Summary
Formation of new blood vessels is required for growth and metastasis of all solid tumors. New blood vessels are established in tumors mainly through angiogenesis. Brain tumors in particular are highly angiogenic. Therefore, interventions designed to prevent angiogenesis may be effective at controlling brain tumors. Indeed, many recent findings from preclinical and clinical studies of antiangiogenic therapy for brain tumors showed that it is a promising approach to managing this deadly disease, especially when combined with other cytotoxic treatments. In this review, we summarize the basic characteristics of brain tumor angiogenesis and role of known angiogenic factors in regulating this angiogenesis, which can be targets of antiangiogenic therapy. We also discuss the current status of antiangiogenic therapy for brain tumors, the suggested mechanisms of this therapy, and the limitations of this strategy.
Keywords: brain tumor angiogenesis, vessel normalization, antiangiogenic therapy, clinical trial, VEGF
Introduction
Brain tumors are much rarer than tumors of other organs. Specifically, less than 2% of all tumors diagnosed each year are primary brain tumors[1]. Furthermore, because of the location and other physiological characteristics of brain tumors, their prognosis is very poor[1]. Whereas primary brain tumors originate in the brain, secondary brain tumors metastasize from other cancers, such as breast, lung, and colon cancer and melanoma. Of the many different types of brain tumors, glioblastoma multiforme (GBM) is the most common, accounting for about 40% of all primary brain tumors and 70% of all malignant gliomas[2-4]. GBM is also one of the most vascular and deadly cancers, with a very low 5-year survival rate of 5%[5]. Patients with high tumor microvascular densities (MVDs) have shorter postoperative survival durations than do patients with a low MVDs, suggesting that the tumor vasculature is important to brain tumor growth[6, 7]. Currently, the majority of the available data on vessel formation in brain tumors in the literature comes from studies of GBMs. In this review, we describe the characteristics of brain tumor angiogenesis and the current status and known mechanisms of antiangiogenic therapy for brain tumors based on published results mainly from studies of primary brain tumors.
Angiogenesis in brain tumors
Tipping the balance between proangiogenic and antiangiogenic factors
When a solid tumor such as a brain tumor grows larger than a critical size (1-2 mm in diameter), it must recruit new blood vessels to have the oxygen and nutrition supply necessary for its survival and growth. This tumor-induced formation of new blood vessels occurs primarily via angiogenesis, the process of development and growth of new blood vessels from pre-existing vasculature[8].
Researchers now widely accept that angiogenesis is tightly controlled by a balance of proangiogenic and antiangiogenic factors [9-11]. These molecules can be secreted by cancer, endothelial, stromal, and blood cells and the extracellular matrix [12, 13]. The proangiogenic factors include vascular endothelial growth factor (VEGF), acidic fibroblast growth factor, basic fibroblast growth factor (bFGF), placental growth factor, angiopoietin-2, and interleukins, whereas the antiangiogenic factors include angiostatin, endostatin, thrombospondin 1, and endothelial monocyte-activating polypeptide 2[14, 15]. In addition, the enzymes serine proteinase and metalloproteinase degrade the extracellular matrix, which has an important role in both induction and suppression of angiogenesis [16]. This biological process that is essential to not only tumor development but also normal development and wound repair is highly regulated. When the expression of proangiogenic molecules is balanced with that of antiangiogenic molecules, the ‘angiogenic switch’ remains off. However, in tumor angiogenesis, the tight regulation of the balance of expression of these molecules is disrupted. Induced expression of proangiogenic molecules leads to uncontrolled and disorganized promotion of angiogenesis [8, 17].
Extracellular signaling promotes brain tumor angiogenesis
Angiogenesis in solid tumors, including brain tumors, is believed to be triggered by low oxygen concentrations (hypoxia) resulting from deficits in the blood supply caused by the tumors' fast growth. Exposure of brain tumor cells to hypoxia induces expression of hypoxia-inducible factor-1 (Hif-1), a transcription factor that regulates the expression of many angiogenesis- and glucose metabolism-related genes. Hif-1 activates transcription of VEGF and other proangiogenic factors in gliomas, in particular [18-20]. Researchers have found high levels of expression of VEGF mRNA in the hypoxic regions of high-grade but not low-grade gliomas. In addition, the VEGF receptors (VEGFR1 and VEGFR2) are highly expressed in gliomas [21]. Expression of VEGF is correlated with MVD in gliomas and meningiomas [22]. bFGF is a potent mitogen of endothelial cells and is required for glioma angiogenesis in vivo [23].
Signaling by neurotrophins and their receptors supports neuronal proliferation, differentiation, and synapse formation. The neurotrophin family consists of four structurally related proteins: nerve growth factor (NGF), brain-derived neurotrophin factor (BDNF), neurotrophin-3, and neurotrophin-4[24]. NGF, BDNF, and neurotrophin-3 bind primarily to the receptor kinases TrkA, TrkB, and TrkC, respectively, to mediate their effects across the cell membrane[25]. Also, NGF and BDNF enhance endothelial cell survival and proliferation [26-29]. In particular, BDNF can enhance the expression of proangiogenic factors (e.g., VEGF) in brain tumor-derived cells through induction of Hif-1 expression [30].
Interleukin-8 (also known as CXCL8) is a chemokine with proangiogenic activity. Authors have reported high levels of expression of hepatocyte growth factor/scatter factor and interleukin-8 in primary and recurrent glial tumors [31, 32]. Expression of another chemokine peptide, CXCL12, and its cognate receptors is induced in brain tumors and promotes angiogenesis [33]. Additionally, a subset of integrins mediates endothelial-cell spread and migration in response to growth factor signaling in brain tumor angiogenesis [34]. mRNA expression profiles in gliomas from patients have shown expression of many proangiogenic factors including insulin-like growth factor-1 (IGF-1) in those tumors [35]. Stem cell factor and its receptor c-Kit pathway play important roles in tumor-induced angiogenesis in the brain, as well [36].
