Abstract
Context: Because GH stimulates lipolysis, an increase in circulating free fatty acid levels, as opposed to a direct effect of high GH levels, could underlie the development of insulin resistance in type 1 diabetes (T1D). Our aim was to explore the relative contributions of GH and free fatty acids to the development of insulin resistance in patients with T1D.
Patients: Seven (four females, three males) nonobese patients with T1D aged 21–30 yr were studied on four occasions in random order. On each visit, overnight endogenous GH production was suppressed by octreotide. Three 1-h pulses of recombinant human GH (rhGH) or placebo were administered on two visits each. Acipimox, an antilipolytic drug, or a placebo were ingested every 4 h on two visits each. Stable glucose and glycerol isotopes were used to assess glucose and glycerol turnover. The overnight protocol was concluded by a two-step hyperinsulinemic euglycemic clamp on each visit.
Main Outcome: rhGH administration led to increases in the insulin infusion rate required to maintain euglycemia overnight (P = 0.008), elevated basal endogenous glucose production (P = 0.007), decreased basal peripheral glucose uptake (P = 0.03), and reduced glucose uptake during step 1 of the clamp (P < 0.0001). Coadministration of rhGH and acipimox reversed these effects and suppression of lipolysis in the absence of GH replacement led to further increases in insulin sensitivity.
Results: GH pulses were associated with an increase in endogenous glucose production and decreased rates of peripheral glucose uptake, which was entirely reversed by acipimox. Therefore, GH-driven decreases in insulin sensitivity are mainly determined by the effect of GH on lipolysis.
Growth hormone decreases insulin sensitivity through increases in free fatty acid levels.
Insulin resistance plays a larger role in the pathophysiology type of 1 diabetes (T1D) than is commonly recognized (1). It contributes to difficulties in targeting insulin therapy (2), and peripheral hyperinsulinemia is associated with excess weight gain (3). The resistance to insulin action in T1D has been linked to the hypersecretion of GH (4), which is particularly marked overnight and therefore implicated in the pathogenesis of the dawn phenomenon (early morning hyperglycemia) (5), leading to increasing insulin requirements as the night progresses (5). GH hypersecretion manifests as increases in pulse amplitude (4), shortening of pulse periodicity (6), and higher basal GH levels (4) and is probably due to a diminished feedback drive from low circulating levels of IGF-I (7). Subcutaneous insulin therapy results in relative portal hypoinsulinemia and therefore impaired IGF-I production (8) because hepatic GH receptors are in part insulin dependent (9). Furthermore, the reciprocal relation of portal insulin levels to circulating levels of IGF binding protein-1, an inhibitor of IGF-I bioactivity, may exacerbate GH hypersecretion (10); only portal administration of insulin results in normalization of the GH/IGF-I axis in T1D (11).
GH antagonizes the action of insulin, and even physiological increases in plasma GH levels as part of an overnight fast have been shown to decrease insulin sensitivity (12,13). Animal models and studies in vitro provided the precedent for a direct adverse effect of GH on insulin sensitivity by showing that there was cross talk between the GH receptor and insulin signaling pathways (14), but a recent study in humans was unable to substantiate this finding (15). In addition, GH promotes lipolysis (16), and after an overnight infusion of recombinant human GH (rhGH), increases in levels of both GH and free fatty acid (FFA) levels are correlated with reductions in insulin sensitivity (17). Thus, GH could also lead to insulin resistance through an indirect mechanism of elevated circulating levels of FFAs, but experimental studies in humans to date have yielded somewhat conflicting results with respect to putative independent effects of GH and FFAs on insulin sensitivity (18,19,20,21,22).
We studied seven volunteers with T1D on four occasions by manipulating GH levels using octreotide and rhGH and suppressing lipolysis with acipimox. Our aims were to explore the effect of GH on insulin sensitivity, find out to what extent this is mediated by FFAs, and determine whether FFAs have an effect on insulin sensitivity that is independent of GH.
Patients and Methods
Patients
Seven participants (four females, three males) aged 21–30 yr with childhood-onset insulin dependent diabetes of more than 2 yr and undetectable C-peptide levels, who are known to the diabetes service at Addenbrooke’s Hospital (Cambridge, UK) were recruited to the study by the research team based at the University Department of Pediatrics, Addenbrooke’s Hospital. Our exclusion criteria included age younger than 18 or above 30 yr, body mass index (BMI) greater than 30 kg/m2, fewer than two insulin injections per day, use of lipid-lowering drugs, abnormal renal and liver function, untreated hypothyroidism, Celiac disease, malignancy, other chronic illnesses, pregnancy, and recurrent episodes of severe unexplained hypoglycemia. The study was approved by the Huntingdon Research Ethics Committee (Cambridge, UK), and written informed consent was obtained from each volunteer.
Study design
In random order, patients attended the Wellcome Trust Clinical Research Facility (WTCRF), Addenbrooke’s Hospital (Cambridge, UK), for four overnight visits. Participants were instructed not to consume alcohol and refrain from vigorous exercise for 48 h before each admission. Patients omitted their own insulin preparations for 36 h before each study visit and controlled blood glucose levels with multiple injections of soluble insulin Actrapid after liaison with a medically qualified member of the study team. In practice, patients injected a dose of Actrapid (Novo Nordisk, Crawley, UK) that was circa one third the dose of their regular long-acting insulin the night before admission to the WTCRF. This regimen disturbed none of the participants’ sleep on any occasion, and during the day patients injected the same dose of Actrapid as they would regular short-acting insulin. No insulin was self-administered after 1400 h on the day of admission.
On admission to the WTCRF, height and weight were measured, and an iv cannula was sited in an antecubital vein of each arm, one of which was warmed with a heating blanket to allow sampling of arterialized blood. At 1800 h, a variable rate iv insulin (Actrapid) infusion was started to maintain patients’ overnight blood glucose levels as close to 5 mmol/liter as possible by adjusting the infusion rate according to glucose levels measured every 15 min. Patients were given a standard meal at 1800 h and then remained fasted until 2400 h. Baseline samples for FFAs, GH, and insulin were taken at 2000 h. Sampling for FFAs and insulin continued overnight at 60-min intervals, whereas samples for GH were taken every 15 min. Final samples for the overnight protocol were taken at 0800 h.
