Abstract
The molecular mechanism for the beneficial effect of fish oil on breast tumor growth is largely undefined. Using the xenograft model in nude mice, we for the first time report that the fish oil diet significantly increased the level of PTEN protein in the breast tumors. In addition, the fish oil diet attenuated the PI 3 kinase and Akt kinase activity in the tumors leading to significant inhibition of NFκB activation. Fish oil diet also prevented the expression of anti-apoptotic proteins Bcl-2 and Bcl-XL in the breast tumors with concomitant increase in caspase 3 activity. To extend these findings we tested the functional effects of DHA and EPA, the two active ω-3 fatty acids of fish oil, on cultured MDA MB-231 cells. In agreement with our in vivo data, DHA and EPA treatment increased PTEN mRNA and protein expression and inhibited the phosphorylation of p65 subunit of NFκB in MDA MB-231 cells. Furthermore, DHA and EPA reduced expression of Bcl-2 and Bcl-XL. NFκB DNA binding activity and NFκB-dependent transcription of Bcl-2 and Bcl-XL genes were also prevented by DHA and EPA treatment. Finally, we showed that PTEN expression significantly inhibited NFκB-dependent transcription of Bcl-2 and Bcl-XL genes. Taken together, our data reveals a novel signaling pathway linking the fish oil diet to increased PTEN expression that attenuates the growth promoting signals and augments the apoptotic signals, resulting in breast tumor regression.
Keywords: PTEN, NFκB, DHA, EPA, Breast tumor growth, Apoptotic signal
Introduction
Beneficial effects of dietary calorie restriction, fiber intake and ω-3 fatty acid supplementation have been extensively studied in experimental models for controlling cancer progression. Though the results presented in a recent survey did not provide evidence to suggest an association between cancer incidence and intake of ω-3 fatty acids [1], the data on the geographic variation in the risk for cancer development suggest a strong association of fish oil diet in preventing breast cancer. For example, breast cancer incidences are 4–7 times higher among women in the United States of America than those in China or Japan [2]. In addition, a higher incidence of breast cancer in Japanese women during the past decade correlates with decreased consumption of fish and increased intake of vegetable oil rich in ω-6 fatty acids [3]. The effect of the docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), the two main ω-3 fatty acids present in fish oil, in inhibition of tumor growth have been tested using animal and cell culture models. Inhibition of tumor cell growth in vivo by fish oil or by DHA and/or EPA in vitro is well documented [4–7]. The mechanism underlying these observations is still not clearly understood and is under extensive research as there is a constant quest for nontoxic adjuvant therapy for cancer patients.
A recent study suggested that these ω-3 fatty acids exert their growth inhibitory effects on cancer cells by altering membrane lipid rafts and the signaling events associated therein [8, 9]. One of the key signaling pathway deregulated in virtually all human cancer cells begins with activation of the phosphatidylinositol (PI) 3 kinase and its downstream target Akt kinase [10–12]. The PI3K/Akt signaling pathway not only aids in uncontrolled cancer cell proliferation but it also helps in blocking apoptosis of these cells. Thus this signaling pathway became “the” target for anticancer drug development ventures [13]. The activated PI 3 kinase converts the plasma membrane phospholipids PI 4,5-bisphosphate (PIP2) to the PI 3,4,5-trisphosphate (PIP3). PIP3 binds to proteins containing either of the two distinct lipid binding domains, namely FYVE and pleckstrin homology (PH) domains [14]. Activation of PI 3 kinase and generation of PIP3 attract PH domain containing Akt to the plasma membrane where it is phosphorylated at Thr-308 and Ser-473 and becomes highly active and translocates to the cytosol and nucleus to phosphorylate a variety of substrates including transcription factors that regulate gene expression [15–18].
In order to understand the mechanism of the breast cancer growth inhibition by dietary fish oil, we tested the involvement of the PI3K/PTEN/Akt signaling axis based on the hypothesis that membrane alteration by the DHA and EPA will alter this signaling pathway towards a better outcome. Using MDA MB-231 breast cancer cells in vitro and in in vivo xenograft studies, we identified that dietary fish oil significantly suppressed the PI 3 kinase activity in the breast tumor, resulting in reduced Akt kinase and NFκB p65 subunit phosphorylation and anti-apoptotic protein expression. In addition, we show that the active constituents of fish oil, DHA and EPA, inhibit NFκB transcriptional activation, resulting in attenuation of the anti-apoptotic gene transcription. Furthermore, we for the first time demonstrate an increase in PTEN level in the fish oil fed mice breast tumor samples. Finally, our results show that in MDA MB-231 breast cancer cells, DHA and EPA increased the level of PTEN, which in turn inhibited the NFκB-dependent anti-apoptotic gene transcription. The data provide a mechanism for the beneficial effects of fish oil on breast tumor cell growth.
Experimental procedures
Cell culture, antibodies, fatty acids and diet
The MDA MB-231 cell line was purchased from the American Type Culture Collection (Rockville, MD) and grown in Dulbecco's modified essential medium (DMEM) with penicillin and streptomycin, supplemented with 10% fetal calf serum (FCS) in 5% CO2 at 37°C. The ZR75-1 breast tumor cell line was kindly provide by Dr. S. Am-manamanchi, Division of Oncology, Department of Medicine, The University of Texas Health Science Center at San Antonio. These cells were grown in RPMI 1640 in the presence of 10% FCS. Antibodies against PTEN, Erk 1/2, Bcl-2 and Bcl-XL were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against Akt and phosphotyrosine were purchased from Upstate; antibodies against p65 and phospho-p65 (recognizing serine-536) were from Cell Signaling (Danvers, MA) and the antibody against caspase 3 was purchased from Calbio-chem (San Diego, CA). Actin and tubulin antibodies and TRIZol RNA isolation kit were purchased from Sigma (St Louis, MO). The ω-3 fatty acids, DHA and EPA were obtained from Cayman Chemical Company (Ann Arbor, MI). The fish oil diet was purchased from Harlan Bio-products (Indianapolis, IN) and prepared as described [19, 20]. The PTEN primer sets (RefSeq Accession #NM_000314.3) and GAPDH primer sets (RefSeq Accession #NM_002046) including forward and reverse primers were obtained from SuperArray Bioscience. Primers to detect human Bcl-2 (F: 5′-CATGTGTGTGGAGAGC GTCAA-3′; R: 5′-GCCGGTTCAGGTACTCAGTCA-3′) and Bcl-XL (F: 5′-TCCTTGTCTACGCTTTCCACG-3′: R: 5′-GGTCGCATTGTGGCCTTT-3′) were synthesized in the core facility at The University of Texas Health Science Center at San Antonio [21]. The reporter plasmids expressing luciferase cDNA driven by Bcl-2 promoter (Bcl-2-Luc) or Bcl-XL promoter (Bcl-XL-Luc) were generously provided by Dr. L. Baxter, Stanford University and Dr. G. Nunez, University of Michigan respectively. PTEN-Luc reporter plasmid containing luciferase gene under the control of PTEN promoter was a gift from Dr. I. de Belle, The Burnham Institute, California [22]. pSGL-PTEN expressing PTEN was described previously [23].
Animal study
The immunocompromised (nu/nu) mice were purchased from NIH animal facilities and used according to the guide of use of small laboratory animals. All animal protocols were approved by the Institutional Animal care and use Committee, The University of Texas Health Science Center at San Antonio. The mice were kept on AIN 93 diet containing 10% fish oil for 7 days before injecting them with the MDA MB-231 breast cancer cells. The control group of mice was kept in normal lab chow diet through out the experiment. For injection in mice, MDA MB-231 cells were trypsinized and 106 cells resuspended in phosphate buffered saline (PBS) were injected in each mammary fat pad of these mice. The experimental group of mice was kept on 10% fish oil diet throughout the experimental period. Mice were sacrificed 22 days following the injection. The length (L) and width (W) of tumor were recorded for each mouse in both groups at 22 days post injection of MDA MB-231 cells. The tumor volume (V) was calculated according to the formula: V = L × W2.
Tumor lysate preparation
Tumor pieces were harvested by controlled motorized homogenization using radioimmunoprecipitation assay (RIPA) buffer (20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM phenyl methyl sulfonyl fluoride, 0.05% aprotinin and 1% Nonidet P-40). Homogenates were centrifuged at 4°C in a microfuge at 10,000g for 30 min. Cleared supernatant was collected for experiments.
Immunoblotting and immunoprecipitation
Tumor tissues or cell lysates were prepared in RIPA buffer and protein concentration was estimated as described previously [23–25]. Equal amounts of proteins were used for immunoprecipitation and immunoblotting, using the required antibodies as described [23–27].
PI 3 kinase assay
PI 3 kinase assay was performed according to the methods described [23–25]. Briefly, the tumor lysates were immunoprecipitated with anti-phosphotyrosine antibody. Washed immunoprecipitates were resuspended in PI 3 kinase assay buffer (20 mM Tris–HCl, pH 7.5, 0.1 M NaCl and 0.5 mM EGTA) on ice. 0.5 μl of PI (20 mg/ml) was added as substrate and incubated at 25°C for 10 min. 1 μl of 1 M MgCl2 and 10 μCi of γ32P-ATP were added followed by incubation at 25°C for 10 more minutes. The reaction was stopped by chloroform–methanol–HCl mixture and processed as described [28]. Finally the reaction product was separated by thin layer chromatography. The spots were visualized by autoradiography.
Akt kinase assay
Akt activity was measured by immunecomplex kinase assay in the anti-Akt immunoprecipitates from the tumor lysates using histone H2B as substrate, according to the methods described previously [23–25].
Caspase activity assay
Caspase 3 activity was measured in the tumor lysates using a Caspase-3 Assay kit from Promega Inc., according to the vendor's protocol. Equal amounts of protein were placed in a 96 well plate (specific for the luminometer). The reagent containing caspase 3 substrate, provided in the kit, was added to the samples and incubated at room temperature for 60 min. The reading was taken using a luminometer and recorded.
Preparation of RNA and real time qRT-PCR
Total RNA was isolated from cells using TRI reagent according to the manufacturer's protocol. Two microgram of total RNA was reverse transcribed to synthesize first strand cDNA using oligo-dT primer in 20 μl. One microliter of this cDNA was amplified using SYBR Green PCR master mix containing the forward and reverse primers. PCR amplification was performed using 7900HT Sequence Detection System (Applied Biosystems) using manufacturer's protocol. The amplification conditions are as follows: 94°C for 10 min followed by 40 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 30 s. Dissociation curve analysis was performed following amplification to confirm the specificity of the primers. Relative mRNA expression was calculated using the ΔΔCt method.