γ-Secretase in brain tumor angiogenesis
Signaling by the transmembrane protein Notch and its ligand Jagged/Delta is indispensible for neural system development and is related to development of many types of tumors [37]. Notch signaling is activated by VEGF signaling and suppresses angiogenesis [38-40]. Accordingly, researchers found that blockade of Delta-like ligand 4 led to increased blood vessel sprouting in a glioma model [41]. Interestingly, such increased vessel sprouting does not support but rather suppresses tumor growth, suggesting that Notch signaling is required for the negative feedback and fine-tuning of the proangiogenic VEGF signaling to establish functional vessels in brain tumors [41]. Notch signaling also downregulates the expression of VEGFR2 and VEGF in endothelial cells [42]. Notch signaling is mediated by cleavage of the Notch molecule by γ-secretase, a presenilin-dependent protease complex [43]. VEGF increases γ-secretase activity-mediated Notch 1 cleavage in endothelial cells. Inhibition of γ-secretase activity blocks VEGF-induced endothelial cell proliferation, migration, and survival and eventually leads to decreased angiogenesis [44]. In addition, presenilin cleaves the erythroblastic leukemia viral oncogene homologue 4, ErbB-4[45], which is widely expressed in gliomas and medulloblastomas and enhances tumor angiogenesis [46]. Moreover, γ-secretase cleaves VEGFR1 [47] and IGF-1 receptor, and both of these receptors' signaling promote angiogenesis in astrocytomas and glioblastomas [35, 48]. These results suggest that γ-secretase has complex but as yet unidentified, important roles in brain tumor angiogenesis.
Intracellular machinery of brain tumor angiogenic signaling
As we described above, researchers have made considerable progress in understanding the interactions among cell surface receptors and ligands that regulate angiogenesis. However, the intracellular machinery that governs the signaling from the receptors on the cell surface to the nucleus to control induction of angiogenesis remains poorly understood. Signaling of VEGFR and that of other receptor tyrosine kinases, such as the platelet-derived growth factor receptors (PDGFRs) and epidermal growth factor receptors, have regulatory mechanisms that are similar in many aspects [49]. VEGFR signaling may induce activation of Ras/Raf/mitogen-activated protein kinase [50, 51] or phospholipase C-γ/protein kinase C signaling [52], which regulates endothelial cell proliferation, migration, and permeability [53]. Also, one of the important signaling pathways activated by VEGFR is the phosphatidylinositol-3 kinase/phosphatase and tensin homologue (PTEN)/Akt/mammalian target of rapamycin (mTOR) pathway. This PI3K/PTEN/mTOR pathway regulates endothelial cell survival, translation, and permeability [53-56]. This pathway is also activated by other proangiogenic stimuli, including PDGF, neurotrophins, IGF, epidermal growth factor, and integrins, and plays a critical role in brain tumor angiogenesis [57]. The pivotal role of this signaling pathway in the proliferation and survival of brain tumor cells strongly suggests the potential use of inhibitors of it to target both brain tumor cells and blood vessel endothelial cells [57].
Characteristics of brain tumor vasculature
The blood-brain barrier in brain tumors
The vasculature in a healthy central nervous system (CNS) tissue is highly specialized and distinguished from the vasculature in other tissues by a unique structure of blood capillaries, the blood-brain barrier (BBB) [58]. Unlike other tissues, in which relatively free diffusion of materials in the blood is allowed through their peripheral capillary walls, the transportation of materials in the blood circulation to the peripheral tissues in CNS is tightly regulated by this barrier. The BBB is an anatomical and physiological barrier that strictly restricts the permeability of blood vessels, suppressing the diffusion of ions, peptides, amino acids, and other substances from the bloodstream to the neural system while supplying the brain with the required nutrients for proper CNS function. This barrier is composed of the walls of vessel endothelia, which are sealed by tight junctions between endothelial cells. Also, the BBB is wrapped with specialized cells (pericytes) and the flattened “end feet” of astrocytes. Pericytes are relatively undifferentiated mesenchyme-like cells that support capillary blood vessels. Astrocytes induce the tight junctions of BBB through decreasing VEGF expression and stimulating angiopoietin release [59]. This tight junction prevents the passive diffusion of hydrophilic molecules whose molecular weight is greater than 500 kDa from the bloodstream to the brain parenchyma. Furthermore, water-soluble materials are not able to pass through the lipophilic membranes of endothelial cells. Thus, the BBB is often the primary obstacle to drug delivery to the CNS [58].
Because the brain is located in a confined space, leakage of fluid into the brain caused by breakage of the BBB results in increased interstitial pressure within the skull and, consequently, vasogenic brain edema. Therefore, keeping the molecules carried in the blood vessels in the brain from breaching the BBB and entering brain tissue is essential for maintaining normal brain physiology. Hence, BBB breakdown is associated with many CNS-associated pathologies, including neurological diseases such as Alzheimer disease.
The BBB in brain tumors is structurally and functionally abnormal [60-63]. Nevertheless, some features of the normal BBB are retained in brain tumor vasculature [61, 63, 64]. Researchers found that, in a murine brain tumor metastasis model, the integrity of the BBB was conserved in small tumors (<0.25 mm in diameter) but not in larger tumors [65]. In addition to loss of BBB integrity, blood vessels in brain tumors exhibit abnormal features similar to those in the vessels in other types of tumors. For example, tumor blood vessels are tortuous, disorganized, and highly permeable because of abnormalities in their endothelial walls [61-63, 66-69]. Therefore, disruption of the BBB and further increases in the permeability of tumor blood vessels in brain tumors because of their loosened endothelial structures result in increased accumulation of fluid peritumorally and in the surrounding brain and bring about vasogenic brain edema. Vasogenic edema is a major cause of morbidity in patients with brain tumors [70, 71]. Hence, tumor angiogenesis must be treated properly to not only prevent brain tumor growth but also suppress the pathological damage caused by changes in the permeability of brain tissue. The blood vessels associated with brain metastases are dilated and contain highly mitotic endothelial cells[65], which may require high concentrations of VEGF for their growth. Increased leakage from blood vessels in brain tumors causes suppressed and irregular blood flow and leads to heterogeneous and inefficient delivery of oxygen, nutrients, and drugs to the brain tumor via the bloodstream [63, 71, 72].
