Abstract
Store-operated calcium channels are plasma membrane Ca2+ channels that are activated by depletion of intracellular Ca2+ stores, resulting in an increase in intracellular Ca2+ concentration, which is maintained for prolonged periods in some cell types. Increases in intracellular Ca2+ concentration serve as signals that activate a number of cellular processes, however, little is known about the regulation of these channels. We have characterized the immuno-suppressant compound BTP, which blocks store-operated channel mediated calcium influx into cells. Using an affinity purification scheme to identify potential targets of BTP, we identified the actin reorganizing protein, drebrin, and demonstrated that loss of drebrin protein expression prevents store-operated channel mediated Ca2+ entry, similar to BTP treatment. BTP also blocks actin rearrangements induced by drebrin. While actin cytoskeletal reorganization has been implicated in store-operated calcium channel regulation, little is known about actin binding proteins that are involved in this process, or how actin regulates channel function. The identification of drebrin as a mediator of this process should provide new insight into the interaction between actin rearrangement and tore-operated channel mediated calcium influx.
1. Introduction
Stimulation of tyrosine kinase coupled receptors such as the T cell receptor in T lymphocytes results in activation of phospholipase Cγ1 (PLCγ) which hydrolyzes phosphoinositol 4,5 bisphosphate (PIP2) to generate the second messengers inositol 1,4,5 trisphosphate (IP3) and diacyl glycerol (DAG) (Winslow et al., 2003). Similarly, IP3 and DAG are generated by PLCβ downstream of G-protein coupled receptors (Hepler and Gilman, 1992). This results in increased intracellular calcium concentration [Ca2+]i due to the action of IP3 on IP3 receptors (IP3R) in the endoplasmic reticulum (ER) membrane which, when activated stimulate the release of ER calcium stores into the cytoplasm. Emptying of the ER calcium stores stimulates entry of extracellular calcium through store-operated channels (SOCs) or CRAC channels, thus maintaining the higher concentration of intracellular Ca2+. [Ca2+]i increases play a critical role in a variety of cellular processes such as transcription factor activation and gene expression, and cytoskeletal reorganization. In T cells, increased [Ca2+]i results in activation of the transcription factor, nuclear factor of activated T cells (NFAT), which is essential for transcription of many cytokine genes important in generating an immune response.
Store-operated channel mediated entry of Ca2+ through CRAC channels is the main mechanism used by many cells to sustain an increased [Ca2+]I. The absence of Ca2+ influx through CRAC channels can severely compromise immune cell activation, proliferation, and effector functions (Feske, 2007, Gwack et al., 2007). This is underscored by the existence of one form of SCID syndrome, whose pathological roots trace to defective CRAC channel function (Gwack et al., 2007). In T-lymphocytes from the patients who are affected by this form of SCID, a missense mutation and an Arginine-to-Tryptophan amino acid (a.a.) substitution at a.a. position 91 in the first transmembrane domain of the Orai1 protein result in the ablation of all CRAC channel activity (Feske, 2007). Stromal Interaction Molecule 1 (STIM1) and Orai are integral parts of the ER-to-plasma membrane (PM) signaling system, necessary for store-operated channel entry (Hogan and Rao, 2007). STIM1 is a single-spanning membrane protein with a Ca2+-binding EF-hand motif and functions as the sensor of ER luminal Ca2+ levels, and its reorganization in the ER allows it to transduce information directly to the plasma membrane. At the plasma membrane, STIM1 may interact with Orai to allow store-operated channel entry of Ca2+. Despite experimental evidence showing that STIM1 and Orai1 are necessary and sufficient for SOCE, many questions remain about the details of the coupling mechanism between these proteins (Hewavitharana et al., 2007). A structural analysis of STIM1-Orai1 interactions by Varnai et al. implicated the presence of additional molecular components within the STIM1-Orai1 complex (Varnai et al., 2007). Therefore, the identification of other molecules, which regulate the operation of CRAC channels, will allow us to better understand how the interactions between STIM1 and Orai1 occur and where they take place within cells. Most importantly, this will allow us to have an impact on the diseases that associate with malfunctioning states of store-operated channel Ca2+ entry.
Actin cytoskeletal changes have been suggested to be essential for the operation of store-operated calcium channels (Patterson et al., 1999b, Hao and August, 2005b). Interestingly, actin cytoskeletal changes appear to be dispensable for ER calcium release (Patterson et al., 1999b, Hao and August, 2005b). Indeed, if actin polymerization is induced with an agent such as jasplakinolide, which prevents actin depolymerization, prior to administration of a calcium ionophore, store-operated channel mediated [Ca2+]i increase is completely blocked (Patterson et al., 1999b). This phenomenon may best be explained by a recent model for activation of store-operated channels that has been proposed wherein the signal between the ER and the plasma membrane that activates store-operated channels involve a secretion-like mechanism which is blocked by thick cortical actin (Patterson et al., 1999b). Along these lines, it was recently demonstrated that treatment of DT40 B cells with the actin depolymerizing agent latrunculin B (LatB) prior to stimulation through the B cell receptor, increased the sensitivity to B cell receptor signals. This was shown to be mediated at least in part by an increase in the intensity and duration of calcium signals (Hao and August, 2005b).
Recently, a class of compounds called BTPs (3,5-bis(trifluoromethyl)pyrazoles) was found to inhibit activation of the calcium regulated transcription factor NFAT (Trevillyan et al., 2001). Other drugs that target NFAT are currently being used clinically to prevent organ-transplant rejection by suppressing the immune system. These drugs, FK506 and cyclosporin A, work by inhibiting the phosphatase activity of the serine/threonine phosphatase, calcineurin. Calcineurin is responsible for activating NFAT by removing inhibitory phosphate groups from serine residues within the NFAT regulatory domain, thus exposing a nuclear localization sequence. Interestingly, BTPs do not inhibit the phosphatase activity of calcineurin in vitro (Djuric et al., 2000b). Thus BTPs represent a unique class of immuno-suppressant compounds. It has since been determined that BTPs prevent NFAT activation by blocking store-operated calcium entry, via an unknown mechanism (Ishikawa et al., 2003a, Zitt et al., 2004b). We have confirmed this finding and, utilizing an affinity purification approach, have identified the actin reorganizing protein drebrin as a likely target of BTP. Drebrin is a member of the ADF–H/cofilin family of actin binding proteins and has been implicated in actin rearrangements driving dendritic spine outgrowth in neurons (Hayashi et al., 1996, Hayashi and Shirao, 1999a, Ishikawa et al., 1994, Sasaki et al., 1996, Shirao et al., 1994a, Takahashi et al., 2003a, Toda et al., 1999a). We show that BTP is able to block drebrin dependent actin rearrangement. We also demonstrate that drebrin expression is essential for activation of store-operated calcium entry in Jurkat T cells, as reduction in drebrin protein expression by siRNA treatment results in a block in store-operated channel mediated [Ca2+]i increase but not ER Ca2+ release, similar to that seen with BTP treatment. Together, these data indicate that BTP blocks store-operated channel activation by binding to the actin-binding protein drebrin, which plays an essential role in store-operated channel activation.
