Abstract
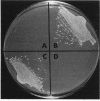
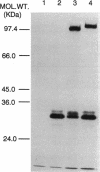
The Pseudomonas aeruginosa DNA gyrase gyrA gene was cloned and sequenced from strain PAO1. An open reading frame of 2,769 bp was found; it coded for a protein of 923 amino acids with an estimated molecular mass of 103 kDa. The derived amino acid sequence shared 67% identity with Escherichia coli GyrA and 54% identity with Bacillus subtilis GyrA, although conserved regions were present throughout the sequences, particularly toward the N terminus. Complementation of an E. coli mutant with a temperature-sensitive gyrA gene with the PAO1 gyrA gene showed that the gene is expressed in E. coli and is able to functionally complement the E. coli DNA gyrase B subunit. Expression of PAO1 gyrA in E. coli or P. aeruginosa with mutationally altered gyrA genes caused a reversion to wild-type quinolone susceptibility, indicating that the intrinsic susceptibility of the PAO1 GyrA to quinolones is comparable to that of the E. coli enzyme. PCR was used to amplify 360 bp of P. aeruginosa gyrA encompassing the so-called quinolone resistance-determining region from ciprofloxacin-resistant clinical isolates from patients with cystic fibrosis. Mutations were found in three of nine isolates tested; these mutations caused the following alterations in the sequence of GyrA: Asp at position 87 (Asp-87) to Asn, Asp-87 to Tyr, and Thr-83 to Ile. The resistance mechanisms in the other six isolates are unknown. The results of the study suggested that mechanisms other than a mutational alteration in gyrA are the most common mechanism of ciprofloxacin resistance in P. aeruginosa from the lungs of patients with cystic fibrosis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colman S. D., Hu P. C., Bott K. F. Mycoplasma pneumoniae DNA gyrase genes. Mol Microbiol. 1990 Jul;4(7):1129–1134. doi: 10.1111/j.1365-2958.1990.tb00687.x. [DOI] [PubMed] [Google Scholar]
- Cullen M. E., Wyke A. W., Kuroda R., Fisher L. M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989 Jun;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimri G. P., Das H. K. Cloning and sequence analysis of gyrA gene of Klebsiella pneumoniae. Nucleic Acids Res. 1990 Jan 11;18(1):151–156. doi: 10.1093/nar/18.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diver J. M., Bryan L. E., Sokol P. A. Transformation of Pseudomonas aeruginosa by electroporation. Anal Biochem. 1990 Aug 15;189(1):75–79. doi: 10.1016/0003-2697(90)90046-c. [DOI] [PubMed] [Google Scholar]
- Diver J. M., Schollaardt T., Rabin H. R., Thorson C., Bryan L. E. Persistence mechanisms in Pseudomonas aeruginosa from cystic fibrosis patients undergoing ciprofloxacin therapy. Antimicrob Agents Chemother. 1991 Aug;35(8):1538–1546. doi: 10.1128/aac.35.8.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasching C. E., Tenover F. C., Slama T. G., Fisher L. M., Sreedharan S., Oram M., Willard K., Sinn L. M., Gerding D. N., Peterson L. R. gyrA mutations in ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus from Indiana, Minnesota, and Tennessee. J Infect Dis. 1991 Nov;164(5):976–979. doi: 10.1093/infdis/164.5.976. [DOI] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes M. L., Dyall-Smith M. L. Mutations in DNA gyrase result in novobiocin resistance in halophilic archaebacteria. J Bacteriol. 1991 Jan;173(2):642–648. doi: 10.1128/jb.173.2.642-648.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopewell R., Oram M., Briesewitz R., Fisher L. M. DNA cloning and organization of the Staphylococcus aureus gyrA and gyrB genes: close homology among gyrase proteins and implications for 4-quinolone action and resistance. J Bacteriol. 1990 Jun;172(6):3481–3484. doi: 10.1128/jb.172.6.3481-3484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. S., Loeb L. A. An E. coli promoter that regulates transcription by DNA superhelix-induced cruciform extrusion. Science. 1988 Aug 5;241(4866):703–705. doi: 10.1126/science.2456617. [DOI] [PubMed] [Google Scholar]