Electrophoretic mobility shift assay (EMSA)
MDA MB-231 cells were grown in the presence or absence of DHA (50 ng/ml) or EPA (50 ng/ml) for 48 h. Nuclear extracts were prepared from these cells as described [24, 27]. The nuclear extracts were incubated with an oligonucleotide probe containing double stranded NFκB binding site (5′-AGTTGAGGGGACTTTCCCAGG C-3′) labeled at the 5′ end using γ32P-ATP and T4 polynucleotide kinase. The DNA–protein complex was analyzed by electrophoresis in a 5% non-denaturing polyacrylamide gel followed by autoradiography [24, 27]. p65 or Erk1/2 antibody was added to the nuclear extract to perform the supershift assays as described previously [24, 27].
Transfection and promoter activity assay
MDA MB-231 cells were transfected with the reporter plasmids using Lipofectamine Plus reagent as described [23, 24, 26, 27]. Twenty-four hours post transfection the cells were treated with 50 ng/ml of DHA or EPA for 24 h as indicated and the cells were harvested and the lysates were assayed for luciferase enzyme activity using an assay kit. The data are presented as mean luciferase activity per microgram protein as arbitrary units ± SE [23, 24, 26, 27].
Statistical analysis of data
Statistical significance of the data was calculated using analysis of variance followed by Student–Newman–Keuls analysis as described previously [23, 27, 28]. Significance level was considered at a P value < 0.05.
Results
Fish oil diet inhibits PI 3 kinase signaling in breast tumor
Efficacy of fish oil diet in reducing tumor growth in animal model has been extensively studied [4–6, 8]. To investigate the mechanism of action of fish oil, we considered the anti-apoptotic cell survival signal transduction pathway. PI 3 kinase signaling plays an important role in survival and resistance to apoptosis of many cancer cells including breast tumor cells [29, 30]. A group of mice fed either lab chow or a diet containing 10% fish oil for 2 weeks were inoculated with the MDA MB-231 breast cancer cells in the mammary fat pads. As expected tumor growth at both breasts at 3 weeks was significantly inhibited in mice fed fish oil diet (Supplementary Fig. S1).
We previously reported that growth factor receptor tyrosine kinase mediated signal transduction is active in MDA MB-231 cells [31]. Activation of PI 3 kinase in tumor cells requires tyrosine phosphorylation of proteins including receptor tyrosine kinases. Anti-phosphotyrosine immunoprecipitates from tumor lysates showed abundant PI 3 kinase activity (Fig. 1a, lanes 1–3). However, tumor lysates obtained from mice, which were treated with fish oil showed significantly reduced PI 3 kinase activity in the anti-phosphotyrosine immunoprecipitates (Fig. 1a, compare lanes 4–6 with lanes 1–3; Fig. 1b).
Fig. 1.
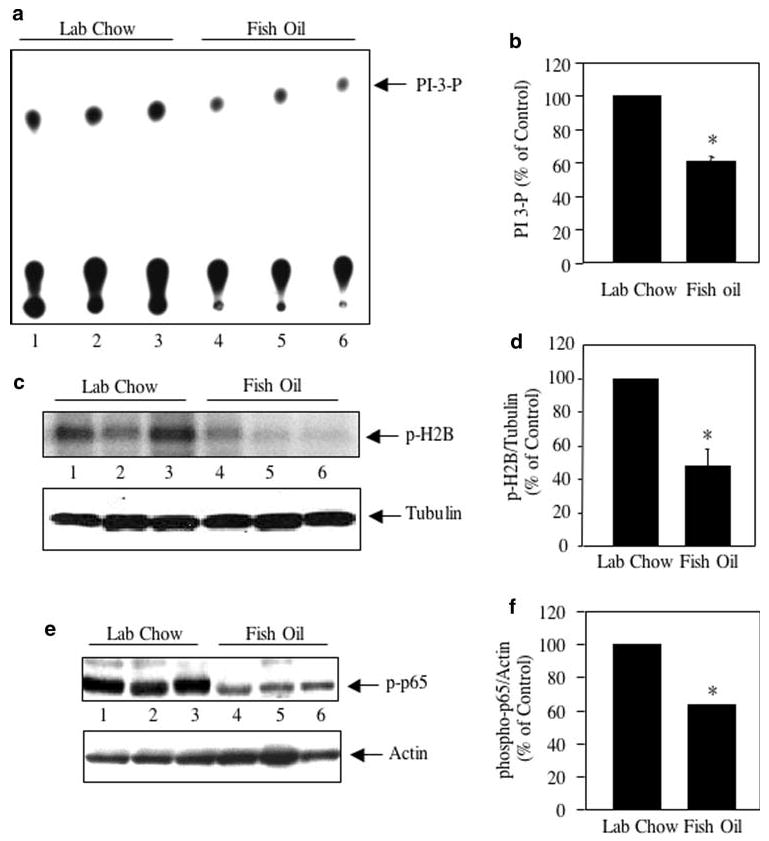
Effect of fish oil on PI 3 kinase signaling in breast cancer. a Tumor lysates from lab chow and fish oil fed mice were immunoprecipitated with anti-phosphotyrosine antibody followed by PI 3 kinase assay as described in the Experimental procedures. The arrow indicates PI 3-P spots. Each lane indicates results from individual animal. b Quantification of the PI 3-P spots in panel a. Mean ± SE of three independent animals is shown; *P < 0.001 vs. lab chow fed animals. c Equal amounts of tumor lysates as indicated were immunoprecipitated with Akt antibody. The immunoprecipitates were used in an immunecomplex kinase assay using histone H2B as substrate as described in the Experimental procedures. Bottom panel shows the immunoblotting of the same samples with tubulin antibody. d Quantification of the phosphorylated histone H2B in panel c. Mean ± SE of three independent animals is shown; *P < 0.05 vs. lab chow fed animals. e Tumor lysates were immunoblotted with anti-phospho-p65 (Ser-536) antibody. The bottom panel shows immuno-blotting of the same samples with actin antibody. f Quantification of the phosphorylated p65 in panel e. Mean ± SE of three independent animals is shown; *P < 0.01 vs. lab chow fed animals
One of the downstream targets of PI 3 kinase is Akt kinase, which plays major roles in the development of cancer by increasing cell survival and decreasing cell death [32–34]. Immunecomplex kinase assay of Akt immunoprecipitates were performed in the presence of histone H2B as substrate. Akt activity was significantly reduced in tumor samples isolated from animals treated with fish oil (Fig. 1c, compare lanes 4–6 with lanes 1–3; Fig. 1d). In cancer cells, Akt utilizes a myriad of transcription factors including NFκB to induce survival signals [35–37]. Activation of Akt induces IKK-mediated phosphorylation of the p65 subunit of NFκB, which is required for its transcriptional activation [36, 38]. Therefore, we tested the phosphorylation of p65 at serine-536 as an output of Akt activation in the breast tumor. As shown in Fig. 1e, abundant phosphorylation of p65 was detected in the breast tumor samples (lanes 1–3). Tumor lysates from fish oil fed animals showed significant reduction in phosphorylation of p65 (Fig. 1e, compare lanes 4–6 with lanes 1–3; Fig. 1f). These data demonstrate that fish oil inhibits the PI 3 kinase signaling pathway that leads to NFκB activation for the prevention of breast tumor burden in mice xenografts (Supplementary Fig. S1).
Fish oil regulates apoptotic signal transduction
Phosphorylation-dependent activation of NFκB induces expression of many anti-apoptotic proteins including Bcl-2 and Bcl-XL [39–42]. We examined the expression of these two proteins in tumor lysates. Abundant expression of Bcl-2 was detected in the tumor lysates (Fig. 2a, lanes 1 and 2). However, expression of Bcl-2 was significantly prevented in the tumors from mice fed a diet containing fish oil (Fig. 2a, compare lanes 3 and 4 with lanes 1 and 2; Fig. 2b). Similarly, the anti-apoptotic Bcl-XL expression in the tumors of fish oil fed mice was significantly inhibited (Fig. 2c, compare lanes 3 and 4 with lanes 1 and 2; Fig. 2d). Apoptosis of cells is carried out by the executor caspase 3. During apoptosis of cells, initiator caspase cleaves procaspase 3 to yield activated caspase 3 [43]. Appearance of low molecular weight cleaved caspase 3 demonstrates the presence of activated caspase 3 during apoptosis. Immunoblot analysis of tumor lysates from control and fish oil fed mice showed increased appearance of activated caspase 3 in the latter samples (Fig. 2e, f). Consequently, caspase 3 protease activity was significantly enhanced in the tumor extracts prepared from fish oil fed animals than the control untreated animals (Fig. 2g). These results indicate that fish oil diet regulates the proteins and enzymes that are involved in apoptosis of breast cancer cells.
Fig. 2.