2. Experimental Procedures
2.1. Cells, antibodies, plasmids, and reagents
Jurkat E6-1 T-cells were grown in complete RPMI supplemented with 5% FCS. HEK293T and CHO cells were grown in complete DMEM supplemented with 5% FCS. Anti-drebrin antibody was from Sigma (St. Louis, MO), anti-GFP and anti-actin antibodies from Santa Cruz Biotech (Santa Cruz, CA). Alexa-fluor 568 conjugated phalloidin was from Molecular Probes (Eugene, OR). Drebrin mutants R236M237M (R mutant), K270MK271M (K mutant) and Q297LQ298L (Q mutant) were generated by standard molecular biology techniques. BTP (N-[4-[3,5-bis(trifluoromethyl)-1H-pyrazole-1-yl] phenyl]-4-methyl-1,2,3-thiadiazol) was synthesized as previously described as well as provided as a kind gift of Drs. James Trevillyan and Stevan Djuric, Abbott Laboratories, Chicago, IL (Djuric et al., 2000a). Synthesis of other BTP compounds is described in supplemental Fig. 1.
2.2. Fluorescent calcium measurement
Changes in [Ca2+]i were measured by loading Jurkat cells with 1 µM Fura-2AM (Sigma, St. Louis, MO) as described in (Hao et al., 2003) except that cells were loaded and assayed in Ringer’s solution (155 mM NaCl, 4.5 mM KCl, 2 mM MgCl2, 10 mM dextrose, 5 mM HEPES, pH 7.4). Cells (1×106/ml in 1 ml) were loaded in the presence of 1 mM CaCl2 and washed with Ringer’s solution without CaCl2 prior to assay. For BTP treatment, cells were treated for 1 hr with 1 µM BTP and loaded with Fura-2AM for the final 30 min. of treatment.
2.3. Transfections and analysis of transfected cells
CHO and 293T cells were grown on glass coverslips, then transfected, followed by analysis 24 to 48 hours later. Cells were imaged live, or fixed for 15 minutes in PBS containing 4% para-formaldehyde and permeabilized with PBS containing 1% triton-x 100 for 2 min. Cells were then blocked in PBS containing 5% BSA. Cells were stained with Alexa-568 phalloidin (Molecular Probes, Eugene, OR) to visualize F-actin. Cells were then analyzed on an Olympus Fluoview 300 confocal laser scanning microscope (Olympus Microscope, Melville, NY). Images were analyzed using ImagePro.
2.4. siRNA and shRNA knockdown
Drebrin expression was knocked down by transfecting 2.0 ×107 Jurkat cells with 200 nM drebrin specific siRNAs (catalog # 011841, accession #s: NM_004395 and NM_080881) or 200 nM siControl #1 non-targeting control siRNAs (Smartpool, Dharmacon, LaFayette, CO). siRNAs were transfected by electroporation using a BTX electrosquare porator 800 (Genetronics, San Diego, CA) at 300V for 20 msec in 400 µl RPMI in a 4 mm electroporation cuvette. Cells were then cultured in RPMI-C + 10% FCS for 48–96 hours prior to assay. Cells were then screened for expression of drebrin by western blot.
2.5. In-gel digest and mass spectrometry
For protein identification, bands were excised from the gel and in-gel tryptic digest was performed following kit instructions (In-gel digestion kit, Pierce Biotech Inc., Rockford, IL) prior to submission to The Proteomics and Mass Spectrometry Core Facility at Penn State University (University Park, PA) for mass spec analysis. Alternatively, excised bands were sent to W.M. Keck Foundation Biotechnology Resource Laboratory at Yale University (New Haven, CT) for in-gel digestion and mass spec analysis. Peptide masses were used to search either the ProFound or Mascot databases as indicated for matching proteins (Zhang and Chait, 2000, Perkins et al., 1999).
3. Results
3.1. Drebrin is a BTP-binding protein
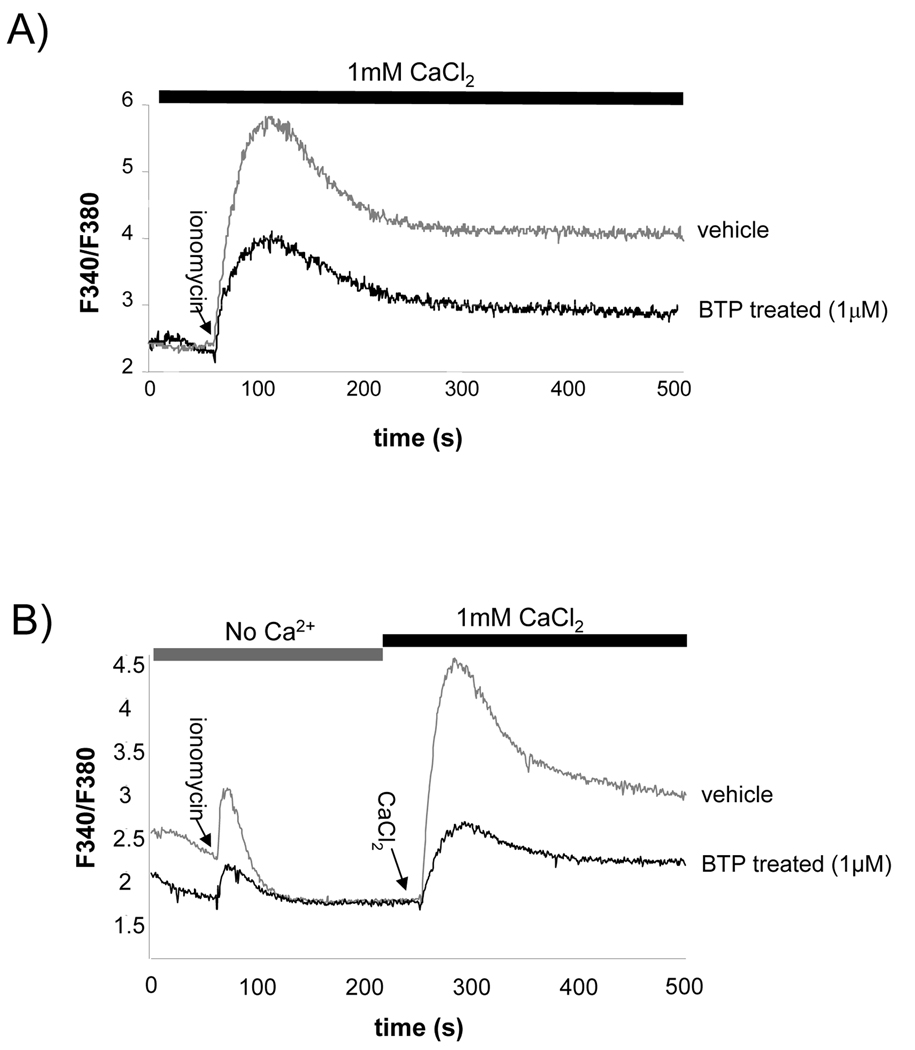
As previously reported by others, when we treated Jurkat T cells with BTP2 prior to stimulation with ionomycin, which triggers an increase in [Ca2+]i, we observed inhibition of this response (Fig. 1a) (Zitt et al., 2004a, Ishikawa et al., 2003b, He et al., 2005). Acute addition of BTP to cells once calcium increase has been induced results in an immediate reduction in calcium influx (Supplementary Fig. 1). Using a calcium add back assay where cells were initially stimulated in the absence of extracellular Ca2+ and then Ca2+ restored to the buffer following the return of [Ca2+]i to baseline, we find that BTP pretreatment results in a significant decrease in Ca2+ entry following addition of extracellular Ca2+. By contrast the initial store release was not consistently affected, indicating that the blunted calcium response caused by BTP is due to inhibition of Ca2+ entry and not Ca2+ store release (Fig. 1b). The ability of BTP to inhibit calcium entry was also observed when cells were treated with the SERCA pump inhibitor thapsigargin (Supplementary Fig. 1).
