Abstract
Human exposure to Florida red tides formed by Karenia brevis, occurs from eating contaminated shellfish and inhaling aerosolized brevetoxins. Recent studies have documented acute symptom changes and pulmonary function responses after inhalation of the toxic aerosols, particularly among asthmatics. These findings suggest that there are increases in medical care facility visits for respiratory complaints and for exacerbations of underlying respiratory diseases associated with the occurrence of Florida red tides.
This study examined whether the presence of a Florida red tide affected the rates of admission with a respiratory diagnosis to a hospital emergency room in Sarasota, FL. The rate of respiratory diagnoses admissions were compared for a 3-month time period when there was an onshore red tide in 2001 (red tide period) and during the same 3-month period in 2002 when no red tide bloom occurred (non-red tide period). There was no significant increase in the total number of respiratory admissions between the two time periods. However, there was a 19% increase in the rate of pneumonia cases diagnosed during the red tide period compared with the non-red tide period. We categorized home residence zip codes as coastal (within 1.6 km from the shore) or inland (>1.6 km from shore). Compared with the non-red tide period, the coastal residents had a significantly higher (54%) rate of respiratory diagnoses admissions than during the red tide period. We then divided the diagnoses into subcategories (i.e. pneumonia, bronchitis, asthma, and upper airway disease). When compared with the non-red tide period, the coastal zip codes had increases in the rates of admission of each of the subcategories during the red tide period (i.e. 31, 56, 44, and 64%, respectively). This increase was not observed seen in the inland zip codes.
These results suggest that the healthcare community has a significant burden from patients, particularly those who live along the coast, needing emergency medical care for both acute and potentially chronic respiratory illnesses during red tide blooms.
Keywords: Asthma, Pneumonia, Bronchitis, Brevetoxins, Sensitive populations, COPD, Harmful algal blooms (HABs), Red tides, Karenia brevis
1. Introduction
Florida red tides occur annually in the Gulf of Mexico from blooms of the marine dinoflagellate, Karenia brevis (K. brevis). These blooms (also known as harmful algal blooms or HABs) result in massive fish kills and mortalities to marine mammals and sea birds due to the production of the natural neurotoxins, the brevetoxins. Human exposure to brevetoxins produced by K. brevis occurs through two routes, either through eating contaminated shellfish, or through inhaling airborne toxins (for review, see Kirkpatrick et al., 2004).
Although neurotoxic shellfish poisoning (NSP), the shellfish poisoning associated with brevetoxin, and other seafood poisoning diseases have been described in the medical literature, these diseases are significantly under reported to public health authorities (Fleming et al., 2002; Backer et al., 2003a, 2005a,b). Even with substantial under reporting of HAB-associated diseases, costs associated with the human health effects of marine toxin diseases have been estimated to account for at least 45% of the total estimated economic impact of HABs nationwide (Anderson et al., 2000).
An additional contributor to the total public health cost of marine and freshwater HABs not included in Anderson et al. (2000) economic report is associated with the inhalation of toxin-contaminated aerosols. In Florida, wave action and on-shore winds incorporate the red tide toxins into marine aerosols (Pierce et al., 1990) that are apparently not observed in other geographic areas. These particular environmental circumstances have resulted in human exposures and subsequent health complaints for >50 years (Woodcock, 1948).
Animal studies have also shown that inhaled brevetoxin aerosols can cause respiratory and systemic effects (Abraham and Baden, 2001; Singer et al., 1998; Abraham et al., 2003, 2004; Baden and Adams, 2000; Benson et al., 2004; Franz and LeClaire, 1989; Kirkpatrick et al., 2004). Benson et al. (1999) found that after mice were exposed to a single aerosolized brevetoxin dose, 80% of the toxins cleared quickly from the lung; however, 20% remained for up to 7 days in the lung, the central nervous system, and elsewhere in the body. Additional research has suggested that brevetoxins may cause immunosuppression, as well as respiratory and neurotoxic effects (Bossart et al., 1998; Kirkpatrick et al., 2004). Therefore, subchronic and even chronic systemic effects are possible from a single acute exposure to aerosolized brevetoxins.