One of the main causes of increased permeability and loss of BBB integrity in brain tumor blood vessels is increased expression of VEGF by the brain tumor cells. Investigators initially purified VEGF for its ability to induce vascular leakage and permeability as well as for its role as a mitogenic factor for endothelial cells. Therefore, it was originally known as vascular permeability factor as well as VEGF [73-75]. The effects of VEGF on vascular permeability in the peripheral circulation appear to occur via modulation of calcium influx, nitric oxide; activation of guanylyl cyclase, protein kinase G, vesiculo-vacuolar organelles, or increased synthesis of platelet-activating factor in endothelial cells [73-78].
Hypoxia and acidosis are hallmarks of solid tumors, but they are not necessarily correlated with each other[79], and low oxygen and pH levels independently upregulate VEGF transcription in brain tumors in vivo [60]. Also, many oncogenes, such as ras and src; cytokines and growth factor receptor signaling; and tumor suppressor genes, such as trp53, regulate transcriptional and translational expression of VEGF in tumors [80, 81] and surrounding tissues [12]. In principle, tumor blood vessels, especially brain tumor blood vessels, are abnormal, which means they are highly permeable and even leaky at many points, resulting in irregular and inefficient blood flow through them. This irregularity and inefficiency are strongly linked with the action of VEGF, the major proangiogenic factor.
Targeting brain tumor vasculature
Therapeutic antiangiogenic agents for brain tumors
In the early 1970s, Folkman and colleagues proposed blockage of tumor vascularization as an approach to treating cancers [9]. Because of the known pivotal role of VEGF in angiogenesis, many antiangiogenic approaches have targeted VEGF and VEGFR signaling. More than 30 years after the seminal findings by Folkman's group, the U.S. Food and Drug Administration approved bevacizumab, a humanized monoclonal antibody against VEGF, as the first drug used for antiangiogenic therapy for cancer (specifically, colon, lung, and breast cancer). Also approved for antiangiogenic therapy for renal carcinoma and other tumors by the U.S. Food and Drug Administration were sorafenib and sunitinib, two small molecules that target VEGFR kinase activity. Unfortunately, none of these agents are approved for therapy for brain tumors yet. However, the number of clinical trials examining the use of antiangiogenic agents to treat brain tumors is increasing. Table 1 summarizes current (as of January 2009) clinical trials targeting angiogenesis in primary and secondary adult brain tumors. More than 70 clinical trials using about 20 anticancer agents that may inhibit angiogenesis in brain tumors are in progress. The majority of these trials are targeting VEGF signaling with monoclonal antibodies against VEGF [20, 68], small-molecule tyrosine kinase inhibitors (TKIs) that inhibit VEGFR2 tyrosine kinase activity [82], and soluble decoy receptors developed from VEGFR1 that selectively inhibit VEGF activity [83]. In addition to VEGFR signaling, PDGFR, αvβ3 integrins [84], and intracellular mediators of angiogenic signaling (protein kinase C and mTOR) are targets reagents used in these clinical trials. Also, several clinical trials are studying modulations of BBB integrity designed to modify the permeability of the blood vessels in brain tumors and eventually improve delivery of cytotoxic agents to the brain tumors.
Table 1. Clinical trials with antiangiogenic agents targeting brain tumorsa.
| Drug | Target | Phase | Tumors |
|---|---|---|---|
| XL184 (TKI) | VEGFR, MET | II | GBM |
| ZK222584 (TKI) | VEGFR, PDGFR | I,II | MG |
| Pazopanib (TKI) | VEGFR/PDGFR/c-Kit | II | Glioma, Meta |
| Sorafenib (TKI) | VEGFR/PDGFR/Raf | I,II | GBM, Meta,GS |
| Sunitinib (TKI) | VEGFR/PDGFR | I,II | GBM,Meta |
| Thalidomide/Lenalidomide | VEGF/bFGF/TNF | I,II | GBM,Meta |
| VEGF Trap (Peptide) | VEGF | II | MG |
| Ceradinib (TKI) | VEGFR | I,II,III | GBM |
| ZD6474 (TKI) | VEGFR | I,II | GBM |
| CT-322 (Peptide) | VEGFR | I,II | GBM |
| Bevacizumab (mAb) | VEGF | I,II | GBM |
| Valproic acid | Potential anti-angiogenic | II | Meningioma, GBM |
| Cilengitide (Peptide) | Integrin | II,III | GBM, MG |
| Everolimus/Sirolimus | FKBP-12/mTOR | I, II | MG |
| II | GBM | ||
| RAD001 (SKI) | mTOR | III | Astrocytoma |
| Temsirolimus (SKI) | mTOR | I | GBM |
| Tandutinib (TKI) | PDGFR | I,II | GB,Astrocytoma,Meta |
| XL765 (SKI) | PI3K/mTOR | I | Oligodedroglioma |
| Enzastaurin (SKI) | PKC | I,II | GBM |
| MPC-6827 | Microtubule (microvessel) | I,II | GBM |
| CY997 | tubulin (microvessel) | I,II | GBM, Gliosarc, Meta |
| Sodiumthiosulfate/Mannitol | BBB | I.II | OG,OA,MB,NB,GBM |
The information in this table was extracted from the National Cancer Institute Web site (http://www.cancer.gov) in a search for currently (Jan. 2009) open clinical trials. Most of the drugs have been used in multiple concurrent clinical trials.
Abbreviations: GBM, glioblastoma multiform; GS, gliosarcoma; MG, malignant glioma; OG, oligoglyoma; OA, oligoastrocytoma; MB, medulloblastoma; NB, neuroblastoma; Meta, metastasized secondary brain tumors; mAb, monoclonal antibody; PKC, protein kinase C; SKI, serine-threonine kinase inhibitor; PI3K, phosphatidylinositol-3 kinase; FKBP-12, FK506 binding protein 1A- 12kDa.
Because Notch signaling suppresses and orchestrates formation of brain tumor vessel sprouts, treatment with γ-secretase inhibitors may inhibit normal Notch signaling and thus inhibits tumor angiogenesis. Indeed, authors reported that treatment with a γ-secretase inhibitor suppressed the growth and vascularization of human GBM xenografted into nude mice [85].