- Inoue Y., Sato K., Fujii T., Hirai K., Inoue M., Iyobe S., Mitsuhashi S. Some properties of subunits of DNA gyrase from Pseudomonas aeruginosa PAO1 and its nalidixic acid-resistant mutant. J Bacteriol. 1987 May;169(5):2322–2325. doi: 10.1128/jb.169.5.2322-2325.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T., Otsuki M., Nishino T. In vitro antibacterial activity of Q-35, a new fluoroquinolone. Antimicrob Agents Chemother. 1992 Aug;36(8):1708–1714. doi: 10.1128/aac.36.8.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuzer K. N., Cozzarelli N. R. Escherichia coli mutants thermosensitive for deoxyribonucleic acid gyrase subunit A: effects on deoxyribonucleic acid replication, transcription, and bacteriophage growth. J Bacteriol. 1979 Nov;140(2):424–435. doi: 10.1128/jb.140.2.424-435.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kureishi A., Bryan L. E. Pre-boiling high GC content, mixed primers with 3' complementation allows the successful PCR amplification of Pseudomonas aeruginosa DNA. Nucleic Acids Res. 1992 Mar 11;20(5):1155–1155. doi: 10.1093/nar/20.5.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel L. S., Rogers L. H., Hill W. E. Survival of recombination-deficient mutants of Escherichia coli during incubation with nalidixic acid. J Bacteriol. 1978 Jun;134(3):1195–1198. doi: 10.1128/jb.134.3.1195-1198.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Modulation of transcription by DNA supercoiling: a deletion analysis of the Escherichia coli gyrA and gyrB promoters. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4185–4189. doi: 10.1073/pnas.84.12.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. V., Scurlock T. R. DNA gyrase (Topoisomerase II) from Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1983 Jan 27;110(2):694–700. doi: 10.1016/0006-291x(83)91205-6. [DOI] [PubMed] [Google Scholar]
- Moriya S., Ogasawara N., Yoshikawa H. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 1985 Apr 11;13(7):2251–2265. doi: 10.1093/nar/13.7.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T., Iyobe S., Hashimoto H., Hirai K. Cloning and characterization of a DNA fragment that complements the nfxB mutation in Pseudomonas aeruginosa PAO. FEMS Microbiol Lett. 1991 Mar 15;63(1):31–35. doi: 10.1016/0378-1097(91)90522-c. [DOI] [PubMed] [Google Scholar]
- Orr E., Staudenbauer W. L. Bacillus subtilis DNA gyrase: purification of subunits and reconstitution of supercoiling activity. J Bacteriol. 1982 Jul;151(1):524–527. doi: 10.1128/jb.151.1.524-527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburne M. S., Maiese W. M., Greenstein M. In vitro inhibition of bacterial DNA gyrase by cinodine, a glycocinnamoylspermidine antibiotic. Antimicrob Agents Chemother. 1990 Jul;34(7):1450–1452. doi: 10.1128/aac.34.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parales R. E., Harwood C. S. Nucleotide sequence of the gyrB gene of Pseudomonas putida. Nucleic Acids Res. 1990 Oct 11;18(19):5880–5880. doi: 10.1093/nar/18.19.5880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. DNA gyrase: structure and function. Crit Rev Biochem Mol Biol. 1991;26(3-4):335–375. doi: 10.3109/10409239109114072. [DOI] [PubMed] [Google Scholar]
- Reece R. J., Maxwell A. The C-terminal domain of the Escherichia coli DNA gyrase A subunit is a DNA-binding protein. Nucleic Acids Res. 1991 Apr 11;19(7):1399–1405. doi: 10.1093/nar/19.7.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robillard N. J. Broad-host-range gyrase A gene probe. Antimicrob Agents Chemother. 1990 Oct;34(10):1889–1894. doi: 10.1128/aac.34.10.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer H. P. Escherichia-Pseudomonas shuttle vectors derived from pUC18/19. Gene. 1991 Jan 2;97(1):109–121. doi: 10.1016/0378-1119(91)90016-5. [DOI] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Stein D. C., Danaher R. J., Cook T. M. Characterization of a gyrB mutation responsible for low-level nalidixic acid resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1991 Apr;35(4):622–626. doi: 10.1128/aac.35.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg S. L., Wang J. C. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987 Oct 20;197(4):729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- Vizán J. L., Hernández-Chico C., del Castillo I., Moreno F. The peptide antibiotic microcin B17 induces double-strand cleavage of DNA mediated by E. coli DNA gyrase. EMBO J. 1991 Feb;10(2):467–476. doi: 10.1002/j.1460-2075.1991.tb07969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Huang W. M., Taylor D. E. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob Agents Chemother. 1993 Mar;37(3):457–463. doi: 10.1128/aac.37.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. E., Iglewski B. H. Codon usage in Pseudomonas aeruginosa. Nucleic Acids Res. 1988 Oct 11;16(19):9323–9335. doi: 10.1093/nar/16.19.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Nakamura M., Bogaki M., Nakamura S. Proportion of DNA gyrase mutants among quinolone-resistant strains of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Jun;34(6):1273–1275. doi: 10.1128/aac.34.6.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]