Recently, several studies identified human respiratory response from exposure to brevetoxin aerosols. People exposed to the toxins in a recreational beach setting reported upper and lower airway symptoms (Backer et al., 2003b). Additional studies reported symptoms ranging from predominantly upper airway symptoms (such as eye and throat irritation) in healthy lifeguards to both upper and lower airway symptoms (eye and throat irritation, cough, shortness of breath and wheezing) in the asthmatic subjects (Backer et al., 2005a,b; Fleming et al., 2005). In these studies air flow parameters measured by spirometry in the healthy subjects revealed no significant changes. However, small but statistically significant decreases in airflow parameters (the amount of the forced vital capacity exhaled in 1 s or FeV1 and the forced expiratory flow in the 25–75% range of the forced vital capacity or FEF25–75) were measured in asthmatic subjects after a 1-h exposure to airborne brevetoxins (36.57 ± 17.51 ng brevetoxin/m3 of air) while on the beach. Even greater decreases in FeV1 and FEF25–75 were measured among severe asthmatics who reported taking their regular medications before participating in study activities. Studies in animals have shown that these same medications both prevent and treat the respiratory effects of brevetoxins (Abraham et al., 2005; Fleming et al., 2005; Benson et al., 2005), and it is not clear why they did not have a similar effect in people.
For many years, the public health message has been that symptoms of exposure to aerosolized Florida red tide would diminish when people left the beach (Baden, 1983). Anecdotal reporting from the community during an onshore bloom suggested that this may not be true for all people, particularly those with underlying respiratory diseases, and that people who live near the shore may be affected more seriously. Quirino et al. (2004) conducted a follow up study on individuals who called the Florida Marine and Freshwater Toxin Hotline (through the South Florida Poison Information Center) with concerns regarding airborne red tide exposure. The study revealed that when compared to a matched cohort of people who called the Hotline for reasons other than to get information about red tide exposure, the people who called about red tide exposure reported significantly more upper and lower airway symptoms, a longer duration of their symptoms (mean 12.84 ± 25.35 days versus 2 ± 1.41 days for cough), a worsening of any underlying respiratory disease, and an increased use of medical care (41% versus 13%) at the time of exposure. This study and the anecdotal reports suggested that exposure to Florida red tide toxins could cause significant subchronic and chronic disease in humans, not just acute effects.
With these recent studies, concerns regarding the potential increased need for health care and the burden on the community healthcare system during onshore toxic blooms have been raised. This study investigated if there was an increased demand for medical care for respiratory disease during an ongoing onshore bloom of Florida red tide in one specific health care venue, the emergency room (ER).
2. Methods
This retrospective cohort study compared the rates of ER visits between two time periods; October 1–December 31, 2001 when there was a red tide (red tide period) and October 1–December 31, 2002 when there was no red tide (non-red tide period) in Sarasota, FL. Sarasota has almost annual onshore Florida red tide events which are documented by extensive ongoing environmental monitoring for K. brevis and brevetoxin.
The facility selected for the study, Sarasota Memorial Hospital (SMH) is one of the four hospitals in the county and is the largest acute care facility in Sarasota County serving 63.3% of the county’s population. It is also the facility that is closest to the coastline. Access to anonymous medical data was provided by the Decision Support Services at SMH after Institutional Review Board (IRB) approval of the study; the study was also approved by the Florida Department of Health IRB.
2.1. Exposure data
The Florida red tide exposure period was during the fall of 2001, and the non-exposure period was during the fall of 2002. The red tide cell count data were provided by the Phytoplankton Ecology Program at Mote Marine Laboratory, Sarasota, FL. This program routinely monitors a minimum of two shore locations on its campus. Water samples are analyzed weekly during non-bloom conditions and daily during blooms. This explains the increased number of samples enumerated in 2001 (red tide period) compared to 2002 (non-red tide period).