Figure 1. BTP blocks intracellular calcium mobilization.
Jurkat T cells were treated with DMSO or BTP and loaded with Fura-2AM in order to measure intracellular calcium concentration. A) Cells were stimulated with ionomycin in Ringer’s solution in the presence of 1 mM extracellular calcium. One of at least 5 experiments. B) Cells were stimulated with ionomycin in Ringer’s solution without extracellular calcium. 1 mM CaCl2 was added as indicated and calcium influx monitored. One of 3 experiments.
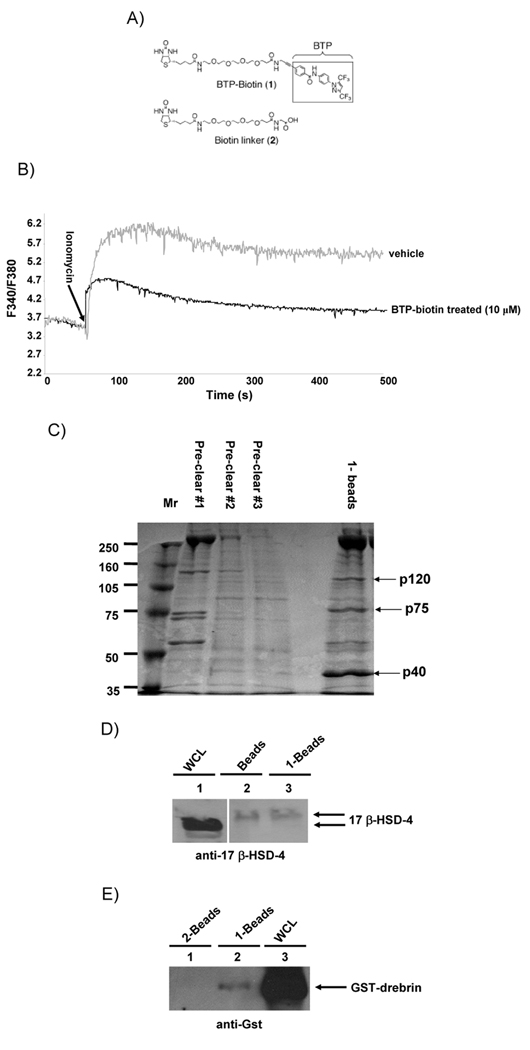
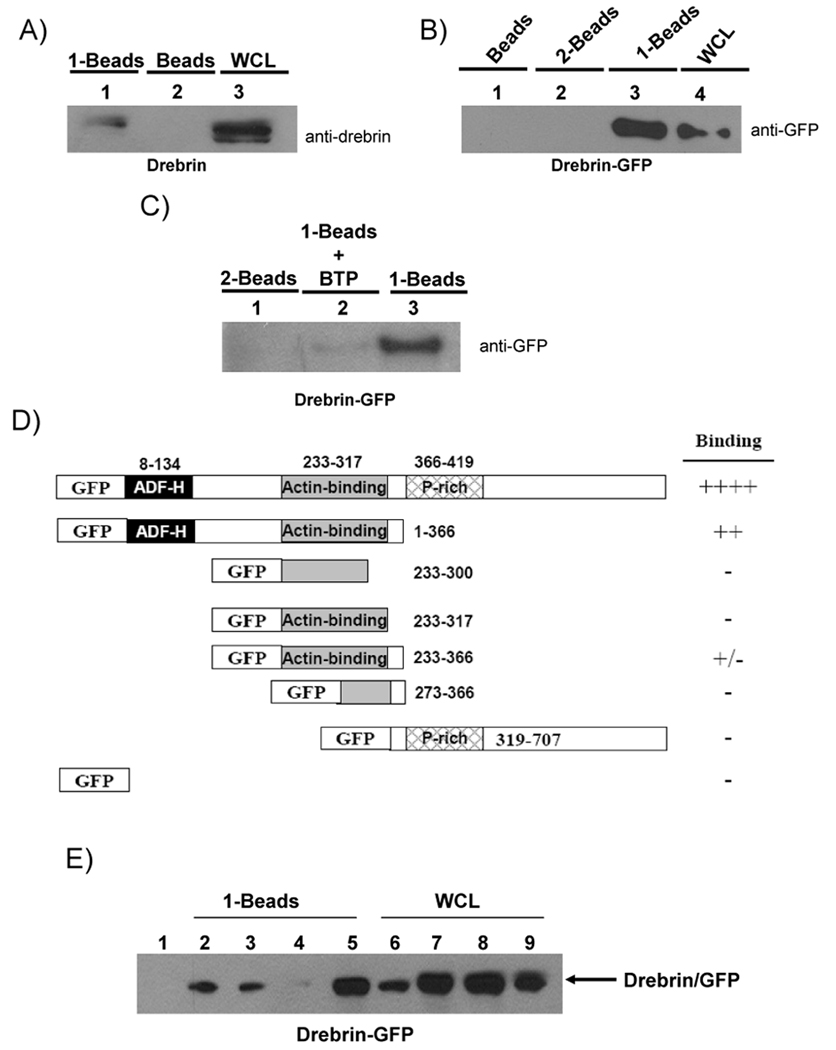
In order to better understand the mechanism by which BTP inhibits store-operated channels, we set up an affinity purification system to purify and identify BTP binding proteins. A derivative of BTP1 was synthesized coupled to biotin as described in supplementary methods and purified to greater than 99% purity (Fig. 2a, see supplementary methods). This biotinylated BTP compound was then tested for its ability to prevent NFAT activation in primary thymocytes carrying a transgenic NFAT luciferase reporter. Addition of the large linker and biotin groups reduced the potency of the biotinylated BTP compared with BTP, however, it still had significant activity in these cells (IC50 ~600 nM compared to ~15 nM for parent compound, data not shown)(Zitt et al., 2004a)). Additionally, at 10 µM, biotinylated BTP was able to inhibit Ca2+ mobilization in response to ionomycin treatment (Fig. 2b). Biotinylated BTP was immobilized onto streptavidin-coated agarose beads and used to purify BTP binding proteins from Jurkat T cell lysates following preclearing with beads coated with the biotinylated linker (Fig. 2a). Purified proteins were then separated by SDS-PAGE and visualized with Coumassie stain (Fig. 2c). Unique bands from biotinylated BTP coated beads were excised from the gel and subjected to in-gel tryptic digestion and MALDI/TOF mass spectrometry based protein identification. This procedure was performed twice and the samples were sent to two different facilities for mass spectrometric analysis. Both facilities identified p120 as the actin binding protein drebrin (Z score 2.32, 99.0 percentile, probability of a match = 1.0e+000 using ProFound in experiment 1, and p<0.05 using Matrixscience in experiment 2, supplementary Tables 1 & 2). Drebrin has been well studied in neuronal cells and appears to be important for actin rearrangements such as those driving neuronal dendritic spine outgrowth (Takahashi et al., 2003b, Toda et al., 1999b, Hayashi and Shirao, 1999b). Binding of drebrin to BTP was confirmed by performing pull-down assays using the biotinylated BTP coupled to streptavidin-coated agarose beads, followed by western blot to detect endogenous drebrin (Fig. 3a). The interaction was further confirmed by over-expressing a GFP-drebrin fusion protein in HEK293T cells and performing the pull-down experiment with lysates from these cells prior to western blotting to detect the GFP tag (Fig. 3b)(Hayashi et al., 1999). The specificity of the drebrin/BTP interaction was confirmed by performing the pull-down assay with streptavidin-agarose beads coated with the biotin-linker compound without the BTP moiety (2) (Fig. 3b & c). In addition, soluble BTP competed with the biotinylated BTP for binding to drebrin, and we estimate the Kd to be in the low nM range (<300 nM based on preliminary binding experiments) (Fig. 3c). We confirmed that drebrin directly interacts with BTP using bacterially expressed gst-drebrin (Fig. 2e). We also sequenced and identified p75 as 17 β-hydroxysteroid dehydrogenase IV, and p40 as actin. We ruled out 17 β-hydroxysteroid dehydrogenase IV as a bona fide BTP binding protein as it interacted non-specifically with the SA-beads used for the pull-downs (Fig. 2d). The identification of actin along with drebrin is not unexpected as drebrin has been demonstrated to interact with and co-immunoprecipitate with actin (Fucini et al., 2000, Fucini et al., 2002, Hayashi et al., 1999).
