Abstract
Chemical nociception, the detection of tissue-damaging chemicals, is important for animal survival and causes human pain and inflammation, but its evolutionary origins are largely unknown. Reactive electrophiles are a class of noxious compounds humans find pungent and irritating, like allyl isothiocyanate (in wasabi) and acrolein (in cigarette smoke)1–3. Insects to humans find reactive electrophiles aversive1–3, but whether this reflects conservation of an ancient sensory modality has been unclear. Here we identify the molecular basis of reactive electrophile detection in flies. We demonstrate that dTRPA1, the Drosophila melanogaster ortholog of the human irritant sensor, acts in gustatory chemosensors to inhibit reactive electrophile ingestion. We show that fly and mosquito TRPA1 orthologs are molecular sensors of electrophiles, using a mechanism conserved with vertebrate TRPA1s. Phylogenetic analyses indicate invertebrate and vertebrate TRPA1s share a common ancestor that possessed critical characteristics required for electrophile detection. These findings support emergence of TRPA1-based electrophile detection in a common bilaterian ancestor, with widespread conservation throughout vertebrate and invertebrate evolution. Such conservation contrasts with the evolutionary divergence of canonical olfactory and gustatory receptors and may relate to electrophile toxicity. We propose human pain perception relies on an ancient chemical sensor conserved across ~500 million years of animal evolution.
Keywords: pungency, electrophile, irritation, pain, TRP channel
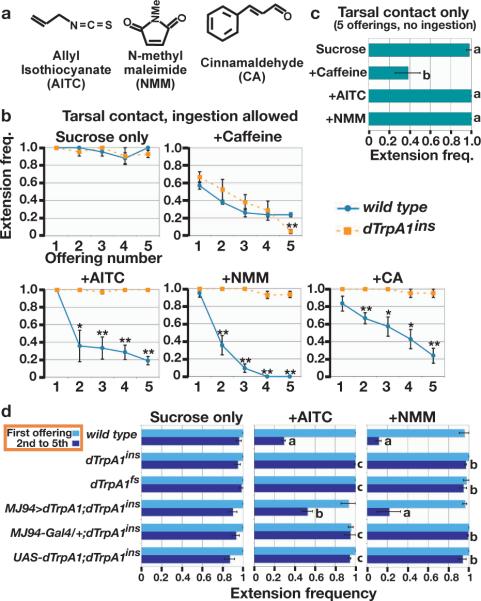
Reactive electrophiles are tissue-damaging agents that modify nucleic acids, proteins and other biomolecules. Reactive electrophiles are aversive to both vertebrates and invertebrates1–3; plants and animals use them as deterrents3. Despite their importance as natural repellents, the cellular and molecular mechanisms by which reactive electrophiles deter insects have not been established. We examined Drosophila responses to reactive electrophiles using feeding. When a droplet of food (350 mM sucrose) contacts the legs of a hungry fly, the fly extends its proboscis to drink. This proboscis extension response (PER) is robust and sustained; >90% of the second through fifth offerings of food elicited PER (Fig. 1b). Adding the reactive electrophile allyl isothiocyanate (AITC, Fig. 1a) to the food dramatically inhibited this response (Fig. 1b). This effect was generalized to other reactive electrophiles using N-methyl maleimide (NMM) and cinnamaldehyde (CA) (Fig. 1a). Both NMM and CA robustly inhibited feeding (Fig. 1b). This inhibitory effect appeared gustatory, not olfactory, because NMM is non-volatile (m.p. 93°C) and avoidance required ingestion. When only leg contact with food was permitted, reactive electrophiles did not affect PER (Fig. 1c), suggesting that chemosensors along the path of food intake rather than the legs mediate their inhibitory effects. The bitter compound caffeine, for which there are tarsal receptors4,5, robustly inhibited PER even when ingestion was not permitted (Fig. 1c).
