Abstract
Atypical antipsychotics are also used in the treatment of anxiety-related disorders. Clinical and preclinical evidence regarding their intrinsic anxiolytic efficacy has been mixed. In this study, we examined the potential anxiolytic-like effects of risperidone and olanzapine, and compared them with haloperidol, chlordiazepoxide (a prototype of sedative-anxiolytic drug) or citalopram (a selective serotonin reuptake inhibitor). We used a composite of two-way avoidance conditioning and acoustic startle reflex model and examined the effects of drug treatments during the acquisition phase (Experiment1) or extinction phase (Experiment2 and 3) on multiple measures of conditioned and unconditioned fear/anxiety-like responses. In Experiment 4, we further compared risperidone, olanzapine, haloperidol, citalopram and chlordiazepoxide in a standard elevated plus maze test. Results revealed three distinct anxiolytic-like profiles associated with risperidone, olanzapine and chlordiazepoxide. Risperidone, especially at 1.0 mg/kg, significantly decreased the number of avoidance responses, 22 kHz ultrasonic vocalization, avoidance conditioning-induced hyperthermia and startle reactivity, but did not affect defecations or time spent on the open arms. Olanzapine (2.0 mg/kg, sc) significantly decreased the number of avoidance responses, 22 kHz vocalization and amount of defecations, but it did not inhibit startle reactivity and time spent on the open arms. Chlordiazepoxide (10 mg/kg, ip) significantly decreased the number of 22 kHz vocalization, avoidance conditioning-induced hyperthermia and amount of defecations, and increased time spent on the open arms, but did not decrease avoidance responses or startle reactivity. Haloperidol and citalopram did not display any anxiolytic-like property in these tests. The results highlight the importance of using multiple measures of fear-related responses to delineate behavioral profiles of psychotherapeutic drugs.
Keywords: risperidone, olanzapine, haloperidol, chlordiazepoxide, citalopram, conditioned avoidance response, stress-induced hyperthermia, startle responses, elevated plus maze, 22 kHz ultrasonic vocalizations
INTRODUCTION
Atypical antipsychotic drugs (APDs) such as risperidone and olanzapine, have been increasingly used for the treatment of anxiety-related disorders with mixed results (Carson and Kitagawa, 2004). Some case reports suggest that clozapine, olanzapine, quetiapine and risperidone improve symptoms of obsessive-compulsive disorder (OCD) and panic disorder, while an equal number of reports indicate the worsening effects of each drug on these disorders (Brooke et al., 2005). For other anxiety disorders, such as post-traumatic stress disorder (PTSD), some studies find that atypicals improve certain symptoms (e.g., hyperarousal and reexperiencing) (Hamner, 1996, Monnelly et al., 2003, Petty et al., 2001), while others failed to reach the same conclusion (Butterfield et al., 2001, Hamner et al., 2003).
Preclinical evidence on the intrinsic anxiolytic-like property of atypical antipsychotics is also inconclusive. Previous studies have found results that suggest an anxiolytic-like, anxiogenic-like or no effect on various anxiety-like measures (Ichihara et al., 1988, Ishida-Tokuda et al., 1996, Karl et al., 2006, Kovacs and de Wied, 1978, Moore et al., 1994, Moore et al., 1992, Thiessen and Upchurch, 1981, Timmerman et al., 1990). We think that at least two factors may contribute to this rather confusing literature. First, a wide variety of behavioral models have been used in different studies, which may not measure the same aspects of fear/anxiety-like responses and may not provide the same assessment of the drug effects. Also, most studies employ only one behavioral task or one response measure of fear, rather than a series of convergent tasks to cross-validate the findings. Thus it is difficult to make comparisons across different studies. Second, there are great variations in the experimental designs (e.g. species, drug doses, timing of drug administrations, etc), which may enhance or mask the effect size of one particular effect and influence data interpretation.
In a recent study (Mead et al., 2008), we evaluated the possible anxiolytic-like property of clozapine and olanzapine, and compared them with the typical antipsychotic haloperidol and chlordiazepoxide (a prototype of sedative-anxiolytic drug). The unique feature of that study was that we employed two different behavioral models (a fear-induced passive avoidance and conditioned place aversion paradigm and a two-way active avoidance conditioning paradigm) and multiple measures of fear/anxiety-like responses (both behavioral as well as physiological) so that our results were not an artifact of a single model or measure. The conditioned avoidance response model (CAR) is a fear-motivated instrumental conditioning model and is commonly used to study anti-“psychotic” activity (Bolles, 1970, Levis and Brewer, 2001, Rescorla and Solomon, 1967, Wadenberg and Hicks, 1999). It was used to study the possible anxiolytic-like effects of antipsychotics because animals tested in this model show various fear or anxiety-like signs, such as increased body temperature, emission of ultrasonic vocalization (termed 22-KHz USV), and defecation and urination, which have been routinely used as reliable measures of conditioned reactive fear as well as to assess anxiolytic-like properties of psychotropic drugs (De Vry et al., 1993, Fanselow, 1986, Godsil et al., 2000, Sanchez, 2003). Thus we were able to use this single behavioral paradigm to assess both antipsychotic (as indexed by anti-avoidance effect) and potential anxiolytic-like effects of antipsychotic drugs. Our results show that clozapine and olanzapine possess an intrinsic anxiolytic-like property, which is not attributable to their anti-“psychotic” effect or favorable effects on motor functions or learning and memory processes. Our findings also suggest that the combined use of multiple models is better in differentiating typical and atypical antipsychotics as well as anxiolytics.
Building on this success, we examined the potential anxiolytic-like property of risperidone and further examined that of olanzapine. We employed a similar multi-measure and multi-task approach (e.g. avoidance conditioning, startle reflex, and elevated plus maze) and tested drugs at both the acquisition and extinction stages of avoidance conditioning. In Experiment 1, we used a composite of avoidance conditioning and an acoustic startle reflex model and examined the dose-dependent effects of risperidone, citalopram and chlordiazepoxide treatment during the acquisition phase on the avoidance response as well as on a host of other fear responses (amount of defecation, ultrasonic vocalization, change in body temperature, fear-intensified startle reflex). In Experiments 2 and 3, we used the same model and examined the effects of risperidone and olanzapine treatment on the extinction of various fear-elicited responses. In Experiment 4, we used a standard elevated plus maze test (EPM), a more widely used animal model of anxiety, to cross-validate the findings from the first three experiments. We also compared risperidone and olanzapine with haloperidol, citalopram and chlordiazepoxide in this task.
MATERIALS AND METHODS
Subjects
Male Sprague-Dawley rats (250–275g upon arrival, Charles River, Portage, MI) were housed two per cage, in 48.3 cm × 26.7 cm × 20.3 cm transparent polycarbonate cages under 12-hr light/dark conditions (light on between 6:00am and 6:00pm). Room temperature was maintained at 22±1° with a relative humidity of 55–60%. Food and water was available ad libitum. Animals were allowed at least one week of habituation to the animal facility before being used in experiments. All procedures were approved by the animal care committees at the University of Nebraska-Lincoln.
Conditioned avoidance response and ultrasonic vocalization recording apparatus
Four identical two-way shuttle boxes custom designed and manufactured by Med Associates (St. Albans, VT) were used. Each box was housed in a ventilated, sound-insulated isolation cubicle (96.52 cm W×35.56 cm D ×63.5 cm H). Each box was 64 cm long, 30 cm high (from grid floor) and 24 cm wide, and divided into two equal-sized compartments by a white PVC partition with an arch style doorway (15cm high × 9cm wide at base). An aluminum hurdle (4cm high) was placed between the two compartments, so the rats had to jump from one compartment to the other. The grid floor consisted of 40 stainless steel rods with a diameter of 0.48 cm, spaced 1.6 cm apart center to center, through which a scrambled footshock (US, 0.8 mA) was delivered by a constant current shock generator (Model ENV-410B) and scrambler (Model ENV-412). The rat location and motor activity was detected by a set of 16 photobeams (ENV-256-8P) affixed at the bottom of the box (3.5 cm above the grid floor). Two houselights (28 volts) were mounted at the top of each compartment. The CS was a 76 dB white noise produced by a speaker (ENV 224AMX) mounted on the ceiling of the cubicle, centered above the shuttle box. All the training and testing procedures were controlled by Med Associates programs running on a computer. Background noise (approximately 74dB) was provided by a ventilation fan affixed at the top corner of each isolation cubicle.
In each CAR box, an ultrasonic vocalization microphone (P48 Avisoft Bioacoustics / Emkay Microphone, Avisoft Bioacoustics, Berlin, Germany) was mounted on the ceiling of the two-compartment chamber. The microphone was connected via an E-MU 0404 USB Audio device to a computer. Acoustic data were displayed in real time by the Avisoft RECORDER, a multi-channel triggering hard-disk recording software (version 3.4; Avisoft Bioacoustics), and were recorded at a sampling rate of 192 kHz in 16 bit format and analyzed by Avisoft SASLab Pro (version 4.51; Avisoft Bioacoustics).
Acoustic startle reflex apparatus
Four startle monitor systems (Kinder Scientific, Julian, CA), controlled by a PC were used as the testing apparatus. They were housed in compact sound attenuation cabinets (35.56 cm wide × 27.62 cm deep × 49.53 cm high). A speaker (diameter: 11 cm) mounted on the cabinet’s ceiling was used to generate acoustic stimuli (70dB-120 dB white noise). The startle activity was measured by a piezoelectric sensing platform on the floor. During testing, rats were placed in a rectangular box made of transparent Plexiglas (19 cm wide × 9.8 cm deep × 14.6 high) with an adjustable ceiling, providing only limited restraint while prohibiting ambulation.