2.1.1. Health endpoint data
Computerized anonymous ER admission data were collected for the months of October–December 2001 and for the same months in 2002 to minimize the effects of variation from respiratory exacerbations or reactions from seasonal exposures (such as pollen, molds, or dander). In addition, since the Sarasota area has a seasonal population, using the same 3-month period adjusted for the fluxes in the population as most seasonal residents have the same visitation pattern year after year.
The study data consisted of the ER admission diagnosis with the international classification of diseases (ICD) diagnosis classification for respiratory disease (codes 460–519) and all other ICD diagnoses, the patient age, the residence zip code, and the date of admission. Due to the use of anonymous data, repeated admissions by the same individual were not identified and removed. The residence zip code was dichotomized into two groups based on zip codes abutting the Gulf of Mexico (i.e. “coastal”) to zip codes with no boundary on the water (i.e. “inland”) residence. With the assistance of three physicians, and using the ICD coding for the primary emergency room diagnosis, the following mutually exclusive respiratory disease diagnoses were selected: pneumonia, bronchitis, asthma, and upper airway disease. These four respiratory diagnoses accounted for 91–92% of all respiratory diagnoses of emergency room admissions reported during the study months. All other diagnoses were grouped as “all other primary diagnoses.”
2.2. Statistical analysis
The total number of emergency room admission diagnoses was evaluated for the periods September 1–December 31, 2001 and September 1–December 31, 2002. Using the ICD-9 codes, these diagnoses were categorized as pneumonia, bronchitis, asthma, upper airway disease, or all other primary diagnoses. SAS, version 9.1 was used for calculations (SAS Institute Inc., Cary, NC).
Standardized ER admission rates were calculated for the above diagnoses and the subgroups of coastal and inland within the county. The Florida population for the year 2000 was used. The population information data were provided by the Florida Cancer Data System at the University of Miami School of Medicine (Miami, FL). Rate ratios were calculated as the standardized 2001 age rate divided by the standardized 2002 age rate, and 95% confidence intervals were calculated for these rate ratios (Clayton and Hills, 1993).
3. Results
3.1. Environmental data
The average daily cell counts of K. brevis by month are presented in Table 1, indicating significantly higher cell counts in September–December 2001 compared to September–December 2002. In Figs. 1 and 2, the true cell counts are displayed on a log scale to account for the large scale changes between the two sampling periods. An arbitrary offset of 120 was added to all values to allow visualization of counts of zero. Although there was a slight spike in cell counts in November 2002, the short duration of the increased counts meant that this was not considered a bloom. The broad definition of a bloom is higher than normal concentrations of cells. However, a toxin level that is measurable and could affect human health requires a minimum of 100,000 cells/L (M. Henry, personal communication). So for this analysis, a bloom is considered when cell counts are over 100,000 cells/L. The ER admissions were assessed between the October and December time period, however the 2001 September cell counts of K. brevis are presented to document that patients reporting to the emergency room October 1, 2001, may have had a significant exposure to Florida red tide in the previous month.
Table 1.
K. brevis cell count averages by month in (cells/L)
| 2001 | 2002 | |
|---|---|---|
| September | 1419435 | 50329 |
| October | 302895 | 207 |
| November | 399020 | 2333 |
| December | 53070 | 173 |
Fig. 1.
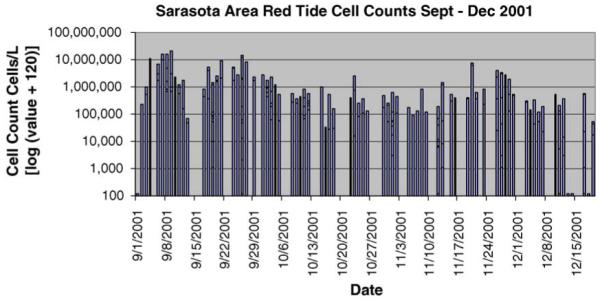
Sarasota area red tide cell counts September–December 2001.
Fig. 2.