Figure 1. dTrpA1 mediates gustatory responses to reactive electrophiles.
a, Chemical structures. b, Proboscis extension response (PER) frequency at five sequential tastant offerings, ingestion permitted. (*p<0.05, **p<0.01, unpaired t-test.) c, PER, tastant contacts only legs. Five sequential offerings combined (n≥10 flies). d, PER, ingestion permitted: light blue, first offering; dark blue, second to fifth offerings combined. Statistically distinct groups marked by different letters (Tukey HSD, α=0.01). Data are mean +/− SEM. All studies use 12% (350mM) sucrose, alone or with 100 mM caffeine, 2 mM AITC, 10 mM NMM, or 6 mM CA. n=3 groups of ≥7 flies, unless noted.
In vertebrates, the cation channel TRPA1 is a molecular receptor for reactive electrophiles, forming covalent adducts with these chemicals and activating sensory neurons to mediate irritation and pain6–11. Previous in vitro physiological analyses suggested that Drosophila TRPA1 relatives dTRPA1 and Painless were not activated by electrophiles6,12, raising the possibility that flies might use different mechanisms to detect these chemicals. We reexamined the possible involvement of dTRPA1 and Painless in vivo, assessing the gustatory response to reactive electrophiles. In contrast to wild type, dTrpA1 loss-of-function mutants showed no reduction in PER when offered food containing AITC, NMM, or CA (Fig. 1b). Similar defects were observed using two loss-of-function dTrpA1 alleles (dTrpA1ins and dTrpA1fs)13 and dTrpA1 cDNA expression rescued this defect (Fig. 1d). Thus this response to reactive electrophiles is entirely TRPA1-dependent. dTrpA1 mutants responded to other deterrents, as caffeine inhibited PER (Fig. 1b). In contrast, painless mutants remained responsive to reactive electrophiles (Supp Fig. 1), although responses were less robust than controls, suggesting a possible auxiliary function consistent with previous report14.
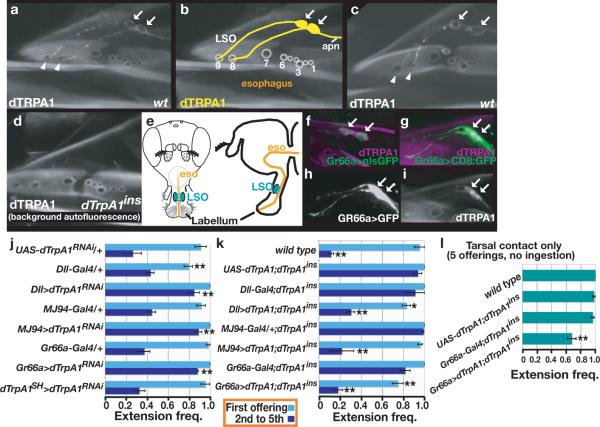
dTRPA1 protein expression was detected in the mouthparts (Fig. 2a–c), but not legs or labellum. Within the mouthparts, dTRPA1 was expressed in neurons innervating sensilla #8 and #9 of the labral sense organ (LSO) (Fig. 2b,e). LSO sensilla contain pores that open onto the esophagus lumen, providing access to chemicals in ingested food. Thus, dTRPA1 is expressed in an appropriate place to mediate ingestion-dependent responses.
Figure 2. dTrpA1 functions in chemosensors.
a–c, dTRPA1 expression. Arrows denote cell bodies, arrowheads distal neurites. Cuticle autofluorescence visible. (b), LSO sensilla numbered. apn: accessory pharyngeal nerve. d. dTrpA1 mutants lack dTRPA1 staining. e. eso, esophagus. f–i, Gr66a-Gal4 and dTRPA1 co-expression in LSO. (f) Nuclear and (g,h) membrane GFPs expressed using Gr66a-Gal4. j–l, PER to 350 mM sucrose containing 10mM NMM. (j, k) Ingestion permitted. l, tarsal contact only. j, dTRPA1 knockdown. k, dTRPA1 rescue. l, dTRPA1 gain-of-function. *: α=0.05, **: α=0.01, differ from Gal4 and UAS controls, Tukey HSD. j,k, n=3 groups of 7–8 flies, l, n≥10 flies.