Elevated plus maze apparatus
The elevated plus maze (EPM) was situated in a room with an illuminance about 52 Lux. It consisted of two open arms (50 cm×10 cm), two enclosed arms (50 cm×10 cm) and a central platform (10 cm×10 cm) made of black polycarbonate plastic. Each arm was supported by a sturdy plastic leg and was elevated 50cm above the floor. The two enclosed arms had high walls (38.5 cm in height), while the two open arms had raised edges (1.0 cm in height) along each side and end to decrease the possibility of falling during drug testing (Fernandes and File, 1996). Behavior was digitally recorded on a computer located in an adjacent room and automatically scored via Biobserve Viewer video tracking software (Biobserve, Germany).
Drugs
The injection solutions of haloperidol (HAL, 5.0 mg/ml ampoules, Sicor Pharmaceuticals, Inc, Irvine, CA) and chlordiazepoxide (CDP, Sigma-Aldrich, St. Louis, MO) were obtained by mixing drugs with sterile water. Olanzapine (OLZ, Toronto Research Chemicals Inc, Ontario, Canada) and risperidone (RIS, a gift from the NIMH drug supply program) were dissolved in 1.0–1.5% glacial acetic acid in sterile water. Citalopram (CIT, Toronto Research Chemicals Inc., Ontario, Canada) was dissolved in 0.9% physiological saline.
Experiment 1: Effects of risperidone, chlordiazepoxide and citalopram treatment during the acquisition phase of a composite CAR and startle reflex task on various fear/anxiety-like responses
Forty-eight rats were first habituated to the CAR boxes and startle boxes for 3 consecutive days (20 min in each box/day). On each habituation day, 1 h after injection of sterile water, rats were placed in the CAR boxes. The number of 22 kHz USV and amount of defecation (in mg) in the CAR boxes were recorded. Body temperature was measured using a probe (lubricated with mineral oil) inserted in the rectum (Thermalert TH-5, Physitemp, Clifton, NJ, USA) immediately before and after the CAR box exposure (20 min interval). Then, they were placed in the startle reflex boxes and exposed to the background noise (70dB) for 20 min before being returned to their home cages.
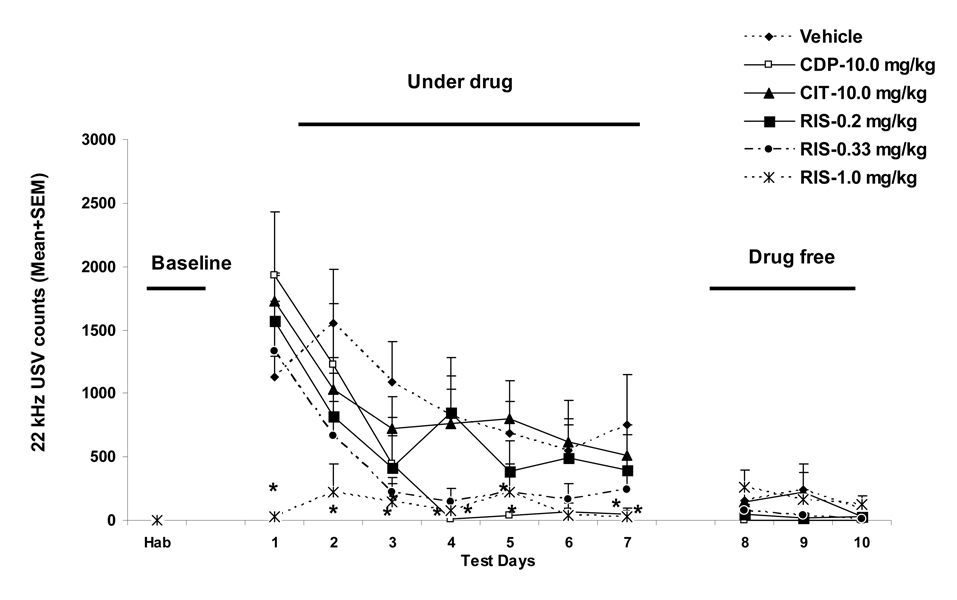
After the habituation, they were randomly assigned to 6 groups (n=8/group): vehicle (sterile water), RIS 0.2 mg/kg, 0.33 mg/kg, 1.0 mg/kg, CDP 10.0 mg/kg or CIT 10.0 mg/kg, and trained/tested under drug for 7 consecutive days, followed by 3 drug-free tests. We tested three doses of RIS, which covered subclinical, clinical and superclinical doses in terms of D2 receptor occupancy (50%–80%) to explore its dose-dependent effect (Kapur et al., 2003). CDP and CIT, two non-antipsychotic drugs were included as comparisons (Griebel et al., 1994, Mead, Li, 2008, Sanchez, 2003). Each daily drug test consisted of two components: a 20-trial two-way CAR session and a startle session. Rats were first injected with RIS, CDP, CIT or vehicle, and their body temperature was measured 0.5 h (in the case of CDP) or 1 h later (in the case of other treatments) (Mead, Li, 2008). Then, they were placed in the CAR boxes and trained for 20 trials. Each trial started by presenting a white noise (CS) for 10 seconds, followed by a continuous scrambled footshock (0.8 mA, US, maximum 5 seconds) on the grid floor. If a subject moved from one compartment into the other within the 10 seconds of CS presentation, it avoided the shock, and this shuttling response was recorded as avoidance. If the rat remained in the same compartment for more than 10s and made a crossing upon receiving the footshock, this response was recorded as escape. If the animal did not respond during the entire 5s presentation of the shock, the trial was terminated and escape failure was recorded. Intertrial intervals varied randomly between 30s and 60s (mean=45s). The number of avoidance responses (max: 20) was calculated as the main dependent variable for avoidance responding. Fecal matter was collected at the end of CAR session and weighed on a Mettler Toledo scale (<0.1 mg). Ultrasonic vocalizations at the 22 kHz range (20–32 kHz) were recorded using Avisoft Recorder software (Version 3.4). Settings included sampling rate at 192 kHz, format 16 bit. For acoustical analysis, recordings were transferred to Avisoft SASLab Pro (Version 4.51) and a fast Fourier transformation (FFT) was conducted. Spectrograms were generated with an FFT-length of 256 points and a time window overlap of 50% (100% Frame, FlatTop window). The spectrogram was produced at a frequency resolution of 750 Hz and a time resolution of 0.6667 ms. Call detection was provided by an automatic single threshold-based algorithm (threshold: −20 dB) and a hold-time mechanism (hold time: 0.02 s).
Immediately after the rats completed the CAR component of testing, they were placed in the acoustic startle boxes and tested for startle responses under 6 trial conditions (Davis, 1986, Walker and Davis, 2002). During the 5 min acclimation period (background noise set at 70dB), activity was sampled for 100 ms every 60 sec. After the acclimation period, 15 startle-eliciting stimuli (leaders) were given in a pseudorandom order, five at each of three different intensities (95, 100, and 105 dB, 50 ms in duration). The leaders were used to familiarize the rats to the acoustic stimuli and were not used for statistical analysis. Following the leaders, another 30 startle stimuli were presented, five for each type at three dB levels (95 dB, 100 dB, 105 dB alone; 76dB+95 dB, 76dB+100 dB, 76dB+105 dB) with a 30s intertrial interval. For the 95 dB, 100 dB and 105 dB alone trials, startle activity was sampled for 50 ms immediately after the white startle noise (95, 100, or 105dB) was presented. For the 76dB+95 dB, 76dB+100 dB, 76dB+105 dB trials, a 76 dB white noise was presented first for 3.2s, followed 10 ms later by one of the startle white noises (95, 100, or 105 dB) for 50 ms. Again, startle activity was sampled for 50 ms immediately after the white startle noise (95, 100, 105 dB) was presented. This 76 dB white noise was identical to the CS used in the CAR. It was hypothesized that startle activity under this condition encompasses an acquired fear component (“conditioned fear”), whereas startle activity under the startle noise-alone condition reflects an innate fear (“unconditioned fear”). Whole body startle responses were recorded in Newtons.
One day after the last training session, all rats were continuously tested drug-free for an additional 3 sessions under the CS-alone (no shock) condition. The procedure described above was employed identically except that the footshock was omitted.
Experiment 2: Effects of risperidone treatment (1.0 mg/kg) on the extinction of various fear/anxiety-like responses in the CAR-trained rats
Experiment 1 showed that RIS at 1.0 mg/kg exhibited a robust and consistent anxiolytic-like effect in the rats that were trained in the CAR. This experiment examined whether RIS at 1.0 mg/kg also exhibits a robust anxiolytic-like effect in rats that had already acquired avoidance behavior.
