Summary
Drosophila melanogaster larvae have a remarkable capacity for regenerative growth: Damage to their imaginal discs, the larval precursors of adult structures, elicits a robust proliferative response from the surviving tissue [1–4]. However, as in other organisms, developmental progression and differentiation can restrict regenerative capacity of Drosophila tissues. Experiments in Drosophila and other holometabolous insects have demonstrated that either damage to imaginal tissues [5, 6] or transplantation of a damaged imaginal disc [7, 8] delays the onset of metamorphosis, a time when the imaginal discs undergo morphogenesis and differentiation into their adult structures. Therefore, in Drosophila there appears to be a mechanism that senses tissue damage and extends the larval phase to coordinate tissue regeneration with the overall developmental program of the organism. However, how such a pathway functions remains unknown. Here we demonstrate that a developmental checkpoint extends larval growth after imaginal disc damage by inhibiting the transcription of the gene encoding PTTH, a neuropeptide that promotes the release of the steroid hormone ecdysone. Using a genetic screen, we identify a previously unsuspected role for retinoid biosynthesis in regulating PTTH expression and delaying development in response to tissue damage. Retinoid signaling plays an important, but poorly defined role in several vertebrate regeneration models [9–11]. Our findings demonstrate that retinoid biosynthesis in Drosophila is important for the maintenance of a permissive condition for regenerative growth.
Keywords: regeneration, ecdysone, retinoid
Results and Discussion
A developmental checkpoint is activated by imaginal disc damage
Damage to Drosophila larval tissues produces an extended larval phase [5, 6, 12]. However, it is not known whether this delay in development occurs immediately after damage or later, at a defined developmental checkpoint prior to pupariation. We irradiated third instar Drosophila larvae at 92 hours after egg deposition (AED) with 2500 or 4000 Rad of X-ray irradiation and found that the median length of the larval phase was extended by approximately 23 and 49 hours respectively (Fig. 1A). When larvae were X-irradiated (4000 Rad) either during the first (48 hours AED) or second (60 hours AED) instar, they still exhibited delayed pupariation, but the earlier molting transition, from second to third larval instar, proceeded without delay (Fig. 1B). Therefore, irradiation of larvae even during the first or second instar delays development only during the third instar stage revealing a mechanism that specifically delays entry into pupariation after tissue damage.
Figure 1.
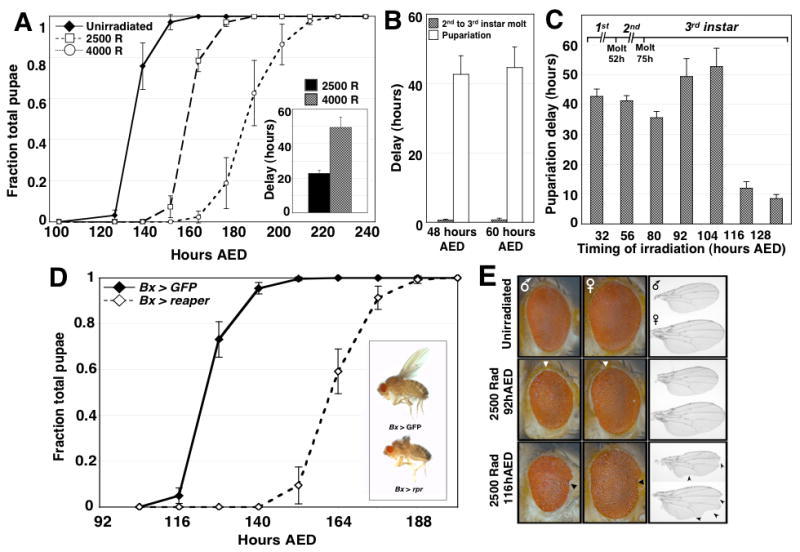
Imaginal tissue damage activates a developmental checkpoint during the third larval instar of Drosophila development. (A) Timing of pupariation for unirradiated larvae, or larvae X-irradiated at 92 hours AED with doses of either 2500 rads or 4000 rads. Developmental timing is represented as both the fraction of total larvae pupariated and in the inset graph as the difference between the median timing to pupariation of irradiated and unirradiated populations of larvae (delay). In all developmental timing experiments described in this paper, n=3 independent populations were assayed for each condition in each genetic background unless otherwise noted. Error bars in all figures represent the standard error for each data point. (B) Duration of delay for both second to third instar molting (gray bars) and pupariation (solid white bars) among larvae irradiated with 4000 rad at either 48 hours AED, during the first larval instar, or at 60 hours AED, during the second larval instar. (C) Pupariation delay for larvae irradiated with 4000 rad at progressively later times throughout larval development. (D) Timing of pupariation for Bx > rpr and Bx > GFP larvae. Bx > rpr larvae exhibit a substantial delay in pupariation timing. (Inset) adult flies expressing either UAS-GFP or UAS-reaper under the control of Bx-GAL4. Eclosed Bx > rpr flies have ablated wings, but exhibit no other developmental or obvious behavioral abnormalities and are fertile. (E) Eyes (shown with posterior to the left) and wings (proximal to the left) from male and female adults irradiated as larvae either before (92 hours AED) or after (116 hours AED) the developmental checkpoint. The boundary of posterior ommatidial disorganization is shown by the white triangles. Anterior notching of eyes is marked with black triangles. Wing notching is marked with arrowheads.