To test the significance of peripheral dTRPA1 expression, tissue-specific RNAi was performed using three promoters whose expression overlaps dTRPA1-positive LSO neurons: Dll-Gal4, expressed broadly within peripheral tissue, MJ94-Gal4, expressed in chemoreceptors and the brain16, and Gr66a-Gal4, expressed in chemoreceptors implicated in aversive responses4,5(Fig. 2 f–i). dTRPA1 knockdown using each promoter robustly reduced NMM's effect on PER, consistent with a requirement for dTRPA1 in peripheral chemoreceptors (Fig. 2j). In contrast, dTRPA1 knockdown in the AC thermosensory neurons of the head using dTrpA1SH-Gal415 had no effect (Fig. 2j). These data cleanly distinguish the sites of action for dTRPA1 in thermotaxis and gustation, with the former involving AC neurons15 and the latter peripheral sensory neurons.
dTRPA1 expression in peripheral chemosensors also sufficed to induce reactive electrophile-dependent PER inhibition. dTRPA1 cDNA expression with Dll-Gal4, MJ94-Gal4, or Gr66a-Gal4 rescued the mutant phenotype (Fig. 2k). In addition, ectopic expression of dTRPA1 in leg chemoreceptors (using Gr66a-Gal4) allowed flies to respond to electrophiles via leg contact (Fig. 2l). Thus, dTRPA1 expression in peripheral chemosensory neurons is both necessary and sufficient for reactive electrophile-induced feeding inhibition.
dTRPA1 has been considered unresponsive to electrophiles6,17; however, we recently found that the original dTRPA1 cDNA contained a partially inactivating mutation15. Using wild-type dTRPA1, we found dTRPA1 was activated by multiple reactive electrophiles when expressed in Xenopus oocytes (Fig. 3a–d; Supp. Figs. 2 and 3). dTRPA1 orthologs from two other Drosophila species, D. mojavensis and D. virilis, and the malaria mosquito Anopheles gambiae also responded to these chemicals (Fig. 3e; Supp. Fig. 2). Combined with the sensitivity of mosquito TRPA1 to AITC in HEK cells17, these findings demonstrate multiple insect TRPA1s respond to electrophiles. Notably, electrophile-activated currents persisted after chemical withdrawal (Fig. 3a–f), contrasting with the transient activation of dTRPA1 by warmth15. Persistent activation by electrophiles has been observed for mammalian TRPA1s, and it is thought to reflect covalent association between agonists and channel10,11. This similarity suggested reactive electrophiles might activate insect and mammalian TRPA1s via similar mechanisms. Finally, we demonstrated that ectopic expression of dTRPA1 in fly neurons can confer physiological sensitivity to electrophiles. In contrast to controls or motorneurons expressing Painless, dTRPA1-expressing motorneurons were cinnamaldehyde-responsive (Fig. 3f; Supp. Fig. 4). Thus, dTRPA1 acts as an electrophile sensor in multiple contexts.
Figure 3. Insect TRPA1s are reactive electrophile sensors.