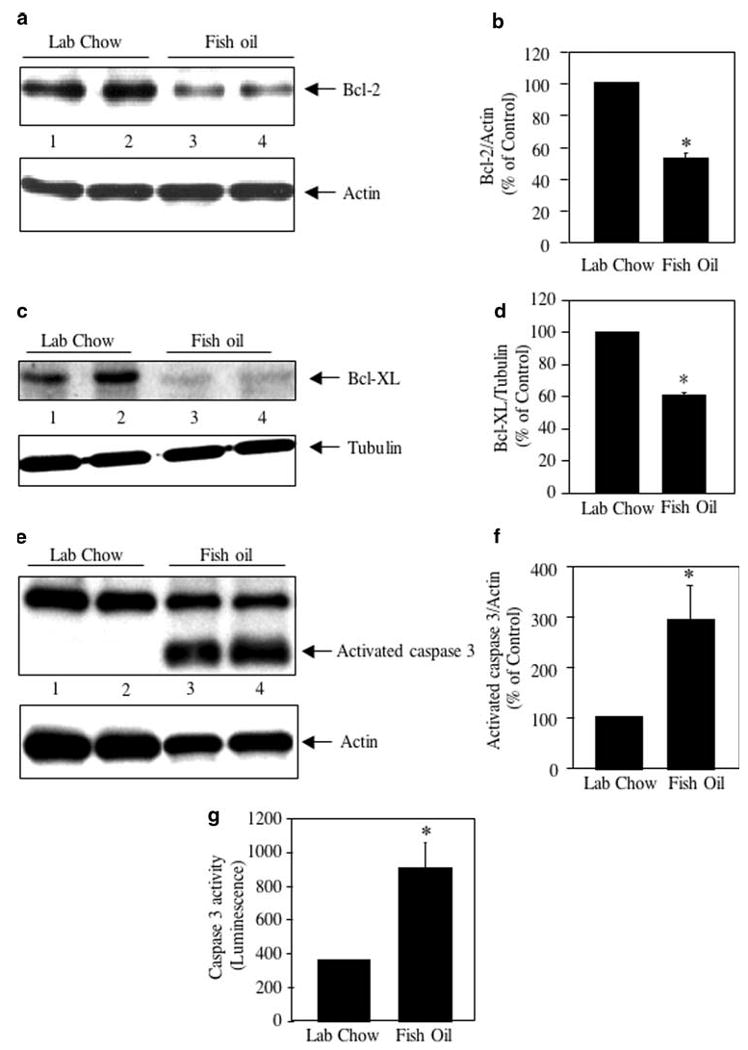
Effect of fish oil on anti- and pro-apoptotic proteins in breast cancer. a, c Tumor lysates from lab chow and fish oil fed mice were immunoblotted with Bcl-2 (a) and Bcl-XL (c) antibodies respectively. Bottom panels show immunoblotting of the same samples with actin (a) and tubulin (c) antibodies. b, d Quantifications of Bcl-2 and Bcl-XL expression. Mean ± SE of four animals is shown. *P < 0.01 for panel b and 0.001 for panel d vs. lab chow fed animals. e Tumor lysates were immunoblotted with an antibody that recognizes both procaspase 3 and activated caspase 3 proteins. Bottom panel shows immunoblotting of the same samples with actin antibody. f Quantification of the activated caspase 3 in panel e. Mean ± SE of four independent animals is shown; *P < 0.03 vs. lab chow fed animals. g Caspase 3 activity in the tumor lysates was determined as described in the Experimental procedures. Mean ± SE of four independent animals are shown; *P < 0.03 vs. lab chow fed animals
DHA and EPA inhibit NFκB transcriptional activity
Two main constituents of fish oil, DHA and EPA are known to induce apoptosis in breast cancer cells [4–7]. Incubation of MDA MB-231 cells with DHA or EPA resulted in significant reduction in viable cells (Supplementary Fig. S2a). Similar results were obtained using ZR75-1 breast tumor cell line (Supplementary Fig. S2b), indicating that fish oil constituents exhibit similar effects on different breast cancer cells. We have shown reduced activating phosphorylation of p65 subunit of NFκB in the breast tumors of fish oil fed mice (Fig. 1e, f). We examined the effect of DHA and EPA on phosphorylation of p65. Nuclear extracts prepared from control MDA MB-231 cells showed abundant phosphorylation of p65 at serine-536 (Fig. 3a, b, lanes 1) concomitant with increased protein–DNA complex formation, as judged by EMSA (Fig. 3c, lane 1). Use of p65 antibody in the supershift analysis confirmed the presence of p65 in this DNA–protein complex (Fig. 3c, lane 2). Incubation of the nuclear extracts with non-specific Erk-1/2 antibody did not have any significant effect on the formation of p65–DNA complex, indicating the specificity of this complex formation (Fig. 3c, lane 3). Incubation of MDA MB-231 cells with DHA or EPA inhibited the phosphorylation of p65 (Fig. 3a, b, compare lanes 2 with lanes 1). This inhibition of p65 phosphorylation resulted in reduction in DNA–protein complex formation in response to DHA and EPA, respectively (Fig. 3d, compare lanes 2 and 3 with lane 1). These results indicate that the active components of fish oil, DHA and EPA, inhibit the activating phosphorylation and DNA binding of NFκB to the cognate target sequence.
Fig. 3.
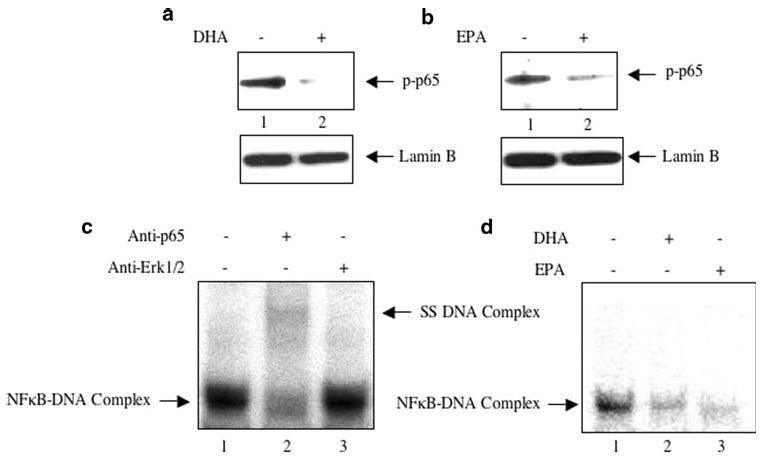
Effect of DHA and EPA on NFκB activation. a, b MDA MB-231 cells were incubated with DHA or EPA as described in the Experimental procedures. Equal amounts of nuclear extracts were immunoblotted with phospho-p65 (serine-536) antibody. Bottom panels show immunoblotting of the nuclear extracts with lamin B antibody. c Nuclear extracts from MDA MB-231 cells were incubated with anti-p65 or anti-Erk1/2 antibody prior to incubation with 32P-labeled doubled stranded NFκB DNA element as described in the Experimental procedures. EMSA was performed using a 5% polyacrylamide gel electrophoresis. Left arrow indicates the protein–DNA complex. p65 antibody inhibited the formation of DNA–protein complex (lane 2). The right arrow indicates the faint supershifted complex in lane 2. d Nuclear extracts prepared from DHA- and EPA-treated MDA MB-231 cells were used in EMSA with the 32P labeled double stranded NFκB DNA element. Arrow indicates the protein–DNA complex
The anti-apoptotic genes Bcl-2 and Bcl-XL are targets of NFκB [39]. Fish oil diet inhibited the expression of Bcl-2 and Bcl-XL (Fig. 2a–d). We tested the effect of DHA and EPA, two constituents of fish oil, on Bcl-2 and Bcl-XL mRNA expression. Incubation of MDA MB-231 cells with DHA and EPA significantly inhibited the expression of Bcl-2 mRNA (Fig. 4a). Also, DHA and EPA attenuated Bcl-XL mRNA expression (Fig. 4b). Similarly, DHA as well as EPA inhibited expression of Bcl-2 and Bcl-XL in ZR75-1 breast tumor cells (Supplemental Fig. S3). Our results above demonstrate that fish oil diet inhibits phosphorylation of p65 in breast tumors (Fig. 1e, f). Also the components of fish oil, DHA and EPA inhibited NFκB DNA binding in MDA MB-231 breast tumor cells (Fig. 3d). Therefore, we tested the effect of these ω-3 fatty acids on the transcription of Bcl-2 and Bcl-XL. For this, reporter plasmids containing the firefly luciferase gene driven by Bcl-2 or Bcl-XL promoter were used [44–46]. MDA MB-231 cells were transiently transfected with Bcl-2-Luc. Incubation of these transiently transfected cells with DHA significantly inhibited the reporter activity (Fig. 4c). Similarly, EPA blocked Bcl-2 promoter activity in MDA MB-231 cells (Fig. 4d). Also, both these ω-3 fatty acids significantly inhibited Bcl-XL promoter activity in the transient transfection assays (Fig. 4e, f). These data suggest that DHA- and EPA-mediated inhibition of NFκB DNA binding may attenuate Bcl-2 and Bcl-XL transcription.
Fig. 4.
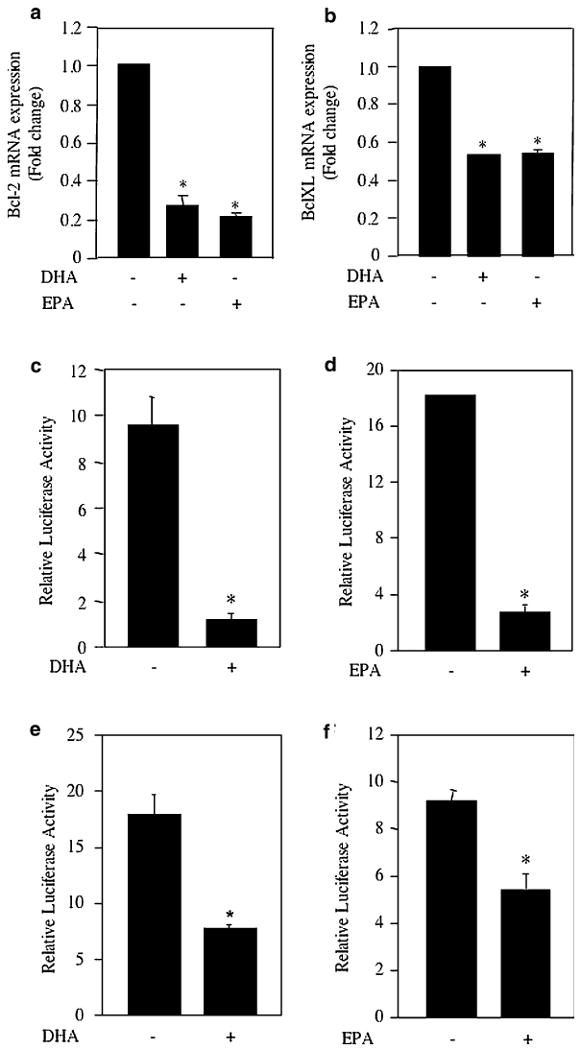
Effect of DHA and EPA on expression of Bcl-2 and Bcl-XL. a, b Two microgram of total RNA isolated from MDA-MB-231 cells incubated with DHA or EPA as indicated were used in real time qRT-PCR to detect Bcl-2 (panel a) and Bcl-XL (panel b) mRNAs as described in the Experimental procedures. The expression of mRNA was normalized to GAPDH. Mean ± SE of three measurements is shown. *P < 0.001 vs. control by ANOVA. c–f Bcl-2-Luc (panels c, d) or Bcl-XL-Luc (panels e, f) was transfected into MDA MB-231 cells. Transiently transfected cells were incubated with DHA or EPA as indicated. Luciferase activity was measured in the cell lysates as described in the Experimental procedures. Mean ± SE of triplicate measurements is shown. *P < 0.01 vs. control for all panels
To confirm the involvement of NFκB, we tested the effect of p65 NFκB subunit on the transcription of these antiapoptotic genes using the reporter constructs. Bcl-2-Luc reporter plasmid was cotransfected with p65 expression construct. Expression of p65 significantly increased Bcl-2 transcription (Fig. 5a, b). Incubation of the transiently transfected cells with DHA or EPA significantly prevented p65-mediated transcription of Bcl-2 (Fig. 5a, b). Similarly, expression of p65 stimulated transcription of Bcl-XL (Fig. 5c, d). DHA as well as EPA significantly inhibited the p65-stimulated Bcl-XL transcription (Fig. 5c, d). These results indicate that the main constituents of the fish oil, DHA and EPA, target the NFκB transcription factor to inhibit the expression of anti-apoptotic genes Bcl-2 and Bcl-XL.