Drosophila larvae achieve a critical size during the third instar, after which neither starvation [13, 14] nor inhibition of tissue growth [15], affects the timing of pupariation. We tested whether, similarly, there is a point in larval development after which tissue damage no longer delays development. Irradiation of larvae at 104 hours AED produces a robust developmental delay, whereas larvae irradiated at 116 hours AED or later pupariate with little delay (Fig. 1C). Thus there is a point late in larval development, between 104–116 hours AED, where larvae commit to a timely developmental schedule for pupariation. This is consistent with earlier work that utilized a temperature-sensitive cell-lethal mutation to produce tissue damage [6]. Together, these results demonstrate that a checkpoint-like mechanism operates in the third larval instar to extend the larval phase of development after tissue damage.
Checkpoint-induced delay results from damage to imaginal tissues
Although X-irradiation has the potential to produce widespread tissue damage, very little apoptosis, was observed in many larval tissues of irradiated animals including the gut, brain, lymph gland, ring gland, salivary glands and fat bodies (Fig. S1A). In contrast, within four hours after irradiation, the larval imaginal discs exhibit elevated caspase activation and cellular apoptosis (Fig. S1A). Consistent with earlier observations [12], irradiation of larvae during the first instar, has an effect on brain growth (Fig S1B–F), but not irradiation after the first larval instar (Figure S1C) which still results in delayed pupariation.
Since X-ray-induced apoptosis is restricted predominantly to imaginal discs, we tested whether damage directed primarily to imaginal discs could activate the developmental checkpoint. The Beadex-GAL4 driver, which expresses primarily in the wing and haltere imaginal discs (Fig. S2A), was used to express the pro-apoptotic gene reaper (Bx > rpr) (Fig. S2E,F). Since Beadex-GAL4 is expressed throughout the third instar of larval development, adult Bx > rpr flies have no wings (Fig. 1D, inset).but appear otherwise normal. However, Bx > rpr larvae exhibit a delay in pupariation that is only slightly shorter than that obtained with 4000 rads of X-irradiation (Fig. 1D). While Beadex-GAL4 was expressed at low levels in the CNS (Fig. S2B), it is unlikely that this expression contributes to the developmental delay in Bx > rpr larvae (Fig S2, S3). Indeed, we also observed a similar delay when reaper expression was directed to the wing disc with either Serrate-GAL4 or rotund-GAL4 (Fig. S3). Therefore, damage directed primarily to imaginal discs can elicit a developmental delay.
Persistent expression of reaper in the wing disc using the Bx-GAL4 driver produces continuing damage. Yet pupariation eventually occurs (Fig. 1D), indicating that the mechanism that delays development can eventually be overcome, similar to cell-cycle checkpoint adaptation in yeast [16].
Checkpoint-induced delay extends the period of regenerative competence
We examined adult flies developing from larvae irradiated either before the developmental checkpoint (which delay their development) or after the developmental checkpoint (which exhibit no delay). Larvae irradiated before the developmental checkpoint, at 92 hours AED, produce adults that have only occasional tissue disorganization evident in the posterior part of the adult eye (Fig. 1D). In contrast, all adults arising from larvae irradiated after the developmental checkpoint, at 116 hours AED or later, exhibit a reduction in tissue size as well as patterning defects in both the eyes and wings (Fig. 1E). Thus, checkpoint-induced delay likely facilitates the efficient regeneration of imaginal discs by extending the period of regenerative competence during larval development.
