Abstract
Voltage-activated sodium (Nav) channels are essential in generating and propagating nerve impulses, placing them amongst the most widely targeted ion channels by toxins from venomous organisms. An increasing number of spider toxins have been shown to interfere with the voltage-driven activation process of mammalian Nav channels, possibly by interacting with one or more of their voltage sensors. This review focuses on our existing knowledge of the mechanism by which spider toxins affect Nav channel gating and the possible applications of these toxins in the drug discovery process.
Introduction
Voltage-activated sodium (Nav) channels are Na+ permeable ion channels that open and close in response to changes in membrane voltage and primarily contribute to the rising phase of the action potential1, 2. In humans, the medical relevance of Nav channels is reflected by mutations that underlie debilitating disorders such as cardiac arrhythmias, epilepsy, muscle weakness, and erythermalgia3-6. In extreme cases, Nav channel abnormalities can even eliminate a person’s ability to feel pain, enabling them to walk on burning coals7. For these reasons, Nav channels are considered to be important therapeutic targets. Yet, our appreciation of these channels is hampered by the lack of insight into their complex structure and working mechanism.
Nine mammalian Nav channel isoforms have been identified (Nav1.1-Nav1.9)8 and share a similar, but complex architecture. The principle channel-forming α-subunit consists of four homologous domains (I-IV), each containing six transmembrane segments (S1-S6). The S5-S6 segments from the four domains collectively form the central ion conduction pore for Na+, with the S1-S4 segments from each domain forming the surrounding voltage sensors (Fig. 1a, b). In a few subtypes where it has been examined (e.g. Nav1.2, Nav1.4 and Nav1.5), each of the four voltage sensors activate in response to changes in membrane voltage, however, those in domains I-III are most important for channel opening, whereas the one in domain IV plays a unique role in inactivating the channel only milliseconds after channel opening9-12. It remains to be seen if this modus operandi applies to all Nav channel subtypes, or whether the voltage sensors in more distantly related Nav channels like Nav1.8 and Nav1.9 will have distinct operational mechanisms.
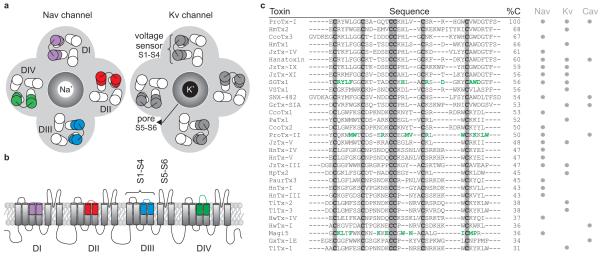
Figure 1. Spider toxins that target voltage-activated ion channels.
a. Cartoon representing a top view of a Nav channel (left) and a Kv channel (right). The central Na+- or K+-selective pore is surrounded by the four voltage sensors of the four domains (DI-DIV). In the Nav channel, the paddles are not identical and are therefore colored differently. In the Kv channel, the paddles are identical and therefore have the same color. b. Cartoon of a side view of a Nav channel imbedded in a lipid membrane. Each domain (DI-DIV) consists of six transmembrane segments (S1-S6) of which S1-S4 form the voltage sensor and the S5-S6 segments of each domain come together to form the Na+-selective pore of the channel. c. Sequence alignment of spider toxins with an ICK motif and three disulfide bridges that target Nav, Kv, and/or Cav channels (indicated by grey circles). Residues that have been shown to be a part of the functionally important surfaces of SGTx138, ProTx-II33, and Magi536 are indicated in green. %C = % conserved residues.
Nav channels are one of the foremost targets of molecules present in animal venoms13. In fact, toxins from scorpion, sea anemone, and cone snail venoms have been used to describe a variety of receptor sites in different regions of the channel14-17. However, the exploration of the mechanism through which spider toxins interact with mammalian Nav channels has only recently begun. This is in contrast to voltage-activated potassium (Kv) channels, where tarantula toxins like hanatoxin from Grammostola spatulata have been used extensively to study the functional properties of these channels18-21. Evidence demonstrating that tarantula toxins modify Kv channel opening and closing, or ‘gating’, by influencing their voltage sensors comes from three observations. First, toxin-bound channels can still open and conduct ions but the energy required to open toxin-bound channels is typically increased22-24. Second, mutagenesis experiments suggest that the toxins interact with defined regions within the voltage sensors21, 25, 26. Third, these toxins have distinct effects on movements of the voltage sensors as reflected in gating current measurements27. (Charged arginine residues in the S4 segment of the voltage sensor move in response to a change in membrane voltage, and this movement can be detected as a non-linear capacitive current or gating current)28.
ProTx-I and ProTx-II from Thrixopelma pruriens are two of the better studied toxins that modify gating of mammalian Nav channels12, 29-34. These tarantula toxins are closely related to hanatoxin (Fig. 1c) and appear to work through similar mechanisms (see below), which fits nicely with the recent discovery that hanatoxin itself can also inhibit Nav channels at concentrations similar to those that modify Kv channel gating12. A large number of spider toxins with related amino acid sequences have now been identified (Fig. 1c), many of which interact with Nav channels and modify gating through what appears to be three distinct mechanisms. The first, and most commonly observed, is for the toxin to inhibit opening of the channel in response to membrane depolarization12, 29-31, 33, 34, as illustrated in Fig. 2a for ProTx-I. A second mechanism is for the toxin to hinder fast inactivation, as observed for SGTx1 from the Scodra griseipes tarantula (Fig. 2a) and JzTx-IV from the Chilobrachys jingzhao tarantula35. A third, as observed in the case of Magi5 from the hexathelid spider Macrothele gigas, is for the toxin to facilitate opening of the channel by shifting activation of Nav channels to more hyperpolarized voltages36 (Fig. 2a). Although these disparate effects of Nav channel toxins seem complex, they can be understood in conceptually simple terms when one considers the voltage sensors they target and how those sensors couple to various aspects of Nav channel gating. We will review what is know about the interaction of spider toxins with the four voltage sensors in Nav channels, the emerging role of the lipid membrane in determining the pharmacology of Nav channels, and potential medicinal applications.