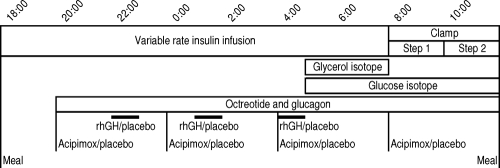
Starting at 2000 h on each study visit, overnight endogenous GH and glucagon production were suppressed using an iv infusion of octreotide (300 ng/kg · h; Fig. 1). Glucagon levels were replaced with an iv infusion of glucagon (60 ng/kg · h; Fig. 1). Intravenous rhGH or placebo (0.9% sodium chloride) was administered as three separate 1-h pulses (12 mU/kg of rhGH per pulse) at 2200, 0100, and 0400 h on two study visits each (Fig. 1). In addition, 250 mg acipimox, an antilipolytic drug, or a placebo were ingested at 2000, 2400, 0400, and 0800 h on two study visits each (Fig. 1). Patients and the study team were blinded to the treatment allocation. The stable isotope d-[6,62H2]glucose (bolus: 0.15 mg/kg, infusion: 0.01 mg/kg · min) was infused between 0500 and 1200 h to determine the rate of glucose turnover, and 2H5-glycerol (bolus: 170 mg, infusion: 1.7 mg/min) was infused between 0500 and 0800 h to calculate the rate of glycerol turnover (Fig. 1).
Figure 1.
Generic protocol for one study visit.
The overnight sampling protocol was followed by a two-step hyperinsulinemic euglycemic clamp between 0800 and 1200 h (step one: 0800–1000 h, insulin bolus of 2.8 mU/kg followed by an infusion of 0.6 mU/kg · min; step two: 1000–1200 h, insulin bolus of 7 mU/kg followed by an infusion of 1.5 mU/kg · min; Fig. 1). During the clamp, blood glucose levels were measured every 5 min, and euglycemia was maintained by an infusion of 20% glucose spiked with 7 mg of d-[6,62H2]glucose per gram of unlabeled glucose in the infusate of 20% glucose. The insulin infusion was stopped at 1200 h, whereas infusion of 20% glucose was allowed to continue until 1230 h, after which patients self-administered a sc injection of Actrapid, had lunch, and were then discharged from the research unit.
Dual-energy x-ray absorptiometry
A dual-energy x-ray absorptiometry scan was performed at the WTCRF after the patients’ final study visit. Data on body composition were gathered with a Lunar Prodigy machine using a constant pixel size of 1.2 × 1.2 cm and Lunar software programs (version 8.1, Lunar Corporation, Madison, WI). The effective radiation dose was 0.2 μSievert. The scan yielded measures of whole-body fat and fat-free mass (FFM) and therefore percent whole-body fat mass.
Biochemical analyses
Glucose levels were measured using 25 μl whole blood samples on a Y.S.I. 2300 stat plus analyser (Lynchford House, UK). The intraassay coefficient of variation (CV) was 1.5% at 4.1 mmol/liter. The equivalent interassay CV at this glucose concentration was 2.8 and 1.7% at 14.1 mmol/liter. Insulin levels were measured using an ELISA (Dako Ltd., High Wycombe, UK) according to the manufacturer’s instructions. The intraassay CV was 4.3% at 82 pmol/liter, 3.0% at 402 pmol/liter, and 5.7% at 907 pmol/liter. The equivalent interassay CVs were 4.3, 5.1, and 5.4%, respectively. Plasma FFA levels were analyzed using a WAKO enzymatic colorimetric kit (α Laboratories, Eastleigh, UK) adapted to an ILab 600 clinical chemistry analyzer (Instrumentation Laboratories, Warrington, UK). The intraassay CV was 2.2% at levels of 559 μmol/liter and 1143 μmol/liter. The equivalent interassay CV was 2.5%. GH levels were measured using an ELISA (Oxford Bio-Innovations, Bicester, UK) according to the manufacturer’s instructions. The assay was calibrated to the World Health Organization First International Standard (80/505). The intra-assay CV was 9.7% at 0.7 ng/ml and 6.5% at 6.4 ng/ml. The equivalent interassay CVs were 10.4 and 5.5%, respectively.
Glucose and glycerol isotope analysis
The use of isotopes for glucose and glycerol allowed direct confirmation of the degree of their endogenous production. Glucose appearance (Ra) and disappearance (Rd) were calculated using equation of Steele and colleagues (23,24). This describes two rapidly exchanging pools in conditions of non-steady state and was specifically derived for studying acute changes in glucose kinetics due to a perturbing hormone infusion. For glycerol, steady-state kinetics have been found more applicable where Ra = Rd. Rd was calculated as the isotope infusion rate divided by the experimentally determined tracer to tracee ratio found in plasma. After solvent extraction and derivatization, samples for glucose and glycerol isotope were analyzed by glass chromatography-mass spectrometry (GC-MS).
GC-MS analysis
Conventional GC-MS measurements were made using an Agilent GCMS 5973N quadrupole mass spectrometer system (Agilent, Wokingham, UK), comprising a HP6890 gas chromatograph with autosampler and injector operated in the splitless mode at 250 C. Chromatographic separation was performed using a DB-1MS capillary column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness; J&W Scientific, Folsom, CA). The mass spectrometer was operated in electron ionization mode, with electron energy of 70 eV. The transfer line to the mass spectrometer was maintained at 280 C, with the ion source at 250 C and quadrupole at 120 C. For deuterated glucose analysis, the initial oven temperature of 90 C was maintained for 1 min before ramping at 30 C/min to 270 C and then held for 0.5 min to clear the column. Selected ion monitoring (SIM) of the isotopomer cluster, m/z 238–243, was performed with a nominal dwell time of 50 msec per mass unit. For deuterated glycerol, the initial oven temperature was 100 C, held for 0.5 min, ramped at 30 C/min to 280 C, and then held for a further 0.5 min. SIM of the cluster, m/z 377–382, was performed again with a dwell time of 50 ms. Isotopomer ratios relative to the most abundant ion in the cluster (m/z 240 for the unlabeled glucose derivative and m/z 377 for the unlabeled glycerol derivative) were calculated using the method of Bluck and Coward (25).