Fig. 5.
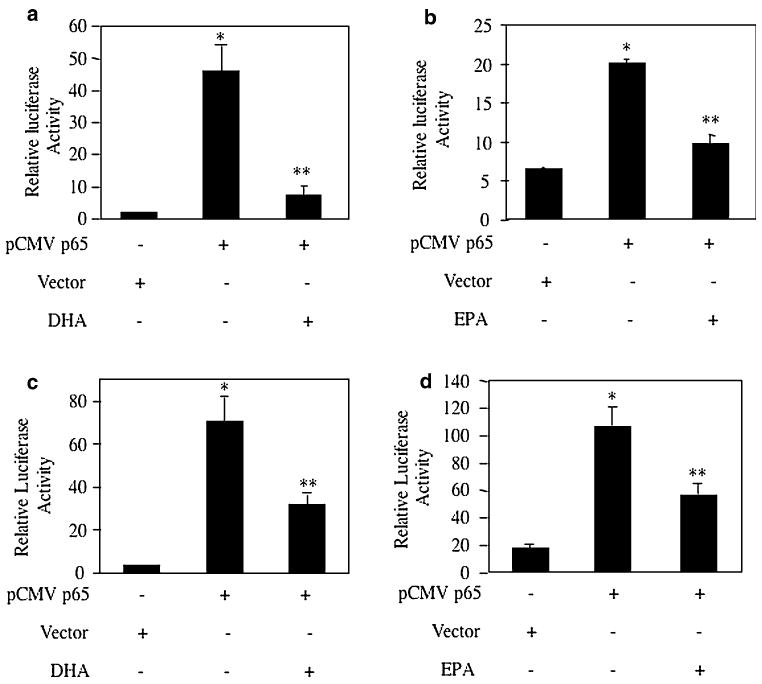
Effects of DHA and EPA on NFκB-mediated Bcl-2 and Bcl-XL transcription. MDA MB-231 cells were transfected with the Bcl-2-Luc (a, b) or Bcl-XL-Luc (c, d) reporter plasmids along with vector or pCMV-p65 expression constructs as indicated. Transiently transfected cells were incubated with DHA (a, c) or EPA (b, d). Luciferase activity was measured in the cell lysates as described in the Experimental procedures. Mean ± SE of triplicate measurements is shown. *P < 0.001 vs. control; **P < 0.05 vs. pCMV-p65-transfected
Fish oil increases PTEN expression
In many cancers, PI 3 kinase/Akt signaling is upregulated [29, 30]. We have shown above that in breast tumors in mouse model, fish oil intercepts the PI 3 kinase/Akt signal transduction involving p65 subunit of NFκB to attenuate the tumor burden (Fig. 1). However, mutation or malfunctioning of the tumor suppressor protein PTEN activates Akt by constitutively maintaining the PI 3 kinase product PIP3 leading to sustained growth of tumor cells [47–49]. We tested the hypothesis whether fish oil regulates expression of PTEN in the breast tumors. MDA MB-231 cells, which were used to produce tumors in mice, express wild type PTEN [50]. However, the tumor lysates from control mice showed significantly low levels of PTEN protein (Fig. 6a). Fish oil treatment significantly increased the expression of PTEN in the breast tumor of mice (Fig. 6a, compare lanes 4–6 with lanes 1–3; Fig. 6b). These results indicate that along with decreased PI 3 kinase activity (Fig. 1a, b), fish oil utilizes an additional mechanism to reduce PI 3 kinase signaling, which result in attenuated Akt activation (Fig. 1c, d). To further examine the role of fish oil, we tested its constituents, DHA and EPA, in expression of PTEN mRNA in the MDA MB-231 cells. Incubation of these breast cancer cells with DHA and EPA increased PTEN mRNA expression (Fig. 6c, d). This increase in mRNA expression was concomitant with elevated PTEN protein expression (Fig. 6e, f). Additionally, DHA as well as EPA increased expression of PTEN mRNA and protein in ZR75-1 breast tumor cells (Supplementary Fig. S4). These results indicate that fish oil and its components DHA and EPA regulate PTEN expression in breast cancer cells.
Fig. 6.
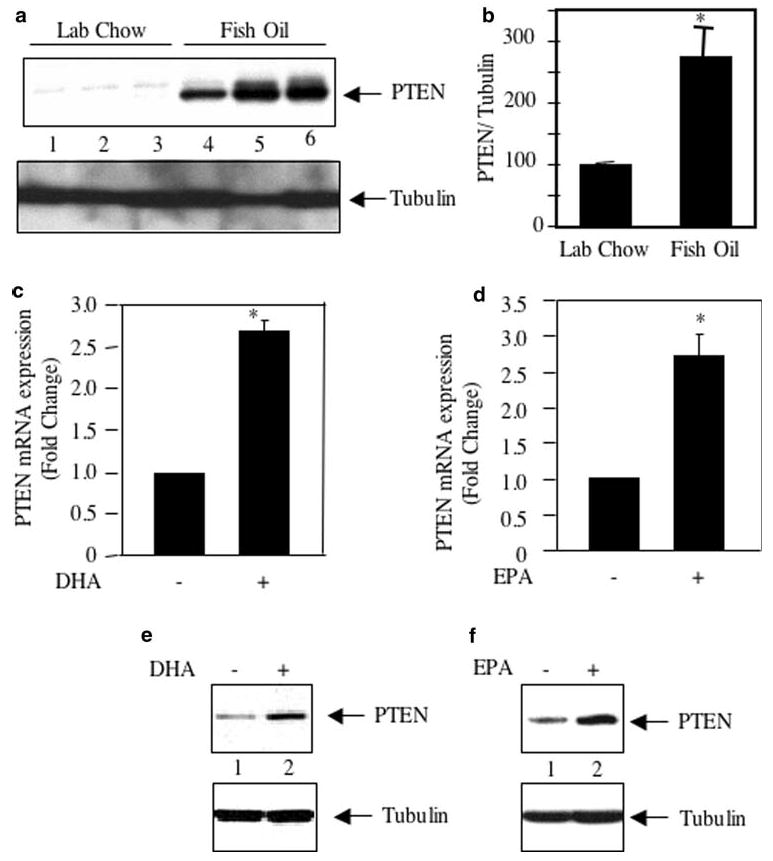
Effect of fish oil and DHA and EPA on PTEN expression. a Tumor lysates from lab chow and fish oil fed mice were immunoblotted with PTEN and tubulin antibodies respectively. b Quantification of the PTEN expression in (panel a). Mean ± SE of three independent animals is shown; *P = 0.02 vs. lab chow fed animals. c, d 2 μg of total RNA isolated from MDA MB-231 cells incubated with DHA (panel c) or EPA panel d as indicated were used in real time qRT-PCR to detect PTEN mRNA as described in the Experimental procedures. The expression of mRNA was normalized to GAPDH. Mean ± SE of three measurements is shown. *P < 0.05 vs. control in (panel c); *P < 0.01 vs. control in panel d; by ANOVA. e, f Lysates of MDA MB-231 cells treated with DHA (panel e) or EPA (panel f) were immunoblotted with PTEN and tubulin respectively
PTEN is known to be regulated by transcriptional mechanism [48]. Therefore, we tested the effect of these ω-3 fatty acids on transcription of PTEN. We tested PTEN promoter by transient transfection assays using a luciferase reporter gene that contained a PTEN genomic fragment including 1,978 bp 5′ of the ATG translation initiation site [22]. Transiently transfected MDA MB-231 cells were incubated with DHA. DHA significantly increased the reporter activity (Fig. 7a). Similarly, EPA enhanced the transcription of PTEN in MDA MB-231 breast cancer cells (Fig. 7b). These results provide a mechanism how these ω-3 fatty acids stimulate expression of PTEN protein.
Fig. 7.