Tissue damage inhibits the endocrine signals that both promote developmental progression and restrict regenerative capacity
The primary signal that promotes developmental progression from the larval to the pupal stage is the rapid increase in levels of the steroid hormone ecdysone. Ecdysone is synthesized by an endocine organ, the larval ring gland and is converted by larval tissues to the active metabolite 20-hydroxyecdysone (20E; summarized in Fig. S4A). In larvae, the level of circulating 20E can be manipulated by feeding 20E [17]. To examine whether irradiation-induced delays could be suppressed by feeding 20E, larvae were irradiated at 92 hours AED. Twelve hours later, irradiated and unirradiated larvae were transferred to food that was either supplemented with 0.3, 0.5 or 1.0 mg/ml 20E in ethanol or an equivalent volume of ethanol as a control. Feeding 20E shortened the duration of the delay in pupariation in irradiated larvae (Fig. 2A), demonstrating that 20E levels are rate limiting for developmental progression in irradiated animals, and that bypassing the regulation of ecdysone synthesis via ectopic feeding of 20E can overcome checkpoint-induced delay.
Figure 2.
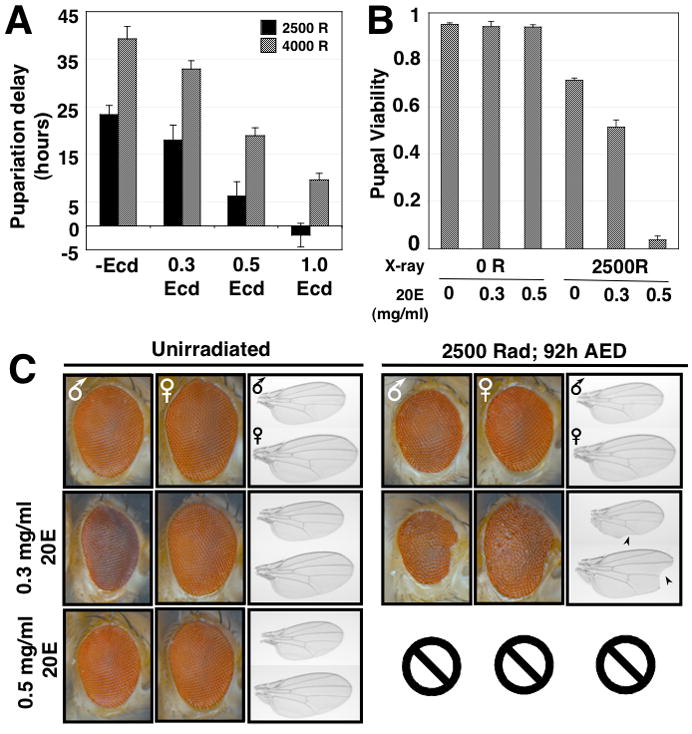
Tissue regeneration and pupal viability in X-irradiated larvae is compromised by 20E addition. (A) Developmental delay of larvae exposed to 2500 or 4000 rad X-irradiation, and fed 0.3, 0.5 or 1.0 mg/ml 20E dissolved in 100% ethanol, or an equivalent volume of ethanol as a control. (B) Pupal viability (as determined by the initiation of adult eclosion from the pupal case) of either unirradiated larvae or larvae irradiated at 92 hours AED, that were either fed different concentrations of 20E (0.3mg/ml and 0.5 mg/ml) in Drosophila molasses-cornmeal media or an equivalent volume of ethanol in molasses-cornmeal media as a negative control. The viability for n=3 independent populations was assayed for each condition (C) Eyes and wings from male and female adult flies irradiated as larvae at 92 hours AED, then either fed 0.3, 0.5 or 1.0 mg/ml 20E or an equivalent volume of ethanol in their normal food. Wing notching is shown with arrowheads. Two larvae irradiated and fed 0.5mg/ml 20E eclosed (Fig. 2B), however one adult died before completing eclosion, and the second adult died immediately in the food. Therefore we were unable to effectively assess the wing and eye phenotypes in these animals.
To determine whether increasing 20E levels would also restrict the capacity of larvae to regenerate tissues damaged by X-irradiation, larvae irradiated at 92 hours AED were then transferred to food supplemented with 20E. These larvae exhibited a dosage-dependent loss of pupal viability, whereas either irradiation or 20E treatment alone produced little to no decrease in pupal viability (Fig. 2B). In addition, 20E-fed larvae exhibited evidence of incomplete repair of irradiation-induced damage in both eyes and wings (Fig. 2C).