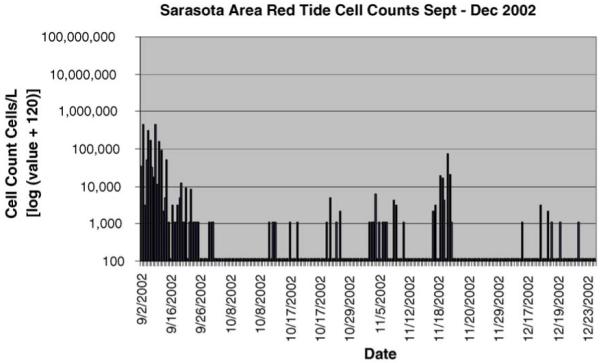
Sarasota area red tide cell counts September–December 2002.
3.2. Emergency room admissions for respiratory disease
The overall number of emergency room admissions during the 2001 and 2002 periods were similar (i.e. 1295 in 2001 and 1235 in 2002) as were the ages (47.51 ± 22.9 years in 2001 and 46.78 ± 22.5 years in 2002), gender distribution (60% female in 2001 and 61% female in 2002), and race distribution (79% white and 21% “other” in both years) of the ER room patients (see Table 2). Of the respiratory diagnoses, the diagnoses of pneumonia, bronchitis, asthma, and upper airway disease comprised 92% of ER admissions in 2001 and 91% in 2002.
Table 2.
Demographics of the 2001 and 2002 ER admissions per 100,000 of Florida population
| 2001 | 2002 | Statistical significance (p-value)* |
|
|---|---|---|---|
| Number of admissions | 1295 | 1235 | 0.12 |
| Mean age ± S.D. (year) | 47.51 ± 22.9 | 46.78 ± 22.5 | 0.42 |
| Residence zip code (%) | |||
| Coastal | 147 (11) | 108 (10) | 0.03 |
| Inland | 1148 (89) | 1127 (90) | |
| Gender (%) | |||
| Female | 781 (60) | 755 (61) | 0.67 |
| Male | 514 (40) | 480 (39) | |
| Race (%) | |||
| White | 1027 (79) | 982 (79) | 0.89 |
| Other | 268 (21) | 253 (21) | |
| Respiratory diagnoses | |||
| All respiratory diagnoses combined (%) | 1192 (92) | 1123 (91) | 0.12 |
| Pneumonia | 181 (14) | 137 (11) | |
| Bronchitis | 457 (35) | 416 (37) | |
| Asthma | 163 (13) | 161 (13) | |
| Upper airways | 391 (30) | 409 (33) | |
| All other diagnosis (%) | 103 (8) | 112 (9) | 0.31 |
Chi-square and t-tests, p > 0.05.
When ER admissions rates were adjusted for age, the overall admission rates for all diagnoses (rate ratio = 1.01; 95% confidence interval = 0.92, 1.10) and for all respiratory diagnoses (rate ratio = 1.01; 95% confidence interval = 0.93, 1.11) were similar for the red tide period and the non-red tide period (see Table 3).
Table 3.