Thirty-six rats were first habituated to the CAR boxes for 2 days (20 min/day). Then, they were trained in the CAR for 10 sessions. The first 9 sessions used 20 trials, while the last session used 30 trials, in which the number of 22 kHz USV and amount of defecation were also recorded. Rats were then semi-randomly assigned to one of four groups matched for avoidance performance on the last training day (baseline): VEH-I (water, sc, n=9), VEH-D (water, sc, n=9), RIS-I (1.0 mg/kg, sc, n=9) and RIS-D (1.0 mg/kg, sc, n=9) and tested daily 1 h after RIS or vehicle treatment under the CS-only (no shock, 30 trials/daily session) condition for 3 consecutive days. In each test, the CS was immediately terminated after a rat made an avoidance response for rats in the VEH-I and RIS-I conditions (“I” stands for “Immediate”), or the CS remained on for another 5 seconds after a rat made an avoidance response for rats in the VEH-D and RIS-D conditions (“D” stands for “Delayed”). This delayed CS condition is known to cause increased fear and facilitate avoidance decline in the CAR-trained rats (Bolles and Grossen, 1970, Kamin, 1956). Each daily test began 60 minutes after RIS or vehicle administration, and each test session consisted of 30 trials. Immediately after being taken out from the CAR boxes, rats were placed in one of four startle boxes for startle activity testing. One day after the 3 days of drug testing, rats were tested drug-free in two additional CS-only sessions (30 trials/session) to examine the post-treatment effect.
Experiment 3: Effects of olanzapine treatment (2.0 mg/kg) on the extinction of various fear/anxiety-like responses in the CAR-trained rats
This experiment was identical to that of Experiment 2 with a couple of exceptions. First, 40 rats instead of 36 were used. Second, OLZ at 2.0 mg/kg was tested. Our previous work shows that when administrated during the acquisition phase of CAR, OLZ at 2.0 mg/kg significantly inhibited the body temperature increase and fear-induced defecation (Mead, Li, 2008). This work further investigated the anxiolytic-like effect of OLZ on the extinction of various fear-elicited responses.
Experiment 4: Effects of risperidone, olanzapine, haloperidol, chlordiazepoxide and citalopram treatment on an elevated plus maze test
Rats were first handled for two days (2 min/day/rat). On the testing day, they were first injected with RIS (0.33 or 1.0 mg/kg, sc, n=8/group), OLZ (0.5 or 1.0 mg/kg, sc, n=9 and 8 respectively), HAL (0.03 or 0.05 mg/kg, sc, n=8 and 9 respectively), CIT (10 mg/kg, sc, n=10), CDP (10 mg/kg, ip, n=18) or vehicle (sterile water, n=18). One hour later (or 0.5 h later for CDP rats), rats were individually placed in the central part of the maze facing one of the enclosed arms. The numbers of entries made into enclosed and open arms and time spent in enclosed and open arms were automatically recorded for 5 min using a video tracking system (Biobserve, Germany). An arm entry was defined as all four paws being placed on an arm. The subjects were run in batches, with a requirement that each batch contained at least 2 vehicle and two CDP controls. RIS and OLZ were assessed first, followed by tests on HAL and CIT.
Statistical analysis
Data were expressed as mean values ± SEM and were analyzed using a factorial repeated measures analysis of variance (ANOVA) with the between-subjects factor being treatment and/or group condition (e.g. RIS vs. VEH, immediate CS vs. delayed CS, etc.) and the within-subject factor being the test sessions (e.g. day 1 test, day 2 test, etc.) or test conditions (e.g. startle levels). Post hoc LSD tests were used to identify any possible drug treatment effect in comparison to vehicle control. To examine group difference on specific test days, one-way ANOVAs and post hoc tests were used. A conventional two-tailed level of significance at the 5% level was required.
RESULTS
Experiment 1: Effects of risperidone, chlordiazepoxide and citalopram treatment during the acquisition phase of a composite CAR and startle reflex task on various fear/anxiety-like responses
Avoidance response
Repeated measures ANOVA revealed a main effect of “treatment” (F(5,42)=8.671, p<0.001), “session” (F(6, 252)= 47.202, p<0.001) and a significant “treatment” × “session” interaction (F(30, 252)=3.555, p<0.001). Post hoc tests indicated that only the RIS 1.0 mg/kg group differed significantly from the vehicle group (p<0.001). Rats treated with CDP (10 mg/kg), CIT (10 mg/kg), RIS (0.2 and 0.33 mg/kg) or vehicle (distilled water) all showed a steady improvement in avoidance responding. On the three subsequent drug-free CS-only test days, once again, only the RIS 1.0 group still showed significantly fewer avoidance responses than the other drug groups (p<0.001). These data were originally reported as Figure 4 in Mead and Li (2009) for the purpose of studying the behavioral mechanisms of antipsychotic action in the CAR. None of the following data was reported before.
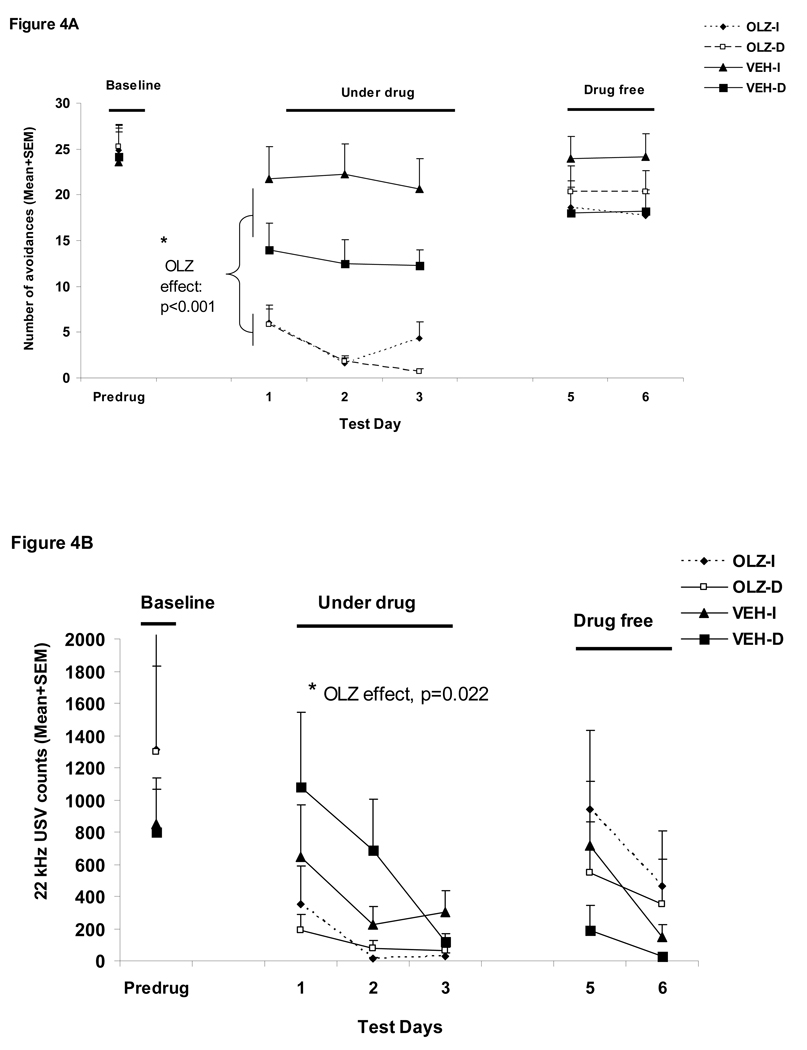
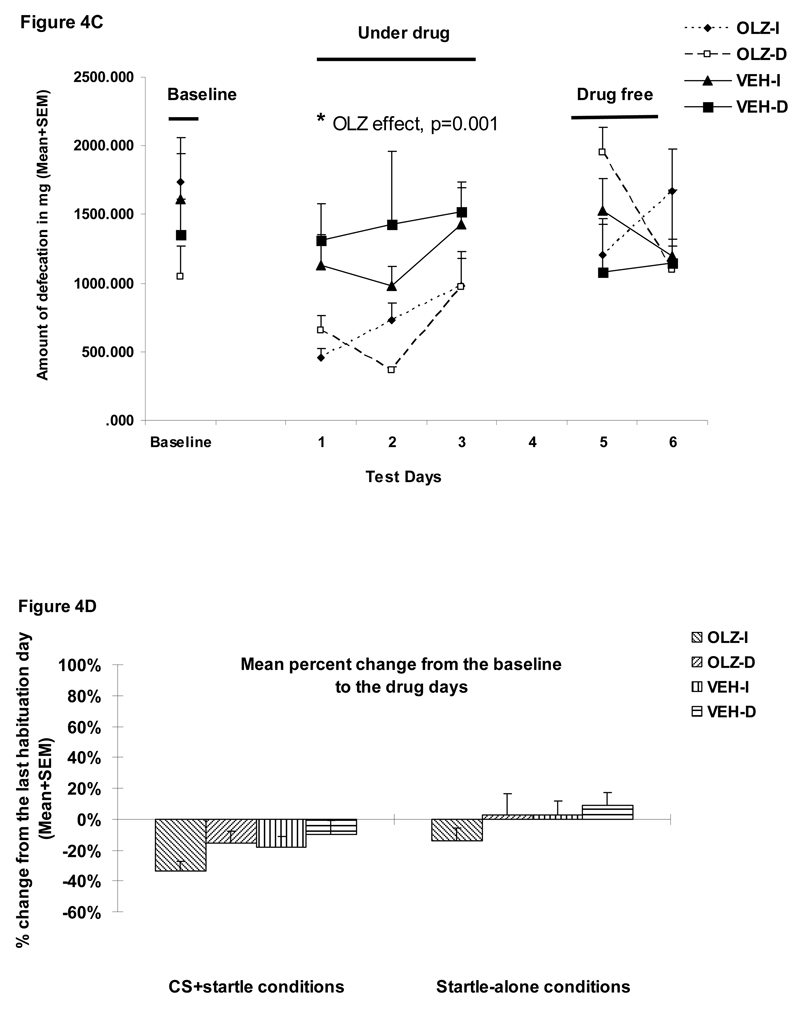
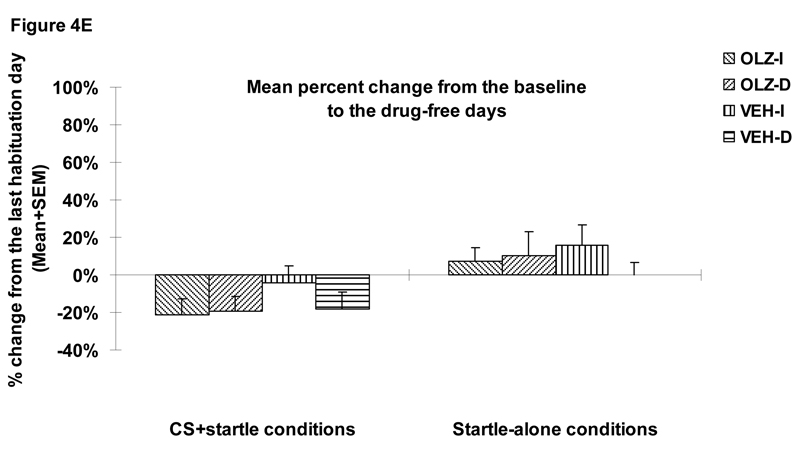
Figure 4.
