebo tablets had no significant effect on the plasma pharmacokinetics of a single oral dose of 300 mg of isoniazid administered to 12 healthy volunteers. These results suggest that isoniazid bioavailability will be unaffected by the antacids in didanosine tablets when the two medications are administered simultaneously to human immunodeficiency virus-seropositive patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berning S. E., Huitt G. A., Iseman M. D., Peloquin C. A. Malabsorption of antituberculosis medications by a patient with AIDS. N Engl J Med. 1992 Dec 17;327(25):1817–1818. doi: 10.1056/NEJM199212173272514. [DOI] [PubMed] [Google Scholar]
- Eidus L., Hodgkin M. M., Schaefer O., Jessamine A. G. Distribution of isoniazid inactivators determined in Eskimos and Canadian college students by a urine test. Rev Can Biol. 1974 Jun;33(2):117–123. [PubMed] [Google Scholar]
- Heifets L. Qualitative and quantitative drug-susceptibility tests in mycobacteriology. Am Rev Respir Dis. 1988 May;137(5):1217–1222. doi: 10.1164/ajrccm/137.5.1217. [DOI] [PubMed] [Google Scholar]
- Hurwitz A., Schlozman D. L. Effects of antacids on gastrointestinal absorption of isoniazid in rat and man. Am Rev Respir Dis. 1974 Jan;109(1):41–47. doi: 10.1164/arrd.1974.109.1.41. [DOI] [PubMed] [Google Scholar]
- Hutchings A., Monie R. D., Spragg B., Routledge P. A. A method to prevent the loss of isoniazid and acetylisoniazid in human plasma. Br J Clin Pharmacol. 1983 Feb;15(2):263–266. doi: 10.1111/j.1365-2125.1983.tb01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings A., Monie R. D., Spragg B., Routledge P. A. High-performance liquid chromatographic analysis of isoniazid and acetylisoniazid in biological fluids. J Chromatogr. 1983 Oct 14;277:385–390. doi: 10.1016/s0378-4347(00)84863-x. [DOI] [PubMed] [Google Scholar]
- Hutchings A., Routledge P. A. A simple method for determining acetylator phenotype using isoniazid. Br J Clin Pharmacol. 1986 Sep;22(3):343–345. doi: 10.1111/j.1365-2125.1986.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knupp C. A., Shyu W. C., Dolin R., Valentine F. T., McLaren C., Martin R. R., Pittman K. A., Barbhaiya R. H. Pharmacokinetics of didanosine in patients with acquired immunodeficiency syndrome or acquired immunodeficiency syndrome-related complex. Clin Pharmacol Ther. 1991 May;49(5):523–535. doi: 10.1038/clpt.1991.63. [DOI] [PubMed] [Google Scholar]
- Midha K. K., Ormsby E. D., Hubbard J. W., McKay G., Hawes E. M., Gavalas L., McGilveray I. J. Logarithmic transformation in bioequivalence: application with two formulations of perphenazine. J Pharm Sci. 1993 Feb;82(2):138–144. doi: 10.1002/jps.2600820205. [DOI] [PubMed] [Google Scholar]
- Männisto P., Mäntylä R., Klinge E., Nykänen S., Koponen A., Lamminsivu U. Influence of various diets on the bioavailability of isoniazid. J Antimicrob Chemother. 1982 Nov;10(5):427–434. doi: 10.1093/jac/10.5.427. [DOI] [PubMed] [Google Scholar]
- Paulsen O., Höglund P., Nilsson L. G., Gredeby H. No interaction between H2 blockers and isoniazid. Eur J Respir Dis. 1986 Apr;68(4):286–290. [PubMed] [Google Scholar]
- Peloquin C. A., MacPhee A. A., Berning S. E. Malabsorption of antimycobacterial medications. N Engl J Med. 1993 Oct 7;329(15):1122–1123. doi: 10.1056/NEJM199310073291513. [DOI] [PubMed] [Google Scholar]
- Sahai J., Gallicano K., Oliveras L., Khaliq S., Hawley-Foss N., Garber G. Cations in the didanosine tablet reduce ciprofloxacin bioavailability. Clin Pharmacol Ther. 1993 Mar;53(3):292–297. doi: 10.1038/clpt.1993.24. [DOI] [PubMed] [Google Scholar]
- Shelton M. J., O'Donnell A. M., Morse G. D. Didanosine. Ann Pharmacother. 1992 May;26(5):660–670. doi: 10.1177/106002809202600511. [DOI] [PubMed] [Google Scholar]
- Sved S., McGilveray I. J., Beaudoin N. Bioavailability of three isoniazid formulations. J Pharm Sci. 1977 Dec;66(12):1761–1764. doi: 10.1002/jps.2600661228. [DOI] [PubMed] [Google Scholar]