Emergency room age adjusted admission rates per 100,000 Florida population and rate ratios (2001 vs. 2002)
| 2001 | 2002 | Rate ratios (95% confidence intervals) 2001/2002 |
|
|---|---|---|---|
| All emergency room admissions | 0.068207 | 0.067639 | 1.01 (0.92, 1.10) |
| Respiratory diagnoses | |||
| ● All respiratory diagnoses | 0.063612 | 0.062692 | 1.01 (0.93, 1.11) |
| ○ Pneumonia | 0.007294 | 0.006156 | 1.18 (0.93, 1.51) |
| ○ Bronchitis | 0.023164 | 0.021888 | 1.06 (0.92, 1.22) |
| ○ Asthma | 0.009467 | 0.009665 | 0.98 (0.77, 1.25) |
| ○ Upper airways | 0.023687 | 0.024983 | 0.95 (0.81, 1.11) |
| All other diagnosis | 0.004595 | 0.004947 | 0.93 (0.70, 1.24) |
| 2001 Coastal | 2002 Coastal | ||
| Respiratory diagnoses | |||
| ● All respiratory diagnoses | 0.072832 | 0.047343 | 1.54 (1.09, 2.18) |
| ○ Pneumonia | 0.009375 | 0.007146 | 1.31 (0.65, 2.66) |
| ○ Bronchitis | 0.024361 | 0.015602 | 1.56 (0.94, 2.59) |
| ○ Asthma | 0.008918 | 0.006174 | 1.44 (0.57, 3.68) |
| ○ Upper airways | 0.030178 | 0.018420 | 1.64 (0.85, 3.16) |
| All other diagnosis | 0.003328 | 0.005647 | 0.59 (0.22, 1.55) |
| 2001 Inland | 2002 Inland | ||
| Respiratory diagnoses | |||
| ● All respiratory diagnoses | 0.062886 | 0.064197 | 0.98 (0.89, 1.08) |
| ○ Pneumonia | 0.007034 | 0.006043 | 1.16 (0.90, 1.51) |
| ○ Bronchitis | 0.022928 | 0.022597 | 1.01 (0.87, 1.18) |
| ○ Asthma | 0.009680 | 0.010028 | 0.97 (0.75, 1.24) |
| ○ Upper airways | 0.023244 | 0.025528 | 0.91 (0.77, 1.08) |
| All other diagnosis | 0.004776 | 0.004862 | 0.98 (0.73, 1.33) |
| 2001 Coastal | 2001 Inland | Coastal/inland | |
| Respiratory diagnoses | |||
| ● All respiratory diagnoses | 0.072832 | 0.062866 | 1.16 (0.92, 1.46) |
| ○ Pneumonia | 0.009375 | 0.007034 | 1.33 (0.79, 2.32) |
| ○ Bronchitis | 0.024361 | 0.022828 | 1.06 (0.76, 1.48) |
| ○ Asthma | 0.008918 | 0.009680 | 0.92 (0.46, 1.83) |
| ○ Upper airways | 0.030178 | 0.023244 | 1.29 (0.85, 1.99) |
| All other diagnosis | 0.0003328 | 0.004776 | 0.70 (0.31, 1.54) |
However, when the respiratory ER admission rates were categorized as coastal (within 1.6 km of the shore) or inland (>1.6 km of the shore) based on the reported home zip code, residents living in the coastal area had a statistically significantly increased ER admission rate than the inland residents during the red tide period (rate ratio = 1.54; 95% confidence interval = 1.09, 2.18). When examined by subcategory (i.e. pneumonia, bronchitis, asthma and upper airway disease), there was a non-significant increase in the admission rate for coastal residents during the red tide (i.e. 31, 56, 44, and 64%, respectively) compared to their admission rates during the non-red tide period (see Table 3).
During the red tide period, there was a small increase (16%) in the risk of having an ER admission for any respiratory diagnosis for coastal residents when compared with the risk for inland residents. In addition, there was an increased risk of ER admission for pneumonia (33%) and upper airway disease (29%) during the red tide period for coastal residents compared to inland residents. However, these increases in risk were not statistically significant.
During the non-red tide period, the inland residents had an increase in ER admissions for respiratory diagnosis compared to coastal residents. A possible cause for this increase is outside the scope of this analysis. Other inland environmental triggers for respiratory illness may have been occurring.
4. Discussion
We found a significant increase in the rate of ER admissions for all selected respiratory diseases taken together during the 2001 red tide exposure period when compared to the 2002 non-red tide period. In particular, during the red tide period, people with recorded home zip codes located within 1.6 km of the coast had increased ER admission rates for all respiratory diagnoses compared to the people with recorded home zip codes further inland. When specific subcategories of respiratory diseases were examined this same pattern was observed, however, the sample sizes were small and the corresponding confidence intervals were wide indicating a considerable amount of variability in the data.
The increased rates of respiratory disease are consistent with the acute response shown in several animal models and human studies (Backer et al., 2003a, 2005a,b; Fleming et al., 2005; Kirkpatrick et al., 2004; Abraham et al., 2001, 2005). Coastal residents presumably experience increased amounts of exposure (dose) and/or increased length of exposure because they live near the ocean.