The intracellular machinery of angiogenic signaling described above can be an effective target in antiangiogenic therapy for brain tumors. As expected from the observation that the PI3K/Akt/mTOR signaling pathway is essential for endothelial cell survival and blood vessel permeability, inhibition of mTOR signaling by small-molecule inhibitors or RNA interference have proven to be efficacious at antiangiogenic therapy in preclinical models of malignant glioma [86, 87].
Efficacy of antiangiogenic therapy for brain tumors
The results of the first series of clinical trials (phase I and II) of treatment of GBM with vatalanib, a small-molecule TKI against of VEGFR2, were disappointing [88, 89]. However, recent clinical trials showed that bevacizumab and a TKI of VEGFR were effective in treatment of brain tumors when combined with standard chemotherapeutic agents [20, 82]. Researchers in one of these studies observed a strong antiedema effect of bevacizumab, suggesting that this antiangiogenic therapy decreased the permeability of brain tumor blood vessels [20]. These results suggested that the antiangiogenic agents used augmented cytotoxic drug efficacy by improving the blood flow in these tumors [20, 82]. The novel hypothesis that ‘antiangiogenic therapy normalizes the abnormal tumor blood vessels’ may explain these results [90]. As described above, intratumoral vessels are leaky because of high local concentrations of VEGF, a potent vascular permeability factor. Blocking VEGF or VEGFRs in tumors suppresses the function of VEGF signaling; the tumor vasculature permeability then returns to normal, leading to restoration of effective blood flow [91]. Accordingly, antiangiogenic therapy enhances the delivery and efficacy of concurrently administered cytotoxic agents [90, 92-94]. This is supported by the results of studies using a number of preclinical models [63, 95-97], including primary and secondary brain tumor models [68, 98]. Authors also reported that VEGFR2 blockade by the monoclonal antibody DC101 temporarily normalized tumor vessel walls, improving vascular function and tumor oxygenation and thus enhancing responses to radiotherapy in a GBM model in nude mice [99]. In human clinical trials, treatment with bevacizumab indeed changed several abnormal characteristics of tumor vessels to resemble normal characteristics of non-tumor vessels [100]. In patients with recurrent GBM treated with a novel TKI, the VEGFR inhibitor AZD2171, the treatment normalized tumor blood vessels, leading to the significant clinical benefit of alleviation of edema [82]. However, such normalization seems to be only temporal and reversible, suggesting that the optimal window of time during which combined cytotoxic and antiangiogenic therapy for brain cancer provides the best outcome is limited. It has been reported that during this window, after treatment with anti-VEGFR2 antibody, tumor oxygenation improved and pericyte coverage and angiopoietin expression increased and thus decreased interstitial pressure and vessel permeability in orthotopic GBM [68].
Researchers have suggested that antiangiogenic therapeutic agents have several mechanisms of action in combined treatment with radiotherapy [101] For example, a study found that blocking VEGF's action enhanced the cytotoxic effect of radiotherapy for tumor cells in vitro [102]. Also, authors reported that the response of brain tumors to radiotherapy depended on endothelial cell apoptosis in the tumors in a murine model [103]. This suggested that blockage of VEGF signaling leading to apoptosis of endothelial cells and thus enhanced the efficacy of radiotherapy. Radiotherapy also induced expression of Hif-1 and genes downstream of it, including VEGF, in an in vivo murine tumor model [104]. In brain tumor models, radiotherapy has induced VEGF expression; this induced expression is essential for endothelial cell survival [105-107]. Therefore, radiotherapy-induced endothelial cell death would be enhanced by blockage of VEGF action by inhibition of newly expressed VEGF in irradiated cancer cells and endothelial cells.
Compelling evidence suggests that brain cancer cells are generated by small fractions of self-renewing, multipotent, tumor-initiating cells termed cancer stem cells (CSCs) in brain tumors as they are in other tumors [108]. Calabrese et al. [109] observed that brain tumor CSCs are found in vascular niches in the tumors as normal brain stem cells are. These authors reported that antiangiogenic therapy disrupted the tumor vasculature and that the CSC niche microenvironment associated with the tumor blood vessels reduced the CSC population in the brain, suggesting that extirpation of brain CSCs may contribute to the efficacy of antiangiogenic cancer therapy.
Current limits of and perspectives on antiangiogenic therapy for brain tumors
The use of antiangiogenic therapy for brain tumors carries several concerns [110]. In spite of the fact that angiogenesis is essential for growth of all tumors, antiangiogenic therapy usually results in only transitory delays of tumor growth and progression, after which tumors begin to regrow and the cancer progresses. This may result from adaptation of tumors to antiangiogenic therapy; evasive resistance of tumors to radiotherapy [111] and intrinsic resistance of tumors to antiangiogenic therapy. Tumor cells self-adjust to antiangiogenic therapy by developing alternative pathways to recruit new blood vessels. Also, tumors may activate and enhance invasion and metastasis of their own cells after antiangiogenic therapy [111]. Therefore, use of antiangiogenic therapy combined with other therapies that may override this adaptive ‘self-adjusting’ pathway would significantly improve the efficacy of the anticancer treatment.
Hemorrhage and thrombosis are potential complications of antiangiogenic treatment of GBM[20]. Also, authors have reported intracerebral hemorrhages in patients with gliomas treated with bevacizumab in combination with irinotecan, a topoisomerase inhibitor [112]. Rebound cerebral edema caused by the reversibility of normalization of blood vessels in brain tumors after stopping antiangiogenic treatment is another potential problem. Additionally, blockage of angiogenesis is potentially cytotoxic to neural stem cells, which reside in the perivascular niches in the subventricular zones of the brain and are supported by the growth factors generated from the endothelial cells. Therefore, antiangiogenic therapy resulting in VEGF-signaling suppression or endothelial cell death may cause neural stem cell death [113]. When more clinical data on the efficacy and mechanism of action of antiangiogenic therapy for brain and other tumors from many of the ongoing clinical trials of this therapy become available, the resulting improvement in understanding of this mechanism will allow us to optimize combinations of antiangiogenic therapy and other cytotoxic treatments and improve the benefits of this therapy in patients with brain cancer.
