Abstract
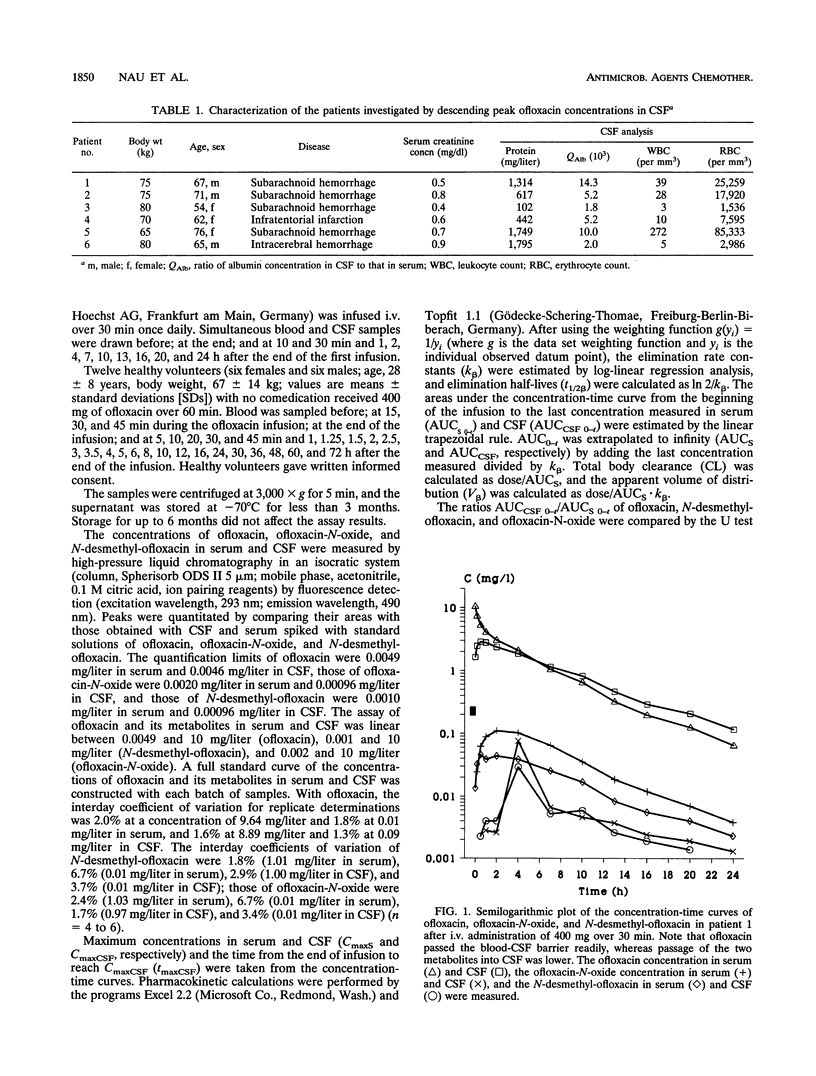
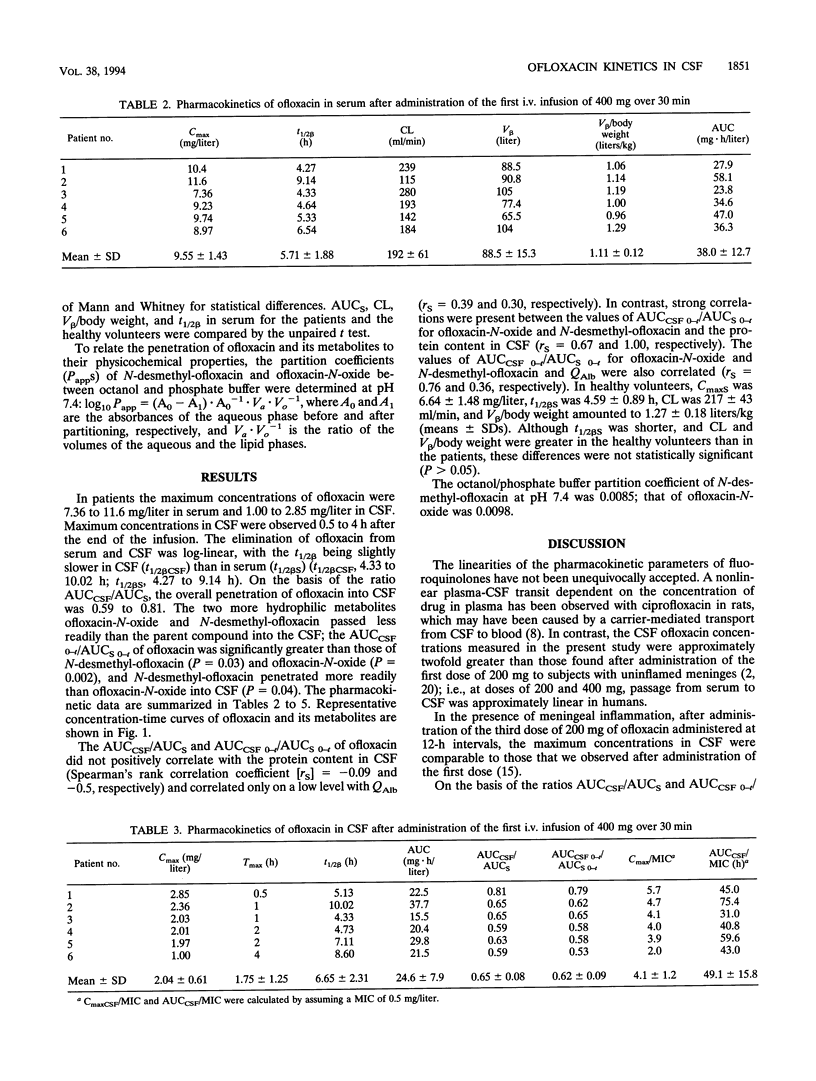
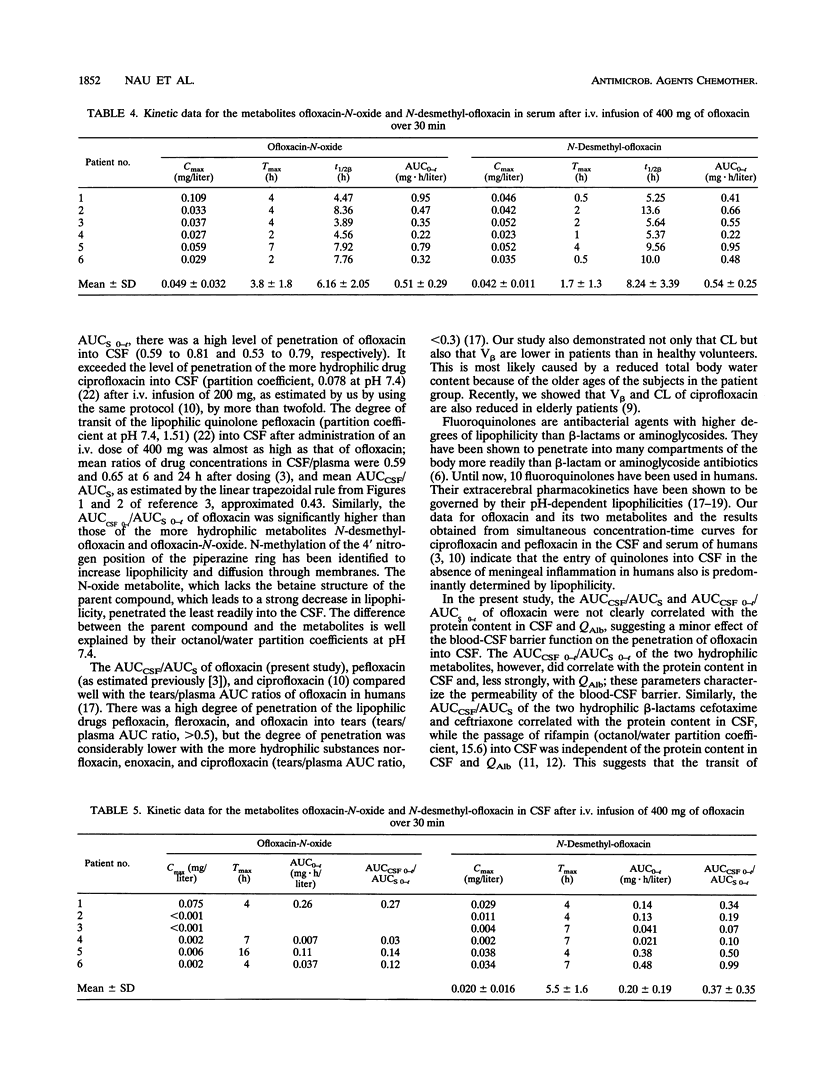
Ofloxacin has been reported to diffuse readily into the cerebrospinal fluid (CSF) in subjects with both inflamed and uninflamed meninges. However, with moderately susceptible bacteria, ofloxacin concentrations in CSF may be subtherapeutic after administration of an intravenous (i.v.) dose of 200 mg. For this reason, the kinetics of a higher dose of ofloxacin in CSF was studied with humans. Six patients with occlusive hydrocephalus caused by cerebrovascular diseases who had undergone external ventriculostomy received 400 mg of ofloxacin i.v. over 30 min. Serum and CSF samples were drawn repeatedly. Serum from 12 healthy volunteers was sampled repeatedly after they had received 400 mg of ofloxacin i.v. over 60 min. Ofloxacin, ofloxacin-N-oxide, and N-desmethyl-ofloxacin concentrations were determined by high-pressure liquid chromatography with fluorescence detection. The maximum ofloxacin concentrations in the serum of the patients ranged from 7.36 to 11.6 mg/liter (mean, 9.55 mg/liter), the apparent volume of distribution/body weight was 0.96 to 1.19 liters/kg (mean, 1.11 liters/kg), and the total body clearance was 115 to 280 ml/min (mean, 192 ml/min). In healthy volunteers, the volume of distribution/body weight and the total body clearance were higher and amounted to 1.27 +/- 0.18 liters/kg and 217 +/- 43 ml/min (means +/- standard deviations), respectively. These differences were attributed to the older ages of the patients than the volunteers. In the CSF of patients, maximum concentrations of 1.00 to 2.85 mg/liter (mean, 2.04 mg/liter) were observed 0.5 to 4 h following the completion of the ofloxacin infusion. Ofloxacin elimination from CSF was slightly