Mean (+SEM) numbers of avoidance responses (A), 22 kHz USV counts (B), amount of defecations (C), percent change of the averaged startle activity under the CS+startle and the startle-alone conditions from the baseline to the drug days (D), and to the drug-free days (E) of the four groups of rats that were treated with olanzapine (2.0 mg/kg, sc) or vehicle (sterile water) and tested under the immediate CS termination (OLZ-I and VEH-I) or the delayed CS termination condition (OLZ-D and VEH-D). *p<0.05 significantly different from the vehicle group.
22 kHz USV
During the 7 CAR training days, the number of USV showed a progressive decline (F(6, 252)= 21.179, p<0.001; Figure 1). RIS (1.0 mg/kg) and CDP (10 mg/kg) decreased the number of 22 kHz USV in comparison to the vehicle treatment (a significant “treatment” × “session” interaction, F(30, 252)=2.299, p<0.001). One-way ANOVAs on each CAR training day showed that RIS (1.0 mg/kg) was significantly different from the vehicle on day 1 (p=0.020), day 2 (p=0.010), day 3 (p=0.014), day 4 (p=0.040), and day 7 (p=0.035). CDP (10 mg/kg) was significantly different from the vehicle on day 4 (p=0.027), day 5 (p=0.048), and day 7 (p=0.040). On the 3 drug-free CS-only test days, all groups exhibited fewer USV and no group difference was detected (“treatment”: F(5, 42)=1.531, p=0.201; “session”: F(2, 84)=2.445, p=0.093; “treatment” × “session” interaction, F(10, 84)=0.714, p=0.709).
Figure 1.
Mean (+SEM) numbers of 22 kHz ultrasonic vocalization (A) that the rats made throughout the habituation, seven CAR training days and three drug-free test days. * p<0.05 significantly different from the vehicle group.
Defecations and body temperature change
During the habituation days, both measures remained low, and no significant group difference was detected (all ps>0.05). During the CAR training phase, RIS (0.2, 1.0 mg/kg) and CDP (10 mg/kg) significantly decreased the avoidance conditioning-induced increase in body temperature (RIS 0.2 vs. VEH, p=0.050; RIS 1.0 vs. VEH, p=0.005; CDP vs. VEH, p=0.017, data not shown). CDP, but not RIS or CIT, also significantly decreased the amount of defecation (p=0.019). During the drug-free test phase, the effects of RIS and CDP were not longer present (all ps>0.05, data not shown). Interestingly, the RIS (1.0 mg/kg) rats defecated significantly more than the vehicle rats (p=0.020), indicating a drug-withdrawal-induced rebound.
Startle responses
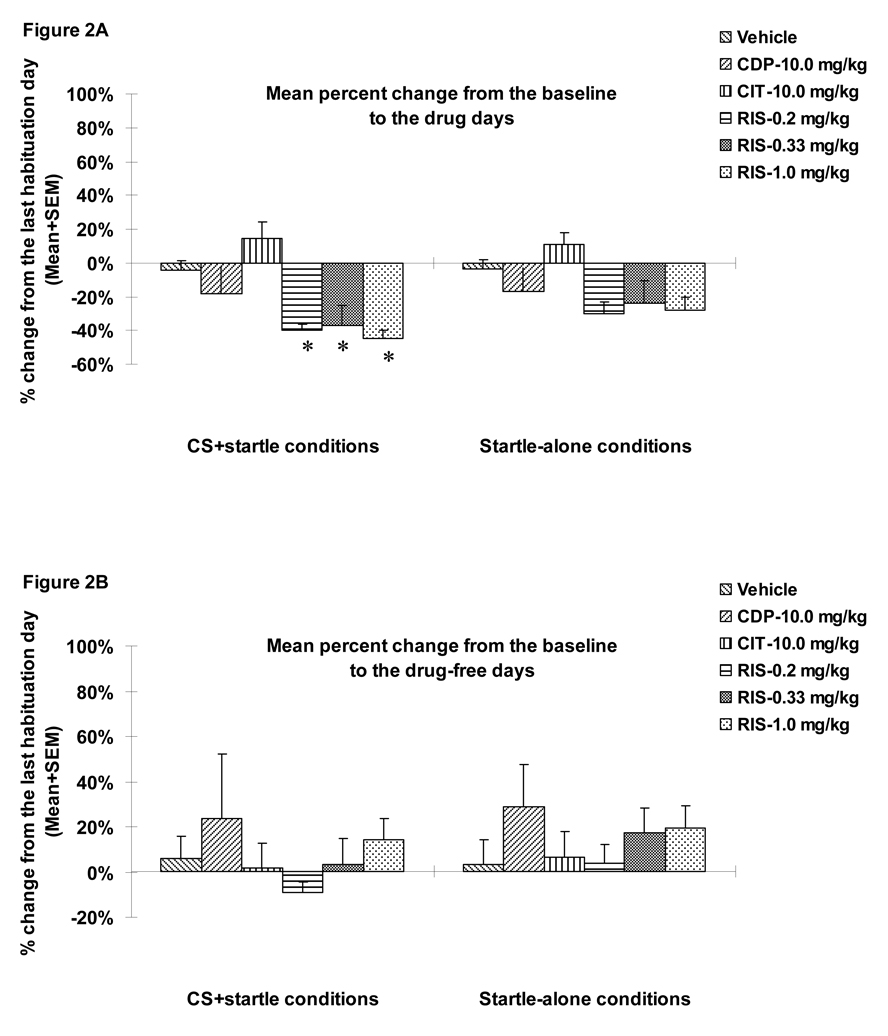
To simplify data presentation, we first averaged each rat’s startle activity data under the three startle-alone conditions (95 dB, 100 dB, 105 dB alone) and three CS+startle conditions (76dB+95 dB, 76dB+100 dB, 76dB+105 dB) separately. We then calculated the percent changes in the average startle activity for each rat from the pre-CAR day (i.e. the 3rd and last habituation day) to each of 7 drug test days and 3 drug-free test days. Finally, we averaged the percent changes for the 7 drug days and 3 drug-free days separately. Results are presented in Figure 2. During the drug test phase, RIS, but not CDP or CIT, suppressed the percent change of the mean startle activity from the baseline, and this suppressive effect of RIS disappeared when the drug treatment stopped. On the drug testing data, repeated measures ANOVA (drug treatment as a between-subjects factor and startle testing conditions (i.e. startle-alone or CS+startle) as a within-subjects factor) revealed a main effect of “testing condition” (F(1, 42)=5.491, p=0.024), “treatment” (F(5, 42)= 4.764, p=0.002), but no significant “testing condition” × “treatment” interaction (F(5, 42)=1.348, p=0.263). One-way ANOVAs on group differences indicated that the three RIS groups differed significantly from the vehicle group only under the CS+startle conditions (p=0.012, 0.023 and 0.005 for RIS 0.2, 0.33 and 1.0 mg/kg respectively), but not under the startle-alone conditions (all ps>0.05). To the extent that startle activity under the CS+startle conditions reflects an acquired fear (“conditioned fear”), whereas startle activity under the startle-alone condition reflects an innate fear (“unconditioned fear”), this result suggests that RIS may preferentially inhibit conditioned fear over unconditioned fear. During the drug-free test phase, the RIS treatment effect was no longer present (ps>0.05).
Figure 2.
Mean (+SEM) percent change from the baseline (the predrug day) to the drug test days of the averaged startle activity under the CS+startle conditions (i.e., 76dB+95 dB, 76dB+100 dB, 76dB+105 dB) and the startle-alone conditions (i.e., 95 dB, 100 dB, 105 dB alone) (A) and drug-free test days (B). * p<0.05 significantly different from the vehicle group.