Calculations
BMI was calculated as weight (kilograms) divided by height (meters) (2). Overnight insulin requirements for euglycemia were calculated as the mean insulin infusion rate per kilogram body weight or FFM per minute between 2200 and 0800 h. The M-value, glucose infusion rate to maintain euglycemia, during the steady state of steps 1 and 2 (0930–1000 and 1130–1200 h) of the hyperinsulinemic euglycemic clamp provided a crude estimate of peripheral insulin sensitivity. M-values were adjusted for FFM. Glycerol Ra per kilogram body weight, a proxy for the rate of lipolysis, was calculated for the euglycemic steady-state period between 0730 and 0800 h. Endogenous glucose production, glucose Ra, per kilogram body weight and peripheral glucose uptake, glucose Rd, per kilogram FFM were calculated for the steady states 0730–0800, 0930–1000 h (clamp step 1) and 1130–1200 h (clamp step 2). Glycerol Ra, glucose Ra, and glucose Rd between 0730 and 0800 h were corrected for plasma insulin levels. The insulin-mediated suppression of glucose Ra and increase in glucose Rd during each step of the clamp was quantified by subtracting basal glucose Ra and Rd from glucose Ra and Rd during steps 1 and 2, respectively. The changes in glucose Ra and Rd during each step were also adjusted for the increment in plasma insulin levels from baseline to clamp steady-state levels.
Statistics
Repeated-measures ANOVA was used to determine trends across all study visits. Given that we were aiming to answer three specific questions as detailed in the introduction and the relatively small size of our cohort, we analyzed differences between individual visits with dependent t tests. Correlations were tested using the Pearson’s correlation coefficient (r). Results are reported as mean ± 1 sem unless stated otherwise. Significance was set to P < 0.05. Analyses were performed using SPSS for Windows version 14.0 (SPSS Inc., Chicago, IL).
Results
Patient characteristics are summarized in Table 1. The study visits (Fig. 1) are encoded GP (rhGH and placebo), PP (placebo and placebo), GA (rhGH and acipimox), and PA (placebo and acipimox).
Table 1.
Patient characteristics
| Characteristic | |
|---|---|
| Sex (female/male) | 4/3 |
| Age (yr) | 26 ± 1.2 (21–30) |
| Duration of T1D (yr) | 10.6 ± 2.0 (3.7–19.5) |
| Insulin dose (U/kg · d) | 0.8 ± 0.1 (0.7–1.1) |
| HbA1c (%) | 8.3 ± 0.2 (7.7–9.1) |
| Weight (kg) | 70.5 ± 4.8 (55.2–90.0) |
| Height (cm) | 170.7 ± 2.7 (161.6–181.1) |
| BMI (kg/m2) | 24.0 ± 0.9 (21.1–27.4) |
| Whole-body fat mass (kg) | 20.4 ± 1.8 (12.7–25.2) |
| FFM (kg) | 48.9 ± 3.7 (41.3–63.4) |
| Whole-body fat mass (%) | 29.4 ± 1.9 (23.5–37.1) |
Insulin doses by body weight are based on an average day. Data are means ± 1 sem and range in parentheses.
Glucose, GH, and FFA levels
Average overnight (2200–0800 h) blood glucose levels were similar on all study visits (Table 2). There was also no difference in glucose and insulin levels during the steady-state of steps 1 and 2 of the hyperinsulinemic euglycemic clamp (Table 3).
Table 2.
Overnight (22:00-08:00) levels of blood glucose and plasma insulin, and insulin infusion rates by body weight and FFM
| Visit GP rhGH+placebo | Visit PP placebo+placebo | Visit GA rhGH+acipimox | Visit PA placebo+acipimox | P value | |
|---|---|---|---|---|---|
| Blood glucose (mmol/liter) | 6.2 ± 0.1 | 6.2 ± 0.2 | 5.8 ± 0.2 | 5.7 ± 0.2 | 0.06 |
| Insulin requirement (mU/kg body weight per minute) | 1.1 ± 0.1 | 0.8 ± 0.1a | 0.6 ± 0.1b | 0.5 ± 0.1 | <0.0001 |
| Insulin requirement (mU/kg FFM per minute) | 1.7 ± 0.2 | 1.1 ± 0.2a | 0.9 ± 0.2b | 0.8 ± 0.1c | <0.0001 |
| Plasma insulin (pmol/liter) | 106.4 ± 18.2 | 85.3 ± 19.1a | 54.5 ± 4.0b | 53.7 ± 9.8 | 0.009 |
Data are means ± 1 sem.
P < 0.05 for GP vs. PP.
P < 0.05 for GP vs. GA.
P < 0.05 for PP vs. PA.
Table 3.