slower than that from serum (half-lives, 4.33 to 10.02 versus 4.27 to 9.14 h). The overall penetration of ofloxacin into CSF, as expressed by the ratios of the areas under the concentration-curves, amounted to 0.59 to 0.81 (mean, 0.65). The more hydrophilic metabolites ofloxacin-N-oxide and N-desmethyl-ofloxacin passed less readily than ofloxacin into the CSF. In conclusion, the concentrations in CSF attained after a single i.v. infusion of 400 mg of ofloxacin in the absence of meningeal inflammation appear to be high enough to inhibit the growth of most staphylococci and members of the family Enterobacteriaceae, which are often involved in CSF shunt infection. Yet, in view of pharmacodynamic studies suggesting a peak concentration in CSF of at least 10-fold the MIC, the use of ofloxacin for central nervous systems infections is optimal only with highly susceptible pathogens (MIC, less than or equal to 0.12 mg/liter).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashby J., Piddock L. J., Wise R. An investigation of the hydrophobicity of the quinolones. J Antimicrob Chemother. 1985 Dec;16(6):805–808. doi: 10.1093/jac/16.6.805-a. [DOI] [PubMed] [Google Scholar]
- Ashraf S. J., Arya S. C., Parande C. M., Sahay R., Ageel A. R. Anti-delta antibody in primary hepatocellular carcinoma patients in the Gizan area of Saudi Arabia. Infection. 1986 Sep-Oct;14(5):250–251. doi: 10.1007/BF01644273. [DOI] [PubMed] [Google Scholar]
- Bitar N., Claes R., Van der Auwera P. Concentrations of ofloxacin in serum and cerebrospinal fluid of patients without meningitis receiving the drug intravenously and orally. Antimicrob Agents Chemother. 1989 Oct;33(10):1686–1690. doi: 10.1128/aac.33.10.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall L., Long L., Stanford J. Poncet's disease: tuberculous rheumatism. Rev Infect Dis. 1989 Jan-Feb;11(1):105–107. doi: 10.1093/clinids/11.1.105. [DOI] [PubMed] [Google Scholar]