An early event in the neuroendocrine pathway that regulates ecdysone production at pupariation, is an increase in the transcription of the gene encoding the neuropeptide PTTH, in two pairs of neurons within the midbrain region of both larval brain lobes (Fig. S4A). The presence of these neurons, and the appropriate transcriptional regulation of the ptth gene are both necessary for the proper timing of the larval-to-pupal transition [18]. The PTTH-expressing neurons innervate the ring gland [18]. PTTH stimulates the ring gland to produce ecdysone promoting the larval to pupal transition [19].
Larvae carrying both a ptth-GAL4 transgene and a UAS-GFP transgene (ptth >GFP) were examined to determine the levels of GFP expression in the PTTH-expressing neurons of the larval brain [18] (Fig. 3A). ptth >GFP larvae were irradiated at 92 hours AED and the larval brains were examined 36 hours after irradiation (128h AED). In irradiated larvae, there was significantly less GFP fluorescence in the PTTH-expressing neurons than was observed in unirradiated controls (Fig. 3B and S4B,C). By 60 hours after irradiation (156h AED), irradiated larvae had begun wandering and a restoration of ptth expression was observed (Fig. S4B). In unirradiated third instar larvae, ptth RNA levels remain low until approximately 12 hours before the median pupariation time, when they increase dramatically. In contrast, larvae exposed to 4000 rad X-irradiation at 92h AED, exhibit a delay in the upregulation of ptth gene expression consistent with their delayed pupariation (Fig 3C). Furthermore, Bx > rpr larvae exhibit a delayed induction of ptth in comparison to Bx > GFP control larvae, consistent with their delayed developmental timing (Fig. 3D), demonstrating that the imaginal-tissue targeted damage is sufficient to delay the upregulation of ptth expression prior to pupariation.
Figure 3.
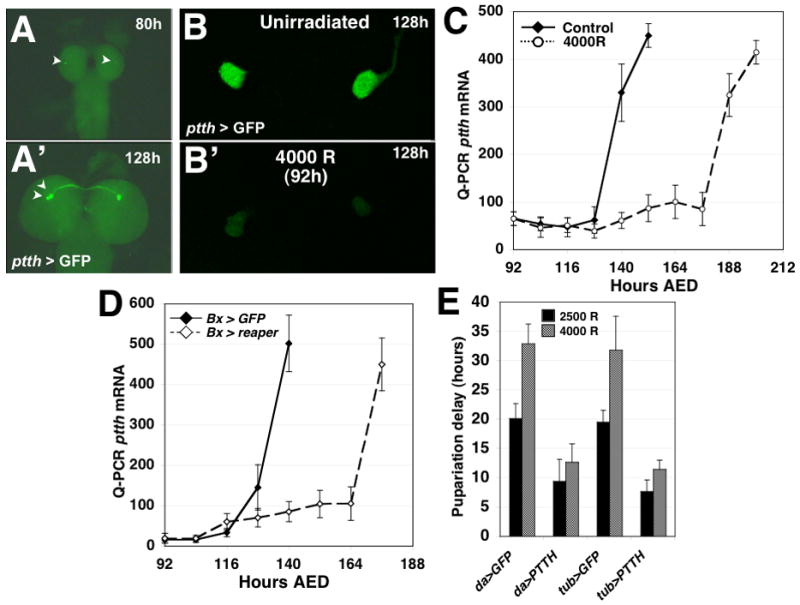
Irradiation-induced tissue damage inhibits PTTH expression and endocrine signaling. (A) Larvae carrying PTTH-GAL4 and UAS-GFP transgenes can be used to visualize PTTH gene activity in PTTH-expressing neurons in the larval brain. The previously described [18] upregulation of PTTH gene activity during developmental progression can be visualized as an increase in GFP fluorescence in the neuron cell bodies (arrowheads) of the PTTH-expressing neurons (A: PTTH > GFP expression in a larval brain fixed at 80 hours AED; A′: PTTH > GFP expression at 128 hours AED). (B) PTTH > GFP expression at 128 hours AED in the brain of an unirradiated larvae or a larva that was X-irradiated at 92 hours AED with 4000 rad. (C) Measurement of ptth transcript levels in unirradiated and X-irradiated (4000 rad) larvae. Larvae were collected every 12 hours after irradiation until the animals had entered pupal phase of development. ptth gene transcript levels were assayed with quantitative RT-PCR with probes targeting the ptth transcript. (D) ptth transcriptional activity in Bx > rpr and Bx > GFP larvae. Bx > rpr larvae exhibit a delayed upregulation of ptth transcript that is consistent with their delayed developmental timing. ptth transcript was detected using quantitative RT-PCR. (E) Ectopic expression of ptth attenuates irradiation-induced developmental delays. Larvae carrying either the daughterless-GAL4 or the tubulin-GAL4 expression transgenes were crossed to either lines carrying UAS-GFP or UAS-ptth. In the progeny of these crosses we assayed the effects of da > PTTH and tub > PTTH expression on the pupariation delay of larvae irradiated at 92 hours AED. Larvae expressing da > GFP or tub > GFP were assayed as controls for developmental delay. n=4 populations were assayed for each set of conditions in each genetic background.