Experiment 2: Effects of risperidone treatment (1.0 mg/kg) on the extinction of various fear/anxiety-like responses in the CAR-trained rats
Avoidance response
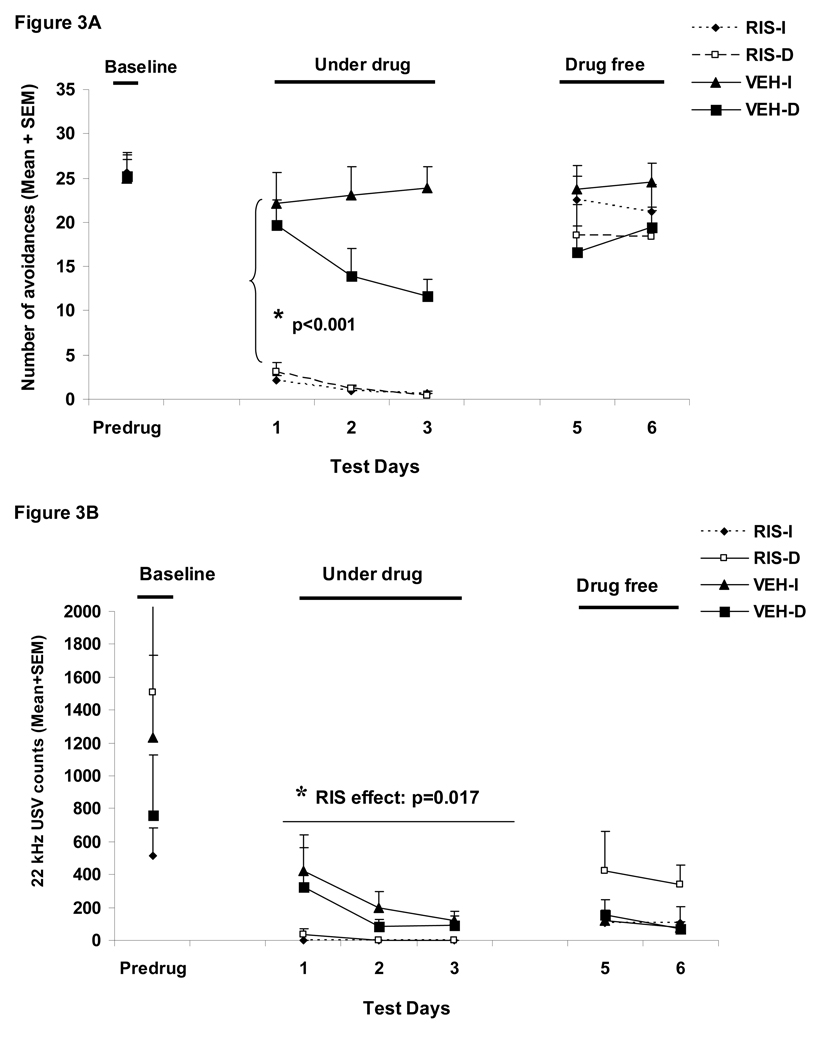
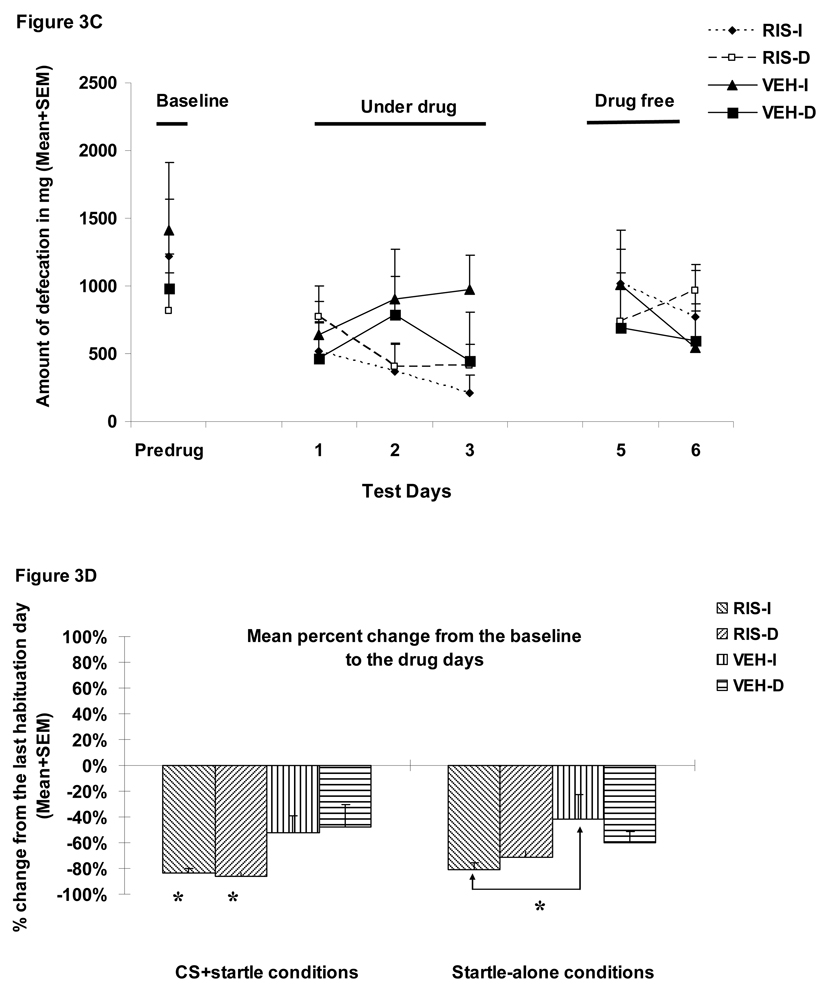
Figure 3A shows the number of conditioned avoidance responses in the four groups over the three phases (baseline, three drug test days and two drug-free days). RIS (1.0 mg/kg) significantly suppressed avoidance responding (F(1,32)=85.787, p<0.001), and the delayed termination of CS also caused a progressive decline in avoidance responding (F(1,32)=4.645, p=0.0390) during the drug test days. There was also a significant 3-way interaction among “treatment”, “condition” and “test day” (day 1, 2 and 3) (F(2, 64)= 6.328, p=0.003). No group difference during the two drug-free test days was detected (ps>0.05).
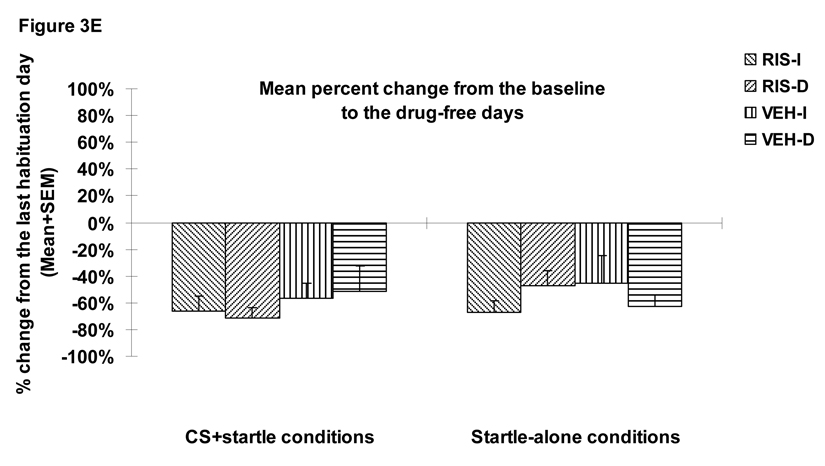
Figure 3.
Mean (+SEM) numbers of avoidance responses (A), 22 kHz USV counts (B), amount of defecations (C), percent change of the averaged startle activity under the CS+startle and the startle-alone conditions from the baseline to the drug days (D), and to the drug-free days (E) of the four groups of rats that were treated with risperidone (1.0 mg/kg, sc) or vehicle (sterile water) and tested under the immediate CS termination (RIS-I and VEH-I) or the delayed CS termination condition (RIS-D and VEH-D). * p<0.05 significantly different from the vehicle group.
22 kHz USV and defecations
During the drug test phase, RIS significantly decreased the number of 22 kHz vocalization calls in the well-trained rats (a main effect of “treatment: F(1, 32)=6.335, p=0.017, Figure 3B), but did not decrease the amount of defecation (F(1, 32)=2.329, p=0.137, Figure 3C). These findings were consistent with what we observed in Experiment 1. The CS testing condition did not impact both measures of fear (22 kHz USVs: F(1, 32)=0.191, p=0.665; defecations: F(1, 32)=0.107, p=0.746), nor did it interact with the RIS treatment on these measures (22 kHz USVs: F(1, 32)=0.332, p=0.569; defecations: F(1, 32)=1.695, p=0.202). No treatment effect or testing condition effect was present on the drug-free test days (ps>0.05).
Startle responses
RIS significantly decreased the mean percent startle activity change from the pre-drug day (the last CAR training day) to the drug test days (Figure 3D). There was a significant main effect of “treatment” (F(1, 32)=9.581, p=0.004), but no main effect of CAR testing condition (immediate vs. delayed CS, p=0.59). No significant interaction effect was found (ps>0.59). The mean percent startle activity change was also not affected by the startle testing conditions (i.e. the CS+startle or startle-alone condition) (F(1, 32)=0.618, p=0.438). One-way ANOVAs on the group differences indicated that the two RIS groups differed significantly from the two vehicle groups, mainly under the CS+startle conditions (ps=0.019–0.049), again suggesting that RIS preferentially inhibits startle activity that encompasses a conditioned fear component. The RIS-I group also showed a significant difference from the VEH-I group under the startle-alone condition (p=0.019). The RIS effect disappeared when the drug treatment stopped as no significant effect with “treatment”, “testing condition” or their interactions was found (Figure 3E, all ps>0.05).