- Dow J., Chazal J., Frydman A. M., Janny P., Woehrle R., Djebbar F., Gaillot J. Transfer kinetics of pefloxacin into cerebro-spinal fluid after one hour i.v. infusion of 400 mg in man. J Antimicrob Chemother. 1986 Apr;17 (Suppl B):81–87. doi: 10.1093/jac/17.suppl_b.81. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Johnson D. E., Rosen M., Standiford H. C. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother. 1993 Mar;37(3):483–490. doi: 10.1128/aac.37.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K. P., Lafredo S. C., Foleno B., Isaacson D. M., Barrett J. F., Tobia A. J., Rosenthale M. E. In vitro and in vivo antibacterial activities of levofloxacin (l-ofloxacin), an optically active ofloxacin. Antimicrob Agents Chemother. 1992 Apr;36(4):860–866. doi: 10.1128/aac.36.4.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaehde U., Langemeijer M. W., de Boer A. G., Breimer D. D. Cerebrospinal fluid transport and disposition of the quinolones ciprofloxacin and pefloxacin in rats. J Pharmacol Exp Ther. 1992 Dec;263(3):1140–1146. [PubMed] [Google Scholar]
- Naber K. G., Sörgel F., Kees F., Jaehde U., Schumacher H. Pharmacokinetics of ciprofloxacin in young (healthy volunteers) and elderly patients, and concentrations in prostatic fluid, seminal fluid, and prostatic adenoma tissue following intravenous administration. Am J Med. 1989 Nov 30;87(5A):57S–59S. doi: 10.1016/0002-9343(89)90023-5. [DOI] [PubMed] [Google Scholar]
- Nau R., Prange H. W., Martell J., Sharifi S., Kolenda H., Bircher J. Penetration of ciprofloxacin into the cerebrospinal fluid of patients with uninflamed meninges. J Antimicrob Chemother. 1990 Jun;25(6):965–973. doi: 10.1093/jac/25.6.965. [DOI] [PubMed] [Google Scholar]