Levels of blood glucose and plasma insulin, M-value (average glucose infusion rate corrected for kilogram FFM), absolute glucose Ra (by kilogram body weight), and Rd (by kilogram FFM) during the euglycemic steady-state period between 0730 and 0800 h and steps 1 and 2 of the hyperinsulinemic euglycemic clamp
| Visit GP rhGH+placebo | Visit PP placebo+placebo | Visit GA rhGH+acipimox | Visit PA placebo+acipimox | P value | |
|---|---|---|---|---|---|
| Preclamp steady state (0730–0800 h) | |||||
| Blood glucose (mmol/liter) | 5.5 ± 0.2 | 5.5 ± 0.3 | 5.4 ± 0.3 | 4.8 ± 0.2 | 0.1 |
| Plasma insulin (pmol/liter) | 104.6 ± 19.2 | 47.2 ± 10.1a | 28.7 ± 7.4b | 32.0 ± 12.5c | <0.0001 |
| Glucose Ra (μmol/kg · min) | 12.5 ± 1.3 | 13.2 ± 1.0a | 17.9 ± 2.4 | 15.7 ± 2.2c | 0.3 |
| Glucose Rd (μmol/kg FFM per minute) | 16.1 ± 2.4 | 18.7 ± 4.2a | 28.4 ± 5.3b | 21.8 ± 4.7c | 0.3 |
| Step 1 steady state (0930–1000 h) | |||||
| Blood glucose (mmol/liter) | 4.9 ± 0.1 | 5.0 ± 0.1 | 4.9 ± 0.04 | 5.0 ± 0.1 | 0.4 |
| Plasma insulin (pmol/liter) | 156.9 ± 23.0 | 167.7 ± 18.9 | 150.4 ± 13.2 | 152.1 ± 16.2 | 0.8 |
| M-value (mg per FFM per minute) | 5.6 ± 0.9 | 8.4 ± 1.1a | 9.8 ± 1.0b | 11.0 ± 1.2 | 0.02 |
| Glucose Ra (μmol/kg · min) | 2.6 ± 1.2 | 4.8 ± 2.2 | 3.1 ± 1.6 | 5.2 ± 1.4 | 0.6 |
| Glucose Rd (μmol/kg FFM per minute) | 35.7 ± 4.3 | 49.0 ± 3.8a | 57.6 ± 4.0b | 68.1 ± 5.8c | <0.0001 |
| Step 2 steady state (1130–1200 h) | |||||
| Blood glucose (mmol/liter) | 4.9 ± 0.08 | 4.9 ± 0.09 | 4.9 ± 0.1 | 5.0 ± 0.07 | 0.5 |
| Plasma insulin (pmol/liter) | 335.6 ± 55.3 | 358.1 ± 52.6 | 355.8 ± 58.0 | 393.5 ± 29.1 | 0.5 |
| M-value (mg per FFM per minute) | 17.1 ± 1.2 | 18.9 ± 1.5 | 20.0 ± 1.0 | 21.0 ± 1.7c | 0.04 |
| Glucose Ra (μmol/kg · min) | −3.2 ± 5.3 | −0.9 ± 1.3 | −1.2 ± 4.0 | −1.2 ± 3.3 | 0.96 |
| Glucose Rd (μmol/kg FFM per minute) | 91.5 ± 10.0 | 104.2 ± 9.7 | 109.0 ± 8.9 | 112.2 ± 7.3 | 0.2 |
Data are means ± 1 sem.
P < 0.05 for GP vs. PP.
P < 0.05 for GP vs. GA.
P < 0.05 for PP vs. PA.
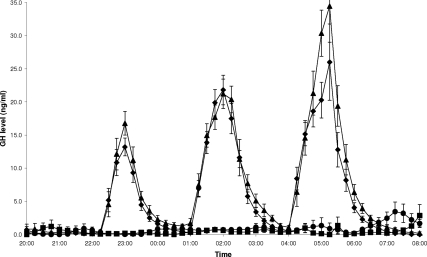
Overnight GH levels were equally well suppressed on study visits PP and PA (0.4 ± 0.1 vs. 0.9 ± 0.4 ng/ml, P = 0.2; Fig. 2). Replacement of GH levels on visits GP and GA resulted in three overnight pulses of GH, which peaked about 1 h after commencement of each infusion, giving similar mean overnight GH levels (6.7 ± 0.7 vs. 8.0 ± 0.6 ng/ml, P = 0.09; Fig. 2). GH levels on visits GP and GA were higher than on visits PP (P < 0.0001 and P < 0.0001, respectively; Fig. 2) and PA (P < 0.0001 and P < 0.0001, respectively; Fig. 2).
Figure 2.
Preclamp GH levels during study visits GP (♦, rhGH + placebo), PP (▪, placebo + placebo), GA (▴, rhGH + acipimox), and PA (•, placebo + acipimox). Data are means ± 1 sem. Repeated-measures analysis showed differences in overnight (2200–0800 h) GH levels across the four study visits (P < 0.0001).
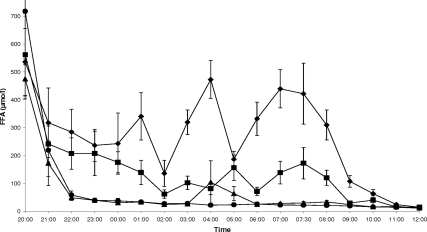
Acipimox reduced average overnight FFA levels during visits GA and PA to a similar extent (41.4 ± 8.8 vs. 30.8 ± 6.5 μmol/liter, P = 0.2; Fig. 3). FFA levels on visit GP were higher than on visits PP (310.6 ± 33.2 vs. 136.8 ± 21.3 μmol/liter, P = 0.01; Fig. 3), GA (P < 0.0001; Fig. 3), and PA (P = 0.02; Fig. 3). After overnight euglycemia, these differences were reflected in a greater rate of lipolysis, as estimated by glycerol Ra between 0730 and 0800 h, on visit GP than on visits PP (P = 0.005; Fig. 4), GA (P = 0.005; Fig. 4) and PA (P = 0.001; Fig. 4). FFA levels and glycerol Ra on visit PP were also higher than on visits GA (P = 0.001 and P = 0.048, respectively; Figs. 3 and 4) and PA (P = 0.02 and P = 0.02, respectively; Figs. 3 and 4).
Figure 3.
FFA levels before and during the two-step hyperinsulinemic euglycemic clamp (step 1: 0800–1000 h, step 2: 1000–1200 h) on study visits GP (♦, rhGH + placebo), PP (▪, placebo + placebo), GA (▴, rhGH + acipimox), and PA (•, placebo + acipimox). Data are means ± 1 sem. Repeated-measures analysis showed differences in overnight (2200–0800 h) FFA levels across the four study visits (P < 0.0001).
Effect of GH on insulin sensitivity (visit GP vs. PP)
The insulin infusion rate required for the maintenance of euglycemia overnight was higher during rhGH administration per kilogram body weight or FFM (P = 0.009 and P = 0.008, respectively; Table 2). There was no difference in overnight plasma insulin levels between visits GP and PP (P = 0.2; Table 2), but insulin levels during the steady-state period between 0730 and 0800 h were higher with rhGH (P = 0.005; Table 3).
GH increased basal (0730–0800 h) glucose Ra (P = 0.007; Fig. 5) and decreased the insulin-mediated suppression of glucose Ra from baseline to the steady state of step 1 of the clamp (P = 0.02; Fig. 6), but the suppression of glucose Ra from baseline to step 2 was similar on visits GP and PP (P = 0.3; Fig. 7). GH decreased basal glucose Rd (P = 0.03; Fig. 8) as well as the M-value and absolute glucose Rd during step 1 (P = 0.005 and P < 0.0001, respectively; Table 3). The M-value and absolute glucose Rd during step 2 were similar on visits GP and PP (P = 0.2 and P = 0.3, respectively; Table 3). The insulin-mediated increase in glucose Rd, corrected for the change in plasma insulin levels, from baseline to step 1 was similar on visits GP and PP (P = 0.4; Fig. 9), whereas the increase from baseline to step 2 tended to be lower with GH (P = 0.1; Fig. 10).