To determine whether ectopic ptth expression can overcome delays induced by X-irradiation, we examined the effect of constitutive expression of a UAS-ptth transgene using either daughterless-GAL4 or tubulin-GAL4 which would express ptth throughout the larva including within the PTTH-expressing neurons. In unirradiated larvae, da > ptth expression did not effect developmental timing as compared with control da > GFP expressing larvae (Fig. S4D). In contrast, tub > ptth larvae pupariated earlier than tub > GFP larvae (Fig. S4E). However both da > ptth and tub > ptth larvae exhibit a significantly reduced delay in pupariation in response to irradiation (Fig. 3E), indicating that maintaining low levels of ptth RNA is necessary for delaying pupariation upon activation of the developmental checkpoint. Taken together with the delay in ptth expression following irradiation, these results indicate that activation of the developmental checkpoint delays pupariation at least in part via the inhibition of ptth expression.
Retinoid activity regulates checkpoint-induced delay
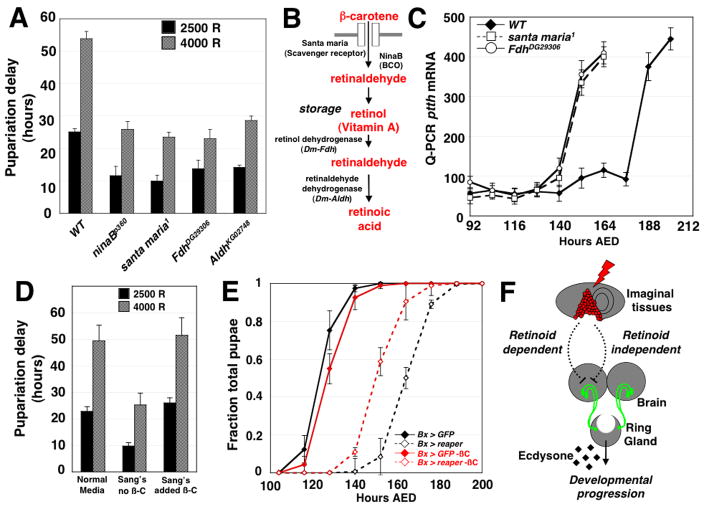
We screened a collection of stocks with chromosomal deletions that together cover at least 65% of the euchromatic portion of chromosomes 2 and 3 to identify heterozygous deletions that reduce the delay in pupariation after irradiation (Fig. S4A). Two deletions, Df(3R)urd and Df(3R)ED5623, that remove an overlapping genomic region each scored positive in the screen. Among the genes in this region is ninaB, which encodes the β-carotene 15,15 monooxygenase (BCO). Larvae that are either heterozygous (Table S1) or homozygous (Fig. 4A) for mutations that specifically disrupt the coding sequence of ninaB gene, have a significantly attenuated delay after irradiation at 92 hours. ninaB mutations also attenuate delay after irradiation early in larval development (Fig. S5B). Since Drosophila BCO has been shown to be important for the initial step of metabolism of carotenoids, such as β-carotene, to produce retinoids [20], this finding implicates retinoids in a pathway that links tissue damage to a delay in pupariation.
Figure 4.