- Nau R., Prange H. W., Menck S., Kolenda H., Visser K., Seydel J. K. Penetration of rifampicin into the cerebrospinal fluid of adults with uninflamed meninges. J Antimicrob Chemother. 1992 Jun;29(6):719–724. doi: 10.1093/jac/29.6.719. [DOI] [PubMed] [Google Scholar]
- Nau R., Prange H. W., Muth P., Mahr G., Menck S., Kolenda H., Sörgel F. Passage of cefotaxime and ceftriaxone into cerebrospinal fluid of patients with uninflamed meninges. Antimicrob Agents Chemother. 1993 Jul;37(7):1518–1524. doi: 10.1128/aac.37.7.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pioget J. C., Wolff M., Singlas E., Laisne M. J., Clair B., Regnier B., Vachon F. Diffusion of ofloxacin into cerebrospinal fluid of patients with purulent meningitis or ventriculitis. Antimicrob Agents Chemother. 1989 Jun;33(6):933–936. doi: 10.1128/aac.33.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiber H., Felgenhauer K. Protein transfer at the blood cerebrospinal fluid barrier and the quantitation of the humoral immune response within the central nervous system. Clin Chim Acta. 1987 Mar 30;163(3):319–328. doi: 10.1016/0009-8981(87)90250-6. [DOI] [PubMed] [Google Scholar]
- Staffensky R. H., Stolke D., Malottke R. Die Liquorgängigkeit von Ofloxacin (Tarivid) bei nicht entzündeten Meningen. Neurochirurgia (Stuttg) 1990 Jul;33(4):110–112. doi: 10.1055/s-2008-1053568. [DOI] [PubMed] [Google Scholar]
- Sörgel F., Jaehde U., Naber K., Stephan U. Pharmacokinetic disposition of quinolones in human body fluids and tissues. Clin Pharmacokinet. 1989;16 (Suppl 1):5–24. doi: 10.2165/00003088-198900161-00004. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Sande M. A. General principles of therapy of pyogenic meningitis. Infect Dis Clin North Am. 1990 Dec;4(4):661–676. [PubMed] [Google Scholar]