Role of FFAs in mediating the effect of GH on insulin sensitivity (visit GP vs. GA)
Coadministration of GH and acipimox reduced overnight insulin requirements per kilogram body weight or FFM (P = 0.002 and P = 0.002, respectively; Table 2), which was mirrored by lower plasma insulin levels overnight and during the preclamp steady-state period (P = 0.02 and P = 0.01, respectively; Tables 2 and 3).
Acipimox tended to reduce basal glucose Ra (P = 0.06; Fig. 5) and enhance the suppression of glucose Ra from baseline to step 1 (P = 0.003; Fig. 6), and there was a trend toward increased suppression of glucose Ra from baseline to step 2 (P = 0.1; Fig. 7). Acipimox increased basal glucose Rd (P = 0.009; Fig. 8) as well as the M-value and absolute glucose Rd during step 1 (P = 0.008 and P = 0.004, respectively; Table 3). The M-value and absolute glucose Rd during step 2 were similar on visits GP and GA (P = 0.2 and P = 0.1, respectively; Table 3). The increase in glucose Rd, corrected for the change in plasma insulin levels, from baseline to step 1 and 2 was greater with acipimox (P = 0.03 and P = 0.047, respectively; Figs. 9 and C).
Effect of FFAs on basal insulin sensitivity (visit PP vs. PA)
Acipimox in the absence of GH reduced overnight insulin requirements per kilogram body weight or FFM (P = 0.047 and P = 0.047, respectively; Table 2). There was no difference in overnight plasma insulin levels between visits PP and PA (P = 0.1; Table 2), but insulin levels during the preclamp steady-state period were lower with acipimox (P = 0.03; Table 3).
Acipimox reduced basal glucose Ra (P = 0.03; Fig. 5), but the suppression of glucose Ra from baseline to steps 1 and 2 of the clamp was unaffected (P = 0.9 and P = 0.9, respectively; Fig. 6C). The M-value during step 1 was similar on visits PP and PA (P = 0.1; Table 3), but acipimox increased the M-value during step 2 (P = 0.01; Table 3). Furthermore, acipimox tended to increase absolute glucose Rd before the clamp (P = 0.06; Fig. 8) and enhance absolute glucose Rd during step 1 (P = 0.04; Table 3), but absolute glucose Rd during step 2 was similar on visits PP and PA (P = 0.2; Table 3). The increase in glucose Rd, corrected for the change in plasma insulin levels, from baseline to step 1 was greater with acipimox (P = 0.03; Fig. 9), whereas the increase from baseline to step 2 tended to higher on visit PA (P = 0.08; Fig. 10).
Correlations between GH, FFAs, and insulin sensitivity
Taking all study visits into account and allowing for repeated measures, average overnight FFA levels correlated positively with basal glucose Ra (r = 0.565, P = 0.002) and inversely with basal glucose Rd (r = −0.42, P = 0.03) and the M-value (r = −0.738, P < 0.0001 and r = −0.543, P = 0.004, respectively) and glucose Rd (r = −0.784, P < 0.0001 and r = −0.484, P = 0.01, respectively) during steps 1 and 2 of the clamp. In contrast, overnight GH levels showed no association with basal glucose Ra and Rd (r = 0.235, P = 0.2 and r = 0.093, P = 0.6, respectively) and the M-value (r = −0.167, P = 0.4 and r = −0.057, P = 0.8, respectively) and glucose Rd (r = −0.276, P = 0.2 and r = −0.022, P = 0.9, respectively) during steps 1 and 2. In addition, FFA levels correlated with overnight insulin requirements for euglycemia per kilogram body weight or FFM (r = 0.677, P < 0.0001 and r = 0.671, P < 0.0001, respectively), whereas GH levels were not associated with insulin infusion rates per kilogram body weight or FFM (r = 0.282, P = 0.2 and r = 0.255, P = 0.2, respectively).
Discussion
In this cohort of nonobese, young adults with T1D, we explored the effects of variation in circulating levels of GH and FFAs on overnight insulin requirements and insulin sensitivity after 10 h of euglycemia overnight. The central finding is that GH-induced decreases in insulin sensitivity, as reflected by increases in insulin requirements for euglycemia and glucose Ra as well as reductions in the M-value and glucose Rd, appear to be mediated through FFAs.
The pattern of GH secretion in T1D is similar to that observed during a prolonged fast, which is also associated with the development of insulin resistance (16). Short-term (≤3 h) infusions of rhGH have been shown to antagonize insulin action in healthy humans without substantial elevations in circulating FFA levels (26,27,28), but this has not been replicated in patients with T1D (29,30). Longer-term (10 h) exposure to rhGH in our study led to elevated plasma insulin levels due to increases in insulin infusion rates required to maintain euglycemia overnight, implicating a state of relative insulin resistance. Treatment with rhGH also increased basal glucose Ra, decreased basal glucose Rd, and reduced the M-value and glucose Rd during step 1 of the clamp. Furthermore, GH attenuated the suppression of glucose Ra from baseline to the steady state of step 1 after adjustment for the increment plasma insulin levels during the clamp, which is in line with several previous studies (12,13,18,21,30) that support the notion of an inhibitory effect of GH on insulin binding at the liver (31).