Figure 2. Identification of BTP binding proteins using a modified BTP affinity probe.
A) Structure of BTP-biotin (1) and biotin-linker (2). B) Jurkat T cells were pretreated with 10 µM biotinylated BTP or DMSO and loaded with Fura-2AM prior to stimulation with ionomycin in the presence of extracellular calcium. One of 3 experiments. C) Jurkat T-cell lysate was incubated with the SA-agarose beads alone 3 times (preclear # 1, 2 and 3), followed by SA-agarose beads coated with (1). Beads were washed extensively, then boiled in SDS-PAGE reducing buffer prior to SDS-PAGE and staining using coumassie blue stain to identify binding proteins. Arrows indicate p120 (identified as drebrin), p75 (identified as 17-β-HSD4) and p40 (identified as actin). D) Jurkat T-cell lysate was incubated with SA-agarose beads alone or SA-beads coated with (1), washed extensively, then boiled in SDS-PAGE reducing buffer prior to SDS-PAGE and western blotting with anti-17-β-HSD4. Lane 1: WCL of Jurkat T cells; lane 2: pull down with SA-beads alone; lane 3: pull down with SA-beads coated with (1). Blot probed with anti-17-β-HSD4. E) Lysates from E. coli expressing Gst-Drebrin were incubated with SA-agarose beads coated with (2) or SA-beads coated with (1), washed extensively, then boiled in SDS-PAGE reducing buffer prior to SDS-PAGE and western blotting with Gst. Lane 1: pulldown with SA-beads coated with (2); lane 2: pulldown with SA-beads coated with (1); lane 3: WCL of Jurkat T cells. Blot probed with anti-Gst.
Figure 3. BTP binds to drebrin.
A) Jurkat T-cell lysate was incubated with the indicated coating of SA-agarose beads. Beads were boiled in SDS-PAGE reducing buffer prior to SDS-PAGE and western blotting. Lane 1, biotinylated BTP; lane 2, DMSO; lane 3, 5% of the input. Blot was probed using anti-drebrin antibody. One of 3 similar experiments. B) CHO cells were transfected with GFP-drebrin plasmid. Cells were then lysed and lysates incubated with the indicated coating of SA-agarose beads as in (A). Lane 1, DMSO; lane 2, Biotinylated linker; lane 3, biotinylated BTP; lane 4, 5% of the input. Blot was probed using anti-GFP antibody. One of 3 similar experiments. C) Lysates from CHO cells transfected with GFP-drebrin incubated as follows: Lane 1, 2-SA-beads; Lane 2, biotinylated BTP-SA-beads along with 10 µM BTP; or lane 3, biotinylated BTP-SA-beads alone. Blot was probed using anti-GFP antibody. One of 3 similar experiments. D) HEK293T cells were transfected with plasmids encoding the indicated fragments of drebrin fused to GFP. Cells were lysed and lysates were incubated with SA-beads alone, biotinylated linker-SA-beads, or biotinylated BTP-SA-beads, washed, separated by SDS-PAGE and probed with anti-GFP. None of the fragments associated with either the SA-beads or biotinylated linker-SA-beads alone. Relative binding of each drebrin fragment to BTP-biotin compared to full-length drebrin with (++++) representing binding the full-length drebrin and (−) representing no binding observed. One of 2 similar experiments. E) Lysates from CHO cells transfected with WT GFP-drebrin incubated with SA-beads alone (lane 1) or WT or the indicated mutants of drebrin incubated with 1-SA-beads as follows: lane 2, WT; lane 3, R236,237 mutant; lane 4, K270,271 mutant; lane 5, Q297,298 mutant. Lanes 6–9 were loaded with lysates from cells expressing WT, R, K and Q mutants respectively. Blot was probed using anti-GFP antibody. One of 2 similar experiments.
3.2. K270 and K271 within drebrin are important for BTP binding
Analysis of fragments of drebrin for binding to the biotinylated BTP coupled to streptavidin-coated agarose beads indicated that the full-length protein had the highest binding capacity, and the N-terminal 1–366 amino acids retained strong binding (Fig. 3d). This region includes the ADF–H and actin-binding domains, but lacks the proline rich region of drebrin (Hayashi and Shirao, 1999b). Further analysis indicated that amino acids 233–366 of drebrin maintained minimal binding, however this binding was much below that observed in the full length or N-terminal 366 amino acids (Fig. 3d). This latter region includes the full actin binding domain and a small region C-terminal to this region (compare to fragment containing amino acids 233–317, which contains just the actin binding domain). Thus the interaction between BTP and drebrin includes the actin-binding domain, but requires residues that flank this region at the N- and C-termini of this domain for optimal binding. To identify residues within this region of drebrin that interacts with BTP, we focused on highly charged residues within the central actin binding domain, and generated several mutants, one of which K270MK271M, destroyed binding to drebrin, while other closely located mutants (R236MR237M, Q297LQ298L) retained binding (Fig. 3e, data not shown).