Experiment 3: Effects of olanzapine treatment (2.0 mg/kg) on the extinction of various fear/anxiety-like responses in the CAR-trained rats
Avoidance response
Figure 4A shows the number of conditioned avoidance responses in the four groups over the three phases (baseline, three drug test days and two drug-free days). OLZ (2.0 mg/kg) significantly suppressed avoidance responding (F(1,36)=40.618, p<0.001), and the delayed termination of CS also caused a decrease in avoidance responding (F(1,36)=5.05, p=0.031). There was also a main effect of “test day” (F(2, 72)= 7.439, p=0.001), and a significant “treatment” × “test day” interaction (F(2, 72)=3.392, p=0.039). There was no group difference during the two drug-free test days (ps>0.4).
22 kHz USV and defecations
During the drug test phase, OLZ significantly decreased the number of 22 kHz calls (a main effect of “treatment: F(1, 36)=5.715, p=0.022, Figure 4B) and the amount of defecation (F(1, 36)=12.513, p=0.001, Figure 4C) in the well-trained rats. The CS testing condition did not impact both measures of fear (22 kHz USVs: F(1, 36)=0.439, p=0.512; defecations: F(1, 36)=0.738, p=0.396), nor did it interact with the OLZ treatment on these measures (22 kHz USVs: F(1, 36)=0.637, p=0.430; defecations: F(1, 36)=1.504, p=0.228). No treatment effect or testing condition effect was present during the drug-free test phase (all ps>0.05).
Startle responses
During the drug test phase, OLZ at 2.0 mg/kg did not significantly decrease the mean percent startle activity change (Figure 4D). There was no significant main effect of “treatment” (F(1, 36)=1.723, p=0.198), no main effect of CAR testing condition (immediate vs. delayed CS, F(1, 36)=2.105, p=0.155) or their interaction (F(1, 36)=0.374, p=0.545). However, the mean percent startle activity change was significantly affected by the startle testing conditions (i.e. the CS+startle or startle-alone condition) (F(1, 36)=25.847, p<0.001), with rats showing less decrease under the startle-alone condition than under the CS+startle condition. During the drug-free test phase, no significant treatment or CAR testing condition was found (Figure 4E, all ps>0.05).
Experiment 4: Effects of risperidone, olanzapine, haloperidol, chlordiazepoxide and citalopram treatment on an elevated plus maze test
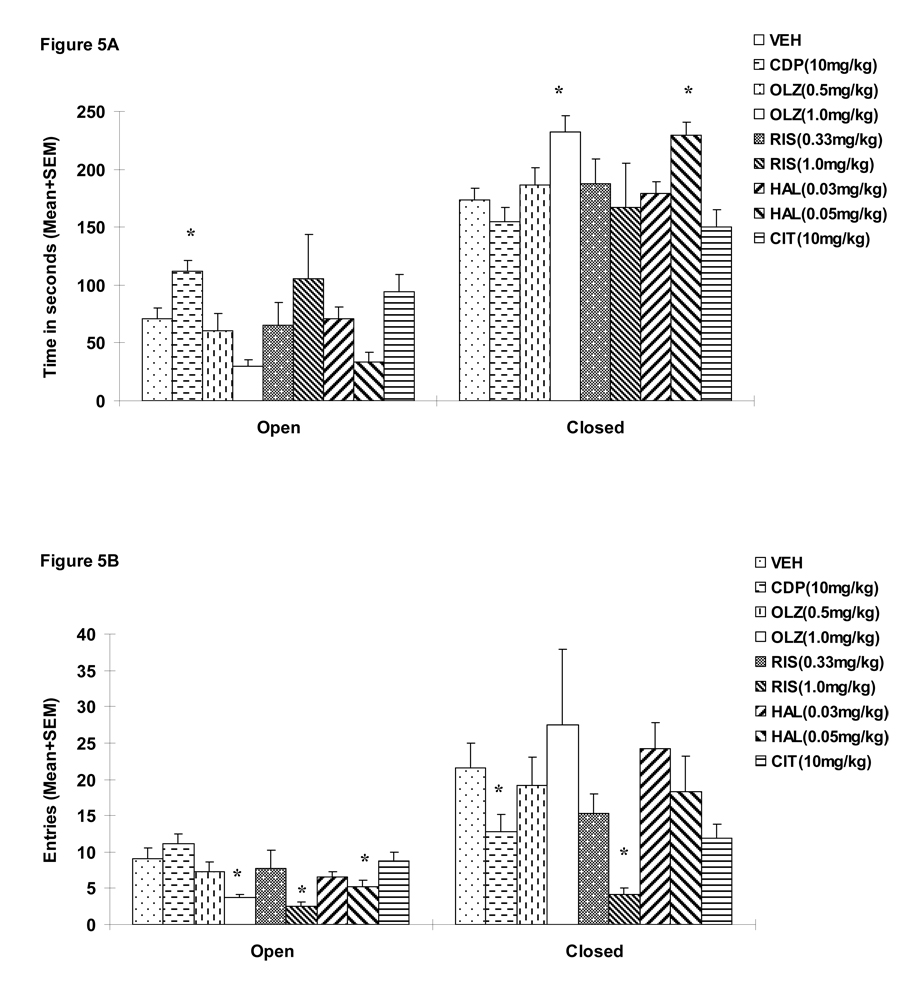
During the test, 1 rat in the CDP group and 1 rat in the OLZ 1.0 mg/kg fell off from the maze, and their data were not included in the analysis (Walf and Frye 2007). A repeated measures ANOVA on the time spent on the open arms (a within-subjects factor) revealed a significant effect of “treatment” (F(8, 85)=2.137, p=0.041), “arm” (F(1, 85)=111.248, p<0.001) and “treatment” × “arm” interaction (F(8, 85)=3.252, p=0.003). Similar analysis on the entries to the open arms also revealed a significant effect of “treatment” (F(8, 85)=2.573, p=0.015), “arm” (F(1, 85)=53.718, p<0.001) and “treatment” × “arm” interaction (F(8, 85)=3.154, p=0.004), suggesting that different drug treatments differentially affected time spent on the open and closed arms and the number of entries to the open and closed arms. One-way ANOVAs showed that only CDP (10.0 mg/kg) significantly increased time spent on the open arms (Figure 5A, ps=0.011 vs. VEH). In addition, OLZ (1.0 mg/kg), RIS (1.0 mg/kg) and HAL (0.05 mg/kg) significantly decreased the number of entries to the open arms (Figure 5B, ps=0.013, 0.02 and 0.049 respectively), suggesting a possible motor impairment effect.
Figure 5.
Mean (+SEM) time in seconds spent on the open and closed arms (A) and mean numbers of entries to the open and closed arms (B) of the rats that were treated with various doses of risperidone (0.33 or 1.0 mg/kg), olanzapine (0.5 or 1.0 mg/kg), haloperidol (0.03 or 0.05 mg/kg), chlordiazepoxide (10 mg/kg), citalopram (10 mg/kg), or vehicle (sterile water). * p<0.05 significantly different from the vehicle group.
DISCUSSIONS
Table 1 summarizes the effects of risperidone, olanzapine and chlordiazepoxide treatment on various fear and anxiety-like responses found in the present study. Three distinct anxiolytic-like profiles are apparent. Risperidone (1.0 mg/kg, sc) was efficacious against an increase in 22 kHz USV, body temperature, and startle reactivity elicited by conditioned fear, but was ineffective in decreasing the amount of defecations and increasing time spent on the open arms. Olanzapine (2.0 mg/kg, sc) was efficacious against an increase in 22 kHz USV and amount of defecations induced by fear, but was ineffective in decreasing startle reactivity and time spent on the open arms. Chlordiazepoxide (10 mg/kg, ip) was efficacious against an increase in 22 kHz USV, body temperature, and amount of defecations. It also increased time spent on the open arms, but was ineffective in decreasing startle reactivity. This study, together with our previous report (Mead, Li, 2008), and others (Moore, Rees, 1994, Moore, Tye, 1992, Wiley et al., 1993), strongly suggests that risperidone and olanzapine possess an anxiolytic-like property. Our results also indicate that haloperidol and citalopram, at least upon acute administration, did not seem to possess any anxiolytic-like properties, as seen in these fear/anxiety-like measures.
Table 1.
Summary of the effects of risperidone, olanzapine and chlordiazepoxide treatment on various fear and anxiety-like responses
| 22 kHz USV |
Defecation | Body temperature increase |
Startle reflex |
Open arm time in EPM |
Active avoidance responding |
|
|---|---|---|---|---|---|---|
| Risperidone | ↓ | _ | ↓ | ↓ | _ | ↓ |
| Olanzapine | ↓ | ↓ | ↓* | _ | _ | ↓ |
| Chlordiazepoxide | ↓ | ↓ | ↓ | _ | ↑ | _ |
“↑”: denotes a significant increasing effect. “↓”: denotes a significant decreasing effect. “_”: denotes no effect. “*”: the finding obtained from Mead et al. (2008).