Retinoid metabolism regulates checkpoint-induced delay in irradiated larvae. (A) Pupariation delay in wild-type larvae or homozygous mutant larvae carrying mutations in genes required for retinoid metabolism. n=3 independent populations were assayed for all timing experiments with all mutants unless otherwise noted. p < 0.01 for each pairwise comparison of wild-type and mutant pupariation delay for all four mutants irradiated at 2500 and 4000 rad, as calculated by two-tailed Student’s t-test. (B) A schematic illustrating retinoid metabolic pathways and the gene products required for each step of retinoid metabolism. The genes responsible for retinol dehydrogenase and retinaldehyde dehydrogenase activities in Drosophila have not been identified. Sequence comparisons suggest that the most similar Drosophila gene to vertebrate retinol dehydrogenase is the Drosophila Formaldehyde dehydrogenase (Fdh) gene; the most similar Drosophila gene to vertebrate retinaldehyde dehydrogenase is the Aldehyde dehydrogenase (Aldh) gene of Drosophila. (C) ptth transcript levels in X-irradiated (4000 R), wild type, FdhDG29306 and santa maria1 larvae. ptth transcript was detected using quantitative RT-PCR. (D) Pupariation delay in irradiated wild-type larvae raised either on normal molasses-cornmeal food, or Sang’s defined medium that is either missing (−A) or supplemented with (+A) β-carotene. p < 0.01 in a pairwise comparison of delay in Normal vs. Sang’s (−A) medium at both at 2500 and 4000 rad. The timing of n=3 independent populations were assayed for each condition. p < 0.01 in a pairwise comparison of delay in Sang’s (−A) vs. Sang’s (+A) medium at both at 2500 and 4000 rad; both p-values were calculated by two-tailed Student’s t-test. (E) Developmental timing of Bx > GFP and Bx > rpr larvae raised on carotenoid-deficient Sang’s defined medium (red) or Sang’s defined medium supplemented with 1.25 g/L β-carotene (black). Bx > rpr larvae grown on carotenoid-deficient Sang’s medium exhibit a shorter larval duration than siblings grown on β-carotene supplemented medium. The timing of n=4 independent populations were assayed for each genetic background under each condition. (F) A model for the developmental checkpoint for tissue repair. Damage to imaginal discs is capable of regulating the expression of the ptth gene in the PTTH-expressing neurons via both a retinoid-dependent pathway, defined by the retinoid biosynthesis mutant phenotypes described in this work, and a second, retinoid-independent pathway.
To date, retinoids have only been shown in Drosophila to be important for vision [21] and not in any other aspect of Drosophila development; ninaB homozygotes are viable and fertile. In contrast, in vertebrates, retinoids in regulate development [22] and promote tissue regeneration within individual tissues [10]. Our studies therefore reveal a novel role for retinoid signaling in coordinating tissue regeneration and developmental progression in Drosophila.
Mutations disrupting other components of the retinoid biosynthesis pathway (Fig. 4B) also reduce the delay in pupariation after irradiation. The scavenger receptor Santa maria, necessary for the transport of carotenoids into cells, acts in concert with BCO for retinoid biosynthesis [23]. Irradiation of homozygous santa maria1 larvae either in the third instar (Fig. 4A, Table S1) or early in larval development (Fig. S5B) have a reduced delay. Cleavage of β-carotene by BCO produces two molecules of retinaldehyde, which can then be converted into vitamin A (retinol). In vertebrates, Vitamin A stored within cells can be mobilized through the activity of Type III alcohol dehydrogenases, which convert Vitamin A to retinaldehyde (retinal). The Drosophila protein most similar to mammalian Type III alcohol dehydrogenase is encoded by the Formaldehyde dehydrogenase (Fdh) gene (Fig. 4B) [24, 25]. A deletion identified in our screen Df(3R) M-kx1, removes the Fdh gene (Table S1). Moreover, two independently-derived P-element insertions within the Fdh gene (FdhDG29306 and FdhBG00983), when homozygous, each has a diminished delay in response to irradiation (Fig. 4A, Fig. S5B and Table S1). In vertebrates, conversion of retinaldehyde into retinoic acid is dependent upon the function of retinaldehyde dehydrogenase. The product of the Drosophila Aldh gene, which has been demonstrated to encode an aldehyde dehydrogenase [26], is most similar to the vertebrate retinaldehyde dehydrogenase proteins. Mutants homozygous for a transcriptional suppressor P-element [27] inserted at the 5′ end of the Aldh coding sequence (AldhKG02748) have an attenuated delay after larval irradiation (Fig. 4A, Fig. S5B, Table S1). Thus, either retinoic acid or a downstream metabolite, likely functions in the damage-induced developmental delay.
Both irradiated FdhDG29306 and santa maria1 larvae upregulate ptth gene expression earlier than irradiated wild-type larvae (Fig. 4C) suggesting that the retinoid biosynthesis pathway plays an important role in the inhibition of ptth expression following irradiation. However, the delay in ptth upregulation following irradiation in santa maria1 and FdhDG29306 mutants is only partially reduced in comparison to unirradiated control larvae, indicating that other mechanisms also operate to delay pupariation after tissue damage.