3.3. BTP blocks drebrin function
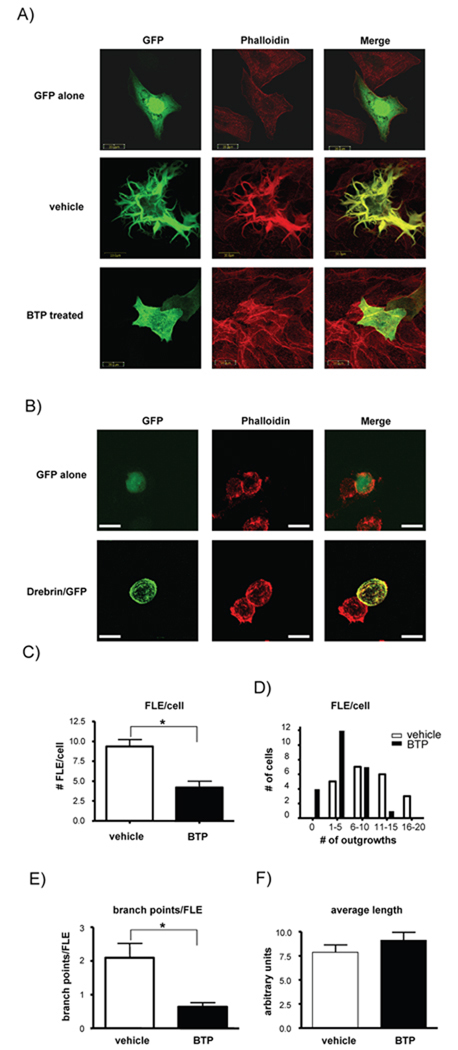
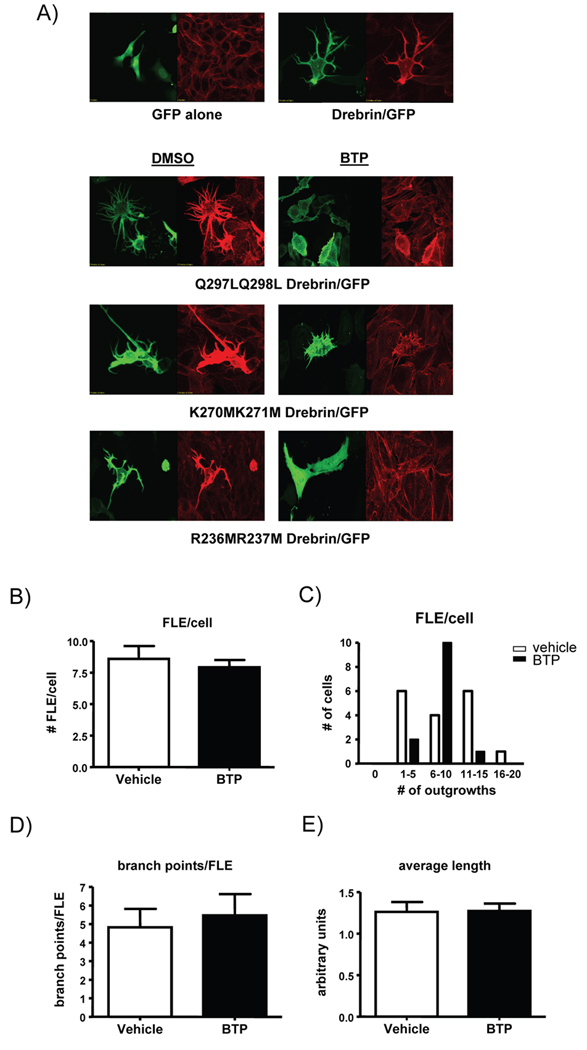
When over-expressed in fibroblasts drebrin causes the formation of long, branched extension and curved, thick actin bundles (Shirao et al., 1994b). In order to assess drebrin as a target of BTP, GFP-tagged drebrin was over-expressed in CHO cells, which caused cells to develop long filopodia-like membrane extensions (FLE) that were highly branched, with drebrin co-localized with actin (Fig. 4). When drebrin over-expressing cells were treated with BTP (or biotinylated BTP, data not shown), there was a drastic reduction in filopodia-like extensions (FLE) and in the number of branch points formed per each FLE but not in the average length of these processes (Fig 4a, c–f). Thus, BTP treated cells had fewer FLE, and the FLE that formed were long and linear as opposed to branched, indicating that BTP affects drebrin’s ability to induce plasticity in the actin cytoskeleton. However, drebrin co-localization with actin was not apparently affected, suggesting that although the actin-binding site within drebrin forms part of the BTP binding domain, this may not affect actin co-localization with drebrin (Fig. 4a, Fig 2c). Overexpression of drebrin in Jurkat T cells did not lead to the development of FLEs, suggesting that drebrin only affected actin superstructure in adherent cells such as CHO cells (Fig. 4b). Of interest, the drebrin mutants R236MR237M, Q297LQ298L, as well as the K270MK271M mutant that lost binding to BTP all retained actin localization and reorganization activity, suggesting that these residues are not critical for this function of drebrin (Fig. 5a). Analysis of the K270MK271M mutant of drebrin indicated that BTP no longer inhibited FLEs in cells expressing this mutant (Fig. 5b–e).
Figure 4. BTP inhibits drebrin function.
A) CHO cells transfected with either GFP (top panel) or GFP-drebrin (middle and bottom panels). Cells were treated with DMSO (vehicle, middle panel) or BTP (bottom panel) prior to staining for F-actin (red) and visualization using confocal laser scanning microscopy. One of 3 similar experiments. B) Jurkat T cells were transfected with either GFP (top panel) or GFP-drebrin (bottom panel). Cells were stained for F-actin (red) and visualized as in (A). One of at least 5 similar experiments. White bar indicate 20 µm. C) Branched cell extensions caused by drebrin over-expression (filopodia-like extensions, FLE) were counted on each cell in DMSO and BTP treated cells and expressed as average FLE per cell, D) the number of cells having a given range of FLEs per cell, E) the average number of branch points per FLE, and F) the average length of each FLE. For all measurements n=25 cells, *p<0.05. One of 3 similar experiments.
Figure 5. BTP binding mutant of drebrin retains actin reorganization function.
A) CHO cells transfected with the indicated mutants of drebrin fused to GFP. Left panels show GFP and right panels show F-actin staining. B–E) CHO cells transfected with K270MK271M mutant of drebrin fused to GFP. One of 3 similar experiments. B) Branched cell extensions caused by drebrin over-expression (filopodia-like extensions, FLE) were counted on each cell in DMSO and BTP treated cells and expressed as average FLE per cell, C) the number of cells having a given range of FLEs per cell, D) the average number of branch points per FLE, and E) the average length of each FLE. For all measurements n=17 for vehicle, n=13 for BTP treated cells. Images were analyzed as in figure 3. One of 2 similar experiments.