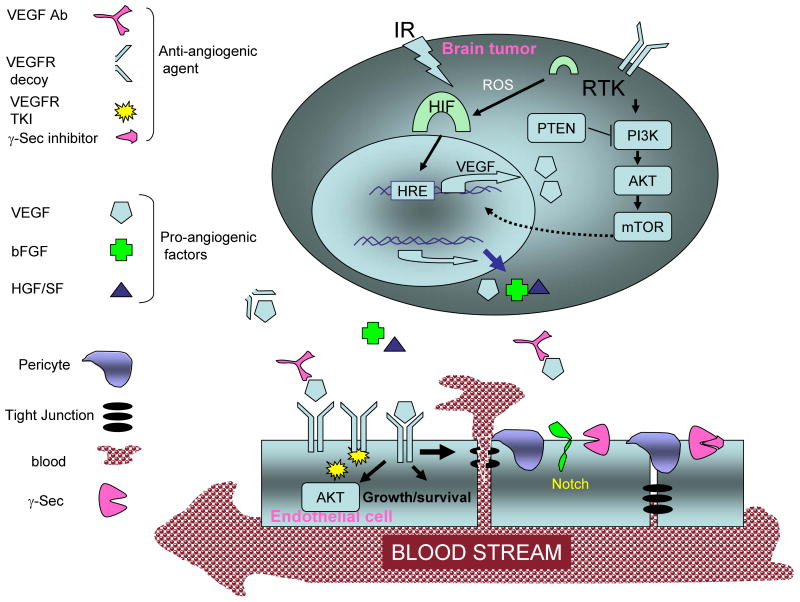
Fig. 1.
Angiogenic interaction between brain tumor and endothelial cells and antiangiogenic therapy. VEGF expression is induced in tumor cells by hypoxia [19], radiation, receptor tyrosine kinase signaling, and Akt pathways [114]. VEGF and other proangiogenic factors are secreted by brain cancer cells in a paracrine and endocrine manner [83]. These factors support endothelial cell survival. Also, VEGF induces the permeability of the BBB [78]. Notch signaling mediated by γ-secretase suppresses angiogenesis but is required for proper vascular development [38-40, 44]. The signaling required for angiogenesis in brain tumors can be targeted using an antibody against VEGF[20, 68], a decoy receptor to VEGF [115], small-molecule TKIs of VEGFR[82], inhibitors of other kinases in the signaling cascade, and inhibitors of γ-secretase [85]. Ab, antibody; HGF/SF, hepatocyte growth factor/scatter factor; IR, ionizing radiation; RTK, receptor tyrosine kinase; PTEN, phosphatase and tensin homologue; PI3K, phosphatidylinositol-3 kinase; HRE, hypoxia-responsive element; ROS, reactive oxygen species; γ-Sec, γ–secretase.
Acknowledgments
This work was supported by National Institutes of Health grants R01 CA109520 and CA100816-01A1 (to H.-Y. Lee).
Addendum
After this article was submitted, U.S. Food and Drug Administration (FDA) granted accelerated approval of bevacizumab (antibody to VEGF) for glioblastoma patients with progressive disease following prior therapy, in May 2009. This is the first FDA approved anti-angiogenic agent for brain tumors.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Ohgaki H, Wiestler OD. The 2007 WHO classification of tumors of the central nervous system. IARC Press; Lyon, France: 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer MA. Malignant gliomas in adults. N Engl J Med. 2008;359:1850. doi: 10.1056/NEJMc086380. author reply 1850. [DOI] [PubMed] [Google Scholar]
- 5.Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- 6.Birlik B, Canda S, Ozer E. Tumour vascularity is of prognostic significance in adult, but not paediatric astrocytomas. Neuropathol Appl Neurobiol. 2006;32:532–538. doi: 10.1111/j.1365-2990.2006.00763.x. [DOI] [PubMed] [Google Scholar]
- 7.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77:362–372. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 8.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 9.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 10.Folkman J. Anti-angiogenesis: new concept for therapy of solid tumors. Ann Surg. 1972;175:409–416. doi: 10.1097/00000658-197203000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folkman J, Szabo S, Stovroff M, McNeil P, Li W, Shing Y. Duodenal ulcer. Discovery of a new mechanism and development of angiogenic therapy that accelerates healing. Ann Surg. 1991;214:414–425. doi: 10.1097/00000658-199110000-00006. discussion 426-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, Selig M, Nielsen G, Taksir T, Jain RK, Seed B. Tumor induction of VEGF promoter activity in stromal cells. Cell. 1998;94:715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 13.Tandle A, Blazer DG, 3rd, Libutti SK. Antiangiogenic gene therapy of cancer: recent developments. J Transl Med. 2004;2:22. doi: 10.1186/1479-5876-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dameron KM, Volpert OV, Tainsky MA, Bouck N. Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science. 1994;265:1582–1584. doi: 10.1126/science.7521539. [DOI] [PubMed] [Google Scholar]
- 15.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang J, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc Natl Acad Sci U S A. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fidler IJ, Ellis LM. Neoplastic angiogenesis--not all blood vessels are created equal. N Engl J Med. 2004;351:215–216. doi: 10.1056/NEJMp048080. [DOI] [PubMed] [Google Scholar]
- 18.Maxwell PH, Dachs GU, Gleadle JM, Nicholls LG, Harris AL, Stratford IJ, Hankinson O, Pugh CW, Ratcliffe PJ. Hypoxia-inducible factor-1 modulates gene expression in solid tumors and influences both angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1997;94:8104–8109. doi: 10.1073/pnas.94.15.8104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 20.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 21.Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992;359:845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- 22.Samoto K, Ikezaki K, Ono M, Shono T, Kohno K, Kuwano M, Fukui M. Expression of vascular endothelial growth factor and its possible relation with neovascularization in human brain tumors. Cancer Res. 1995;55:1189–1193. [PubMed] [Google Scholar]
- 23.Auguste P, Gursel DB, Lemiere S, Reimers D, Cuevas P, Carceller F, Di Santo JP, Bikfalvi A. Inhibition of fibroblast growth factor/fibroblast growth factor receptor activity in glioma cells impedes tumor growth by both angiogenesis-dependent and -independent mechanisms. Cancer Res. 2001;61:1717–1726. [PubMed] [Google Scholar]
- 24.Hallbook F, Wilson K, Thorndyke M, Olinski RP. Formation and evolution of the chordate neurotrophin and Trk receptor genes. Brain Behav Evol. 2006;68:133–144. doi: 10.1159/000094083. [DOI] [PubMed] [Google Scholar]