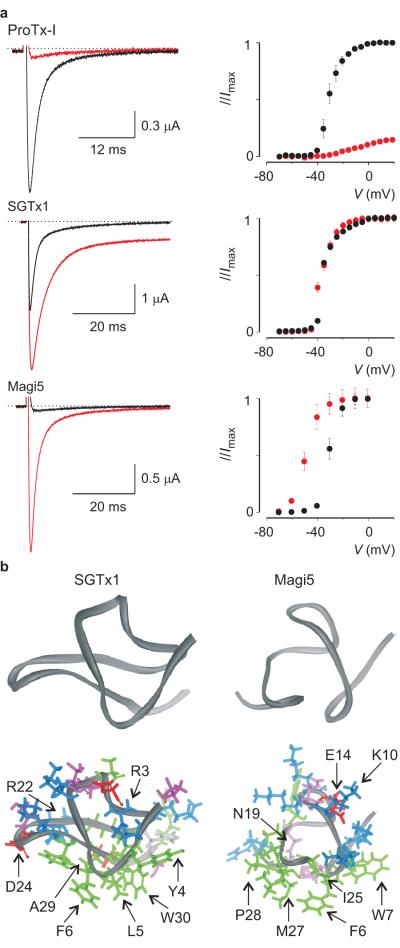
Figure 2. Interactions between spider toxins and Nav channels.
a. Effects of 100nM ProTx-I, 100nM SGTx1, and 1μM Magi5 on rNav1.2a channels expressed in Xenopus laevis oocytes and recorded with the two-electrode voltage-clamp technique. Left, sodium currents elicited by a depolarization to a suitable membrane voltage before (black) and after toxin addition (red), are shown. Right, corresponding conductance-voltage relationships are shown (n=3; error bars are s.e.m.). b. NMR solution structures of SGTx1 and Magi5. Residue coloring is as follows: blue, basic; red, acidic; green, hydrophobic; white, histidine; pink, serine/threonine/asparagine. Backbone fold is shown on top in dark grey. Images were created using DSViewer Pro and Protein Data Bank accession IDs 1LA4 for SGTx138 and 2GX1 for Magi536.
Structural features of Nav channel voltage-sensor toxins
Most spider toxins targeting mammalian Nav channels consist of 30 to 40 amino acids, with the core of the molecule stabilized by three or four disulfide bridges. The ICK (Inhibitory Cystine Knot) motif 37 seems to be the most commonly used fold, but does not specify the mechanism of action, as some ICK toxins act as pore blockers and others as gating-modifiers20. Studies on tarantula toxins interacting with voltage sensors in Kv channels have provided a comprehensive map of their functionally important surfaces. For example, alanine scanning mutagenesis of SGTx1 has revealed that the active face of the molecule consists of a hydrophobic protrusion surrounded by a ring-like assembly of highly polar residues38 (Fig. 2b). Since the affinity of SGTx1 for Nav channels is even higher than for Kv channels12, these structural features are likely important for most spider toxins interacting with Nav channels. Indeed, amphipatic structures are found in a range of spider toxins that interact with voltage sensors in Nav channels (e.g. hanatoxin39, ProTx-II29, PaurTx340, CcoTx140, and HwTx-IV41) and Kv channels (e.g. hanatoxin39, VSTx142, PaTx143, and HpTx244).
It is interesting that toxins having seemingly distinct effects on Nav channel gating (Fig. 2a) are similar when comparing functionally important surfaces. The NMR structures of SGTx138 and Magi536 show that both toxins contain a cluster of hydrophobic residues surrounded by basic, acidic and other highly polar residues (Fig. 2b). Although the overall fold and amphipatic nature of the functionally important surfaces are similar, there are subtle differences in the residues that are crucial for activity. The active face of SGTx1 towards Kv channels contains the solvent exposed hydrophobic surface in which mutations of L5, F6 and W30 result in dramatic weakening of toxin affinity. Of the polar residues, the positively charged R3 and R22 greatly decrease toxin affinity, probably by removing electrostatic interactions with polar residues within the voltage sensor. In Magi5 it seems that F6, W7, I25, M27 and P28, which are all on the hydrophobic face of the molecule, are essential for toxin activity towards Nav channels. Furthermore, polar residues K10, E14 and N19 greatly reduce toxin effects on channel activation when replaced with alanine. Interestingly, Magi5 resembles the surface of the differently folded β-scorpion toxin CssIV36. Moreover, both these toxins cause Nav channels to open at more negative voltages and competition studies suggest an interaction with a common receptor site45. Previous work on CssIV revealed an important role for the negatively charged E15 in stabilizing the voltage sensor of domain II in an activated state thereby promoting channel activation46. Remarkably, E14 in Magi5 is essential for toxin affinity as well, and may therefore enable it to affect channel activation through the same mechanism45.