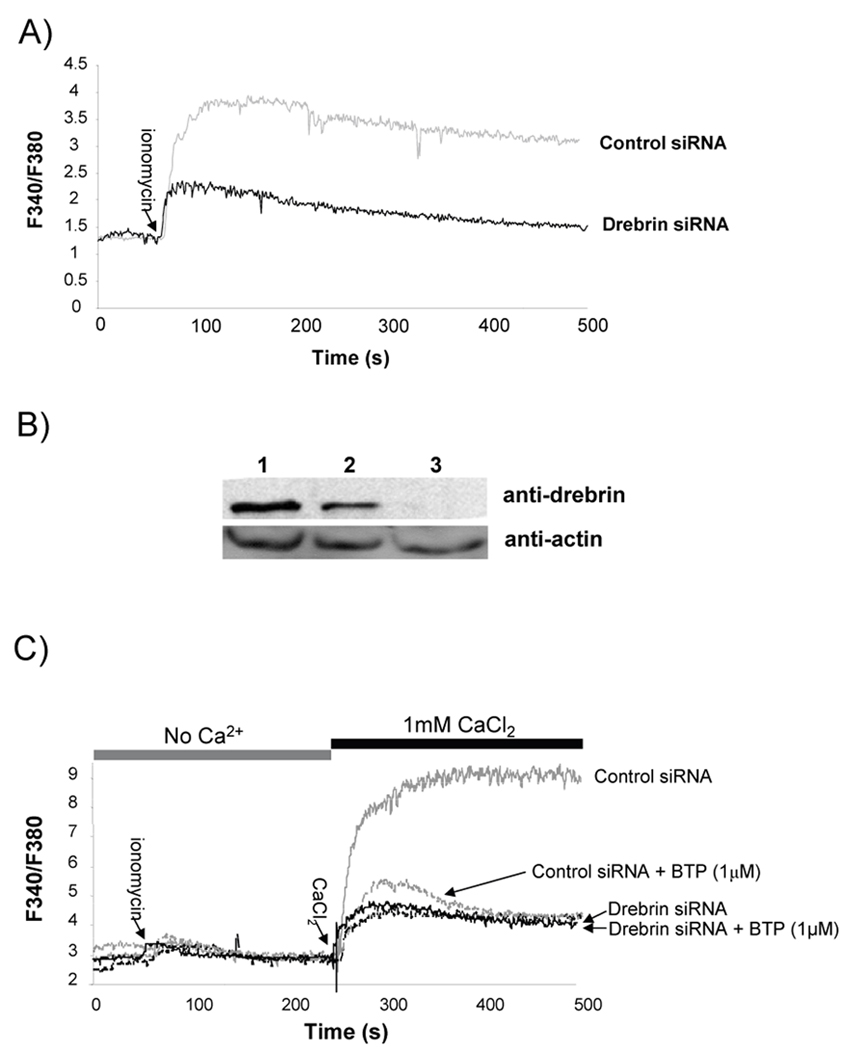
3.4. Drebrin expression is required for calcium influx into cells
Since actin rearrangement has previously been linked to store-operated channel regulation we were interested in determining if this actin reorganizing protein was important for store-operated channel operation (Patterson et al., 1999a, Hao and August, 2005a). When we used drebrin specific siRNA to reduce drebrin protein expression in Jurkat T cells, we observed a reduction in [Ca2+]i increase following ionomycin treatment, which was not observed in cells transfected with control siRNA (Fig. 6a). This defect was a result of reduced drebrin expression and not the transfection procedure itself as the drebrin specific siRNA significantly reduced the amount of drebrin protein whereas control siRNA had much less of an effect on the protein levels of drebrin (Fig. 6b). Drebrin knockdown appears to inhibit only store-operated channel mediated Ca2+ entry, as when these cells are stimulated in Ca2+ free buffer we continued to see an initial spike in Ca2+ corresponding to intracellular stores (Fig. 6c). However, when we added extracellular calcium we observed only a slight increase in [Ca2+]i in cells transfected with the drebrin siRNA compared with control siRNA transfected cells (Fig. 6c). We treated the drebrin-specific RNA transfected cells with BTP prior to performing the Ca2+ add-back assay and found no further reduction in Ca2+ entry indicating that BTP does not act on a separate pathway and further suggesting that drebrin is the target of BTP in regulating Ca2+ entry (Fig. 6c). By contrast, overexpression of drebrin did not affect calcium influx, suggesting that drebrin expression is not limiting (supplementary Fig. 2a, b).
Figure 6. Loss of drebrin expression prevents extracellular calcium influx.
A) Jurkat T cells were transfected with either control siRNAs or drebrin-specific siRNAs and grown for 48 hours following transfection. Cells were then loaded with Fura-2AM and intracellular calcium concentration monitored in the presence of 1 mM extracellular Ca2+ before and after ionomycin treatment. One of 3 similar experiments. B) Representative drebrin knockdown 48 hours post-transfection in Jurkat T cells. Lane 1, Jurkat lysate no siRNA; Lane 2, Jurkat lysates from cells treated with control siRNA; or lane 3, Jurkat lysates from cells treated with drebrin-specific siRNA. One of 3 similar experiments. C) Jurkat T cells were transfected with either control or drebrin-specific siRNA. 48 hours post-transfection, cells were treated with DMSO or 1 µM BTP and then loaded with Fura-2AM. Intracellular calcium concentration was monitored following initial stimulation with ionomycin in the absence of extracellular calcium. After [Ca2+]i returned to baseline levels, 1 mM CaCl2 was added and calcium influx determined by monitoring the change in Fura-2 fluorescence. One of 3 similar experiments.
4. Discussion
The small-molecule immuno-suppressant BTP has recently been shown to block activation of store-operated calcium channels (He et al., 2005). In this study, we have identified the actin binding protein drebrin as a BTP binding protein, and shown that reduction in drebrin expression results in similar effects to that seen with BTP treatment. Using inhibitors of actin polymerization/depolymerization, others have established a link between cytoskeletal rearrangement and store-operated channel regulation or calcium influx into cells (Patterson et al., 1999a, Hao and August, 2005a). Specifically, following intracellular store depletion, actin filaments must be depolymerized and then repolymerized in order to activate store-operated channels. In DT40 B cells, treatment with low concentrations of actin depolymerizing agents such as latrunculin B caused sustained activation of Ca2+ influx (Hao and August, 2005a). These concentrations used are thought to be low enough so as to favor actin depolymerization, but not fully prevent repolymerization, thus the net effect is to increase actin filament turn-over (Hao and August, 2005a). Thus increasing the plasticity of the actin cytoskeleton seems to favor store-operated channel activation. Although a clear role for actin rearrangement has been established, few actin-regulating proteins have been implicated in this process. Recently, the actin modulating protein WAVE2 was shown to modulate store-operated channel in T cells, further supporting the idea that modulating the actin cytoskeleton modulate store-operated channel and calcium influx (Nolz et al., 2006). Similar to our work with drebrin, reduction in the expression of WAVE2 results in selective block in calcium influx into cells. Therefore, our data indicating that drebrin is involved in store-operated channel regulation should provide further insight into the relationship between the cytoskeleton and store-operated channel regulation.
The identity of proteins that make up the store operated channels have recently been revealed with the cloning and characterization of Orai, along with STIM1, which seems to act as a modulator of Orai’s function (Mercer et al., 2006, Soboloff et al., 2006, Peinelt et al., 2006, Zhang et al., 2005, Liou et al., 2005, Roos et al., 2005, Yeromin et al., 2006, Prakriya et al., 2006 ). STIM1 seems to function by translocating near to or into the plasma membrane, and seems to provide a link between the ER, where calcium is released, to the plasma membrane, where Orai acts as a channel to allow calcium into the cell (Hauser and Tsien, 2007, Ross et al., 2007, Wu et al., 2006, Spassova et al., 2006, Zhang et al., 2005). Of interest is the observation that STIM1 can interact with TRPC proteins, as well as Orai, and TRPC proteins may modulate calcium channel activity (Liao et al., 2007, Ong et al., 2007, Huang et al., 2006, Lopez et al., 2006).
Drebrin may regulate calcium entry by facilitating actin rearrangement in association with store-operated channels following store depletion. This may be mediated by formation of a complex at the IP3R where drebrin could interact with homer adaptor proteins, which have been shown to bind both IP3R and drebrin, as well as the TRPC family of ion-channels (Yuan et al., 2003, Shiraishi et al., 1999). Drebrin has also recently been shown to exist in a complex and co-immunoprecipitate with TRPC5 and TRPC6 (Goel et al., 2005). In addition, it was recently reported that BTP2 modulates the function of TRPM4, which has been shown to modulate store-operated channel (Takezawa et al., 2006). By recruiting drebrin to such complexes, the associated actin structure could be induced to rearrange, thus regulating store-operated channel function. Further research dissecting multi-protein complexes that drebrin participates in may lead to a better understanding of the mechanisms regulating store-operated channel function.