To address the possibility that the retinoid biosynthesis genes might be important for the metabolism of a non-retinoid substrate necessary for checkpoint-induced delay, we examined whether the availability of a carotenoid substrate for retinoid biosynthesis affects the checkpoint-induced delay. Flies reared on Sang’s defined Drosophila medium, which lacks any carotenoid substrates for retinoid biosynthesis [28], have not been found to have any developmental abnormalities but exhibit a substantially attenuated delay when compared to controls (Fig. 4D). The normal delay can be restored by the addition of β-carotene (Fig. 4D), which can be utilized by larvae as a dietary source of retinoids. Similarly, Bx > rpr larvae exhibit a substantially attenuated delay on carotenoid-deficient medium in comparison to Bx > rpr larvae raised on carotenoid-supplemented Sang’s medium (Fig. 4E). Thus retinoids function to delay pupariation after damage directed primarily to imaginal discs as well. Importantly, in each case depletion of retinoids only partially offsets the delay in pupariation, indicating that retinoid-independent mechanisms also exist. In addition, the relative contribution of the retinoid-mediated mechanism to the developmental delay may vary with specific types of tissue damage and repair (Fig. 4F).
Our observations may provide insights into mechanisms that operate in a variety of organisms to delay development in response to tissue damage. For instance, pediatric patients with chronic inflammatory diseases are often observed to experience a delay in the onset of puberty [29–31]. Further analysis of this developmental checkpoint in Drosophila may provide a tractable model for understanding how individual tissues communicate the presence of damage to the entire organism.
Supplementary Material
Acknowledgments
We thank C. Montell, J. O’Tousa, M. O’Connor, L. Jan, and Y-N Jan for Drosophila fly stocks, D. Bilder and members of the Hariharan and Bilder labs for useful suggestions, A. Gerhold for assistance with experiments and screening and D. Gould-Halme for editorial advice. A.H. has been supported by a National Institute of Health (NIH) Ruth L. Kirschstein NRSA Postdoctoral Fellowship (F32 GM07333) and an American Heart Association (AHA) Postdoctoral Fellowship (0825261F). This work was funded in part by NIH grants RO1 GM61672 and RO1 GM85576 to I.K.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bryant P. Regeneration and duplication in imaginal discs. Ciba Foundation symposium; 1975. [DOI] [PubMed] [Google Scholar]
- 2.Bryant P. Pattern formation in the imaginal wing disc of Drosophila melanogaster: fate map, regeneration and duplication. J Exp Zool. 1975;193:49–77. doi: 10.1002/jez.1401930106. [DOI] [PubMed] [Google Scholar]
- 3.Schubiger G. Regeneration, duplication and transdetermination in fragments of the leg disc of Drosophila melanogaster. Dev Biol. 1971;26:277–295. doi: 10.1016/0012-1606(71)90127-8. [DOI] [PubMed] [Google Scholar]
- 4.Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Dev Cell. 2009;16:797–809. doi: 10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussey R, Thompson W, Calhoun E. The influence of x-rays on the development of drosophila larvae. Science. 1927;66:65–66. doi: 10.1126/science.66.1698.65. [DOI] [PubMed] [Google Scholar]
- 6.Simpson P, Berreur P, Berreur-Bonnenfant J. The initiation of pupariation in Drosophila: dependence on growth of the imaginal discs. J Embryol Exp Morphol. 1980;57:155–165. [PubMed] [Google Scholar]
- 7.Dewes E. Entwicklungsleistungen implantierter ganzer und halbierter männlicher …. Dev Genes Evol. 1975 doi: 10.1007/BF00848395. [DOI] [PubMed] [Google Scholar]
- 8.Rahn P. Untersuchungen zur Entwicklung von Ganz-und Teilimplantaten der Flügelimaginalscheibe …. Dev Genes Evol. 1972 doi: 10.1007/BF00575521. [DOI] [PubMed] [Google Scholar]
- 9.Hind M, Maden M. Retinoic acid induces alveolar regeneration in the adult mouse lung. Eur Respir J. 2004;23:20–27. doi: 10.1183/09031936.03.00119103. [DOI] [PubMed] [Google Scholar]
- 10.Maden M, Hind M. Retinoic acid, a regeneration-inducing molecule. Dev Dyn. 2003;226:237–244. doi: 10.1002/dvdy.10222. [DOI] [PubMed] [Google Scholar]
- 11.Tsonis PA, Trombley MT, Rowland T, Chandraratna RA, del Rio-Tsonis K. Role of retinoic acid in lens regeneration. Dev Dyn. 2000;219:588–593. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1082>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 12.Poodry C, Woods D. Control of the developmental timer for Drosophila pupariation. Roux’s Arch Dev Biol. 1990;199:219–227. doi: 10.1007/BF01682081. [DOI] [PubMed] [Google Scholar]