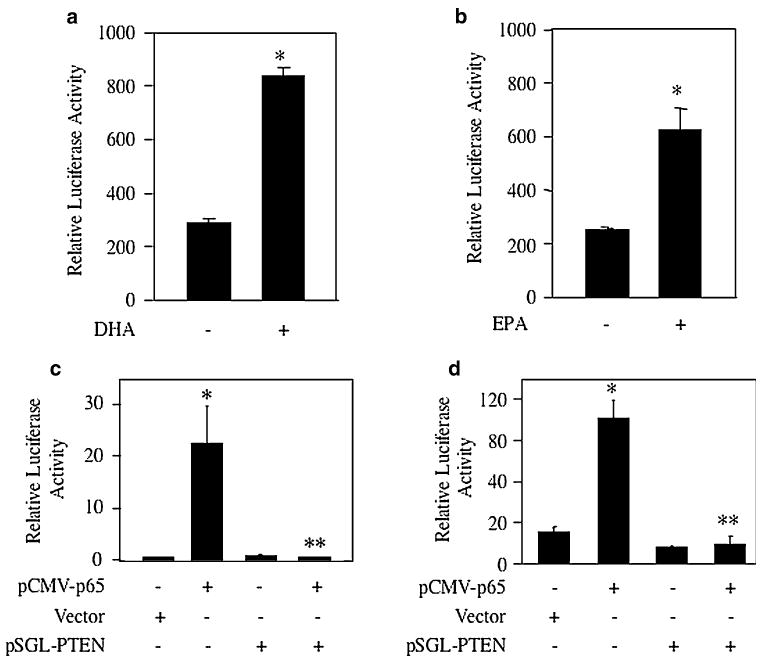
a, b MDA MB-231 cells were transfected with PTEN-Luc reporter plasmid. Transiently transfected cells were incubated with DHA (panel a) or EPA (panel b). Luciferase activity was determined as described in the Experimental procedures. Mean ± SE of triplicate measurements is shown. *P < 0.001 vs. control in a; *P < 0.01 vs. control in panel b. c, d MDA MB-231 cells were transfected with the Bcl-2-Luc (panel c) or Bcl-XL-Luc (panel d) along with pCMV-p65, vector or pSGL-PTEN expression constructs as indicated. Luciferase activity was measured as described in the Experimental procedures. Mean ± SE of triplicate measurements is shown. Panel c *P < 0.05 vs. control and **P < 0.05 vs. pCMV-p65-transfected. Panel d *P < 0.001 vs. control and **P < 0.001 vs. p65-transfected
We have shown above that the proapoptotic effects of the ω-3 fatty acids, DHA and EPA, on breast tumor cells is due to inhibition of NFκB-dependent Bcl-2 and Bcl-XL expression (Figs. 4, 5; Supplemental Fig. S3). Also, we demonstrated increased expression of PTEN in response to DHA and EPA (Figs. 6c–f, 7a, b; Supplemental Fig. S4). We tested the effect of PTEN on p65-mediated transcription of these anti-apoptotic genes. As expected cotransfection of p65 subunit of NFκB with Bcl-2-Luc increased the reporter activity (Fig. 7c). Cotransfection of PTEN significantly inhibited p65-mediated Bcl-2 transcription (Fig. 7c). Furthermore, expression of PTEN blocked NFκB-dependent Bcl-XL transcription in MDA MB-231 cells (Fig. 7d). These results demonstrate that increased PTEN in ω-3 fatty acid-treated breast tumor cells may downregulate the expression of anti-apoptotic genes, thus providing a mechanism of reduced tumor cell growth in vivo and in vitro.
Discussion
For humans, intakes up to 12–21 g of fish oil per day have been reported [51, 52]. In experimental animal studies, 10–23% fish oil is added to the diet for comparison with chow [53 and references therein]. In this study, we provide the first evidence that 10% fish oil diet exerts its beneficial effect on breast tumor growth by simultaneously inhibiting PI 3 kinase activity and increasing the level of PTEN. Consequently, reduced PI 3 kinase signaling is sufficient to inhibit NFκB activity and to block expression of anti-apoptotic Bcl-2 and Bcl-XL mRNAs and proteins. We demonstrate that DHA and EPA, two active components of fish oil, prevent NFκB-dependent Bcl-2 and Bcl-XL transcription by increasing PTEN mRNA and protein in breast cancer cells. Our results provide a mechanism of action of fish oil on breast cancer growth.
Class IA PI 3 kinases containing different isotypes of p110 catalytic subunits with 85 kDa SH2-domain containing regulatory subunit occupy a nodal position in cell signaling [54–57]. The first indication that PI 3 kinase plays a role in cellular transformation came from the discovery of activating mutation in the PIK3CA gene encoding for p110α [58]. Mutation of PIK3CA occurs in many cancers including breast tumor [59–62]. Similarly, mutation in PIK3CB coding for the p110β subunit has been associated with various cancers including breast cancer [55, 63]. However, in the absence of the activating mutations, increased activity of PI 3 kinase due to amplification of different catalytic subunits result in tumorigenesis [63–65]. Furthermore, in the absence of genetic alterations, class IA PI 3 kinases are activated by association with receptor and non-receptor tyrosine kinases, which are activated by autocrine growth factor receptor signaling in tumor cells [54, 55]. We detected increased PI 3 kinase activity in the anti-phosphotyrosine immunoprecipitates of the breast tumor lysates (Fig. 1a). Fish oil diet, which reduced the breast tumor burden in the mice (Supplementary Fig. S1), significantly prevented the PI 3 kinase activity (Fig. 1b). To our knowledge, this is the first direct demonstration of fish oil-induced inhibition of PI 3 kinase activity.
One of the downstream targets of PI 3 kinase is the Akt kinase, which exists in three isoforms (Akt1-3) [33, 34]. Hyperactivation of Akt kinases in human cancer has been associated with increased tumor cell survival and enhanced resistance to apoptosis [32, 33]. Although amplification of Akt2 has been identified in different cancers including breast cancer, mutation in any Akt isoforms for its hyperactivation has not been reported until recently [66–68]. Evaluation of complete coding region of Akt1 in many clinical tumor specimens including breasts revealed mutation of glutamic acid in the codon 17 to lysine (E17K) [69, 70]. This mutation in the PH domain constitutively localizes Akt1 in the plasma membrane [70]. Production of PIP3 due to increased PI 3 kinase activity binds to the PH domain of Akt, resulting in its translocation and activation in the plasma membrane [33]. In the MDA MB-231 xenograft breast tumor model, we identified increased PI 3 kinase activity with concomitant increase in Akt kinase activity (Fig. 1c, d). However, fish oil diet reduced the Akt kinase activity in the breast tumors (Fig. 1c). This effect of fish oil may result from the inhibition of PI 3 kinase activity we observed. Furthermore, our results are in accordance with the recently reported inhibition of Akt kinase in MDA MB-231 cells by DHA and EPA, two main constituents of fish oil [7].
Akt kinase regulates proliferation of cells by phosphorylating a myriad of substrates including proteins such as p27, p21, tuberin and PRAS40 which directly take part in cycling of tumor cells [71–76]. Similarly, cell survival is enhanced by Akt-mediated phosphorylation of proapoptotic protein Bad [77, 78]. Akt also blocks expression of proapoptotic proteins by its inhibitory effects on transcription factors such as FoxO and p53 [79–84]. In addition, Akt positively regulates the transcription factor NFκB, which targets many anti-apoptotic genes such as Bcl-2 and Bcl-XL [37, 39–41, 85–90]. The canonical NFκB heterodimer (p65/p50) complexed with IκB is present as a latent complex in the cytoplasm. Upon activation by proliferating/anti-apoptotic signals, IκB kinase (IKK) complex, consisting of catalytic IKKα/IKKβ subunits and a scaffold NFκB essential modulator (NEMO), phosphorylates the inhibitor IκB. Phosphorylated IκB undergoes degradation enabling nuclear translocation of NFκB to activate expression of target genes [91, 92]. Activation of NFκB further results from the phosphorylation of p65 subunit at serine-536 in the c-terminal transactivation domain 1 [42]. Both IKKα and IKKβ have been shown to phosphorylate this residue in an Akt-dependent manner [41, 85, 86, 93–95]. Serine-536 phosphorylated p65 subunit enables the NFκB to be localized in the nucleus in a sustained manner to induce transcription of target antiapoptotic genes [42]. In the present study, we detected constitutive serine-536 phosphorylation of p65 subunit in the breast tumor and in the MDA MB-231 cells. Breast tumors from fish oil fed mice possessed significantly low serine phosphorylation of p65 (Fig. 1e). Furthermore, our data demonstrate that DHA and EPA, two active ω-3 fatty acids of fish oil, reduce the serine-536 phosphorylation of p65 subunit of NFκB, resulting in attenuation of its DNA binding (Fig. 3).
Tumor cell death is initiated by the interaction of specific proapoptotic proteins such as BAX and BAK with the mitochondrial compartment to induce mitochondrial outer membrane permeabilization (MOMP) [96]. The ratio of anti-apoptotic BH-domain containing Bcl-2 and Bcl-XL proteins to the proapoptotic proteins regulates the cancer cell death [43]. Thus increased expression of Bcl-2 and Bcl-XL in the tumor cells poses resistance to apoptosis of these cells. Fish oil fed mice showed significant reduction in expression of Bcl-2 and Bcl-XL proteins, which resulted in increased activation of caspase 3 (Fig. 2). These results indicate that the active components of the fish oil may target the expression of Bcl-2 and Bcl-XL, two antiapoptotic proteins, to activate the executor caspase 3, resulting in reduced growth of tumor. Both these proteins are known to be regulated by transcriptional mechanism. In fact, both DHA and EPA inhibited expression of Bcl-2 and Bcl-XL by a transcriptional mechanism (Fig. 4).
Expression of both Bcl-2 and Bcl-XL is regulated by NFκB [39, 40, 88, 89, 97–99]. Our data demonstrating increased transcriptional activation of Bcl-2 and Bcl-XL by p65 subunit of NFκB support this notion (Fig. 5). Furthermore, we demonstrate that both DHA and EPA significantly block p65-mediated transcription of Bcl-2 and Bcl-XL (Fig. 5). These results provide a mechanism how fish oil may intercept the expression of these two antiapoptotic proteins to prevent tumor growth in the mice.
Using primary human tumor tissues and established cell lines, high frequency of PTEN tumor suppressor mutation/deletion was demonstrated in a number of tumors including breast cancer [48, 56, 100, 101]. PTEN is a lipid phosphatase that dephosphorylates the D3 position of PIP3, the product of PI 3 kinase [102, 103]. Thus negative regulation of PI 3 kinase results in constitutive activation of Akt, which acts as prosurvival kinase [101]. Recently, a significant role of PTEN in cancer stem cell proliferation has been identified. Deletion of PTEN increased leukemia generating stem cells [104]. Also PTEN deficiency resulted in intestinal tumorigenesis [105]. Women with germ line PTEN mutation suffer from bilateral hypertrophy of the virginal breasts and early malignant transformation [101]. PTEN heterozygous mice exhibit increased penitrence to breast cancer [106]. Targeted mutation of PTEN in the mammary gland of mice displayed tumorigenesis early in life demonstrating a significant role of this tumor suppressor in breast cancer [107]. Breast cancer stem cells have been identified which form tumor when inoculated in mice [108]. Downregulation of PTEN was associated with breast cancer stem cell proliferation [109]. Recently, it was shown that in breast cancers caused by the deficiency of BRCA1 tumor suppressor gene, loss of PTEN is a common event [50].