- 25.Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 26.Nico B, Mangieri D, Benagiano V, Crivellato E, Ribatti D. Nerve growth factor as an angiogenic factor. Microvasc Res. 2008;75:135–141. doi: 10.1016/j.mvr.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Kraemer R, Hempstead BL. Neurotrophins: novel mediators of angiogenesis. Front Biosci. 2003;8:s1181–1186. doi: 10.2741/1169. [DOI] [PubMed] [Google Scholar]
- 28.Cantarella G, Lempereur L, Presta M, Ribatti D, Lombardo G, Lazarovici P, Zappala G, Pafumi C, Bernardini R. Nerve growth factor-endothelial cell interaction leads to angiogenesis in vitro and in vivo. FASEB J. 2002;16:1307–1309. doi: 10.1096/fj.01-1000fje. [DOI] [PubMed] [Google Scholar]
- 29.Donovan MJ, Lin MI, Wiegn P, Ringstedt T, Kraemer R, Hahn R, Wang S, Ibanez CF, Rafii S, Hempstead BL. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development. 2000;127:4531–4540. doi: 10.1242/dev.127.21.4531. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Martin KC, Jackson JK, Beppu K, Woo CW, Thiele CJ. Brain-derived neurotrophic factor activation of TrkB induces vascular endothelial growth factor expression via hypoxia-inducible factor-1alpha in neuroblastoma cells. Cancer Res. 2006;66:4249–4255. doi: 10.1158/0008-5472.CAN-05-2789. [DOI] [PubMed] [Google Scholar]
- 31.Salmaggi A, Eoli M, Frigerio S, Silvani A, Gelati M, Corsini E, Broggi G, Boiardi A. Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol. 2003;62:297–303. doi: 10.1023/a:1023367223575. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt NO, Westphal M, Hagel C, Ergun S, Stavrou D, Rosen EM, Lamszus K. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Li M, Ransohoff RM. The roles of chemokine CXCL12 in embryonic and brain tumor angiogenesis. Semin Cancer Biol. 2008;19:111–115. doi: 10.1016/j.semcancer.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 34.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 35.Tso CL, Freije WA, Day A, Chen Z, Merriman B, Perlina A, Lee Y, Dia EQ, Yoshimoto K, Mischel PS, Liau LM, Cloughesy TF, Nelson SF. Distinct transcription profiles of primary and secondary glioblastoma subgroups. Cancer Res. 2006;66:159–167. doi: 10.1158/0008-5472.CAN-05-0077. [DOI] [PubMed] [Google Scholar]
- 36.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Koch U, Radtke F. Notch and cancer: a double-edged sword. Cell Mol Life Sci. 2007;64:2746–2762. doi: 10.1007/s00018-007-7164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diez H, Fischer A, Winkler A, Hu CJ, Hatzopoulos AK, Breier G, Gessler M. Hypoxia-mediated activation of Dll4-Notch-Hey2 signaling in endothelial progenitor cells and adoption of arterial cell fate. Exp Cell Res. 2007;313:1–9. doi: 10.1016/j.yexcr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 39.Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, Lindblom P, Alva J, Nilsson AK, Karlsson L, Gaiano N, Yoon K, Rossant J, Iruela-Arispe ML, Kalen M, Gerhardt H, Betsholtz C. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 40.Ridgway J, Zhang G, Wu Y, Stawicki S, Liang WC, Chanthery Y, Kowalski J, Watts RJ, Callahan C, Kasman I, Singh M, Chien M, Tan C, Hongo JA, de Sauvage F, Plowman G, Yan M. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 41.Noguera-Troise I, Daly C, Papadopoulos NJ, Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD, Thurston G. Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 42.Boulton ME, Cai J, Grant MB. gamma-Secretase: a multifaceted regulator of angiogenesis. J Cell Mol Med. 2008;12:781–795. doi: 10.1111/j.1582-4934.2008.00274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Selkoe D, Kopan R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu Rev Neurosci. 2003;26:565–597. doi: 10.1146/annurev.neuro.26.041002.131334. [DOI] [PubMed] [Google Scholar]
- 44.Takeshita K, Satoh M, Ii M, Silver M, Limbourg FP, Mukai Y, Rikitake Y, Radtke F, Gridley T, Losordo DW, Liao JK. Critical role of endothelial Notch1 signaling in postnatal angiogenesis. Circ Res. 2007;100:70–78. doi: 10.1161/01.RES.0000254788.47304.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 46.Russell KS, Stern DF, Polverini PJ, Bender JR. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol. 1999;277:H2205–2211. doi: 10.1152/ajpheart.1999.277.6.H2205. [DOI] [PubMed] [Google Scholar]
- 47.Boulton ME, Cai J, Grant MB, Zhang Y. Gamma-secretase regulates VEGFR-1 signalling in vascular endothelium and RPE. Adv Exp Med Biol. 2008;613:313–319. doi: 10.1007/978-0-387-74904-4_36. [DOI] [PubMed] [Google Scholar]
- 48.Hirano H, Lopes MB, Laws ER, Jr, Asakura T, Goto M, Carpenter JE, Karns LR, VandenBerg SR. Insulin-like growth factor-1 content and pattern of expression correlates with histopathologic grade in diffusely infiltrating astrocytomas. Neuro Oncol. 1999;1:109–119. doi: 10.1093/neuonc/1.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling - in control of vascular function. Nat Rev Mol Cell Biol. 2006;7:359–371. doi: 10.1038/nrm1911. [DOI] [PubMed] [Google Scholar]
- 50.Jones MK, Itani RM, Wang H, Tomikawa M, Sarfeh IJ, Szabo S, Tarnawski AS. Activation of VEGF and Ras genes in gastric mucosa during angiogenic response to ethanol injury. Am J Physiol. 1999;276:G1345–1355. doi: 10.1152/ajpgi.1999.276.6.G1345. [DOI] [PubMed] [Google Scholar]
- 51.Takahashi T, Ueno H, Shibuya M. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene. 1999;18:2221–2230. doi: 10.1038/sj.onc.1202527. [DOI] [PubMed] [Google Scholar]