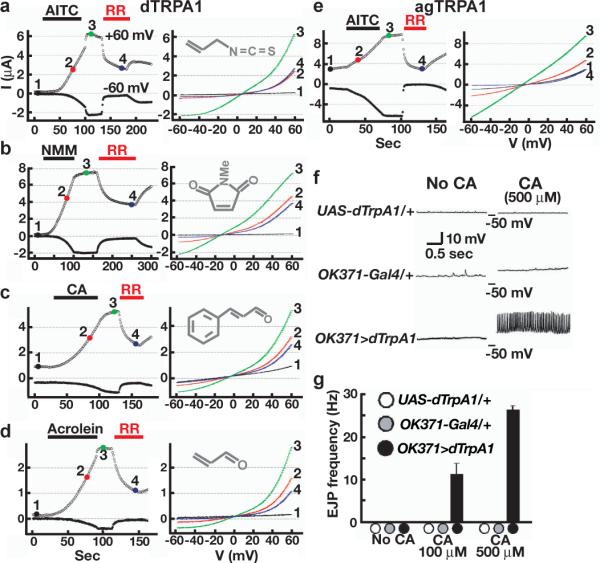
a–e. Representative responses of dTRPA1 (a–d) and agTRPA1 (e) expressed in oocytes. Left panels, currents at −60 and +60 mV. Perfusion buffer containing indicated chemical (a, c, d, 100 μM; b, e, 40 μM) was applied for 60–80 sec. 100 μM ruthenium red (RR) applied as noted. Right panels show I–V relationships at points marked on left panels. f, g, Ectopic dTRPA1 expression confers electrophile sensitivity upon motor neurons. f, Motor neuron-driven excitatory junction potentials (EJPs) from third instar larval muscles. g. Mean EJP frequencies. In controls, no EJPs were observed.
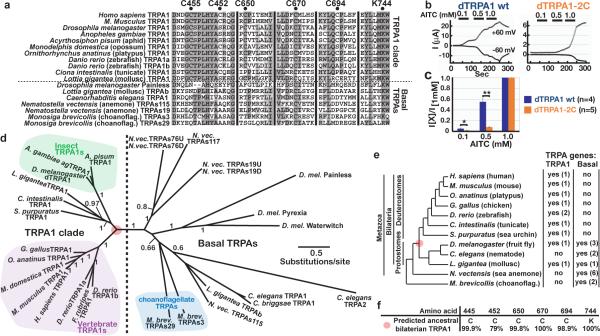
Reactive electrophiles activate mammalian TRPA1s by forming covalent bonds with cysteine and lysine residues in the channel; six residues (five cysteines and one lysine) are implicated in electrophile detection and mutations in these residues decrease electrophile sensitivity10,11. Insect TRPA1s conserve five of these six residues (Fig. 4a). Mutating dTRPA1 cysteines 650 and 670 to serines (dTRPA1-2C) significantly decreased AITC sensitivity (Fig. 4b–c); this dTRPA1-2C mutant remained robustly warmth-activated (Supp. Fig. 5). The shared requirement for these residues further supports a common mechanism for reactive electrophile sensing by fly and vertebrate TRPA1s. TRPA1s also exhibit some species-specific differences in chemical sensitivity; 2-aminoethoxydiphenyl borate (2-APB) and nicotine, conserved cysteine-independent agonists of mammalian TRPA1s10,18, did not activate dTRPA1 (Supp. Fig. 6).
Figure 4. TRPA phylogeny.
a, Conservation of residues implicated in electrophile detection. choannoflag, choannoflagellate. b, TRPA1-wt (wild type) and dTRPA1-2C channels in Xenopus oocytes. 60 sec pulses of AITC (0.1, 0.5, and 1.0 mM) were applied with 25 sec intervals. c. +60 mV currents normalized to channel's response to 1.0 mM AITC. *p<0.05, **p<0.001, unpaired t-test. d, Bayesian consensus phylogeny for TRPAs. Internal branches labeled by posterior probability (<0.5 branches collapsed). Red dot denotes ancestor of TRPA1 clade. e, Cladogram showing TRPA complements, including numbers of channels. Red dot denotes bilaterian ancestor. f, PAML residue identity estimates for ancestral TRPA1.