Toxin-channel interactions
Classic studies on scorpion venom have established the presence of toxins that interact with the voltage sensors in Nav channels45, 47-51. In the conventional view, these toxins interact with extracellular loops between S3 and S4 to stabilize the voltage sensors in particular states45, 50, 52, 53. In parallel, spider toxins interacting with Kv channels were shown to interact with a specific structural motif within the voltage sensors, composed of S3b and S4 helices, also referred to as the paddle motif (see box)21, 22, 25, 26, 54-59.
Box.
Unlike Nav channels, Kv channels are composed of four identical subunits that come together to form a functional voltage-activated K+ ion selective channel. Each subunit possesses a voltage-sensing domain (S1-S4) and a region (S5-S6) that forms the central pore domain55. The external four arginine (or lysine) residues in S4 are positively charged and carry most of the gating charge, thereby driving conformational changes of the voltage sensor in response to changes in membrane voltage84-86. It is thought that all four voltage sensors must activate before the channels opens87. Extensive studies on the voltage sensor in Kv channels have identified a specific S3b-S4 helix-turn-helix structural motif, also known as the voltage sensor paddle, which moves in contact with the surrounding lipid membrane in response to changes in membrane voltage21, 54-59. It was recently shown that paddle motifs are modular units and can be transferred between ion channels and proteins with a voltage-sensing domain without losing their functional properties, suggesting that this motif resides in a relatively unconstrained environment12, 21. Moreover, the paddle is an important pharmacological target in ion channels since various tarantula toxins were shown to interact with this region12, 24, 25, 66-68. Hanatoxin is the founding member of a family of toxins that bind to the paddle motif in Kv channels and inhibit opening of these channels by stabilizing a resting conformation of the voltage sensor22-24. Although one hanatoxin molecule can inhibit Kv channel activation, it is probable that toxin occupancy of the channel can be as high as four22, 24.
Defining the protein-protein interface between voltage-sensor toxins from spiders and mammalian Nav channels has turned out to be a challenge. Initially, it was suggested that the functional similarities between hanatoxin and the protoxins (ProTx-I and ProTx-II) implied a toxin receptor in the S3-S4 region of Nav channels29. However, Nav channels have four of those, one in each voltage sensor and all have similar amino acid sequences. Therefore, the authors hypothesized that voltage-sensor toxins from tarantulas might simultaneously interact with different voltage sensors within one Nav channel. However, there were no tools available yet to explore this possibility. Even extensive mutagenesis and the swapping of S3-S4 regions between Nav1.5 domains by Smith and colleagues did not reveal the binding site of ProTx-II33.
The first indication of an interaction site and a working mechanism of these tarantula toxins came only recently when Catterall’s group showed that ProTx-II impedes movement of the gating charges in Nav1.2, thereby providing evidence that this toxin can actually influence voltage sensor movement in Nav channels34. The Leu833Cys mutation in the S3-S4 region in domain II reduced affinity for ProTx-II by about two-fold, and mutation of the outermost two arginines to glutamine weakened voltage-dependent reversal of toxin action and toxin inhibition of gating current. They also reported substantially different inhibition characteristics of ProTx-II between Nav1.2 and Nav1.5, raising the possibility that the toxin may interact with distinct receptors on different Nav channels subtypes. Shortly after this study, two groups reported that certain amino acids in the domain II voltage sensor of Nav1.7 are involved in the interaction between this particular channel and two voltage sensor toxins, ProTx-II31 and HwTx-IV60. However, mutating equivalent residues in Nav1.2 did not significantly influence sensitivity to ProTx-II, suggesting other interaction sites in different regions of the channel.
A common theme in all of these studies is that the interaction between Nav channels and voltage-sensor toxins from spiders is multi-faceted and therefore difficult to define29, 30, 33, 34. A significant contribution to our understanding of this interaction was made when S3b-S4 paddle motifs were identified within the four Nav channel voltage sensors and transplanted into four-fold symmetric Kv channels to individually examine their interactions with toxins from tarantulas and scorpions12. An advantage of this paddle transfer approach is that it allows individual toxin-paddle interactions to be studied in isolation, eliminating the masking effects of toxins interacting with multiple paddle motifs in Nav channels. In this study, it was demonstrated that the paddle motif in each of the four Nav channel voltage sensors can interact with toxins from tarantulas or scorpions and that multiple paddle motifs are often targeted by a single toxin. For example, it was shown that ProTx-II can interact with the voltage sensor in domain I, II and IV, whereas ProTx-I only interacts with domain II and IV12 (Fig. 3). It is also interesting that the profiles of toxin-paddle interactions vary for different subtypes of Nav channels, which could help to explain the disparate effects of mutations in Nav1.7 and Nav1.2 on inhibition by ProTx-II31.
Figure 3. Sensitivity of rNav1.2a paddle chimeras to ProTx-I and ProTx-II.
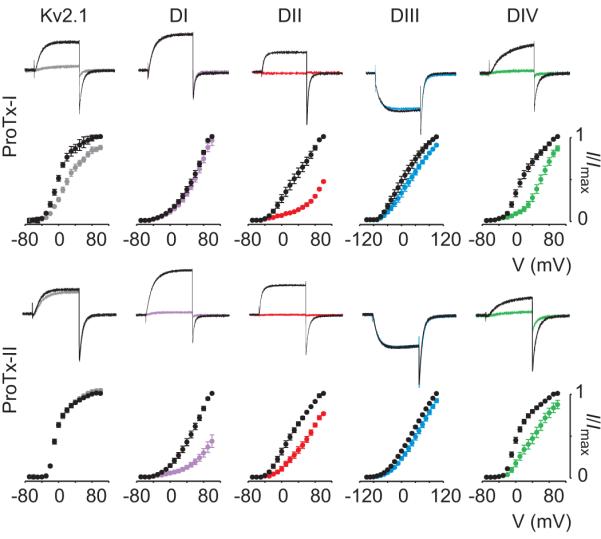
Effects of 100nM ProTx-I and ProTx-II on Kv2.1 and rNav1.2 paddle chimeras are shown where paddle motifs were transferred from each voltage-sensing domain from rNav1.2a into Kv2.112. For each toxin and construct, potassium currents were elicited by depolarizations near the foot of the voltage-activation curve (top). Currents are shown before (black) and in the presence of toxin (colored). Normalized tail current voltage-activation relationships are also shown (bottom), where tail current amplitude (I/Imax) is plotted against test voltage before (black) and in the presence of toxins (colored). n=3-5; error bars are s.e.m.