Supplementary Material
ACKNOWLEDGEMENTS
We thank members of the August and Peterson labs, and the Center for Molecular Immunology & Infectious Disease at Penn State for feedback and discussion. We also thank Elaine Kunze and Susan Magargee in the Center for Quantitative Cell Analysis at Penn State, as well as Dr. Carol Gay for help and access to instruments, and Drs. Jerzy Adamski and Gabriele Moeller (Institute for Experimental Genetics, Neuherberg, Germany) for the 17 β-hydroxysteroid dehydrogenase IV antibodies and expression plasmids. We also thank those investigators mentioned in the materials and methods section for sharing reagents. This work was supported in part by grants from the National Institutes of Health (AI51626), the American Heart Association (# 0330036N), and from the College of Agricultural Sciences at Penn State to AA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: IP3, Inositol 1,4,5 trisphosphate; DAG, Diacyl glycerol; ER, Endoplasmic reticulum; FLE, Filopodia-like-extensions; SA. Streptavidin; SOC, Store-operated channel; [Ca2+]i, Intracellular Ca2+ concentration; BTP, 3,5-bistrifluoromethyl pyrazole; NFAT, Nuclear Factor of Activated T cells.
References
- Djuric S, BaMaung N, Basha A, Liu H, Luly J, Madar D, Sciotti R, Tu N, Wagenaar F, Wiedeman P, Zhou X, Ballaron S, Bauch J, Chen Y, Chiou X, Fey T, Gauvin D, Gubbins E, Hsieh G, Marsh K, Mollison K, Pong M, Shaughnessy T, Sheets M, Smith M, Trevillyan J, Warrior U, Wegner C, Carter G. 3,5-Bis(trifluoromethyl)pyrazoles: a novel class of NFAT transcription factor regulator. J Med Chem. 2000a;43:2975–2981. doi: 10.1021/jm990615a. [DOI] [PubMed] [Google Scholar]
- Djuric SW, BaMaung NY, Basha A, Liu H, Luly JR, Madar DJ, Sciotti RJ, Tu NP, Wagenaar FL, Wiedeman PE, Zhou X, Ballaron S, Bauch J, Chen YW, Chiou XG, Fey T, Gauvin D, Gubbins E, Hsieh GC, Marsh KC, Mollison KW, Pong M, Shaughnessy TK, Sheets MP, Smith M, Trevillyan JM, Warrior U, Wegner CD, Carter GW. 3,5-Bis(trifluoromethyl)pyrazoles: a novel class of NFAT transcription factor regulator. J Med Chem. 2000b;43:2975–2981. doi: 10.1021/jm990615a. [DOI] [PubMed] [Google Scholar]
- Feske S. Calcium signalling in lymphocyte activation and disease. Nat Rev Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- Fucini R, Chen J, Sharma C, Kessels M, Stamnes M. Golgi vesicle proteins are linked to the assembly of an actin complex defined by mAbp1. Mol Biol Cell. 2002;13:621–631. doi: 10.1091/mbc.01-11-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fucini R, Navarrete A, Vadakkan C, Lacomis L, Erdjument-Bromage H, Tempst P, Stamnes M. Activated ADP-ribosylation factor assembles distinct pools of actin on golgi membranes. J Biol Chem. 2000;275:18824–18829. doi: 10.1074/jbc.M000024200. [DOI] [PubMed] [Google Scholar]
- Goel M, Sinkins W, Keightley A, Kinter M, Schilling W. Proteomic analysis of TRPC5- and TRPC6-binding partners reveals interaction with the plasmalemmal Na(+)/K(+)-ATPase. Pflugers Arch. 2005;451:87–98. doi: 10.1007/s00424-005-1454-y. [DOI] [PubMed] [Google Scholar]
- Gwack Y, Feske S, Srikanth S, Hogan PG, Rao A. Signalling to transcription: store-operated Ca2+ entry and NFAT activation in lymphocytes. Cell Calcium. 2007;42:145–156. doi: 10.1016/j.ceca.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Hao S, August A. Actin depolymerization transduces the strength of B-cell receptor stimulation. Mol Biol Cell. 2005a;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, August A. Actin Depolymerization Transduces the Strength of B Cell Receptor Stimulation. Mol. Biol. Cell. 2005b;16:2275–2284. doi: 10.1091/mbc.E04-10-0881. (E2204-2210-0881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S, Kurosaki T, August A. Differential regulation of NFAT and SRF by the B cell Receptor via a PLCγ/Ca2+ dependent pathway. EMBO J. 2003;22:4166–4177. doi: 10.1093/emboj/cdg401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser C, Tsien R. A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc Natl Acad Sci U S A. 2007;104:3693–3697. doi: 10.1073/pnas.0611713104. Epub 2007 Feb 3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Ishikawa R, Kawai-Hirai R, Takagi T, Taketomi A, Shirao T. Domain analysis of the actin-binding and actin-remodeling activities of drebrin. Exp Cell Res. 1999;253:673–680. doi: 10.1006/excr.1999.4663. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ishikawa R, Ye LH, He XL, Takata K, Kohama K, Shirao T. Modulatory role of drebrin on the cytoskeleton within dendritic spines in the rat cerebral cortex. J Neurosci. 1996;16:7161–7170. doi: 10.1523/JNEUROSCI.16-22-07161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Shirao T. Change in the Shape of Dendritic Spines Caused by Overexpression of Drebrin in Cultured Cortical Neurons. J. Neurosci. 1999a;19:3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Shirao T. Change in the shape of dendritic spines caused by overexpression of drebrin in cultured cortical neurons. J Neurosci. 1999b;19:3918–3925. doi: 10.1523/JNEUROSCI.19-10-03918.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Hewavitharana T, Soboloff J, Spassova M, Gill D. A functional link between store-operated and TRPC channels revealed by the 3,5-bistrifluoromethyl-pyrazole derivative, BTP2. J Biol Chem. 2005 Jan 12; doi: 10.1074/jbc.M411797200. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-t. [DOI] [PubMed] [Google Scholar]
- Hewavitharana T, Deng X, Soboloff J, Gill DL. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32:235–245. doi: 10.1016/j.tibs.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Huang G, Zeng W, Kim J, Yuan J, Han L, Muallem S, Worley P. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. Epub 2006 Aug 1013. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Ohga K, Yoshino T, Takezawa R, Ichikawa A, Kubota H, Yamada T. A Pyrazole Derivative, YM-58483, Potently Inhibits Store-Operated Sustained Ca2+ Influx and IL-2 Production in T Lymphocytes. J Immunol. 2003a;170:4441–4449. doi: 10.4049/jimmunol.170.9.4441. [DOI] [PubMed] [Google Scholar]
- Ishikawa J, Ohga K, Yoshino T, Takezawa R, Ichikawa A, Kubota H, Yamada T. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J Immunol. 2003b;170:4441–4449. doi: 10.4049/jimmunol.170.9.4441. [DOI] [PubMed] [Google Scholar]
- Ishikawa R, Hayashi K, Shirao TC, Xue Y, Takagi T, Sasaki Y, Kohama K. Drebrin, a development-associated brain protein from rat embryo, causes the dissociation of tropomyosin from actin filaments. J Biol Chem. 1994;269:29928–29933. [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong D, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci U S A. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. Epub 2007 Mar 4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou J, Kim M, Heo W, Jones J, Myers J, Ferrell JJ, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Salido G, Pariente J, Rosado J. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. Epub 22006 Jul 28226. [DOI] [PubMed] [Google Scholar]