- 52.Takahashi T, Shibuya M. The 230 kDa mature form of KDR/Flk-1 (VEGF receptor-2) activates the PLC-gamma pathway and partially induces mitotic signals in NIH3T3 fibroblasts. Oncogene. 1997;14:2079–2089. doi: 10.1038/sj.onc.1201047. [DOI] [PubMed] [Google Scholar]
- 53.Lal BK, Varma S, Pappas PJ, Hobson RW, 2nd, Duran WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res. 2001;62:252–262. doi: 10.1006/mvre.2001.2338. [DOI] [PubMed] [Google Scholar]
- 54.Li B, Xu W, Luo C, Gozal D, Liu R. VEGF-induced activation of the PI3-K/Akt pathway reduces mutant SOD1-mediated motor neuron cell death. Brain Res Mol Brain Res. 2003;111:155–164. doi: 10.1016/s0169-328x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 55.Six I, Kureishi Y, Luo Z, Walsh K. Akt signaling mediates VEGF/VPF vascular permeability in vivo. FEBS Lett. 2002;532:67–69. doi: 10.1016/s0014-5793(02)03630-x. [DOI] [PubMed] [Google Scholar]
- 56.Takahashi M, Matsui A, Inao M, Mochida S, Fujiwara K. ERK/MAPK-dependent PI3K/Akt phosphorylation through VEGFR-1 after VEGF stimulation in activated hepatic stellate cells. Hepatol Res. 2003;26:232–236. doi: 10.1016/s1386-6346(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 57.Castellino RC, Durden DL. Mechanisms of disease: the PI3K-Akt-PTEN signaling node--an intercept point for the control of angiogenesis in brain tumors. Nat Clin Pract Neurol. 2007;3:682–693. doi: 10.1038/ncpneuro0661. [DOI] [PubMed] [Google Scholar]
- 58.Deeken JF, Loscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 59.Lee SW, Kim WJ, Choi YK, Song HS, Son MJ, Gelman IH, Kim YJ, Kim KW. SSeCKS regulates angiogenesis and tight junction formation in blood-brain barrier. Nat Med. 2003;9:900–906. doi: 10.1038/nm889. [DOI] [PubMed] [Google Scholar]
- 60.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 2001;61:6020–6024. [PubMed] [Google Scholar]
- 61.Hobbs SK, Monsky WL, Yuan F, Roberts WG, Griffith L, Torchilin VP, Jain RK. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc Natl Acad Sci U S A. 1998;95:4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Monsky WL, Fukumura D, Gohongi T, Ancukiewcz M, Weich HA, Torchilin VP, Yuan F, Jain RK. Augmentation of transvascular transport of macromolecules and nanoparticles in tumors using vascular endothelial growth factor. Cancer Res. 1999;59:4129–4135. [PubMed] [Google Scholar]
- 63.Yuan F, Salehi HA, Boucher Y, Vasthare US, Tuma RF, Jain RK. Vascular permeability and microcirculation of gliomas and mammary carcinomas transplanted in rat and mouse cranial windows. Cancer Res. 1994;54:4564–4568. [PubMed] [Google Scholar]
- 64.Monsky WL, Mouta Carreira C, Tsuzuki Y, Gohongi T, Fukumura D, Jain RK. Role of host microenvironment in angiogenesis and microvascular functions in human breast cancer xenografts: mammary fat pad versus cranial tumors. Clin Cancer Res. 2002;8:1008–1013. [PubMed] [Google Scholar]
- 65.Fidler IJ, Yano S, Zhang RD, Fujimaki T, Bucana CD. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol. 2002;3:53–57. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 66.Bullitt E, Zeng D, Gerig G, Aylward S, Joshi S, Smith JK, Lin W, Ewend MG. Vessel tortuosity and brain tumor malignancy: a blinded study. Acad Radiol. 2005;12:1232–1240. doi: 10.1016/j.acra.2005.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plate KH, Mennel HD. Vascular morphology and angiogenesis in glial tumors. Exp Toxicol Pathol. 1995;47:89–94. doi: 10.1016/S0940-2993(11)80292-7. [DOI] [PubMed] [Google Scholar]
- 68.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 69.Zagzag D, Hooper A, Friedlander DR, Chan W, Holash J, Wiegand SJ, Yancopoulos GD, Grumet M. In situ expression of angiopoietins in astrocytomas identifies angiopoietin-2 as an early marker of tumor angiogenesis. Exp Neurol. 1999;159:391–400. doi: 10.1006/exnr.1999.7162. [DOI] [PubMed] [Google Scholar]
- 70.Jain RK, di Tomaso E, Duda DG, Loeffler JS, Sorensen AG, Batchelor TT. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 71.Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67:2729–2735. doi: 10.1158/0008-5472.CAN-06-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grizzi F, Russo C, Colombo P, Franceschini B, Frezza EE, Cobos E, Chiriva-Internati M. Quantitative evaluation and modeling of two-dimensional neovascular network complexity: the surface fractal dimension. BMC Cancer. 2005;5:14. doi: 10.1186/1471-2407-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM. Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation. 1998;97:99–107. doi: 10.1161/01.cir.97.1.99. [DOI] [PubMed] [Google Scholar]
- 74.Sirois MG, Edelman ER. VEGF effect on vascular permeability is mediated by synthesis of platelet-activating factor. Am J Physiol. 1997;272:H2746–2756. doi: 10.1152/ajpheart.1997.272.6.H2746. [DOI] [PubMed] [Google Scholar]
- 75.Wu HM, Huang Q, Yuan Y, Granger HJ. VEGF induces NO-dependent hyperpermeability in coronary venules. Am J Physiol. 1996;271:H2735–2739. doi: 10.1152/ajpheart.1996.271.6.H2735. [DOI] [PubMed] [Google Scholar]
- 76.Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49:419–432. doi: 10.1177/002215540104900401. [DOI] [PubMed] [Google Scholar]
- 77.Hippenstiel S, Krull M, Ikemann A, Risau W, Clauss M, Suttorp N. VEGF induces hyperpermeability by a direct action on endothelial cells. Am J Physiol. 1998;274:L678–684. doi: 10.1152/ajplung.1998.274.5.L678. [DOI] [PubMed] [Google Scholar]
- 78.Mayhan WG. VEGF increases permeability of the blood-brain barrier via a nitric oxide synthase/cGMP-dependent pathway. Am J Physiol. 1999;276:C1148–1153. doi: 10.1152/ajpcell.1999.276.5.C1148. [DOI] [PubMed] [Google Scholar]