A particularly fascinating problem with toxins targeting voltage sensors is that the effects on Nav channel gating are remarkably diverse. One rule emerging from the paddle transfer approach is that toxins targeting the voltage sensors in domains I-III influence opening of Nav channels, whereas toxins need to specifically interact with the voltage sensor in domain IV to impede inactivation12. Thus, ProTx-I inhibits channel opening because it interacts with the paddle motif in domain II and IV, whereas SGTx1 hinders inactivation because it interacts exclusively with the paddle motif in domain IV. Although the domains targeted by Magi5 have yet to be identified, a simple hypothesis would be that the toxin interacts with paddle motifs in domains I, II or III to stabilize at least one of the voltage sensors in an activated state. The competition observed between Magi5 and a CssIV61, β-scorpion toxin known to interact with the paddle motif in domain II45, suggests that Magi5 may interact with the voltage sensor in domain II to facilitate opening.
Toxin-lipid interactions
In the classical view, toxins that affect the gating process of voltage-activated ion channels are thought to interact with voltage sensors through direct protein-protein interactions22, 25, 45, 50. However, recent X-ray structures of Kv channels predict that these voltage sensors are extensively exposed to surrounding lipids when embedded in a membrane55-57 and functional studies demonstrate that the composition of the lipid membrane affects how Kv channels open and close in response to changes in voltage62-65. Motivated by these new ideas about voltage sensors, several groups have explored the possibility that tarantula toxins interact with voltage-sensors in Kv channels by partitioning into the membrane and binding to paddle motifs at the protein lipid interface. The amphipatic character observed in the structures of many of these tarantula toxins39, 42-44 is consistent with the notion that membrane partitioning may be required for the toxin to reach the channel. VSTx1, hanatoxin and SGTx1 can partition into model membranes24, 42, 66, 67, and in the case of SGTx1, partitioning was observed under physiologically relevant conditions67. In addition, modification of native lipid membranes alters the apparent affinity of tarantula toxins, suggesting that these toxins interact with Kv channels within the membrane67, 68. To explore the possibility that the inhibitory effects of tarantula toxins might result from indirect membrane perturbations without the toxin actually interacting with the Kv channel, the effects of the D- and L-enantiomers of SGTx1 were compared67. The interaction of the two enantiomers with membranes is indistinguishable, yet the D-enantiomer is pharmacologically inactive, indicating that the toxin–membrane interaction itself is not sufficient to inhibit Kv channels.
Although these studies examining membrane interactions focused on toxins that interact with voltage sensors in Kv channels, it was subsequently discovered that both hanatoxin and SGTx1 also interact with paddle motifs in Nav channels, implying that partitioning is also involved in toxins interacting with Nav channels. Indeed, ProTx-II can partition into membranes32 and the interaction of ProTx-I with the paddle motif from domain IV of Nav1.4 is sensitive to lipid modification68, consistent with the involvement of membrane partitioning. In contrast, studies with HwTx-IV failed to detect partitioning of the toxin into either negatively charged or neutral phospholipid bilayers41. However, this does not preclude the involvement of membrane partitioning of the toxin in channel inhibition because the method used is not sensitive enough to detect weak partitioning. For example, Milescu and colleagues showed that SGTx1 partitions into native cell membranes with a mole-fraction partitioning coefficient as low as ~103, an interaction that is barely detectable, but sufficient for the toxin to access its channel interaction site within the membrane67.
One of the more intriguing discoveries with tarantula toxins is that they can actually detect the intimate interaction between a specific membrane lipid and voltage sensors in Kv and Nav channels68. The apparent affinity of tarantula toxins increases following conversion of the membrane lipid sphingomyelin to ceramide-1-phosphate, and the magnitude of the effects varies for different toxins and paddle motifs. Moreover, the effect of lipid modification differs greatly for mutations in the paddle motif, suggesting that the lipid interacts in a specific fashion with this structural motif and that the toxin receptor in voltage-activated ion channels is actually a tri-molecular complex consisting of the voltage sensor, the toxin and the surrounding membrane lipids. These results also imply that the toxin pharmacology of the ion channel is not determined by the protein alone, but by the lipids in the surrounding membrane and how they interact with the channel protein. For this reason, an exciting goal will be to explore whether the membrane properties vary in different cell types or pathophysiological conditions, thereby influencing the gating properties and pharmacological sensitivities of their voltage-activated ion channels.
Promiscuity of voltage-sensor toxins
A unique feature of voltage-sensor toxins is that they can interact with different families of voltage-activated ion channels. For example, hanatoxin was initially isolated during a search for new inhibitors of Kv2.169. In addition to inhibiting other Kv channel subtypes, such as Kv4.2, hanatoxin can also interact with certain subtypes of voltage-activated calcium (Cav) and Nav channels12, 70. Similar promiscuous activity has been observed for both ProTx-I, ProTx-II and SGTx112, 29, 71, 72. Since it is not a common practice to screen spider toxins for activity on different families of ion channels, other toxins might also be promiscuous. In this respect, it would be interesting to see if Nav channels are affected by closely related peptides that interact with entirely different ion channels (e.g. GsMTx-473 and VaTx1-374).