Because GH is a potent stimulator of lipolysis (16), as demonstrated by marked elevations in glycerol Ra during rhGH administration in our study, and increases in plasma FFA levels are associated with the development of insulin resistance (32,33,34), FFAs may be the mediators of GH-induced decreases in insulin sensitivity. Numerous studies have explored the effects of short-term experimental reductions in circulating FFA levels by suppressing lipolysis using acipimox with an almost consistent finding of improved insulin sensitivity in patients who are healthy (18,19,20,35,36,37) and have a family history of diabetes (38,39), type 2 diabetes (37,40,41,42), or even GH deficiency (21,22). Five of these studies attempted to separate the effect of FFAs on insulin sensitivity from those of GH with the following partially conflicting results. In adults with GH deficiency, the M-value and insulin-stimulated glucose Rd were lowest during rhGH treatment without acipimox (21,22). Administration of acipimox on and off rhGH in these patients increased glucose Rd during the clamp in one study (21), but Nielsen et al. were unable to confirm this finding with respect to the M-value (22). Norrelund et al. (20) studied seven healthy men aged 23 ± 1 yr to show that acipimox during constant infusions of somatostatin and rhGH increased basal glucose Rd after a 34-h fast, whereas omission of rhGH during treatment with acipimox had no effect on glucose Rd. The M-value followed a similar pattern, but there was no difference in glucose Rd during the clamp. In their study of nine healthy men aged 23 ± 1 yr after 7 d of daily rhGH injections, Krag et al. (19) were unable to replicate the effects of acipimox on the M-value described by Norrelund et al. possibly due to rebound increases in FFA levels, which have been described before in association with the use of acipimox (42,43,44,45,46) but were not evident in our study. During a third, different treatment arm in the study by Krag et al., which involved injections of placebo instead of rhGH, the M-value was greater than that measured during administration of rhGH with and without acipimox (19). Finally, Piatti et al. (18) studied seven healthy men aged 25 ± 3 yr to show that the M-value was lower after a 6-h rhGH infusion than without the infusion, concomitant antilipolysis with acipimox, or while plasma FFAs were clamped at basal levels.
We complement and extend these findings in our study that was aimed to systematically delineate the dependent and independent effects of GH and FFAs on insulin sensitivity in patients with T1D, who provide a useful model for such investigations due to the lack of endogenous insulin secretion that, if present, is bound to hinder accurate assessments of artificially induced changes in insulin sensitivity. We showed that acipimox-induced decreases in circulating FFA levels entirely reversed the effects of GH: coadministration of acipimox and rhGH reduced overnight insulin requirements for euglycemia and plasma insulin levels, lowered basal glucose Ra, and increased basal glucose Rd as well as the M-value and glucose Rd during step 1 of the clamp. Furthermore, after adjustment for the increment in plasma insulin levels during the clamp, acipimox enhanced the suppression of glucose Ra from baseline to step 1 and led to a greater increase in glucose Rd from baseline to steps 1 and 2. These results suggest that GH-induced decreases in insulin sensitivity are, by and large, mediated through increases in circulating FFA levels, which in our study showed close positive correlations with overnight insulin requirements and basal glucose Ra and negative correlations with the M-value and glucose Rd, whereas GH levels showed no association to these measures of insulin sensitivity. In addition, acipimox-induced suppression of lipolysis in the absence of GH replacement was associated with a further reduction in basal glucose Ra and increases in glucose Rd during step 1 and the M-value during step 2 of the clamp, suggesting that FFAs may reduce insulin sensitivity independent of GH. However, an effect inherent to acipimox cannot be ruled out because there is no evidence in the literature to the contrary.
In conclusion, systematic analysis of the effects of GH and FFAs, dependent and independent of each other, on insulin sensitivity in patients with T1D showed that changes in circulating FFA levels have an intimate inverse relation to hepatic and peripheral insulin sensitivity, which we were able to elicit in response to low-dose insulin infusions (step 1). Given these findings, an even lower-dose insulin infusion to analyze the insulin sensitivity of adipocytes across the various study conditions would have been of interest, but this may prove difficult in light of the relatively high insulin requirements for euglycemia when GH, or rather FFAs, are present. High doses of insulin (step 2) tended to mask the effects of changes in circulating levels of GH and FFAs. However, all of our analyses may have been limited by the relatively small size of the cohort. For example, there was an apparent trend toward better glycemic control in the presence of acipimox, yet the relatively large interval of 15 min between successive measurements of blood glucose levels may have been a confounding factor in the interpretation of this trend. Moreover, an element of hepatic resistance to GH in T1D (47,48) could have minimized, if not negated, changes in glucose Ra in particular. Furthermore, we obtained negative values for glucose Ra during step 2 of the clamp despite a consistent tracer to tracee ratio for the glucose stable isotope, suggesting that the high infusion rates of glucose during this step of the clamp resulted in an underestimation of glucose Ra by the Steele model (30,49,50). Overall, our data support the notion that GH-driven decreases in insulin sensitivity are mainly determined by the effect of GH on lipolysis and are therefore the consequence of elevated plasma FFA levels, which provides a precedent for further examining means of safe and consistent reductions in FFA levels that may involve a study of the effect of longer-term inhibitions of GH function using, for example, the GH receptor antagonist pegvisomant (51).
Supplementary Material
Acknowledgments
We thank the study participants and research nurses at the WTCRF. We also thank Novo Nordisk for the provision of rhGH and glucagon and Novartis Pharmaceuticals for octreotide. At the time of the study, B.S. was a MB/PhD student at the School of Clinical Medicine, University of Cambridge, and M.L.M. was a European Society for Pediatric Endocrinology research fellow sponsored by Novo Nordisk A/S.
Footnotes
This work was supported by the National Institute for Health Research Cambridge Comprehensive Biomedical Research Centre.
Disclosure Summary: The authors have nothing to declare.
First Published Online June 30, 2009
Abbreviations: BMI, Body mass index; CV, coefficient of variation; FFA, free fatty acid; FFM, fat-free mass; rhGH, recombinant human GH; T1D, type 1 diabetes.