While functional similarities between insect and vertebrate TRPA1s could reflect conservation of an ancestral mechanism for electrophile detection, the electrophile insensitivity of invertebrate TRPA1 relatives like Painless12 and C. elegans TRPA1 (ceTRPA1)19 raised the possibility that some insect and vertebrate TRPA1s recently converged on similar mechanisms. To test these alternatives, a phylogeny of TRPA proteins was constructed using three different approaches, Bayesian inference20, maximum likelihood21, and neighbor joining22. Trees were rooted using TRPAs from the unicellular choanoflagellate M. brevicollis. All methods indicated with high confidence that the electrophile-activated TRPA1 channels of invertebrates and vertebrates belong to a monophyletic clade, the TRPA1 clade, distinct from other TRPAs (termed basal TRPAs) by both tree topology and branch lengths (Fig. 4d, e; Supp. Fig. 7, 8 and 9). The TRPA1 clade channels derive from a common ancestral TRPA1 present in the common ancestor of vertebrates and invertebrates (Fig. 4d,e). Consistent with a common evolutionary origin of electrophile detection, sequence reconstruction23 suggested this ancestral TRPA1 contained all six critical residues associated with electrophile sensing (Fig. 4f). This mode of electrophile detection appears specific to TRPA1 clade members, as no known basal TRPAs conserve more than one of the five cysteines implicated in electrophile detection (Fig. 4a).
These analyses also suggest revisions to proposed relationships among TRPAs. Painless has been called the fly homolog of mammalian TRPA1, and ceTRPA1 considered the nematode TRPA1 ortholog. However, all analyses indicated that neither Painless nor ceTRPA1 descend from the ancestral TRPA1; both are closer to anemone and choannoflagellate TRPAs (Fig. 4d). Consistent with their electrophile insensitivity12,19, Painless and ceTRPA1 lack most cysteines implicated in electrophile detection (Fig. 4a). During evolution, nematodes appear to have lost their TRPA1 ortholog and vertebrates their basal TRPA(s) (Fig. 4d–e).
Functional conservation of TRPA1 provides a simple molecular foundation for the widespread aversion to reactive electrophiles across the animal kingdom. The conservation of reactive electrophile detection differs from other chemical senses like olfaction and gustation whose origins are molecularly diverse and evolutionarily distinct24,25. For example, many fly olfactory receptors are ion channels rather than the G-protein coupled receptors of vertebrates25. Reactive electrophile detection also contrasts with capsaicin detection; capsaicin activates mammalian nociceptors1, but elicits no acute response in flies or nematodes. The exceptional conservation of TRPA1-mediated nociception could relate to the toxicity of reactive electrophiles26, which could provide selective pressure for maintaining an effective monitoring system.
dTRPA1's ability to mediate aversive responses to natural deterrents suggests insect TRPA1s as targets for developing new deterrents. Insect TRPA1 agonists could be useful against an array of pests, as disease vectors from mosquitoes to lice and agricultural pests from flour beetles to aphids15 contain dTRPA1 relatives. While natural electrophilic deterrents also activate mammalian TRPA1s, deterring insects and mammals alike, the differential responsiveness of insect and mammalian TRPA1s to other stimuli like temperature27 and nicotine raises the possibility of identifying insect-specific TRPA1 agonists. Such selective agonists could maximize pest deterrence while minimizing irritation to other animals.
TRPA1 activation by reactive electrophiles is a key component of mammalian nociceptor function, eliciting pungency, irritation, inflammation and pain6–9. Our findings demonstrate that the molecular mechanism that initiates these perceptions is not a recent evolutionary innovation and that it is not specific to vertebrates. Rather, we propose that reactive electrophile detection represents an ancient sensory modality conserved in molecular detail across ~500 million years of animal evolution.
Methods summary
Proboscis extension behavior
The proboscis extension assay was modified from ones previously described4,5 as detailed in Methods.
Physiology
Oocyte and larval physiology were performed largely as described15,28, with additional details provided in Methods. Chemical sensitivities of wild type and mutant (dTRPA1-2C) channels were assessed by normalizing all currents to currents observed at 1mM AITC. Chemically unrelated insect repellents like DEET, IR-3535, and deltamethrin failed to activate dTRPA1 (K.K. and P.G., unpublished).