Our results highlight the importance of using multiple measures of fear/anxietylike responses from several animal models to delineate behavioral profiles of psychotherapeutic drugs. As our results show, no two drugs are the same. A drug may be efficacious in lowering levels of fear shown in some measures but not in others. Therefore, it is insufficient to rely on a single measure of fear or anxiety-like behavior to determine an anxiolytic-like profile of a drug. With multiple measures of fear/anxietylike behavior, we not only determined that risperidone and olanzapine have an anxiolytic-like property, but also shed light against which aspects of fear or anxiety-like responses that risperidone, olanzapine or chlordiazepoxide is most effective. This work may be potentially useful in helping better understand the neural basis of the specific drug effects given the fact that each measurement of fear or anxiety-like responses (body temperature, ultrasonic vocalizations, defecation and urination) may be subserved by a distinct pathway from the central amygdala to a variety of brainstem regions (Davis and Whalen, 2001).
The present study extended our previous work (Mead, Li, 2008) in the following three directions. First, we examined risperidone, another widely prescribed second generation antipsychotic drug with a billion dollar revenue comparable to that of olanzapine. Second, we examined risperidone at both the acquisition and extinction phases of avoidance conditioning, and olanzapine at the extinction phase. Mead et al. (2008) only examined olanzapine at the acquisition phase. In Mead et al.(2008), we did not observe any decreasing effect of olanzapine on 22 kHz USV, whereas in the present study we did. This discrepancy may be due to the methodological differences. In Mead et al. (2008), olanzapine was given to rats that still experienced footshock at the acquisition phase, whereas in the present study, olanzapine was given to rats that were tested with only the CS at the extinction phase. Thus, the 22 kHz USV in Mead et al. (2008) still consisted of those that were elicited by footshock (an unconditioned fear), on which olanzapine may be less effective. Siemiatkowski et al. (2001) also reported that acute treatment with olanzapine (1.0 mg/kg, ip) reduced the pre-shock contextual 22 kHz USV (a measurement of a “conditioned fear”) but not the shock-elicited ultrasonic vocalizations. These findings suggest that olanzapine may be less effective against measurements of unconditioned fear than those of conditioned fear, a notion supported by its lack of effects on the EPM and startle reflex task (see below), and on the open field and holeboard test (Frye and Seliga, 2003).
Third, we incorporated an acoustic startle reflex test component in the two-way conditioned avoidance task. In each acoustic startle test, the rats was tested under both CS+startle and startle-alone conditions, reflecting putative “conditioned fear” and “unconditioned fear” respectively. Our results indicate that risperidone preferentially inhibits the conditioned fear-elicited startle reactivity over the innate fear-elicited one, whereas olanzapine does not inhibit either component. These profiles of risperidone and olanzapine are consistent with clinical (Wynnn et al., 2007) and preclinical work (Le Pen and Moreau, 2002, Moore, Rees, 1994, Moore, Tye, 1992, Swerdlow et al., 1996, Wiley, Compton, 1993) showing that risperidone and olanzapine generally do not decrease startle reactivity under the startle-alone conditions. These results are also consistent with the findings from the EPM test (Experiment 4) in which both risperidone and olanzapine did not affect the time spent and number of entries to the open arms, two measures of “unconditioned” fear/anxiety-like behavior (Walf and Frye, 2007). We are not aware of any previous work that has investigated the risperidone effect on the EPM. Our finding of olanzapine on the EPM is consistent with a recent study showing that olanzapine (0.5 mg/kg, ip) has little effect on anxiety-like behavior in the EPM test in normal rats, but shows an attenuation effect only in rats that have received an inescapable stress prior to the test on EPM (Locchi et al., 2008). However, this finding was inconsistent with that of Frye and Seliga (2003) who reported that olanzapine (5.0 or 10.0 mg/kg, i.p.) significantly increased time on the open arms. This difference may simply be due to dose differences. It is possible that only at a much higher dose does olanzapine exhibit an anxiolytic-like effect in the EPM. Because olanzapine at 1.0 mg/kg was effective on several measures of conditioned fear (e.g., CS-elicited 22 kHz USV, body temperature change, and defections), the EPM studies may indicate that in order to inhibit unconditioned fear (as measured in the EPM), a much higher dose of olanzapine may be required. More studies are needed to further determine whether risperidone and olanzapine at clinical relevant doses (Kapur et al. 2003) are truly more efficacious against conditioned fear versus unconditioned fear.
The neurobiological mechanism(s) of the anxiolytic-like action of olanzapine has implicated allopregnanolone, a metabolite of progesterone as an important molecule (Frye and Seliga, 2003, Ugale et al., 2004). Marx et al. (2006b; 2003) found that olanzapine can dose-dependently increase allopregnanolone in the rat cerebral cortex and hippocampus (Marx et al., 2006a, Marx et al., 2006b, Marx et al., 2003). Frye and Seliga (2003) also found that olanzapine’s anxiolytic-like effect coincides with its enhancing action on brain allopregnanolone (Frye and Seliga, 2003). Since allopregnanolone acts as a positive modulator of the GABAA receptor (Majewska, 1990) and shows a strong anxiolytic-like effect in the EPM task and the Geller-Seifter conflict test (Akwa et al., 1999, Bitran et al., 2000, Brot et al., 1997), it is therefore possible that olanzapine-induced elevations in allopregnanolone may contribute to their anxiolytic-like effect. On the other hand, the neurobiological mechanisms that mediate the anxiolytic-like action of risperidone are less clear. Because risperidone has a complex multiple-receptor binding profile, with higher affinities for D2, D3, and 5-HT2A, 5-HT7 receptors but weaker affinities to D4, 5-HT6, histaminic H1, and muscarinic m1 receptors (Horacek et al., 2006, Miyamoto et al., 2005, Seeman, 2006), it is difficult to pinpoint which receptor action or actions account for its anxiolytic-like action. Since risperidone’s antagonist action on 5-HT2A receptors has been suggested to be important for the improved therapeutic effects of risperidone on negative symptoms of schizophrenia, it is possible that risperidone may exhibit an anti-anxiety-like effect by antagonizing 5-HT2A receptors, especially the ones located on glutamatergic pyramidal neurons and GABAergic interneurons in the cortex and hippocampus (Meltzer et al., 2003). Future research should direct attention to test this hypothesis.
In summary, the present study demonstrates that atypical APDs such as risperidone and olanzapine do possess an anxiolytic-like efficacy in addition to their antipsychotic efficacy. This additional efficacy is different from that of chlordiazepoxide, a traditional anxiolytic drug. Our findings are important because they can be utilized to develop methods of ameliorating anxiety or fear in schizophrenics (Blin et al. 1996) and provide some guidelines on which drug to use for different manifestations (e.g., behavioral, emotional, and physiological, etc) of anxiety symptoms.