More recently, alternative mechanism of PTEN inactivation in cancer is reported where genetic background of this tumor suppressor is normal. PTEN downregulation was observed in tumors with activated Notch signaling [110]. Also, increased level of the E3 ubiquitin ligase NEDD4-1 negatively regulates PTEN stability in many cancers [47]. Similarly, in breast cancer, where there is no genetic mutation is observed, immunoreactive PTEN was shown to be reduced by overexpression of a redox sensitive protein DJ-1 [49].
Along with the regulation of PTEN at the level of protein, expression of this tumor suppressor protein is transcriptionally regulated. For example, p53, Egr1, and PPARγ have been shown to increase expression of PTEN under various cell signaling condition [22, 111, 112]. On the other hand, activated NFκB in many cancer cells acts as a transcriptional repressor for PTEN [113–115]. We have found very low expression of PTEN in the breast tumor of mice concomitant with increased activating phosphorylation of NFκB (Figs. 1e, 6a). Also, activated NFκB was detected in the MDA MB-231 cells (Fig. 3). Administration of fish oil increased the level of PTEN in the breast tumor of mice in the xenograft model (Fig. 6a, b). Furthermore, our results show increased expression of PTEN in the breast cancer cells treated with DHA and EPA (Fig. 6c–e; Supplementary Fig. S4). In line with these observations, both DHA and EPA reduced DNA binding of NFκB, which increased transcription of PTEN (Figs. 3d, 7a, b). These data indicate that inactivation of NFκB may result derepression of PTEN expression in the mice treated with fish oil as well as in the breast tumor cells incubated with DHA and EPA. Increased expression of PTEN inhibits Akt activity [48, 56, 101]. Fish oil diet suppressed Akt activity in the tumor lysates (Fig. 1c, d), demonstrating a possible role of increased PTEN. Additionally, reduced PI 3 kinase activity by fish oil might have contributed to reduced Akt activation (Fig. 1a, b). Thus, fish oil utilizes two alternative pathways to reduce Akt activity, which resulted in inhibition of activating serine-536 phosphorylation of p65 NFκB subunit (Figs. 1e, f, 3a, b).
Expression of PTEN has been shown to be associated with increased apoptosis of tumor cells [116, 117]. Furthermore, increased apoptosis results from attenuated expression of the anti-apoptotic proteins Bcl-2 and Bcl-XL [40, 118]. In accordance with these results, fish oil decreased expression of both these anti-apoptotic proteins (Fig. 2a–d). Also, DHA and EPA inhibited the transcription of these genes induced by NFκB (Figs. 4, 5). We show that expression of PTEN inhibited NFκB-induced transcription of Bcl-2 and Bcl-XL (Fig. 7c, d). These results demonstrate that DHA and EPA-mediated expression of PTEN may downregulate the antiapoptotic gene expression, thus enabling the breast cancer cells undergoing apoptosis.
In summary, we, for the first time, show that fish oil diet inhibits PI 3 kinase activity in the breast tumor in the mice. Our results demonstrate a role of p65 NFκB subunit to regulate the expression of Bcl-2 and Bcl-XL, which become the targets of fish oil ω-3 fatty acids, DHA and EPA, which induce apoptosis in the breast cancer cells. Furthermore, we provide the first evidence that fish oil and its active components DHA and EPA increase PTEN, which in turn downregulates NFκB-mediated expression of Bcl-2 and Bcl-XL. These results represent a novel mechanism for the beneficial effect of fish oil on breast tumor growth.
Supplementary Material
Acknowledgments
This work was supported by the NIH RO1 AR52425, DOD Breast cancer Concept Award, Morrison Trust Fund and VA Merit Review grants to NGC. GGC is supported by NIH RO1 DK 50190, VA Merit Review and Juvenile Diabetes Research Foundation Regular Research Grants. GGC is recipient of the Research Career Scientist Award from the Department of Veterans Affairs. GF is supported by NIH RO1 AG023648.
Abbreviations
- DHA
Docosahexaenoic acid
- EPA
Eicosapentaenoic acid
- PI
Phosphatidylinositol
- PH
Pleckstrin homology
- EMSA
Electrophoretic mobility shift assay
- LUC
Luciferase
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-008-0227-7) contains supplementary material, which is available to authorized users.
Contributor Information
Triparna Ghosh-Choudhury, Department of Pathology, University of Texas Health Science, Center at San Antonio, San Antonio, TX, USA.
Chandi C. Mandal, Department of Pathology, University of Texas Health Science, Center at San Antonio, San Antonio, TX, USA
Kathleen Woodruff, Department of Pathology, University of Texas Health Science, Center at San Antonio, San Antonio, TX, USA.
Patricia St Clair, Department of Pathology, University of Texas Health Science, Center at San Antonio, San Antonio, TX, USA.
Goutam G. Choudhury, Geriatric Research, Education and Clinical Center, San Antonio, TX, USA; South Texas Veterans Health Care System, San Antonio, TX, USA
Nandini Ghosh-Choudhury, Email: choudhury@uthscsa.edu, Department of Pathology, University of Texas Health Science, Center at San Antonio, San Antonio, TX, USA; South Texas Veterans Health Care System, San Antonio, TX, USA.
References
- 1.MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim YW, Traina SB, Hilton L, Garland R, et al. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295(4):403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 2.Kelsey JL, Horn-Ross PL. Breast cancer: magnitude of the problem and descriptive epidemiology. Epidemiol Rev. 1993;15(1):7–16. doi: 10.1093/oxfordjournals.epirev.a036118. [DOI] [PubMed] [Google Scholar]
- 3.Lands WE, Hamazaki T, Yamazaki K, Okuyama H, Sakai K, Goto Y, Hubbard VS. Changing dietary patterns. Am J Clin Nutr. 1990;51(6):991–993. doi: 10.1093/ajcn/51.6.991. [DOI] [PubMed] [Google Scholar]
- 4.Chajes V, Sattler W, Stranzl A, Kostner GM. Influence of n-3 fatty acids on the growth of human breast cancer cells in vitro: relationship to peroxides and vitamin-E. Breast Cancer Res Treat. 1995;34(3):199–212. doi: 10.1007/BF00689711. [DOI] [PubMed] [Google Scholar]
- 5.Grammatikos SI, Subbaiah PV, Victor TA, Miller WM. n-3 and n-6 fatty acid processing and growth effects in neoplastic and non-cancerous human mammary epithelial cell lines. Br J Cancer. 1994;70(2):219–227. doi: 10.1038/bjc.1994.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83(3):217–244. doi: 10.1016/S0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 7.Schley PD, Jijon HB, Robinson LE, Field CJ. Mechanisms of omega-3 fatty acid-induced growth inhibition in MDA-MB-231 human breast cancer cells. Breast Cancer Res Treat. 2005;92(2):187–195. doi: 10.1007/s10549-005-2415-z. [DOI] [PubMed] [Google Scholar]
- 8.Schley PD, Brindley DN, Field CJ. (n-3) PUFA alter raft lipid composition and decrease epidermal growth factor receptor levels in lipid rafts of human breast cancer cells. J Nutr. 2007;137(3):548–553. doi: 10.1093/jn/137.3.548. [DOI] [PubMed] [Google Scholar]
- 9.Stillwell W, Shaikh SR, Zerouga M, Siddiqui R, Wassall SR. Docosahexaenoic acid affects cell signaling by altering lipid rafts. Reprod Nutr Dev. 2005;45(5):559–579. doi: 10.1051/rnd:2005046. [DOI] [PubMed] [Google Scholar]
- 10.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14(5):381–395. doi: 10.1016/S0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 11.Testa JR, Bellacosa A. AKT plays a central role in tumorigenesis. Proc Natl Acad Sci USA. 2001;98(20):10983–10985. doi: 10.1073/pnas.211430998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 13.Marone R, Cmiljanovic V, Giese B, Wymann MP. Targeting phosphoinositide 3-kinase: moving towards therapy. Biochim Biophys Acta. 2008;1784(1):159–185. doi: 10.1016/j.bbapap.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Pawson T, Nash P. Protein–protein interactions define specificity in signal transduction. Genes Dev. 2000;14(9):1027–1047. [PubMed] [Google Scholar]
- 15.Alessi DR, Deak M, Casamayor A, Caudwell FB, Morrice N, Norman DG, Gaffney P, Reese CB, MacDougall CN, Harbison D, et al. 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7(10):776–789. doi: 10.1016/S0960-9822(06)00336-8. [DOI] [PubMed] [Google Scholar]
- 16.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 17.Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280(49):40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 18.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279(5351):710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 19.Troyer DA, Chandrasekar B, Barnes JL, Fernandes G. Calorie restriction decreases platelet-derived growth factor (PDGF)-A and thrombin receptor mRNA expression in autoimmune murine lupus nephritis. Clin Exp Immunol. 1997;108(1):58–62. doi: 10.1046/j.1365-2249.1997.d01-970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Venkatraman JT, Chandrasekar B, Kim JD, Fernandes G. Effects of n-3 and n-6 fatty acids on the activities and expression of hepatic antioxidant enzymes in autoimmune-prone NZBxNZW F1 mice. Lipids. 1994;29(8):561–568. doi: 10.1007/BF02536628. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka K, Rocchi P, Miyake H, Fazli L, Vessella B, Zangemeister-Wittke U, Gleave ME. A novel antisense oligonucleotide inhibiting several antiapoptotic Bcl-2 family members induces apoptosis and enhances chemosensitivity in androgen-independent human prostate cancer PC3 cells. Mol Cancer Ther. 2005;4(11):1689–1698. doi: 10.1158/1535-7163.MCT-05-0064. [DOI] [PubMed] [Google Scholar]
- 22.Virolle T, Adamson ED, Baron V, Birle D, Mercola D, Mustelin T, de Belle I. The Egr-1 transcription factor directly activates PTEN during irradiation-induced signalling. Nat Cell Biol. 2001;3(12):1124–1128. doi: 10.1038/ncb1201-1124. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh-Choudhury N, Mandal CC, Choudhury GG. Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem. 2007;282(7):4983–4993. doi: 10.1074/jbc.M606706200. [DOI] [PubMed] [Google Scholar]
- 24.Ghosh-Choudhury N, Abboud SL, Mahimainathan L, Chandrasekar B, Choudhury GG. Phosphatidylinositol 3-kinase regulates bone morphogenetic protein-2 (BMP-2)-induced myocyte enhancer factor 2A-dependent transcription of BMP-2 gene in cardiomyocyte precursor cells. J Biol Chem. 2003;278(24):21998–22005. doi: 10.1074/jbc.M302277200. [DOI] [PubMed] [Google Scholar]