Phylogeny
TRPA sequences were assembled from available genomic and EST data. Multiple sequence alignment was performed using ProbCons29 for region from ~310 amino acids N-terminal of transmembrane regions (containing the residues implicated in chemical sensing) to ~50 amino acids C-terminal of transmembrane regions (Supp. Fig. 5). Bayesian analysis was calculated with the parallel version of MrBayes 3.1.2 using mixed substitution rate matrices and gamma distributed rate variation across sites (8 categories). An exponential prior (mean = 1.0) was assumed for shape parameter of the gamma distribution, an unconstrained exponential prior (mean = 1.0) assumed for branch lengths, and a uniform prior assumed for all labeled topologies. Two independent MCMC analyses were performed (each with one cold and three heated chains), with other parameters set to defaults. Chains were run for 10,000,000 generations, and convergence inferred after cold chain topologies reached a standard deviation of split frequencies of less than 0.005 (~250,000 generations). After convergence, the first half of the chain was discarded as “burnin”. Maximum likelihood analysis was performed with PhyML 3.0, using LG substitution rate matrix, gamma distributed rate variation (8 categories) and was bootstrapped 1000 times. A BioNJ distance-based phylogenetic analysis was performed with PAUP 4b1030 and bootstrapped 1000 times. Ancestral sequence reconstruction was performed with PAML 4.2b23 using the consensus Bayesian phylogenetic tree and mean alpha rate parameter. Branch lengths were fixed.
Methods
Fly strains and immunohistochemistry
dTrpA1SH-Gal4, UAS-dTRPA1, and UAS-dTRPA1dsRNA transgenic strains15, as well as Dll-Gal432, MJ94-Gal416,33, Gr66a-Gal434, UAS-PainlessAR914, and painless35 mutants have been previously described. UAS-nls:GFP and UAS-mCD8:GFP fly strains were obtained from Bloomington. Anti-dTRPA1 immunohistochemistry was performed as described31. Details of the creation of dTrpA1fs and dTrpA1ins were previously reported13,15. Briefly, dTrpA1fs has a 2-bp insertion creating frameshift mutation within the third ankyrin repeat of dTRPA1, prior to the transmembrane regions. dTrpA1ins contains two mutated copies of dTrpA1 that flank vector targeting sequences: one copy lacks the ion pore and sixth transmembrane domain, while the other copy lacks the promoter and upstream sequences, all of exon 1, part of exon 2, and contains the 2bp insertion mutation present in dTrpA1fs.
PER behavioral assays
Two to seven day old flies were starved overnight on wet Kim wipes, anaesthetized on ice, and affixed to a glass slide. Flies recovered in a humidified chamber for at least 2 hrs at room temperature prior to testing. During the PER assay, the fly was first satiated with water, then a solution containing tastants was touched to the forelegs as a liquid ball on a pipette tip. If the proboscis was extended and contact with the food was maintained for 2–3 sec, the response was scored as 1. If the proboscis stuttered on the tastant, or contact was brief, a 0.5 was awarded. If the proboscis failed to contact the solution within 5 sec of offering, a 0 was awarded. Each fly was offered tastants five times per experiment, and between offerings water was given to satiation. Because AITC, cinnamaldehyde and NMM were usually accepted on first offering, PER frequency was calculated for the second through fifth offerings (sum of four scores per fly divided by 4). Responses to sucrose resumed within ~10 minutes after pungent chemical exposure, indicating that feeding was not permanently impaired (K.K. and P.G., unpublished). For leg only PER assays, the procedures were as above except flies were not allowed to contact the food with their proboscis. We found that NMM had no effect on ingestion when using a previously published ingestion-independent PER assay for chemical sensitivity14, suggesting the inhibitory effects of AITC in that assay were not gustatory.