The widespread targeting of paddle motifs by animal toxins emphasizes the pharmacological importance of this part of the voltage sensor. However, their amino acid sequence homology is rather low, suggesting that toxins probably recognize the conserved three-dimensional structure of the paddle as opposed to a specific sequence of residues12, 21, 70. In addition, the prevalent amphipatic structure of voltage-sensor toxins suggests that toxin-lipid interactions constitute a major part of the interaction mechanism and may contribute to the promiscuous character of these toxins. We are only beginning to explore the role of membrane lipids in forming the toxin receptor, so their function in toxin promiscuity is an aspect that warrants future study.
Future applications
Although spider toxins have been valuable tools to probe the structure and functional mechanisms of Nav channels, opportunities also exist for using these toxins therapeutically. A vast number of people experience moderate to severe chronic pain, which affects their quality of life and their ability to work. Recent reports concerning the key roles of Nav1.7, Nav1.8 and Nav1.9 in pain perception offers exciting prospects for the discovery of new approaches to analgesia75. In fact, ProTx-I and ProTx-II were purified on the basis of their ability to reversibly inhibit the activation of Nav1.829. Unfortunately, these toxins are promiscuous and inhibit other voltage-activated ion channels. However, at the time, these toxins were the first inhibitors of Nav1.8 and provided an important incentive to continue spider venom exploration. Interestingly, Schmalhofer and colleagues have shown afterwards that ProTx-II has the highest affinity for Nav1.7, thereby blocking action potential propagation in nociceptors31. Recently, three more gating-modifier toxins (CcoTx1, CcoTx2 and PaurTx3) were isolated40. All of them inhibit Nav1.7 and Nav1.8, albeit with low affinity. Although peptide degradation may limit the use of these toxins in vivo to cases warranting local application of anesthetics, they can still be valuable tools in proof-of-concept experiments in experimental pain models that require the development of subtype selective Nav channel modulators.
Nav1.5 is known to be involved in cardiovascular function, as demonstrated by the catastrophic effects that mutations can cause4, 76. However, no drug lead has emerged from toxin research for direct application in cardiovascular pathologies. Nevertheless, several tarantula toxins have been shown to posses a certain degree of selectivity for this Nav channel subtype. For example, the recently discovered CcoTx3 was tested on most Nav channel subtypes40, 77 (except Nav1.9) and shown to preferentially inhibit the activation of Nav1.5 (albeit with a weak affinity). Also, JzTx-I78 and -III79 modulate cardiac Na+ currents, presumably by targeting Nav1.5. However, like many other toxins, JzTX-I and -III were not tested on all Nav channel subtypes.
One very important contribution of Nav channel toxins is that they can be used to identify and characterize important relationships between domain-specific interactions and the effect of certain molecules on Nav channel gating. It was recently shown that toxins which slow inactivation of Nav channels only bind to domain IV, whereas toxins that influence opening can do so by interacting with the paddle motifs in domains I-III12. So, influencing opening of the channel can be accomplished by designing molecules to interact with one or more of the first three voltage sensors in Nav channels, while influencing inactivation requires that a molecule only interacts with the domain IV voltage sensor. The influence of domain-specific interactions has important implications for designing drugs to reshape Nav channel activity: diseases associated with accelerated Nav channel inactivation, like certain congenital heart disorders, could be managed by drugs that selectively target the domain IV paddle80. In contrast, the abnormal opening of Nav channels as seen in epilepsy disorders could be controlled with drugs targeting any paddle within the first three voltage sensors81.
Challenges still remain between the initial drug discovery phase and the use of spider toxins in a clinical context since their inherent biochemical instability limits oral availability. Nevertheless, synthetic methods such as cyclization, minimization and diselenide or fluorous bridges have been proposed for stabilizing and locking the conformation of small peptides82, 83 which may expand the future uses of these toxins as therapeutic drugs.
Box figure.
Ribbon representation of the X-ray structure of a paddle chimera between the Kv2.1 and Kv1.2 channel viewed from the external side of the membrane (top view) and from within the membrane (side view)57. The S3b-S4 paddle motif is colored blue, the pore domain (S5-S6) is colored yellow and possible lipid molecules are colored grey. Basic residues in S4 are shown as stick representations (Protein Data Bank accession ID is 2R9R). The side view of the chimeric channel shows the S1–S4 voltage-sensing domain and its interface with the pore domain together with the possible location of lipid molecules.