- 13.Layalle S, Arquier N, Léopold P. The TOR pathway couples nutrition and developmental timing in Drosophila. Dev Cell. 2008;15:568–577. doi: 10.1016/j.devcel.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Shingleton AW, Das J, Vinicius L, Stern DL. The temporal requirements for insulin signaling during development in Drosophila. Plos Biol. 2005;3:e289. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stieper BC, Kupershtok M, Driscoll MV, Shingleton AW. Imaginal discs regulate developmental timing in Drosophila melanogaster. Developmental Biology. 2008;321:18–26. doi: 10.1016/j.ydbio.2008.05.556. [DOI] [PubMed] [Google Scholar]
- 16.Sandell LL, Zakian VA. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993;75:729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- 17.Neubueser D, Warren J, Gilbert L. molting defective is required for ecdysone biosynthesis. Developmental Biology. 2005 doi: 10.1016/j.ydbio.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 18.McBrayer Z, Ono H, Shimell M, Parvy JP, Beckstead RB, Warren JT, Thummel CS, Dauphin-Villemant C, Gilbert LI, O’connor MB. Prothoracicotropic hormone regulates developmental timing and body size in Drosophila. Dev Cell. 2007;13:857–871. doi: 10.1016/j.devcel.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warren JT, Yerushalmi Y, Shimell MJ, O’connor MB, Restifo LL, Gilbert LI. Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: correlations with changes in gene activity. Dev Dyn. 2006;235:315–326. doi: 10.1002/dvdy.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.von Lintig J, Dreher A, Kiefer C, Wernet MF, Vogt K. Analysis of the blind Drosophila mutant ninaB identifies the gene encoding the key enzyme for vitamin A formation invivo. Proc Natl Acad Sci USA. 2001;98:1130–1135. doi: 10.1073/pnas.031576398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montell C. Visual transduction in Drosophila. Annu Rev Cell Dev Biol. 1999;15:231–268. doi: 10.1146/annurev.cellbio.15.1.231. [DOI] [PubMed] [Google Scholar]
- 22.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang T, Jiao Y, Montell C. Dissection of the pathway required for generation of vitamin A and for Drosophila phototransduction. The Journal of Cell Biology. 2007;177:305–316. doi: 10.1083/jcb.200610081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner M. Speculations on the subject of alcohol dehydrogenase and its properties in Drosophila and …. Bioessays. 1998 doi: 10.1002/(SICI)1521-1878(199811)20:11<949::AID-BIES10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Luque T, Atrian S, Danielsson O, Jörnvall H, Gonzàlez-Duarte R. Structure of the Drosophila melanogaster glutathione-dependent formaldehyde dehydrogenase/octanol dehydrogenase gene (class III alcohol dehydrogenase). Evolutionary pathway of the alcohol dehydrogenase genes. Eur J Biochem. 1994;225:985–993. doi: 10.1111/j.1432-1033.1994.0985b.x. [DOI] [PubMed] [Google Scholar]
- 26.Fry JD, Saweikis M. Aldehyde dehydrogenase is essential for both adult and larval ethanol resistance in Drosophila melanogaster. Genet Res. 2006;87:87–92. doi: 10.1017/S0016672306008032. [DOI] [PubMed] [Google Scholar]
- 27.Roseman RR, Johnson EA, Rodesch CK, Bjerke M, Nagoshi RN, Geyer PK. A P element containing suppressor of hairy-wing binding regions has novel properties for mutagenesis in Drosophila melanogaster. Genetics. 1995;141:1061–1074. doi: 10.1093/genetics/141.3.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang J. The quantitative nutritional requirements of Drosophila melanogaster. Journal of Experimental Biology 1956 [Google Scholar]
- 29.Azooz OG, Farthing MJ, Savage MO, Ballinger AB. Delayed puberty and response to testosterone in a rat model of colitis. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1483–1491. doi: 10.1152/ajpregu.2001.281.5.R1483. [DOI] [PubMed] [Google Scholar]
- 30.Ballinger AB, Savage MO, Sanderson IR. Delayed puberty associated with inflammatory bowel disease. Pediatric Research. 2003;53:205–210. doi: 10.1203/01.PDR.0000047510.65483.C9. [DOI] [PubMed] [Google Scholar]
- 31.Simon D. Puberty in chronically diseased patients. Horm Res. 2002;57(Suppl 2):53–56. doi: 10.1159/000058102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.