Acknowledgments
This study was funded in part by a support from the NIMH (R21MH079894) to Dr. Ming Li. We thank Natashia Swalve for her help on proofreading this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akwa Y, Purdy RH, Koob GF, Britton KT. The amygdala mediates the anxiolytic-like effect of the neurosteroid allopregnanolone in rat. Behav Brain Res. 1999;106:119–25. doi: 10.1016/s0166-4328(99)00101-1. [DOI] [PubMed] [Google Scholar]
- Bitran D, Klibansky DA, Martin GA. The neurosteroid pregnanolone prevents the anxiogenic-like effect of inescapable shock in the rat. Psychopharmacology (Berl) 2000;151:31–7. doi: 10.1007/s002130000472. [DOI] [PubMed] [Google Scholar]
- Bolles RC. Species-specific defense reactions and avoidance learning. Psychological Review. 1970;77:32–48. [Google Scholar]
- Bolles RC, Grossen NE. Function of the CS in shuttle-box avoidance learning by rats. J Comp Physiol Psychol. 1970;70:165–9. doi: 10.1037/h0028412. [DOI] [PubMed] [Google Scholar]
- Brooke NS, Wiersgalla M, Salzman C. Atypical uses of atypical antipsychotics. Harv Rev Psychiatry. 2005;13:317–39. doi: 10.1080/10673220500433148. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABA(A) receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Butterfield MI, Becker ME, Connor KM, Sutherland S, Churchill LE, Davidson JR. Olanzapine in the treatment of post-traumatic stress disorder: a pilot study. Int Clin Psychopharmacol. 2001;16:197–203. doi: 10.1097/00004850-200107000-00003. [DOI] [PubMed] [Google Scholar]
- Carson WH, Kitagawa H. Drug development for anxiety disorders: new roles for atypical antipsychotics. Psychopharmacol Bull. 2004;38:38–45. [PubMed] [Google Scholar]
- Davis M. Pharmacological and anatomical analysis of fear conditioning using the fear-potentiated startle paradigm. Behav Neurosci. 1986;100:814–24. doi: 10.1037//0735-7044.100.6.814. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Vry J, Benz U, Schreiber R, Traber J. Shock-induced ultrasonic vocalization in young adult rats: a model for testing putative anti-anxiety drugs. Eur J Pharmacol. 1993;249:331–9. doi: 10.1016/0014-2999(93)90530-u. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned fear-induced opiate analgesia: a competing motivational state theory of stress analgesia. Ann N Y Acad Sci. 1986;467:40–54. doi: 10.1111/j.1749-6632.1986.tb14617.x. [DOI] [PubMed] [Google Scholar]
- Fernandes C, File SE. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol Biochem Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- Frye CA, Seliga AM. Olanzapine's effects to reduce fear and anxiety and enhance social interactions coincide with increased progestin concentrations of ovariectomized rats. Psychoneuroendocrinology. 2003;28:657–73. doi: 10.1016/s0306-4530(02)00049-5. [DOI] [PubMed] [Google Scholar]
- Godsil BP, Quinn JJ, Fanselow MS. Body temperature as a conditional response measure for pavlovian fear conditioning. Learn Mem. 2000;7:353–6. doi: 10.1101/lm.32800. [DOI] [PubMed] [Google Scholar]
- Griebel G, Moreau JL, Jenck F, Misslin R, Martin JR. Acute and chronic treatment with 5-HT reuptake inhibitors differentially modulate emotional responses in anxiety models in rodents. Psychopharmacology (Berl) 1994;113:463–70. doi: 10.1007/BF02245224. [DOI] [PubMed] [Google Scholar]
- Hamner MB. Clozapine treatment for a veteran with comorbid psychosis and PTSD. Am J Psychiatry. 1996;153:841. doi: 10.1176/ajp.153.6.841. [DOI] [PubMed] [Google Scholar]
- Hamner MB, Faldowski RA, Ulmer HG, Frueh BC, Huber MG, Arana GW. Adjunctive risperidone treatment in post-traumatic stress disorder: a preliminary controlled trial of effects on comorbid psychotic symptoms. Int Clin Psychopharmacol. 2003;18:1–8. doi: 10.1097/00004850-200301000-00001. [DOI] [PubMed] [Google Scholar]
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389–409. doi: 10.2165/00023210-200620050-00004. [DOI] [PubMed] [Google Scholar]
- Ichihara K, Nabeshima T, Kameyama T. Effects of haloperidol, sulpiride and SCH 23390 on passive avoidance learning in mice. Eur J Pharmacol. 1988;151:435–42. doi: 10.1016/0014-2999(88)90540-7. [DOI] [PubMed] [Google Scholar]
- Ishida-Tokuda K, Ohno Y, Sakamoto H, Ishibashi T, Wakabayashi J, Tojima R, et al. Evaluation of perospirone (SM-9018), a novel serotonin-2 and dopamine-2 receptor antagonist, and other antipsychotics in the conditioned fear stress-induced freezing behavior model in rats. Jpn J Pharmacol. 1996;72:119–26. doi: 10.1254/jjp.72.119. [DOI] [PubMed] [Google Scholar]
- Kamin LJ. The effects of termination of the CS and avoidance of the US on avoidance learning. J Comp Physiol Psychol. 1956;49:420–4. doi: 10.1037/h0088011. [DOI] [PubMed] [Google Scholar]
- Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–31. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, O'Brien E, Matsumoto I, Dedova I. Behavioural effects of chronic haloperidol and risperidone treatment in rats. Behav Brain Res. 2006 doi: 10.1016/j.bbr.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Kovacs GL, de Wied D. Effects of amphetamine and haloperidol on avoidance behavior and exploratory activity. Eur J Pharmacol. 1978;53:103–7. doi: 10.1016/0014-2999(78)90272-8. [DOI] [PubMed] [Google Scholar]
- Le Pen G, Moreau JL. Disruption of prepulse inhibition of startle reflex in a neurodevelopmental model of schizophrenia: reversal by clozapine, olanzapine and risperidone but not by haloperidol. Neuropsychopharmacology. 2002;27:1–11. doi: 10.1016/S0893-133X(01)00383-9. [DOI] [PubMed] [Google Scholar]
- Levis DJ, Brewer KE. The neurotic paradox: Attempts by two-factor fear theory and alternative avoidance models to resolve the issues associated with sustained avoidance responding in extinction. In: Mowrer RR, Klein SB, editors. Handbook of contemporary learning theories. Mahwah, NJ: Lawrence Erlbaum Associates; 2001. pp. 561–97. [Google Scholar]
- Locchi F, Dall'olio R, Gandolfi O, Rimondini R. Olanzapine counteracts stress-induced anxiety-like behavior in rats. Neurosci Lett. 2008;438:146–9. doi: 10.1016/j.neulet.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Majewska MD. Steroid regulation of the GABAA receptor: ligand binding, chloride transport and behaviour. Ciba Found Symp. 1990;153:83–97. doi: 10.1002/9780470513989.ch5. discussion −106. [DOI] [PubMed] [Google Scholar]
- Marx CE, Shampine LJ, Duncan GE, VanDoren MJ, Grobin AC, Massing MW, et al. Clozapine markedly elevates pregnenolone in rat hippocampus, cerebral cortex, and serum: candidate mechanism for superior efficacy? Pharmacol Biochem Behav. 2006a;84:598–608. doi: 10.1016/j.pbb.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Marx CE, Shampine LJ, Khisti RT, Trost WT, Bradford DW, Grobin AC, et al. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol Biochem Behav. 2006b;84:609–17. doi: 10.1016/j.pbb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28:1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- Mead A, Li M. Avoidance-suppressing effect of antipsychotic drugs is progressively potentiated after repeated administration: an interoceptive drug state mechanism. J Psychopharmacol. 2009 doi: 10.1177/0269881109102546. [DOI] [PubMed] [Google Scholar]
- Mead A, Li M, Kapur S. Clozapine and olanzapine exhibit an intrinsic anxiolytic property in two conditioned fear paradigms: contrast with haloperidol and chlordiazepoxide. Pharmacol Biochem Behav. 2008;90:551–62. doi: 10.1016/j.pbb.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Li Z, Kaneda Y, Ichikawa J. Serotonin receptors: their key role in drugs to treat schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1159–72. doi: 10.1016/j.pnpbp.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Monnelly EP, Ciraulo DA, Knapp C, Keane T. Low-dose risperidone as adjunctive therapy for irritable aggression in posttraumatic stress disorder. J Clin Psychopharmacol. 2003;23:193–6. doi: 10.1097/00004714-200304000-00012. [DOI] [PubMed] [Google Scholar]
- Moore NA, Rees G, Sanger G, Tye NC. Effects of olanzapine and other antipsychotic agents on responding maintained by a conflict schedule. Behav Pharmacol. 1994;5:196–202. doi: 10.1097/00008877-199404000-00011. [DOI] [PubMed] [Google Scholar]
- Moore NA, Tye NC, Axton MS, Risius FC. The behavioral pharmacology of olanzapine, a novel "atypical" antipsychotic agent. J Pharmacol Exp Ther. 1992;262:545–51. [PubMed] [Google Scholar]
- Petty F, Brannan S, Casada J, Davis LL, Gajewski V, Kramer GL, et al. Olanzapine treatment for post-traumatic stress disorder: an open-label study. Int Clin Psychopharmacol. 2001;16:331–7. doi: 10.1097/00004850-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychol Rev. 1967;74:151–82. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Sanchez C. Stress-induced vocalisation in adult animals. A valid model of anxiety? Eur J Pharmacol. 2003;463:133–43. doi: 10.1016/s0014-2999(03)01277-9. [DOI] [PubMed] [Google Scholar]
- Seeman P. Targeting the dopamine D2 receptor in schizophrenia. Expert Opin Ther Targets. 2006;10:515–31. doi: 10.1517/14728222.10.4.515. [DOI] [PubMed] [Google Scholar]
- Siemiatkowski M, Maciejak P, Sienkiewicz-Jarosz H, Czlonkowska AI, Szyndler J, Gryczynska A, et al. Opposite effects of olanzapine and haloperidol in rat ultrasonic vocalization test. Pol J Pharmacol. 2001;53:669–73. [PubMed] [Google Scholar]
- Swerdlow NR, Bakshi V, Geyer MA. Seroquel restores sensorimotor gating in phencyclidine-treated rats. J Pharmacol Exp Ther. 1996;279:1290–9. [PubMed] [Google Scholar]
- Thiessen DD, Upchurch M. Haloperidol and clonidine increase, and apomorphine decreases ultrasonic vocalizations by gerbils. Psychopharmacology (Berl) 1981;75:287–90. doi: 10.1007/BF00432440. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Tepper PG, Bohus BG, Horn AS. The potential antipsychotic activity of the partial dopamine receptor agonist (+)N-0437. Eur J Pharmacol. 1990;181:253–60. doi: 10.1016/0014-2999(90)90086-l. [DOI] [PubMed] [Google Scholar]
- Ugale RR, Hirani K, Morelli M, Chopde CT. Role of neuroactive steroid allopregnanolone in antipsychotic-like action of olanzapine in rodents. Neuropsychopharmacology. 2004;29:1597–609. doi: 10.1038/sj.npp.1300460. [DOI] [PubMed] [Google Scholar]
- Wadenberg ML, Hicks PB. The conditioned avoidance response test re-evaluated: is it a sensitive test for the detection of potentially atypical antipsychotics? Neurosci Biobehav Rev. 1999;23:851–62. doi: 10.1016/s0149-7634(99)00037-8. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc. 2007;2:322–8. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–92. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Compton AD, Porter JH. Effects of four antipsychotics on punished responding in rats. Pharmacol Biochem Behav. 1993;45:263–7. doi: 10.1016/0091-3057(93)90237-n. [DOI] [PubMed] [Google Scholar]
- Wynn JK, Green MF, Sprock J, Light GA, Widmark C, Reist C, et al. Effects of olanzapine, risperidone and haloperidol on prepulse inhibition in schizophrenia patients: a double-blind, randomized controlled trial. Schizophr Res. 2007;95:134–42. doi: 10.1016/j.schres.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]