Acknowledgements
We thank the members of the Swartz laboratory for helpful discussions and B. Billen, J. Tytgat, and G. Corzo for making the Magi5 data available. This work was supported by the Intramural Research Program of the NINDS, NIH and by a NIH-FWO postdoctoral fellowship to F.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Catterall WA. From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 2.Hille B. Ion channels of excitable membranes. Sinauer Associates, Inc.; 2001. [Google Scholar]
- 3.Cannon SC. Pathomechanisms in Channelopathies of Skeletal Muscle and Brain. Annu Rev Neurosci. 2006;29:387–415. doi: 10.1146/annurev.neuro.29.051605.112815. [DOI] [PubMed] [Google Scholar]
- 4.George AL., Jr. Inherited disorders of voltage-gated sodium channels. J Clin Invest. 2005;115:1990–1999. doi: 10.1172/JCI25505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldin AL. Resurgence of sodium channel research. Annu Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 6.Waxman SG, Dib-Hajj S. Erythermalgia: molecular basis for an inherited pain syndrome. Trends Mol Med. 2005;11:555–562. doi: 10.1016/j.molmed.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Cox J, et al. An SCN9A channelopathy causes congenital inability to experience pain. Nature. 2006;444(7121):894–898. doi: 10.1038/nature05413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catterall WA, et al. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- 9.Chanda B, Bezanilla F. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J Gen Physiol. 2002;120:629–645. doi: 10.1085/jgp.20028679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horn R, et al. Immobilizing the moving parts of voltage-gated ion channels. J Gen Physiol. 2000;116:461–476. doi: 10.1085/jgp.116.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheets MF, et al. The Na channel voltage sensor associated with inactivation is localized to the external charged residues of domain IV, S4. Biophys J. 1999;77:747–757. doi: 10.1016/S0006-3495(99)76929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosmans F, et al. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008;456:202–208. doi: 10.1038/nature07473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mebs D. Venomous and Poisonous Animals: A Handbook for Biologists, Toxicologists and Toxinologists, Physicians and Pharmacists. 2002. Medpharm. [Google Scholar]
- 14.Catterall WA, et al. Voltage-gated ion channels and gating modifier toxins. Toxicon. 2007;49:124–141. doi: 10.1016/j.toxicon.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- 16.Honma T, Shiomi K. Peptide toxins in sea anemones: structural and functional aspects. Mar Biotechnol (NY) 2006;8:1–10. doi: 10.1007/s10126-005-5093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez de la Vega RC, Possani LD. Overview of scorpion toxins specific for Na+ channels and related peptides: biodiversity, structure-function relationships and evolution. Toxicon. 2005;46:831–844. doi: 10.1016/j.toxicon.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Escoubas P, et al. Novel tarantula toxins for subtypes of voltage-dependent potassium channels in the Kv2 and Kv4 subfamilies. Mol Pharmacol. 2002;62:48–57. doi: 10.1124/mol.62.1.48. [DOI] [PubMed] [Google Scholar]
- 19.Ruta V, et al. Functional analysis of an archaebacterial voltage-dependent K+ channel. Nature. 2003;422:180–185. doi: 10.1038/nature01473. [DOI] [PubMed] [Google Scholar]
- 20.Swartz KJ. Tarantula toxins interacting with voltage sensors in potassium channels. Toxicon. 2007;49:213–230. doi: 10.1016/j.toxicon.2006.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alabi AA, et al. Portability of paddle motif function and pharmacology in voltage sensors. Nature. 2007;450:370–375. doi: 10.1038/nature06266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swartz KJ, MacKinnon R. Mapping the receptor site for hanatoxin, a gating modifier of voltage-dependent K+ channels. Neuron. 1997;18:675–682. doi: 10.1016/s0896-6273(00)80307-4. [DOI] [PubMed] [Google Scholar]
- 23.Swartz KJ, MacKinnon R. Hanatoxin modifies the gating of a voltage-dependent K+ channel through multiple binding sites. Neuron. 1997;18:665–673. doi: 10.1016/s0896-6273(00)80306-2. [DOI] [PubMed] [Google Scholar]
- 24.Phillips LR, et al. Voltage-sensor activation with a tarantula toxin as cargo. Nature. 2005;436:857–860. doi: 10.1038/nature03873. [DOI] [PubMed] [Google Scholar]
- 25.Li-Smerin Y, Swartz KJ. Localization and molecular determinants of the Hanatoxin receptors on the voltage-sensing domains of a K(+) channel. J Gen Physiol. 2000;115:673–684. doi: 10.1085/jgp.115.6.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruta V, MacKinnon R. Localization of the voltage-sensor toxin receptor on KvAP. Biochemistry. 2004;43:10071–10079. doi: 10.1021/bi049463y. [DOI] [PubMed] [Google Scholar]
- 27.Lee HC, et al. Interaction between extracellular Hanatoxin and the resting conformation of the voltage-sensor paddle in Kv channels. Neuron. 2003;40:527–536. doi: 10.1016/s0896-6273(03)00636-6. [DOI] [PubMed] [Google Scholar]
- 28.Bezanilla F, Stefani E. Gating currents. Methods Enzymol. 1998;293:331–352. doi: 10.1016/s0076-6879(98)93022-1. [DOI] [PubMed] [Google Scholar]
- 29.Middleton RE, et al. Two tarantula peptides inhibit activation of multiple sodium channels. Biochemistry. 2002;41:14734–14747. doi: 10.1021/bi026546a. [DOI] [PubMed] [Google Scholar]