- 79.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 80.Dvorak HF. Vascular permeability factor/vascular endothelial growth factor: a critical cytokine in tumor angiogenesis and a potential target for diagnosis and therapy. J Clin Oncol. 2002;20:4368–4380. doi: 10.1200/JCO.2002.10.088. [DOI] [PubMed] [Google Scholar]
- 81.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 82.Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov. 2007;6:273–286. doi: 10.1038/nrd2115. [DOI] [PubMed] [Google Scholar]
- 84.Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25:1651–1657. doi: 10.1200/JCO.2006.06.6514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paris D, Quadros A, Patel N, DelleDonne A, Humphrey J, Mullan M. Inhibition of angiogenesis and tumor growth by beta and gamma-secretase inhibitors. Eur J Pharmacol. 2005;514:1–15. doi: 10.1016/j.ejphar.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 86.Newton HB. Molecular neuro-oncology and development of targeted therapeutic strategies for brain tumors. Part 2: PI3K/Akt/PTEN, mTOR, SHH/PTCH and angiogenesis. Expert Rev Anticancer Ther. 2004;4:105–128. doi: 10.1586/14737140.4.1.105. [DOI] [PubMed] [Google Scholar]
- 87.Iwamaru A, Kondo Y, Iwado E, Aoki H, Fujiwara K, Yokoyama T, Mills GB, Kondo S. Silencing mammalian target of rapamycin signaling by small interfering RNA enhances rapamycin-induced autophagy in malignant glioma cells. Oncogene. 2007;26:1840–1851. doi: 10.1038/sj.onc.1209992. [DOI] [PubMed] [Google Scholar]
- 88.Conrad C, F H, Reardon D, Conrad C, Friedman H, Reardon D, Provenzale J, Jackson E, Serajuddin H, Laurent D, Chen B, Yung WKA. A phase I/II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM) J Clin Oncol. 2004;22:1512. abstract. [Google Scholar]
- 89.Reardon D, Friedman H, Yung WKA, Brada M, Conrad C, Provenzale J, Jackson E, Serajuddin H, C B, Laurent D. A phase I/II trial of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM) J Clin Oncol. 2004;22:1513. abstract. [Google Scholar]
- 90.Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7:987–989. doi: 10.1038/nm0901-987. [DOI] [PubMed] [Google Scholar]
- 91.Baluk P, Hashizume H, McDonald DM. Cellular abnormalities of blood vessels as targets in cancer. Curr Opin Genet Dev. 2005;15:102–111. doi: 10.1016/j.gde.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Jain RK. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology (Williston Park) 2005;19:7–16. [PubMed] [Google Scholar]
- 93.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 94.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 95.Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: role of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64:3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- 97.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci U S A. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Izumi Y, Xu L, di Tomaso E, Fukumura D, Jain RK. Tumour biology: herceptin acts as an anti-angiogenic cocktail. Nature. 2002;416:279–280. doi: 10.1038/416279b. [DOI] [PubMed] [Google Scholar]
- 99.Weichselbaum RR. How does antiangiogenic therapy affect brain tumor response to radiation? Nat Clin Pract Oncol. 2005;2:232–233. doi: 10.1038/ncponc0177. [DOI] [PubMed] [Google Scholar]
- 100.Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 102.Gorski DH, Beckett MA, Jaskowiak NT, Calvin DP, Mauceri HJ, Salloum RM, Seetharam S, Koons A, Hari DM, Kufe DW, Weichselbaum RR. Blockage of the vascular endothelial growth factor stress response increases the antitumor effects of ionizing radiation. Cancer Res. 1999;59:3374–3378. [PubMed] [Google Scholar]
- 103.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, Fuks Z, Kolesnick R. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. doi: 10.1126/science.1082504. [DOI] [PubMed] [Google Scholar]
- 104.Moeller BJ, Cao Y, Li CY, Dewhirst MW. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 105.Jiang F, Zhang ZG, Katakowski M, Robin AM, Faber M, Zhang F, Chopp M. Angiogenesis induced by photodynamic therapy in normal rat brains. Photochem Photobiol. 2004;79:494–498. doi: 10.1562/2003-11-19-rc.1. [DOI] [PubMed] [Google Scholar]
- 106.Kim JH, Chung YG, Kim CY, Kim HK, Lee HK. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. 2004;19:879–886. doi: 10.3346/jkms.2004.19.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tsao MN, Li YQ, Lu G, Xu Y, Wong CS. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999;58:1051–1060. doi: 10.1097/00005072-199910000-00003. [DOI] [PubMed] [Google Scholar]
- 108.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 109.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 110.Wong ET, Brem S. Antiangiogenesis treatment for glioblastoma multiforme: challenges and opportunities. J Natl Compr Canc Netw. 2008;6:515–522. doi: 10.6004/jnccn.2008.0039. [DOI] [PubMed] [Google Scholar]
- 111.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 113.Li Q, Ford MC, Lavik EB, Madri JA. Modeling the neurovascular niche: VEGF- and BDNF-mediated cross-talk between neural stem cells and endothelial cells: an in vitro study. J Neurosci Res. 2006;84:1656–1668. doi: 10.1002/jnr.21087. [DOI] [PubMed] [Google Scholar]
- 114.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 115.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]