References
- Greenbaum CJ 2002 Insulin resistance in type 1 diabetes. Diabetes Metab Res Rev 18:192–200 [DOI] [PubMed] [Google Scholar]
- Edge JA, Matthews DR, Dunger DB 1990 Failure of current insulin regimes to meet the overnight requirements of adolescents with insulin-dependent diabetes. Diabetes Res 15:109–112 [PubMed] [Google Scholar]
- Ahmed ML, Ong KK, Watts AP, Morrell DJ, Preece MA, Dunger DB 2001 Elevated leptin levels are associated with excess gains in fat mass in girls, but not boys, with type 1 diabetes: longitudinal study during adolescence. J Clin Endocrinol Metab 86:1188–1193 [DOI] [PubMed] [Google Scholar]
- Edge JA, Dunger DB, Matthews DR, Gilbert JP, Smith CP 1990 Increased overnight growth hormone concentrations in diabetic compared with normal adolescents. J Clin Endocrinol Metab 71:1356–1362 [DOI] [PubMed] [Google Scholar]
- Edge JA, Matthews DR, Dunger DB 1990 The dawn phenomenon is related to overnight growth hormone release in adolescent diabetics. Clin Endocrinol (Oxf) 33:729–737 [DOI] [PubMed] [Google Scholar]
- Pal BR, Matthews DR, Edge JA, Mullis PE, Hindmarsh PC, Dunger DB 1993 The frequency and amplitude of growth hormone secretory episodes as determined by deconvolution analysis are increased in adolescents with insulin dependent diabetes mellitus and are unaffected by short-term euglycaemia. Clin Endocrinol (Oxf) 38:93–100 [DOI] [PubMed] [Google Scholar]
- Wurzburger MI, Sonksen PH 1996 Natural course of growth hormone hypersecretion in insulin-dependent diabetes mellitus. Med Hypotheses 46:145–149 [DOI] [PubMed] [Google Scholar]
- Boni-Schnetzler M, Schmid C, Meier PJ, Froesch ER 1991 Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol 260:E846–E851 [DOI] [PubMed] [Google Scholar]
- Baxter RC, Bryson JM, Turtle JR 1980 Somatogenic receptors of rat liver: regulation by insulin. Endocrinology 107:1176–1181 [DOI] [PubMed] [Google Scholar]
- Lee PD, Conover CA, Powell DR 1993 Regulation and function of insulin-like growth factor-binding protein-1. Proc Soc Exp Biol Med 204:4–29 [DOI] [PubMed] [Google Scholar]
- Shishko PI, Kovalev PA, Goncharov VG, Zajarny IU 1992 Comparison of peripheral and portal (via the umbilical vein) routes of insulin infusion in IDDM patients. Diabetes 41:1042–1049 [DOI] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Gerich JE 1982 Effects of growth hormone on insulin action in man. Mechanisms of insulin resistance, impaired suppression of glucose production, and impaired stimulation of glucose utilization. Diabetes 31:663–669 [DOI] [PubMed] [Google Scholar]
- Bratusch-Marrain PR, Smith D, DeFronzo RA 1982 The effect of growth hormone on glucose metabolism and insulin secretion in man. J Clin Endocrinol Metab 55:973–982 [DOI] [PubMed] [Google Scholar]
- Dominici FP, Argentino DP, Munoz MC, Miquet JG, Sotelo AI, Turyn D 2005 Influence of the crosstalk between growth hormone and insulin signalling on the modulation of insulin sensitivity. Growth Horm IGF Res 15:324–336 [DOI] [PubMed] [Google Scholar]
- Nielsen C, Gormsen LC, Jessen N, Pedersen SB, Moller N, Lund S, Jorgensen JO 2008 Growth hormone signaling in vivo in human muscle and adipose tissue: impact of insulin, substrate background, and growth hormone receptor blockade. J Clin Endocrinol Metab 93:2842–2850 [DOI] [PubMed] [Google Scholar]
- Norrelund H 2005 The metabolic role of growth hormone in humans with particular reference to fasting. Growth Horm IGF Res 15:95–122 [DOI] [PubMed] [Google Scholar]
- Neely RD, Rooney DP, Bell PM, Bell NP, Sheridan B, Atkinson AB, Trimble ER 1992 Influence of growth hormone on glucose-glucose 6-phosphate cycle and insulin action in normal humans. Am J Physiol 263:E980–E987 [DOI] [PubMed] [Google Scholar]
- Piatti PM, Monti LD, Caumo A, Conti M, Magni F, Galli-Kienle M, Fochesato E, Pizzini A, Baldi L, Valsecchi G, Pontiroli AE 1999 Mediation of the hepatic effects of growth hormone by its lipolytic activity. J Clin Endocrinol Metab 84:1658–1663 [DOI] [PubMed] [Google Scholar]
- Krag MB, Gormsen LC, Guo Z, Christiansen JS, Jensen MD, Nielsen S, Jorgensen JO 2007 Growth hormone-induced insulin resistance is associated with increased intramyocellular triglyceride content but unaltered VLDL-triglyceride kinetics. Am J Physiol Endocrinol Metab 292:E920–E927 [DOI] [PubMed] [Google Scholar]
- Norrelund H, Nielsen S, Christiansen JS, Jorgensen JO, Moller N 2004 Modulation of basal glucose metabolism and insulin sensitivity by growth hormone and free fatty acids during short-term fasting. Eur J Endocrinol 150:779–787 [DOI] [PubMed] [Google Scholar]
- Segerlantz M, Bramnert M, Manhem P, Laurila E, Groop LC 2003 Inhibition of lipolysis during acute GH exposure increases insulin sensitivity in previously untreated GH-deficient adults. Eur J Endocrinol 149:511–519 [DOI] [PubMed] [Google Scholar]
- Nielsen S, Moller N, Christiansen JS, Jorgensen JO 2001 Pharmacological antilipolysis restores insulin sensitivity during growth hormone exposure. Diabetes 50:2301–2308 [DOI] [PubMed] [Google Scholar]
- Debodo RC, Steele R, Altszuler N, Dunn A, Bishop JS 1963 On the hormonal regulation of carbohydrate metabolism: studies with C14 glucose. Recent Prog Horm Res 19:445–488 [PubMed] [Google Scholar]
- Steele R 1959 Influences of glucose loading and of injected insulin on hepatic glucose output. Ann NY Acad Sci 82:420–430 [DOI] [PubMed] [Google Scholar]
- Bluck LJC, Coward WA 1997 Peak measurement in gas chromatographic/mass spectrometric isotope studies. J Mass Spectrom 32:1212–1218 [Google Scholar]
- Rabinowitz D, Klassen GA, Zierler KL 1965 Effect of human growth hormone on muscle and adipose tissue metabolism in the forearm of man. J Clin Invest 44:51–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller N, Jorgensen JO, Schmitz O, Moller J, Christiansen J, Alberti KG, Orskov H 1990 Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol 258:E86–E91 [DOI] [PubMed] [Google Scholar]