- 30.Edgerton GB, et al. Evidence for multiple effects of ProTxII on activation gating in Na(V)1.5. Toxicon. 2008;52:489–500. doi: 10.1016/j.toxicon.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmalhofer WA, et al. ProTx-II, a selective inhibitor of NaV1.7 sodium channels, blocks action potential propagation in nociceptors. Mol Pharmacol. 2008;74:1476–1484. doi: 10.1124/mol.108.047670. [DOI] [PubMed] [Google Scholar]
- 32.Smith JJ, et al. Differential phospholipid binding by site 3 and site 4 toxins. Implications for structural variability between voltage-sensitive sodium channel domains. J Biol Chem. 2005;280:11127–11133. doi: 10.1074/jbc.M412552200. [DOI] [PubMed] [Google Scholar]
- 33.Smith JJ, et al. Molecular interactions of the gating modifier toxin ProTx-II with NaV 1.5: implied existence of a novel toxin binding site coupled to activation. J Biol Chem. 2007;282:12687–12697. doi: 10.1074/jbc.M610462200. [DOI] [PubMed] [Google Scholar]
- 34.Sokolov S, et al. Inhibition of sodium channel gating by trapping the domain II voltage sensor with protoxin II. Mol Pharmacol. 2008;73:1020–1028. doi: 10.1124/mol.107.041046. [DOI] [PubMed] [Google Scholar]
- 35.Wang M, et al. JZTX-IV, a unique acidic sodium channel toxin isolated from the spider Chilobrachys jingzhao. Toxicon. 2008;52:871–880. doi: 10.1016/j.toxicon.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 36.Corzo G, et al. Solution structure and alanine scan of a spider toxin that affects the activation of mammalian voltage-gated sodium channels. J Biol Chem. 2007;282:4643–4652. doi: 10.1074/jbc.M605403200. [DOI] [PubMed] [Google Scholar]
- 37.Escoubas P, Rash L. Tarantulas: eight-legged pharmacists and combinatorial chemists. Toxicon. 2004;43:555–574. doi: 10.1016/j.toxicon.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Wang JM, et al. Molecular surface of tarantula toxins interacting with voltage sensors in K(v) channels. J Gen Physiol. 2004;123:455–467. doi: 10.1085/jgp.200309005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi H, et al. Solution structure of hanatoxin1, a gating modifier of voltage-dependent K(+) channels: common surface features of gating modifier toxins. J Mol Biol. 2000;297:771–780. doi: 10.1006/jmbi.2000.3609. [DOI] [PubMed] [Google Scholar]
- 40.Bosmans F, et al. Four novel tarantula toxins as selective modulators of voltage-gated sodium channel subtypes. Mol Pharmacol. 2006;69:419–429. doi: 10.1124/mol.105.015941. [DOI] [PubMed] [Google Scholar]
- 41.Xiao Y, et al. Synthesis and characterization of huwentoxin-IV, a neurotoxin inhibiting central neuronal sodium channels. Toxicon. 2008;51:230–239. doi: 10.1016/j.toxicon.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 42.Jung HJ, et al. Solution structure and lipid membrane partitioning of VSTx1, an inhibitor of the KvAP potassium channel. Biochemistry. 2005;44:6015–6023. doi: 10.1021/bi0477034. [DOI] [PubMed] [Google Scholar]
- 43.Diochot S, et al. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Ito1 in cardiac electrogenesis. Br J Pharmacol. 1999;126:251–263. doi: 10.1038/sj.bjp.0702283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanguinetti MC, et al. Heteropodatoxins: peptides isolated from spider venom that block Kv4.2 potassium channels. Mol Pharmacol. 1997;51:491–498. [PubMed] [Google Scholar]
- 45.Cestele S, et al. Voltage sensor-trapping: enhanced activation of sodium channels by beta-scorpion toxin bound to the S3-S4 loop in domain II. Neuron. 1998;21:919–931. doi: 10.1016/s0896-6273(00)80606-6. [DOI] [PubMed] [Google Scholar]
- 46.Cohen L, et al. Common features in the functional surface of scorpion beta-toxins and elements that confer specificity for insect and mammalian voltage-gated sodium channels. J Biol Chem. 2005;280:5045–5053. doi: 10.1074/jbc.M408427200. [DOI] [PubMed] [Google Scholar]
- 47.Cahalan MD. Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J Physiol. 1975;244:511–534. doi: 10.1113/jphysiol.1975.sp010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koppenhofer E, Schmidt H. [Effect of scorpion venom on ionic currents of the node of Ranvier. II. Incomplete sodium inactivation] Pflugers Arch. 1968;303:150–161. doi: 10.1007/BF00592632. [DOI] [PubMed] [Google Scholar]
- 49.Koppenhofer E, Schmidt H. [Effect of scorpion venom on ionic currents of the node of Ranvier. I. The permeabilities PNa and PK] Pflugers Arch. 1968;303:133–149. doi: 10.1007/BF00592631. [DOI] [PubMed] [Google Scholar]
- 50.Rogers JC, et al. Molecular determinants of high affinity binding of alpha-scorpion toxin and sea anemone toxin in the S3-S4 extracellular loop in domain IV of the Na+ channel alpha subunit. J Biol Chem. 1996;271:15950–15962. doi: 10.1074/jbc.271.27.15950. [DOI] [PubMed] [Google Scholar]
- 51.Rochat H, et al. Interaction of scorpion toxins with the sodium channel. J Physiol (Paris) 1984;79:334–337. [PubMed] [Google Scholar]
- 52.Campos FV, et al. Alpha-scorpion toxin impairs a conformational change that leads to fast inactivation of muscle sodium channels. J Gen Physiol. 2008;132:251–263. doi: 10.1085/jgp.200809995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campos FV, et al. beta-Scorpion toxin modifies gating transitions in all four voltage sensors of the sodium channel. J Gen Physiol. 2007;130:257–268. doi: 10.1085/jgp.200609719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chakrapani S, et al. Structural dynamics of an isolated voltage-sensor domain in a lipid bilayer. Structure. 2008;16:398–409. doi: 10.1016/j.str.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang Y, et al. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 56.Jiang Y, et al. The principle of gating charge movement in a voltage-dependent K+ channel. Nature. 2003;423:42–48. doi: 10.1038/nature01581. [DOI] [PubMed] [Google Scholar]