- 25.Ghosh-Choudhury N, Abboud SL, Nishimura R, Celeste A, Mahimainathan L, Choudhury GG. Requirement of BMP-2-induced phosphatidylinositol 3-kinase and Akt serine/threonine kinase in osteoblast differentiation and Smad-dependent BMP-2 gene transcription. J Biol Chem. 2002;277(36):33361–33368. doi: 10.1074/jbc.M205053200. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh-Choudhury N, Choudhury GG, Harris MA, Wozney J, Mundy GR, Abboud SL, Harris SE. Autoregulation of mouse BMP-2 gene transcription is directed by the proximal promoter element. Biochem Biophys Res Commun. 2001;286(1):101–108. doi: 10.1006/bbrc.2001.5351. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh-Choudhury N, Singha PK, Woodruff K, St Clair P, Bsoul S, Werner SL, Choudhury GG. Concerted action of Smad and CREB-binding protein regulates bone morphogenetic protein-2-stimulated osteoblastic colony-stimulating factor-1 expression. J Biol Chem. 2006;281(29):20160–20170. doi: 10.1074/jbc.M511071200. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesan B, Mahimainathan L, Das F, Ghosh-Choudhury N, Ghosh Choudhury G. Downregulation of catalase by reactive oxygen species via PI 3 kinase/Akt signaling in mesangial cells. J Cell Physiol. 2007;211(2):457–467. doi: 10.1002/jcp.20953. [DOI] [PubMed] [Google Scholar]
- 29.Baker SJ. PTEN enters the nuclear age. Cell. 2007;128(1):25–28. doi: 10.1016/j.cell.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmstrom PO, Mansukhani M, Enoksson J, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65(7):2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 31.Ghosh-Choudhury N, Woodruff K, Qi W, Celeste A, Abboud SL, Ghosh Choudhury G. Bone morphogenetic protein-2 blocks MDA MB 231 human breast cancer cell proliferation by inhibiting cyclin-dependent kinase-mediated retinoblastoma protein phosphorylation. Biochem Biophys Res Commun. 2000;272(3):705–711. doi: 10.1006/bbrc.2000.2844. [DOI] [PubMed] [Google Scholar]
- 32.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24(50):7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 33.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Testa JR, Tsichlis PN. AKT signaling in normal and malignant cells. Oncogene. 2005;24(50):7391–7393. doi: 10.1038/sj.onc.1209100. [DOI] [PubMed] [Google Scholar]
- 35.Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr Biol. 1999;9(11):601–604. doi: 10.1016/S0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 36.Madrid LV, Mayo MW, Reuther JY, Baldwin AS., Jr Akt stimulates the transactivation potential of the RelA/p65 Subunit of NF-kappa B through utilization of the Ikappa B kinase and activation of the mitogen-activated protein kinase p38. J Biol Chem. 2001;276(22):18934–18940. doi: 10.1074/jbc.M101103200. [DOI] [PubMed] [Google Scholar]
- 37.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401(6748):82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 38.Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. Distinct roles of the Ikappa B kinase alpha and beta subunits in liberating nuclear factor kappa B (NF-kappa B) from Ikappa B and in phosphorylating the p65 subunit of NF-kappa B. J Biol Chem. 2002;277(6):3863–3869. doi: 10.1074/jbc.M110572200. [DOI] [PubMed] [Google Scholar]
- 39.Konishi T, Sasaki S, Watanabe T, Kitayama J, Nagawa H. Overexpression of hRFI inhibits 5-fluorouracil-induced apoptosis in colorectal cancer cells via activation of NF-kappaB and upregulation of BCL-2 and BCL-XL. Oncogene. 2006;25(22):3160–3169. doi: 10.1038/sj.onc.1209342. [DOI] [PubMed] [Google Scholar]
- 40.Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits nuclear factor-kappaB signaling pathway through inhibition of receptor-interacting protein and TAK1 leading to suppression of antiapoptotic gene products and potentiation of apoptosis. J Biol Chem. 2007;282(1):809–820. doi: 10.1074/jbc.M610028200. [DOI] [PubMed] [Google Scholar]
- 41.Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005;24(44):6719–6728. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- 42.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25(51):6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 43.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 44.Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275(15):10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- 45.Gascoyne DM, Kypta RM, Vivanco MM. Glucocorticoids inhibit apoptosis during fibrosarcoma development by transcriptionally activating Bcl-xL. J Biol Chem. 2003;278(20):18022–18029. doi: 10.1074/jbc.M301812200. [DOI] [PubMed] [Google Scholar]
- 46.Grillot DA, Gonzalez-Garcia M, Ekhterae D, Duan L, Inohara N, Ohta S, Seldin MF, Nunez G. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol. 1997;158(10):4750–4757. [PubMed] [Google Scholar]
- 47.Wang X, Trotman LC, Koppie T, Alimonti A, Chen Z, Gao Z, Wang J, Erdjument-Bromage H, Tempst P, Cordon-Cardo C, et al. NEDD4-1 is a proto-oncogenic ubiquitin ligase for PTEN. Cell. 2007;128(1):129–139. doi: 10.1016/j.cell.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6(3):184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 49.Kim RH, Peters M, Jang Y, Shi W, Pintilie M, Fletcher GC, DeLuca C, Liepa J, Zhou L, Snow B, et al. DJ-1, a novel regulator of the tumor suppressor PTEN. Cancer Cell. 2005;7(3):263–273. doi: 10.1016/j.ccr.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Saal LH, Gruvberger-Saal SK, Persson C, Lovgren K, Jump-panen M, Staaf J, Jonsson G, Pires MM, Maurer M, Holm K, et al. Recurrent gross mutations of the PTEN tumor suppressor gene in breast cancers with deficient DSB repair. Nat Genet. 2008;40(1):102–107. doi: 10.1038/ng.2007.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anti M, Marra G, Armelao F, Bartoli GM, Ficarelli R, Percesepe A, De Vitis I, Maria G, Sofo L, Rapaccini GL, et al. Effect of omega-3 fatty acids on rectal mucosal cell proliferation in subjects at risk for colon cancer. Gastroenterology. 1992;103(3):883–891. doi: 10.1016/0016-5085(92)90021-p. [DOI] [PubMed] [Google Scholar]
- 52.Burns CP, Halabi S, Clamon GH, Hars V, Wagner BA, Hohl RJ, Lester E, Kirshner JJ, Vinciguerra V, Paskett E. Phase I clinical study of fish oil fatty acid capsules for patients with cancer cachexia: cancer, leukemia group B study 9473. Clin Cancer Res. 1999;5(12):3942–3947. [PubMed] [Google Scholar]
- 53.Hardman WE, Avula CP, Fernandes G, Cameron IL. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clin Cancer Res. 2001;7(7):2041–2049. [PubMed] [Google Scholar]
- 54.Wymann MP, Marone R. Phosphoinositide 3-kinase in disease: timing, location, and scaffolding. Curr Opin Cell Biol. 2005;17(2):141–149. doi: 10.1016/j.ceb.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 55.Zhao JJ, Roberts TM. PI3 kinases in cancer: from oncogene artifact to leading cancer target. Sci STKE. 2006;2006(365):pe52. doi: 10.1126/stke.3652006pe52. [DOI] [PubMed] [Google Scholar]
- 56.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96(8):4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rameh LE, Cantley LC. The role of phosphoinositide 3-kinase lipid products in cell function. J Biol Chem. 1999;274(13):8347–8350. doi: 10.1074/jbc.274.13.8347. [DOI] [PubMed] [Google Scholar]
- 58.Chang HW, Aoki M, Fruman D, Auger KR, Bellacosa A, Tsichlis PN, Cantley LC, Roberts TM, Vogt PK. Transformation of chicken cells by the gene encoding the catalytic subunit of PI 3-kinase. Science. 1997;276(5320):1848–1850. doi: 10.1126/science.276.5320.1848. [DOI] [PubMed] [Google Scholar]
- 59.Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3(8):772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 60.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304(5670):554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 61.Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, De-long L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7(6):561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 62.Isakoff SJ, Engelman JA, Irie HY, Luo J, Brachmann SM, Pearline RV, Cantley LC, Brugge JS. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005;65(23):10992–11000. doi: 10.1158/0008-5472.CAN-05-2612. [DOI] [PubMed] [Google Scholar]
- 63.Benistant C, Chapuis H, Roche S. A specific function for phosphatidylinositol 3-kinase alpha (p85alpha–p110alpha) in cell survival and for phosphatidylinositol 3-kinase beta (p85alpha–p110beta) in de novo DNA synthesis of human colon carcinoma cells. Oncogene. 2000;19(44):5083–5090. doi: 10.1038/sj.onc.1203871. [DOI] [PubMed] [Google Scholar]
- 64.Knobbe CB, Reifenberger G. Genetic alterations and aberrant expression of genes related to the phosphatidyl-inositol-3′-kinase/protein kinase B (Akt) signal transduction pathway in glioblastomas. Brain Pathol. 2003;13(4):507–518. doi: 10.1111/j.1750-3639.2003.tb00481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang S, Denley A, Vanhaesebroeck B, Vogt PK. Oncogenic transformation induced by the p110beta, -gamma, and -delta isoforms of class I phosphoinositide 3-kinase. Proc Natl Acad Sci USA. 2006;103(5):1289–1294. doi: 10.1073/pnas.0510772103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene. 2005;24(50):7455–7464. doi: 10.1038/sj.onc.1209085. [DOI] [PubMed] [Google Scholar]
- 67.Bellacosa A, de Feo D, Godwin AK, Bell DW, Cheng JQ, Altomare DA, Wan M, Dubeau L, Scambia G, Masciullo V, et al. Molecular alterations of the AKT2 oncogene in ovarian and breast carcinomas. Int J Cancer. 1995;64(4):280–285. doi: 10.1002/ijc.2910640412. [DOI] [PubMed] [Google Scholar]
- 68.Cheng JQ, Godwin AK, Bellacosa A, Taguchi T, Franke TF, Hamilton TC, Tsichlis PN, Testa JR. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci USA. 1992;89(19):9267–9271. doi: 10.1073/pnas.89.19.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brugge J, Hung MC, Mills GB. A new mutational AK-Tivation in the PI3K pathway. Cancer Cell. 2007;12(2):104–107. doi: 10.1016/j.ccr.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 70.Carpten JD, Faber AL, Horn C, Donoho GP, Briggs SL, Robbins CM, Hostetter G, Boguslawski S, Moses TY, Savage S, et al. A transforming mutation in the pleckstrin homology domain of AKT1 in cancer. Nature. 2007;448(7152):439–444. doi: 10.1038/nature05933. [DOI] [PubMed] [Google Scholar]