Physiology
Two-electrode voltage clamping on Xenopus laevis oocytes
Agonist-evoked dTRPA1 currents were recorded as previously described15, with the following modifications. Agonists of interest were added to the oocyte perfusion buffer (96 mM NaCl, 1 mM MgCl2, 4 mM KCl, and 5 mM HEPES, pH 7.6). Voltage was initially held at −60 mV, and a 300-ms voltage ramp (−60 mV to 60 mV) per sec was applied to dTRPA1- or AgTRPA1-expressing oocytes during perfusion of agonist-containing buffer. Typical oocyte resting membrane potentials were between −25 and −60 mV. Agonist-elicited currents were specific and TRPA1-dependent; they were absent from uninjected or water-injected oocytes and were significantly reduced by mutation of two cysteine residues within dTRPA1 (Fig. 3a–e, Fig. 4b, data not shown). Furthermore, they were inhibited by ruthenium red, which partially inhibits warmth-activated dTRPA1 and agTRPA1 currents, and they exhibited the reversal potential and rectification properties previously associated with warmth-activated dTRPA1 and agTRPA1 currents15. EC50s for wild type dTRPA1 channels were obtained at −60mV, with AITC provided for 60 sec with 30 sec intervals between increasing concentrations. The low sensitivity of dTRPA1-2C to AITC precluded EC50 analysis of the mutant channel.
Larval neuromuscular junction electrophysiology
TRPA channels were expressed in larval motor neurons using OK371-GAL4, a driver specific for glutamatergic neurons, as described28. In all preparations, the ventral ganglion was dissected away, leaving only motor axons and terminals. Larval muscle 6 (m6) was impaled with a sharp electrode (10–20 MΩ) containing 3M KCl. Resting membrane potentials were typically between −40 and −50 mV. Saline was perfused over the preparation, then increasing concentrations of cinnamaldehyde applied using a custom built gravity perfusion system. EJP frequency was measured~30 sec after application of each concentration using analysis scripts in Spike 2 (Cambridge Electronic Design, Cambridge, UK). Painless was overexpressed using the functional rescue construct UAS-PainlessAR914.
Molecular biology
Substitutions of cysteine/lysine residues in dTrpA1 were made by swapping a region of wild type cDNA sequence including codons of cysteine or lysine with mutated cassettes. A pair of mutually complementary oligonucleotide primers with a desired mutation were prepared, and each of them was paired with upstream or downstream primers for the first two PCR reactions. The resulting two PCR fragments overlap in the mutant primer-annealing region that contains the replaced codons, and served as template for the second PCR reaction amplified only with the upstream and down stream primers. The upstream and down stream primers were designed to be just outside of specific restriction endonuclease target sites that were used to clone the second PCR products back in the wild type dTrpA1 cDNA background sequence. The fragments amplified by PCR were confirmed by sequencing after cloning to make sure that only desired mutations were introduced in the final cDNA constructs.
Sequence alignment and phylogeny
Multiple sequence alignments were visualized using JAL2.436. Conservation reflects conservation of physico-chemical properties of residues calculated as described37. Quality is inversely proportional to the cost of mutations in a residue, measure of likelihood of observing mutations36. Consensus reflects percentage of modal residue. The LG substitution matrix was as described38. The input data for the ancestral reconstruction was the consensus Bayesian phylogenetic tree depicted in Figure 4D. We used the “marginal reconstruction” method (RateAncestor = 1) in PAML425, which determines the posterior probability of each amino acid at each site in the protein alignment for a given node. We fixed the alpha parameter (for gamma distributed rate variation across sites) to the Bayesian expected value as determined by MrBayes.
Supplementary Material
Acknowledgments
We thank H. Amrein, G. Boekh, K. Scott, D. Tracey, and Bloomington Stock center for flies, C. Miller-Patterson and D. Zeiger for assistance, and the Garrity lab, J.P. Garrity, L. Huang, E. Marder, S. Nelson, G. Turrigiano and M. Rosbash for comments. This work was supported by grants from the NIMH (R21 MH080206, P.A.G., RO1 MH067284 , L.C.G.) and NINDS (PO1 NS044232).
Footnotes
The authors have no competing interests.