These data also suggest that the respiratory response to Florida red tide toxins may not be a transient response that is resolved upon leaving the beach. Although recent findings have revealed an acute response in asthmatics from Florida red tide toxins (Fleming et al., 2005), as with Quirino et al. (2004), the data in this study also demonstrated increased rates of more chronic respiratory illnesses such as bronchitis and pneumonia for people living in coastal areas during red tide blooms. These illnesses may be associated with an infectious etiology, and could be caused directly or indirectly by exposure to Florida red tide toxins. Computer modeling has suggested that brevetoxin is a possible enzymatic binding inhibitor of cysteine cathepsins. Cathepsins are powerful lysosomal proteinases and epitope-presenting enzymes. They are found within cytosol or lysosomes of macrophages, lymphoid tissues and other cells (Bossart et al., 1998; Sudarsanam et al., 1992). Based on the chronic exposure and effects in Florida manatees from Florida red tide toxins (Bossart et al., 1998) and in laboratory rodents (J. Benson, personal communication), it is possible that the effects of aerosolized brevetoxins may induce chronic, as well as acute effects. Based on the effects of chronic Florida red tide toxin exposure in manatees, these chronic effects could begin with the initial phagocytosis by macrophages, inhibition of cathepsins, and apoptosis of these cells, followed by the phagocytosis of the debris by new macrophages. This response could ultimately result in chronic neuro-intoxication, hemolytic anemia, and/or immunologic compromise (Bossart et al., 1998). Therefore, exposure, particularly chronic exposure, to aerosolized brevetoxins may lead to immunosuppression that is sufficient to cause the increased rates of pneumonia and bronchitis seen in this study, especially among persons with underlying respiratory disease (Bossart et al., 1998; Kirkpatrick et al., 2004).
4.1. Limitations
No individual exposure information was collected for this analysis; this study only reports an association of exposure with the time periods selected. The zip codes reported as place of residence do not necessarily identify the actual location of Florida red tide exposure, nor do they document that exposure actually occurred. Furthermore, since the study did not assess individual exposure, it is not possible assess the latency (i.e. the time of exposure to the time when people reported to the emergency room for medical care) period associated with the observed health effects.
The amount of toxins in the air can vary within the day and over time based on the direction and speed of the wind (Cheng et al., 2005). The daily toxin level in the air was not assessed. Exposure was assessed through water analysis of the number of red tide cells present.
Although the data were adjusted for age, no individual health assessment was made to explore other patterns that might have affected the increase in specific respiratory admissions in 2001 compared to 2002. For example, an early influenza season in 2001 could have lead to increased respiratory admissions during October–December 2001. Furthermore, individual underlying pre-disposing conditions (such as chronic obstructive pulmonary disease) were not examined.
5. Conclusions
This study indicated a significant increase in the rates of respiratory disease admissions to an ER for coastal residents during a year when there was a red tide bloom over several months compared to respiratory disease admissions during no red tide. This information is consistent with responses in the animal models after chronic Florida red tide toxin exposure, as well as anecdotal reports and initial epidemiologic studies in humans. In addition, the increased admission rate for all the different respiratory diseases taken together was particularly high among people who lived close to the shore, possibly reflecting their higher or more chronic exposure to the Florida red tide toxins.
This study was a preliminary investigation to examine if there is an increased health care burden to the medical community when an onshore bloom occurs over a period of several months. Since the ER is only one of various possible access points for people seeking medical care, assessment of other medical venues (such as walk in clinics, private practice, and state health departments) is warranted to determine the true burden to the healthcare community. In addition, it is important to assess the individual dose and duration of Florida red tide exposure with follow up over time to establish the onset and severity of disease.
Acknowledgements
This research was supported by the Centers for Disease Control and Prevention and the Florida Department of Health (Cooperative Agreement: U50/CCU423360-02), as well as by the P01 ES 10594 DHHS NIH of the National Institute of Environmental Health Sciences. The authors thank Ms. Carla Laney from Decision Support Services and Ms. Patricia A. Rensing, IRB Coordinator, at Sarasota Memorial Hospital for their outstanding support of this project.[SES].