- 71.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, Lee JH, Ciarallo S, Catzavelos C, Beniston R, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002;8(10):1153–1160. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 72.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8(10):1145–1152. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 73.Viglietto G, Motti ML, Bruni P, Melillo RM, D'Alessio A, Califano D, Vinci F, Chiappetta G, Tsichlis P, Bellacosa A, et al. Cytoplasmic relocalization and inhibition of the cyclindependent kinase inhibitor p27(Kip1) by PKB/Akt-mediated phosphorylation in breast cancer. Nat Med. 2002;8(10):1136–1144. doi: 10.1038/nm762. [DOI] [PubMed] [Google Scholar]
- 74.Zhou BP, Liao Y, Xia W, Spohn B, Lee MH, Hung MC. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat Cell Biol. 2001;3(3):245–252. doi: 10.1038/35060032. [DOI] [PubMed] [Google Scholar]
- 75.Kwiatkowski DJ, Manning BD. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet. 2005;14:R251–R258. doi: 10.1093/hmg/ddi260. Spec No. 2. [DOI] [PubMed] [Google Scholar]
- 76.Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25(6):903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 77.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–241. doi: 10.1016/S0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 78.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. -3 Proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6(1):14–3. 41–51. doi: 10.1016/S1097-2765(00)00006-X. [DOI] [PubMed] [Google Scholar]
- 79.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3(11):973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 80.Mayo LD, Donner DB. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA. 2001;98(20):11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/S0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 82.Dijkers PF, Birkenkamp KU, Lam EW, Thomas NS, Lammers JW, Koenderman L, Coffer PJ. FKHR-L1 can act as a critical effector of cell death induced by cytokine withdrawal: protein kinase B-enhanced cell survival through maintenance of mitochondrial integrity. J Cell Biol. 2002;156(3):531–542. doi: 10.1083/jcb.200108084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 2005;24(50):7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 84.Tran H, Brunet A, Griffith EC, Greenberg ME. The many forks in FOXO's road. Sci STKE. 2003;2003(172):RE5. doi: 10.1126/stke.2003.172.re5m. [DOI] [PubMed] [Google Scholar]
- 85.Chapman NR, Europe-Finner GN, Robson SC. Expression and deoxyribonucleic acid-binding activity of the nuclear factor kappaB family in the human myometrium during pregnancy and labor. J Clin Endocrinol Metab. 2004;89(11):5683–5693. doi: 10.1210/jc.2004-0873. [DOI] [PubMed] [Google Scholar]
- 86.Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, Kracht M, Schmitz ML. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol. 2004;172(10):6336–6344. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- 87.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64:5–6. 963–970. doi: 10.1016/S0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 88.Angileri FF, Aguennouz M, Conti A, La Torre D, Cardali S, Crupi R, Tomasello C, Germano A, Vita G, Tomasello F. Nuclear factor-kappaB activation and differential expression of survivin and Bcl-2 in human grade 2–4 astrocytomas. Cancer. 2008;112(10):2258–2266. doi: 10.1002/cncr.23407. [DOI] [PubMed] [Google Scholar]
- 89.Upadhyay AK, Singh S, Chhipa RR, Vijayakumar MV, Ajay AK, Bhat MK. Methyl-beta-cyclodextrin enhances the susceptibility of human breast cancer cells to carboplatin and 5-fluorouracil: involvement of Akt, NF-kappaB and Bcl-2. Toxicol Appl Pharmacol. 2006;216(2):177–185. doi: 10.1016/j.taap.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Takase O, Minto AW, Puri TS, Cunningham PN, Jacob A, Hayashi M, Quigg RJ. Inhibition of NF-kappaB-dependent Bcl-xL expression by clusterin promotes albumin-induced tubular cell apoptosis. Kidney Int. 2008;73(5):567–577. doi: 10.1038/sj.ki.5002563. [DOI] [PubMed] [Google Scholar]
- 91.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 92.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25(51):6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 93.Yeh YY, Chiao CC, Kuo WY, Hsiao YC, Chen YJ, Wei YY, Lai TH, Fong YC, Tang CH. TGF-beta1 increases motility and alphavbeta3 integrin up-regulation via PI3K, Akt and NF-kappaB-dependent pathway in human chondrosarcoma cells. Biochem Pharmacol. 2008;75(6):1292–1301. doi: 10.1016/j.bcp.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 94.Haller D, Russo MP, Sartor RB, Jobin C. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277(41):38168–38178. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 95.Strassheim D, Asehnoune K, Park JS, Kim JY, He Q, Richter D, Kuhn K, Mitra S, Abraham E. Phosphoinositide 3-kinase and Akt occupy central roles in inflammatory responses of Toll-like receptor 2-stimulated neutrophils. J Immunol. 2004;172(9):5727–5733. doi: 10.4049/jimmunol.172.9.5727. [DOI] [PubMed] [Google Scholar]
- 96.Spierings D, McStay G, Saleh M, Bender C, Chipuk J, Maurer U, Green DR. Connected to death: the (unexpurgated) mitochondrial pathway of apoptosis. Science. 2005;310(5745):66–67. doi: 10.1126/science.1117105. [DOI] [PubMed] [Google Scholar]
- 97.Watson MR, Wallace K, Gieling RG, Manas DM, Jaffray E, Hay RT, Mann DA, Oakley F. NF-kappaB is a critical regulator of the survival of rodent and human hepatic myofibroblasts. J Hepatol. 2008;48(4):589–597. doi: 10.1016/j.jhep.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 98.Toruner M, Fernandez-Zapico M, Sha JJ, Pham L, Urrutia R, Egan LJ. Antianoikis effect of nuclear factor-kappaB through up-regulated expression of osteoprotegerin, BCL-2, and IAP-1. J Biol Chem. 2006;281(13):8686–8696. doi: 10.1074/jbc.M512178200. [DOI] [PubMed] [Google Scholar]
- 99.Tsukahara T, Kannagi M, Ohashi T, Kato H, Arai M, Nunez G, Iwanaga Y, Yamamoto N, Ohtani K, Nakamura M, et al. Induction of Bcl-x(L) expression by human T-cell leukemia virus type 1 Tax through NF-kappaB in apoptosis-resistant T-cell transfectants with Tax. J Virol. 1999;73(10):7981–7987. doi: 10.1128/jvi.73.10.7981-7987.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275(5308):1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 101.Stiles B, Groszer M, Wang S, Jiao J, Wu H. PTENless means more. Dev Biol. 2004;273(2):175–184. doi: 10.1016/j.ydbio.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 102.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273(22):13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 103.Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9(4):125–128. doi: 10.1016/S0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 104.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. PTEN dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441(7092):475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 105.He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, Dirisina R, Porter-Westpfahl KS, Hembree M, Johnson T, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39(2):189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten +/− mice. Cancer Res. 2000;60(13):3605–3611. [PubMed] [Google Scholar]
- 107.Li G, Robinson GW, Lesche R, Martinez-Diaz H, Jiang Z, Rozengurt N, Wagner KU, Wu DC, Lane TF, Liu X, et al. Conditional loss of PTEN leads to precocious development and neoplasia in the mammary gland. Development. 2002;129(17):4159–4170. doi: 10.1242/dev.129.17.4159. [DOI] [PubMed] [Google Scholar]
- 108.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou J, Wulfkuhle J, Zhang H, Gu P, Yang Y, Deng J, Mar-golick JB, Liotta LA, Petricoin E, III, Zhang Y. Activation of the PTEN/mTOR/STAT3 pathway in breast cancer stem-like cells is required for viability and maintenance. Proc Natl Acad Sci USA. 2007;104(41):16158–16163. doi: 10.1073/pnas.0702596104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Palomero T, Sulis ML, Cortina M, Real PJ, Barnes K, Ciofani M, Caparros E, Buteau J, Brown K, Perkins SL, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13(10):1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8(2):317–325. doi: 10.1016/S1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 112.Patel L, Pass I, Coxon P, Downes CP, Smith SA, Macphee CH. Tumor suppressor and anti-inflammatory actions of PPARgamma agonists are mediated via upregulation of PTEN. Curr Biol. 2001;11(10):764–768. doi: 10.1016/S0960-9822(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 113.Vasudevan KM, Gurumurthy S, Rangnekar VM. Suppression of PTEN expression by NF-kappa B prevents apoptosis. Mol Cell Biol. 2004;24(3):1007–1021. doi: 10.1128/MCB.24.3.1007-1021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang Q, Zhou Y, Wang X, Chung DH, Evers BM. Regulation of PTEN expression in intestinal epithelial cells by c-Jun NH2-terminal kinase activation and nuclear factor-kappaB inhibition. Cancer Res. 2007;67(16):7773–7781. doi: 10.1158/0008-5472.CAN-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xia D, Srinivas H, Ahn YH, Sethi G, Sheng X, Yung WK, Xia Q, Chiao PJ, Kim H, Brown PH, et al. Mitogen-activated protein kinase kinase-4 promotes cell survival by decreasing PTEN expression through an NF kappa B-dependent pathway. J Biol Chem. 2007;282(6):3507–3519. doi: 10.1074/jbc.M610141200. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura N, Ramaswamy S, Vazquez F, Signoretti S, Loda M, Sellers WR. Forkhead transcription factors are critical effectors of cell death and cell cycle arrest downstream of PTEN. Mol Cell Biol. 2000;20(23):8969–8982. doi: 10.1128/MCB. 20.23.8969-8982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR. Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA. 1999;96(5):2110–2115. doi: 10.1073/pnas.96.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu M, Min C, Wang X, Yu Z, Kirsch KH, Trackman PC, Sonenshein GE. Repression of BCL2 by the tumor suppressor activity of the lysyl oxidase propeptide inhibits transformed phenotype of lung and pancreatic cancer cells. Cancer Res. 2007;67(13):6278–6285. doi: 10.1158/0008-5472.CAN-07-0776. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