References
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bessac BF, Jordt SE. Breathtaking TRP Channels: TRPA1 and TRPV1 in Airway Chemosensation and Reflex Control. Physiology (Bethesda) 2008;23:360–370. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisner T. In: Chemical Ecology. Sondheimer E, Simeone JB, editors. Academic Press; New York: 1970. Vol. [Google Scholar]
- 4.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 7.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 8.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 10.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103:19564–19568. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445:541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 12.Sokabe T, Tsujiuchi S, Kadowaki T, Tominaga M. Drosophila painless is a Ca2+-requiring channel activated by noxious heat. J Neurosci. 2008;28:9929–9938. doi: 10.1523/JNEUROSCI.2757-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenzweig M, Kang K, Garrity PA. Distinct TRP channels are required for warm and cool avoidance in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2008;105:14668–14673. doi: 10.1073/pnas.0805041105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Anzi B, Tracey WD, Jr., Benzer S. Response of Drosophila to wasabi is mediated by painless, the fly homolog of mammalian TRPA1/ANKTM1. Curr Biol. 2006;16:1034–1040. doi: 10.1016/j.cub.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gendre N, Luer K, Friche S, Grillenzoni N, Ramaekers A, Technau GM, Stocker RF. Integration of complex larval chemosensory organs into the adult nervous system of Drosophila. Development. 2004;131:83–92. doi: 10.1242/dev.00879. [DOI] [PubMed] [Google Scholar]
- 17.Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talavera K, et al. Nicotine activates the chemosensory cation channel TRPA1. Nat Neurosci. 2009;12:1293–1299. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 19.Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- 20.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 21.Guindon S, Delsuc F, Dufayard JF, Gascuel O. Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol. 2009;537:113–137. doi: 10.1007/978-1-59745-251-9_6. [DOI] [PubMed] [Google Scholar]
- 22.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Yang Z. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 2007;24:1586–1591. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 24.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa T, Vosshall LB. Controversy and consensus: noncanonical signaling mechanisms in the insect olfactory system. Curr Opin Neurobiol. 2009;19:284–292. doi: 10.1016/j.conb.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes R, Meek ME, Eggleton M. Concise International Chemical Assessment Document No 43. World Health Organization; Geneva: 2002. [Google Scholar]
- 27.Viswanath V, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 28.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do CB, Mahabhashyam MS, Brudno M, Batzoglou S. ProbCons: Probabilistic consistency-based multiple sequence alignment. Genome Res. 2005;15:330–340. doi: 10.1101/gr.2821705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swofford DL. Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4 Sinauer Associates; Sunderland, Massachusetts: 2003. [Google Scholar]
- 31.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 2005;19:419–424. doi: 10.1101/gad.1278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Calleja M, Moreno E, Pelaz S, Morata G. Visualization of gene expression in living adult Drosophila. Science. 1996;274:252–255. doi: 10.1126/science.274.5285.252. [DOI] [PubMed] [Google Scholar]
- 33.Joiner Ml A, Griffith LC. CaM kinase II and visual input modulate memory formation in the neuronal circuit controlling courtship conditioning. J Neurosci. 1997;17:9384–9391. doi: 10.1523/JNEUROSCI.17-23-09384.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunipace L, Meister S, McNealy C, Amrein H. Spatially restricted expression of candidate taste receptors in the Drosophila gustatory system. Curr Biol. 2001;11:822–835. doi: 10.1016/s0960-9822(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 35.Tracey WD, Jr., Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 36.Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ. Jalview version 2: A multiple sequence alignment and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livingstone CD, Barton GJ. Protein sequence alignments: a strategy for the hierarchical analysis of residue conservation. CABIOS. 1993;9:745–756. doi: 10.1093/bioinformatics/9.6.745. [DOI] [PubMed] [Google Scholar]
- 38.Le SQ, Gascuel O. An improved general amino acid replacement matrix. Mol Biol Evol. 2008;25:1307–1320. doi: 10.1093/molbev/msn067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.