Abbreviations
- K. brevis
Karenia brevis
- NSP
neurotoxic shellfish poisoning
References
- Abraham WM, Baden DG. Mechanisms of red tide-induced bronchial responses. Int. Soc. Expo. Anal. 2001 November;4–8:126. [Google Scholar]
- Abraham WM, Bourdelais AJ, Ahmed A, Sereberiakov I, Baden DG. Effects of inhaled brevetoxins in allergic airways: toxin–allergen interactions and treatment. Environ. Health Perspect. 2005 May 5;113:632–637. doi: 10.1289/ehp.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham WM, Ahmed A, Bourdelais AJ, Baden DG. Pathophysiologic airway responses to inhaled red tide brevetoxin in allergic sheep. The Toxicologist. 2003;72:115. [Google Scholar]
- Abraham WM, Ahmed A, Bourdelais A, Baden DG. Effects of novel antagonists of polyether brevetoxin (PbTx)-induced bronchoconstriction in allergic sheep. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, Intergovernmental Oceanographic Commission of UNESCO; St. Petersburg, FL: 2004. pp. 496–498. [Google Scholar]
- Anderson DM, Hoagland P, Kaoru Y, White A. Estimated Annual Economic Impacts from Harmful Algal Blooms (HABs) in the United States. Woods Hole, MA: 2000. Woods Hole Oceanog. Inst. Tech. Rep., WHOI-2000–11. [Google Scholar]
- Backer L, Fleming LE, Rowan A, Baden D. Epidemiology and public health of human illnesses associated with harmful marine phytoplankton. In: Hallegraeff GM, Anderson DM, Cembella AD, editors. UNESCO Manual on Harmful Marine Algae. UNESCO/WHO; Geneva, Switzerland: 2003a. pp. 725–750. [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng Y, Bensen J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized brevetoxins during Florida red tide events. Harmful Algae. 2003b;2:19–28. [Google Scholar]
- Backer LC, Kirkpatrick B, Fleming LE, Cheng YS, Pierce R, Bean JA, Clark R, Johnson D, Wanner A, Tamer R, Baden D. Occupational exposure to aerosolized brevetoxins during Florida red tide events: impacts on a healthy worker population. Environ. Health Perspect. 2005a May 5;113:644–649. doi: 10.1289/ehp.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer LC, Schurz Rogers H, Fleming LE, Kirkpatrick B, Benson J. Phycotoxins in marine seafood. In: Dabrowski W, editor. Chemical and Functional Properties of Food Components: Toxins in Food. CRC Press; Boca Raton, FL: 2005b. pp. 155–190. [Google Scholar]
- Baden DG. Marine food-borne dinoflagellate toxins. Int. Rev. Cytol. 1983;82:99–150. doi: 10.1016/s0074-7696(08)60824-4. [DOI] [PubMed] [Google Scholar]
- Baden DG, Adams DJ. Brevetoxins: Chemistry, mechanism of action, and methods of detection. In: Botana L, editor. Seafood and Freshwater Toxins: Pharmacology, Physiology and Detection. Marcel Dekker; NY: 2000. pp. 505–532. [Google Scholar]
- Benson J, Tischler D, Baden D. Uptake, tissue distribution, and excretion of brevetoxin-3 administered to rats by intratracheal instillation. J. Toxicol. Environ. Health. 1999;56:345–355. doi: 10.1080/009841099157656. [DOI] [PubMed] [Google Scholar]
- Benson J, Hahn FF, Tibbetts BM, Bowen LE, March TF, Langley R, Murray TF, Bourdelais AJ, Naar J, Zaias J, Baden DG. Florida red tide: inhalation toxicity of Karenia brevis extract in rats. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA, editors. Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography, Intergovernmental Oceanographic Commission of UNESCO; St. Petersburg, FL: 2004. pp. 502–504. [PMC free article] [PubMed] [Google Scholar]