- Mercer J, Dehaven W, Smyth V, Wedel B, Boyles R, Bird G, Putney JJ. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. Epub 22006 Jun 24928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolz J, Gomez T, Zhu P, Li S, Medeiros R, Shimizu Y, Burkhardt J, Freedman B, Billadeau D. The WAVE2 complex regulates actin cytoskeletal reorganization and CRAC-mediated calcium entry during T cell activation. Curr Biol. 2006;16:24–34. doi: 10.1016/j.cub.2005.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong H, Cheng K, Liu X, Bandyopadhyay B, Paria B, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh B, Gill D, Ambudkar I. Dynamic assembly of TRPC1-STIM1-Orai1 ternary complex is involved in store-operated calcium influx. Evidence for similarities in store-operated and calcium release-activated calcium channel components. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. Epub 2007 Jan 9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R, van Rossum D, Gill D. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999a;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Patterson RL, van Rossum DB, Gill DL. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999b;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa D, Beck A, Nadler M, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet J. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. Epub 2006 May 2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins D, Pappin D, Creasy D, Cottrell J. Probability-based protein identification by searching sequence databases using mass spectrometry data. Electrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan P. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. Epub 2006 Aug 2020. [DOI] [PubMed] [Google Scholar]
- Roos J, DiGregorio P, Yeromin A, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak J, Wagner S, Cahalan M, Velicelebi G, Stauderman K. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. Epub 2005 May 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K, Whitaker M, Reynolds N. Agonist-induced calcium entry correlates with STIM1 translocation. J Cell Physiol. 2007;211:569–576. doi: 10.1002/jcp.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Hayashi K, Shirao T, Ishikawa R, Kohama K. Inhibition by Drebrin of the Actin-Bundling Activity of Brain Fascin, a Protein Localized in Filopodia of Growth Cones. J Neurochem. 1996;66:980–988. doi: 10.1046/j.1471-4159.1996.66030980.x. [DOI] [PubMed] [Google Scholar]
- Shiraishi Y, Mizutani A, Bito H, Fujisawa K, Narumiya S, Mikoshiba K, Furuichi T. Cupidin, an Isoform of Homer/Vesl, Interacts with the Actin Cytoskeleton and Activated Rho Family Small GTPases and Is Expressed in Developing Mouse Cerebellar Granule Cells. J. Neurosci. 1999;19:8389–8400. doi: 10.1523/JNEUROSCI.19-19-08389.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirao T, Hayashi K, Ishikawa R, Isa K, Asada H, Ikeda K, Uyemura K. Formation of Thick, Curving Bundles of Actin by Drebrin A Expressed in Fibroblasts. Experimental Cell Research. 1994a;215:145–153. doi: 10.1006/excr.1994.1326. [DOI] [PubMed] [Google Scholar]
- Shirao T, Hayashi K, Ishikawa R, Isa K, Asada H, Ikeda K, Uyemura K. Formation of thick, curving bundles of actin by drebrin A expressed in fibroblasts. Exp Cell Res. 1994b;215:145–153. doi: 10.1006/excr.1994.1326. [DOI] [PubMed] [Google Scholar]
- Soboloff J, Spassova M, Tang X, Hewavitharana T, Xu W, Gill D. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. Epub 22006 Jun 20669. [DOI] [PubMed] [Google Scholar]
- Spassova M, Soboloff J, He L, Xu W, Dziadek M, Gill D. STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels. Proc Natl Acad Sci U S A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. Epub 2006 Mar 4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Drebrin-Dependent Actin Clustering in Dendritic Filopodia Governs Synaptic Targeting of Postsynaptic Density-95 and Dendritic Spine Morphogenesis. J. Neurosci. 2003a;23:6586–6595. doi: 10.1523/JNEUROSCI.23-16-06586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Sekino Y, Tanaka S, Mizui T, Kishi S, Shirao T. Drebrin-dependent actin clustering in dendritic filopodia governs synaptic targeting of postsynaptic density-95 and dendritic spine morphogenesis. J Neurosci. 2003b;23:6586–6595. doi: 10.1523/JNEUROSCI.23-16-06586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takezawa R, Cheng H, Beck A, Ishikawa J, Launay P, Kubota H, Kinet J, Fleig A, Yamada T, Penner R. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol Pharmacol. 2006;69:1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- Toda M, Shirao T, Uyemura K. Suppression of an actin-binding protein, drebrin, by antisense transfection attenuates neurite outgrowth in neuroblastoma B104 cells. Developmental Brain Research. 1999a;114:193–200. doi: 10.1016/s0165-3806(99)00030-9. [DOI] [PubMed] [Google Scholar]
- Toda M, Shirao T, Uyemura K. Suppression of an actin-binding protein, drebrin, by antisense transfection attenuates neurite outgrowth in neuroblastoma B104 cells. Brain Res Dev Brain Res. 1999b;114:193–200. doi: 10.1016/s0165-3806(99)00030-9. [DOI] [PubMed] [Google Scholar]
- Trevillyan JM, Chiou XG, Chen YW, Ballaron SJ, Sheets MP, Smith ML, Wiedeman PE, Warrior U, Wilkins J, Gubbins EJ, Gagne GD, Fagerland J, Carter GW, Luly JR, Mollison KW, Djuric SW. Potent inhibition of NFAT activation and T cell cytokine production by novel low molecular weight pyrazole compounds. J Biol Chem. 2001;276:48118–48126. doi: 10.1074/jbc.M107919200. [DOI] [PubMed] [Google Scholar]
- Varnai P, Toth B, Toth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1-Orai1 Complex. J Biol Chem. 2007;282:29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- Winslow MM, Neilson JR, Crabtree GR. Calcium signalling in lymphocytes. Curr Opin Immunol. 2003;15:299–307. doi: 10.1016/s0952-7915(03)00050-5. [DOI] [PubMed] [Google Scholar]
- Wu M, Buchanan J, Luik R, Lewis R. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin A, Zhang S, Jiang W, Yu Y, Safrina O, Cahalan M. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. Epub 2006 Aug 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Kiselyov K, Shin D, Chen J, Shcheynikov N, Kang S, Dehoff M, Schwarz M, Seeburg P, Muallem S, Worley P. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zhang S, Yu Y, Roos J, Kozak J, Deerinck T, Ellisman M, Stauderman K, Cahalan M. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Chait B. ProFound: an expert system for protein identification using mass spectrometric peptide mapping information. Anal Chem. 2000;72:2482–2489. doi: 10.1021/ac991363o. [DOI] [PubMed] [Google Scholar]
- Zitt C, Strauss B, Schwarz E, Spaeth N, Rast G, Hatzelmann A, Hoth M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004a;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- Zitt C, Strauss B, Schwarz EC, Spaeth N, Rast G, Hatzelmann A, Hoth M. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J Biol Chem. 2004b;279:12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.