- Moller N, Butler PC, Antsiferov MA, Alberti KG 1989 Effects of growth hormone on insulin sensitivity and forearm metabolism in normal man. Diabetologia 32:105–110 [DOI] [PubMed] [Google Scholar]
- Moller N, Schmitz O, Moller J, Butler PC 1992 Effects of a physiological growth hormone pulse on substrate metabolism in insulin-dependent (type 1) diabetic subjects. J Clin Endocrinol Metab 75:432–436 [DOI] [PubMed] [Google Scholar]
- Simpson HL, Jackson NC, Shojaee-Moradie F, Jones RH, Russell-Jones DL, Sonksen PH, Dunger DB, Umpleby AM 2004 Insulin-like growth factor I has a direct effect on glucose and protein metabolism, but no effect on lipid metabolism in type 1 diabetes. J Clin Endocrinol Metab 89:425–432 [DOI] [PubMed] [Google Scholar]
- Kahn CR, Goldfine ID, Neville Jr DM, De Meyts P 1978 Alterations in insulin binding induced by changes in vivo in the levels of glucocorticoids and growth hormone. Endocrinology 103:1054–1066 [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA 1983 Effect of fatty acids on glucose production and utilization in man. J Clin Invest 72:1737–1747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI 1996 Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest 97:2859–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Chen X, Ruiz J, White JV, Rossetti L 1994 Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest 93:2438–2446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigazio S, Lehto HR, Tuunanen H, Nagren K, Kankaanpaa M, Simi C, Borra R, Naum AG, Parkkola R, Knuuti J, Nuutila P, Iozzo P 2008 The lowering of hepatic fatty acid uptake improves liver function and insulin sensitivity without affecting hepatic fat content in humans. Am J Physiol Endocrinol Metab 295:E413–E419 [DOI] [PubMed] [Google Scholar]
- Gormsen LC, Jessen N, Gjedsted J, Gjedde S, Norrelund H, Lund S, Christiansen JS, Nielsen S, Schmitz O, Moller N 2007 Dose-response effects of free fatty acids on glucose and lipid metabolism during somatostatin blockade of growth hormone and insulin in humans. J Clin Endocrinol Metab 92:1834–1842 [DOI] [PubMed] [Google Scholar]
- Santomauro AT, Boden G, Silva ME, Rocha DM, Santos RF, Ursich MJ, Strassmann PG, Wajchenberg BL 1999 Overnight lowering of free fatty acids with Acipimox improves insulin resistance and glucose tolerance in obese diabetic and nondiabetic subjects. Diabetes 48:1836–1841 [DOI] [PubMed] [Google Scholar]
- Cusi K, Kashyap S, Gastaldelli A, Bajaj M, Cersosimo E 2007 Effects on insulin secretion and insulin action of a 48-h reduction of plasma free fatty acids with acipimox in nondiabetic subjects genetically predisposed to type 2 diabetes. Am J Physiol Endocrinol Metab 292:E1775–E1781 [DOI] [PubMed] [Google Scholar]
- Bajaj M, Medina-Navarro R, Suraamornkul S, Meyer C, DeFronzo RA, Mandarino LJ 2007 Paradoxical changes in muscle gene expression in insulin-resistant subjects after sustained reduction in plasma free fatty acid concentration. Diabetes 56:743–752 [DOI] [PubMed] [Google Scholar]
- Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA 2005 Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes 54:3148–3153 [DOI] [PubMed] [Google Scholar]
- Worm D, Vinten J, Vaag A, Henriksen JE, Beck-Nielsen H 2000 The nicotinic acid analogue acipimox increases plasma leptin and decreases free fatty acids in type 2 diabetic patients. Eur J Endocrinol 143:389–395 [DOI] [PubMed] [Google Scholar]
- Worm D, Henriksen JE, Vaag A, Thye-Ronn P, Melander A, Beck-Nielsen H 1994 Pronounced blood glucose-lowering effect of the antilipolytic drug acipimox in noninsulin-dependent diabetes mellitus patients during a 3-day intensified treatment period. J Clin Endocrinol Metab 78:717–721 [DOI] [PubMed] [Google Scholar]
- Carlson LA, Oro L 1962 The effect of nicotinic acid on the plasma free fatty acid; demonstration of a metabolic type of sympathicolysis. Acta Med Scand 172:641–645 [DOI] [PubMed] [Google Scholar]
- Pinter EJ, Pattee CJ 1967 Biphasic nature of blood glucose and free fatty acid changes following intravenous nicotinic acid in man. J Clin Endocrinol Metab 27:440–443 [DOI] [PubMed] [Google Scholar]
- Wang W, Basinger A, Neese RA, Shane B, Myong SA, Christiansen M, Hellerstein MK 2001 Effect of nicotinic acid administration on hepatic very low density lipoprotein-triglyceride production. Am J Physiol Endocrinol Metab 280:E540–E547 [DOI] [PubMed] [Google Scholar]
- Saloranta C, Taskinen MR, Widen E, Harkonen M, Melander A, Groop L 1993 Metabolic consequences of sustained suppression of free fatty acids by acipimox in patients with NIDDM. Diabetes 42:1559–1566 [DOI] [PubMed] [Google Scholar]
- Lanes R, Recker B, Fort P, Lifshitz F 1985 Impaired somatomedin generation test in children with insulin-dependent diabetes mellitus. Diabetes 34:156–160 [DOI] [PubMed] [Google Scholar]
- Wurzburger MI, Prelevic GM, Sonksen PH, Wheeler M, Balint-Peric L 1995 Effect of recombinant human growth hormone treatment on insulin-like growth factor (IGF-I) levels in insulin-dependent diabetic patients. Acta Diabetol 32:131–134 [DOI] [PubMed] [Google Scholar]
- Finegood DT, Bergman RN, Vranic M 1988 Modeling error and apparent isotope discrimination confound estimation of endogenous glucose production during euglycemic glucose clamps. Diabetes 37:1025–1034 [DOI] [PubMed] [Google Scholar]
- Levy JC, Brown G, Matthews DR, Turner RC 1989 Hepatic glucose output in humans measured with labeled glucose to reduce negative errors. Am J Physiol 257:E531–E540 [DOI] [PubMed] [Google Scholar]
- Williams RM, Amin R, Shojaee-Moradie F, Umpleby AM, Acerini CL, Dunger DB 2003 The effects of a specific growth hormone antagonist on overnight insulin requirements and insulin sensitivity in young adults with type 1 diabetes mellitus. Diabetologia 46:1203–1210 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.