- Benson JM, Hahn FF, March TH, McDonald JD, Gomez AP, Sopori MJ, Bourdelais AJ, Naar J, Zaias J, Bossart GD, Baden DG. Inhalation toxicity of brevetoxin 3 in rats exposed for twenty-two days. Environ. Health Perspect. 2005;113:626–631. doi: 10.1289/ehp.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossart GD, Baden DG, Ewing R, Roberts B, Wright S. Brevetoxicosis in Manatees (Trichechus manatus latirostris) from the 1996 epizootic: gross, histopathologic and immunocytochemical features. Toxicol. Pathol. 1998;26(2):276–282. doi: 10.1177/019262339802600214. [DOI] [PubMed] [Google Scholar]
- Cheng YS, Zhou Y, Irvin CM, Pierce RH, Naar J, Backer LC, Fleming LE, Kirkpatrick B, Baden D. Characterization of marine aerosol for assessment of human exposure to brevetoxins. Environ. Health Perspect. 2005 May 5;113:638–643. doi: 10.1289/ehp.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton D, Hills M. Statistical Methods in Epidemiology. Oxford Science Publications; New York: 1993. [Google Scholar]
- Fleming LE, Backer L, Rowan A. The epidemiology of human illnesses associated with harmful algal blooms. In: Baden D, Adams D, editors. Neurotoxicology Handbook. Vol. 1. Humana Press Inc.; Totowa, NJ: 2002. pp. 363–381. [Google Scholar]
- Fleming LE, Kirkpatrick B, Backer LC, Bean JA, Wanner A, Dalpra D, Tamer R, Zaias J, Cheng YS, Pierces R, Naar J, Abraham W, Clark R, Zhou Y, Henry MS, Johnson D, Van de Bogart G, Bossart GD, Harrington M, Baden DG. Initial evaluation of the effects of aerosolized Florida red tide toxins (brevetoxins) in persons with asthma. Environ. Health Perspect. 2005 May 5;113:650–657. doi: 10.1289/ehp.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz DR, LeClaire RD. Respiratory effects of brevetoxin and saxitoxin in awake guinea pigs. Toxicon. 1989;27:647–654. doi: 10.1016/0041-0101(89)90015-9. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Fleming L, Squicciarini D, Backer LC, Clark R, Abraham W, Benson J, Cheng YS, Johnson D, Pierce R, Zaias J, Bossart G, Baden DG. Literature review of Florida red tide: implications for human health. Harmful Algae. 2004;3(2):99–115. doi: 10.1016/j.hal.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RH, Henry MS, Proffitt LS, Hasbrouck PA. Red tide toxin (brevetoxin) enrichment in marine aerosol. In: Graneli E, Sundstron S, Elder L, Anderson DM, editors. Toxic Marine Phytoplankton. Elsevier Scientific Publishing; New York: 1990. pp. 397–402. [Google Scholar]
- Quirino W, Fleming LE, Weisman R, Backer L, Kirkpatrick B, Clark R, Dalpra D, Van de Bogart G, Gaines M. Follow up study of red tide associated respiratory illness. Fl. J. Environ. Health. 2004;186:18–22. [Google Scholar]
- Singer LJ, Lee T, Rosen KA, Baden DG, Abraham WM. Inhaled Florida red tide toxins induce bronchoconstriction (BC) and airway hyperresponsiveness (AHR) in sheep. Am. J. Respir. Crit. Care Med. 1998;157(3):A158. [Google Scholar]
- Sudarsanam S, Duke-Virca G, March CJ, Srinivasan S. An approach to computer aided inhibitor design: application to Cathepsin L. J. Comput. Aided Mol. Des. 1992;6:223–233. doi: 10.1007/BF00123378. [DOI] [PubMed] [Google Scholar]
- Woodcock AH. Note concerning human respiratory irritation associated with high concentrations of plankton and mass mortality of marine organisms. J. Mar. Res. 1948;7:56–62. [Google Scholar]