- 57.Long SB, et al. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 58.Ruta V, et al. Calibrated measurement of gating-charge arginine displacement in the KvAP voltage-dependent K+ channel. Cell. 2005;123:463–475. doi: 10.1016/j.cell.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 59.Swartz KJ. Sensing voltage across lipid membranes. Nature. 2008;456:891–897. doi: 10.1038/nature07620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao Y, et al. Tarantula huwentoxin-IV inhibits neuronal sodium channels by binding to receptor site 4 and trapping the domain ii voltage sensor in the closed configuration. J Biol Chem. 2008;283:27300–27313. doi: 10.1074/jbc.M708447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corzo G, et al. Distinct primary structures of the major peptide toxins from the venom of the spider Macrothele gigas that bind to sites 3 and 4 in the sodium channel. FEBS Lett. 2003;547:43–50. doi: 10.1016/s0014-5793(03)00666-5. [DOI] [PubMed] [Google Scholar]
- 62.Ramu Y, et al. Enzymatic activation of voltage-gated potassium channels. Nature. 2006;442:696–699. doi: 10.1038/nature04880. [DOI] [PubMed] [Google Scholar]
- 63.Schmidt D, et al. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444:775–779. doi: 10.1038/nature05416. [DOI] [PubMed] [Google Scholar]
- 64.Schmidt D, MacKinnon R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci U S A. 2008;105:19276–19281. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xu Y, et al. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature. 2008;451:826–829. doi: 10.1038/nature06618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee SY, MacKinnon R. A membrane-access mechanism of ion channel inhibition by voltage sensor toxins from spider venom. Nature. 2004;430:232–235. doi: 10.1038/nature02632. [DOI] [PubMed] [Google Scholar]
- 67.Milescu M, et al. Tarantula toxins interact with voltage sensors within lipid membranes. J Gen Physiol. 2007;130:497–511. doi: 10.1085/jgp.200709869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milescu M, et al. Interactions between lipids and voltage sensor paddles detected with tarantula toxins. Nat Struct Mol Biol. 2009;16:1080–1085. doi: 10.1038/nsmb.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Swartz KJ, MacKinnon R. An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron. 1995;15:941–949. doi: 10.1016/0896-6273(95)90184-1. [DOI] [PubMed] [Google Scholar]
- 70.Li-Smerin Y, Swartz KJ. Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels. Proc Natl Acad Sci U S A. 1998;95:8585–8589. doi: 10.1073/pnas.95.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edgerton GB, et al. Modification of gating kinetics in Cav3.1 by the tarantula toxin ProTxII. Biophysical Journal. 2007:601a–601a. [Google Scholar]
- 72.Lee CW, et al. Solution structure and functional characterization of SGTx1, a modifier of Kv2.1 channel gating. Biochemistry. 2004;43:890–897. doi: 10.1021/bi0353373. [DOI] [PubMed] [Google Scholar]
- 73.Suchyna TM, et al. Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol. 2000;115:583–598. doi: 10.1085/jgp.115.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Siemens J, et al. Spider toxins activate the capsaicin receptor to produce inflammatory pain. Nature. 2006;444:208–212. doi: 10.1038/nature05285. [DOI] [PubMed] [Google Scholar]
- 75.Momin A, Wood JN. Sensory neuron voltage-gated sodium channels as analgesic drug targets. Curr Opin Neurobiol. 2008;18:383–388. doi: 10.1016/j.conb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 76.Ashcroft F. Ion channels and disease. Elsevier; 1999. [Google Scholar]
- 77.Bosmans F, et al. Animal Toxins: State of the Art. Editora UFMG; 2009. [Google Scholar]
- 78.Xiao Y, et al. Jingzhaotoxin-I, a novel spider neurotoxin preferentially inhibiting cardiac sodium channel inactivation. J Biol Chem. 2005;280:12069–12076. doi: 10.1074/jbc.M411651200. [DOI] [PubMed] [Google Scholar]
- 79.Liao Z, et al. Solution structure of Jingzhaotoxin-III, a peptide toxin inhibiting both Nav1.5 and Kv2.1 channels. Toxicon. 2007;50:135–143. doi: 10.1016/j.toxicon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 80.Bennett PB, et al. Molecular mechanism for an inherited cardiac arrhythmia. Nature. 1995;376:683–685. doi: 10.1038/376683a0. [DOI] [PubMed] [Google Scholar]
- 81.Spampanato J, et al. Generalized epilepsy with febrile seizures plus type 2 mutation W1204R alters voltage-dependent gating of Na(v)1.1 sodium channels. Neuroscience. 2003;116:37–48. doi: 10.1016/s0306-4522(02)00698-x. [DOI] [PubMed] [Google Scholar]
- 82.Bulaj G. Integrating the discovery pipeline for novel compounds targeting ion channels. Curr Opin Chem Biol. 2008;12:441–447. doi: 10.1016/j.cbpa.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Craik DJ, Adams DJ. Chemical modification of conotoxins to improve stability and activity. ACS Chem Biol. 2007;2:457–468. doi: 10.1021/cb700091j. [DOI] [PubMed] [Google Scholar]
- 84.Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- 85.Ahern CA, Horn R. Specificity of charge-carrying residues in the voltage sensor of potassium channels. J Gen Physiol. 2004;123:205–216. doi: 10.1085/jgp.200308993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seoh SA, et al. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 1996;16:1159–1167. doi: 10.1016/s0896-6273(00)80142-7. [DOI] [PubMed] [Google Scholar]
- 87.Islas LD, Sigworth FJ. Voltage sensitivity and gating charge in Shaker and Shab family potassium channels. J Gen Physiol. 1999;114:723–742. doi: 10.1085/jgp.114.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]