Summary
Bone morphogenetic proteins (BMPs) have multiple roles during embryogenesis. Current data indicate that the dosage of BMPs is tightly regulated for normal development in mice. Since Bmp2 or Bmp4 homozygous mutant mice show early embryonic lethality, we generated compound heterozygous mice for Bmp2 and Bmp4 to explore the impact of lowered dosage of these BMP ligands. Genotyping pups bred between Bmp2 and Bmp4 heterozygous mice revealed that the ratio of adult compound heterozygous mice for Bmp2 and Bmp4 is much lower than expected. During embryogenesis, the compound heterozygous embryos showed several abnormalities, including defects in eye formation, body wall closure defects, and ventricular septal defects (VSD) in the heart. However, the ratio of the compound heterozygous embryos was the same as expected. Caesarean sections at E18.5 revealed that half of the compound heterozygotes died soon after birth, and the majority of the dead individuals exhibited VSD. Survivors were able to grow to adults, but their body weight was significantly lower than control littermates. They demonstrated progressive abnormalities in the heart, eventually showing a branched leaflet in atrioventricular valves. These results suggest that the dosage of both BMP2 and 4 is critical for functional heart formation during embryogenesis and after birth.
Keywords: haploid insufficiency, ventricular septum defect, mitral valve, tricaspid valve, microphthalmia
INTRODUCTION
Bone morphogenetic proteins (BMPs) are the members of the TGF-β superfamily and were found by their osteogenic activity when placed in ectopic locations (de Caestecker, 2004; Kishigami and Mishina, 2005). However, recent studies of several organisms demonstrated that BMPs have other roles during embryogenesis, including the dorsoventral and/or anterior-posterior axis formation and processes of organogenesis, such as cardiac and lung development (Mishina, 2003; Zhao, 2003). BMP2 and BMP4 are the first discovered members of the BMP family and are rediscovered to have these alternate roles. Homozygous mutant embryos for Bmp2 show developmental abnormalities in fusion of the preammniotic canal at E7.5 and defects in cardiac development and embryonic turning, and die by E9.5 (Zhang and Bradley, 1996). BMP2 is necessary for migration but not for induction of neural crest cells (Correia et al., 2007). It is also necessary for PGC formation (Ying and Zhao, 2001). Homozygous mutant embryos for Bmp4 show abnormal development in the posterior half of the embryo and defects in germ cell development causing death by E10.5 (Lawson et al., 1999; Winnier et al., 1995; Ying et al., 2001). Severity of the phenotype of Bmp4 homozygous mutant embryos varies, depending partly on genetic background. For example, no mesoderm induction is observed in a C57BL6 background but the mutant embryos show a much milder phenotype in an outbred background and develop an apparent anterior-posterior axis (Furuta and Hogan, 1998; Winnier et al., 1995). Recent studies using a conditional null allele revealed important roles of BMP2 and BMP4 in later stages of embryogenesis. BMP4 is required for the endocardial epithelial-to-mesenchymal transformation (EMT) in the atrioventricular canal (AVC) (Jiao et al., 2003) and outflow tract septation (Liu et al., 2004). BMP4 also plays critical roles in morphogenesis of lung epithelial cells (Eblaghie et al., 2006). BMP2 is critical for the formation of the endocardial cushions in the AVC and subsequent endocardial EMT (Ma et al., 2005; Rivera-Feliciano and Tabin, 2006).
BMP ligands bind to both Type I and Type II receptors to form heteromultimers for their signal transduction. Three Type I receptors (BMPRIA, BMPRIB, and ACVRI), and three Type II receptors (BMPRII, ActRIIA, and ActRIIB) behave as receptors for BMPs. Homozygous mutants for Bmpr1a, which encodes a Type I receptor for BMP2 and BMP4, develop no mesoderm and die by E8.5 (Mishina et al., 1995). Homozygous mutants for Bmpr2 also show a phenotype identical to homozygous mutants for Bmpr1a (Beppu et al., 2000). Homozygous mutant embryos for Acvr1, which encodes a Type I receptor for BMP2 and BMP7, start to gastrulate, but stop developing at the late streak stage of gastrulation (Gu et al., 1999; Komatsu et al., 2007; Mishina et al., 1999). The fact that homozygous mutants for BMP receptors show more severe phenotypes than their ligands prompted us to hypothesize that loss of Bmp2 may be compensated, in part, by BMP4, and loss of Bmp4 may be compensated by BMP2. Both genes are highly conserved with over 90% similarity in the mature proteins, thus it is reasonable to hypothesize that Bmp2 and Bmp4 may play synergistic roles for proper embryonic development, and generation of compound mutant embryos may further reveal functions of these genes.
The amount of BMP signaling is regulated at different levels. Recent findings demonstrated that extracellular antagonists including noggin and chordin tightly regulate the amount of signaling (Balemans and Van Hul, 2002; Yanagita, 2005). Indeed, compound mutant embryos of noggin and chordin display a wide range of severity in their phenotype depending on the numbers of functional copies of BMP antagonists (Anderson et al., 2002; Bachiller et al., 2000), suggesting that tight regulation of the amount of BMP signaling is critical for normal development. It is reported that a small portion of heterozygous mutant mice for Bmp4 in a C57BL6 background demonstrate some phenotypes including polydactyl, a shorter head, and circling behavior (Dunn et al., 1997), but their survivability was not affected. A hypomorphic allele for Bmp4 was recently reported (Goldman et al., 2006). It was generated by introduction of a mutation in the cleavage site of the BMP4 precursor. Analyses of mice homozygous for this mutation revealed that the proper amount of BMP4 is critical for placental vasculature formation and ventral body wall closure (Goldman et al., 2006). No heterozygous phenotype was reported originally for Bmp2 (Zhang and Bradley, 1996), however, we recently found that some heterozygous embryos for Bmp2 exhibited closure defects in the cephalic region of the neural tube (Castranio and Mishina, 2008). Also, we recently generated a floxed allele for Bmp2 and the presence of a neo selection cassette in the locus dramatically reduced levels of Bmp2 (Singh et al., 2008). This leads to closure defects in cephalic region of the neural tube as well as in the ventral body wall (Singh et al., 2008). Therefore, it would be a reasonable hypothesis that mice that are compound heterozygous mutant for Bmp2 and Bmp4 would develop additional phenotypes during development.
Here, we found that compound heterozygous mutant mice for Bmp2 and Bmp4 were underrepresented at the time of weaning. Approximately half of the compound heterozygous fetuses showed defects in formation of the interventricular septum (ventricular septal defects, VSD) during development and died soon after birth. A small portion of the compound heterozygous fetuses showed a variety of phenotypes including closure defects in the ventral abdominal body wall, suggesting that those organs are sensitive for a decreased dose of BMPs during development. Interestingly, the survivors progressively developed abnormal valve structure in the heart by 2 months after birth. These results suggest that the dosage of both of BMP2 and 4 is critical for functional heart formation during embryogenesis and after birth, and that lowered BMP signaling is a potential cause of congenital and postnatal heart abnormalities such as VSD.
RESULTS
Bmp2/Bmp4 Compound Heterozygotes Show Disadvantage for Survival
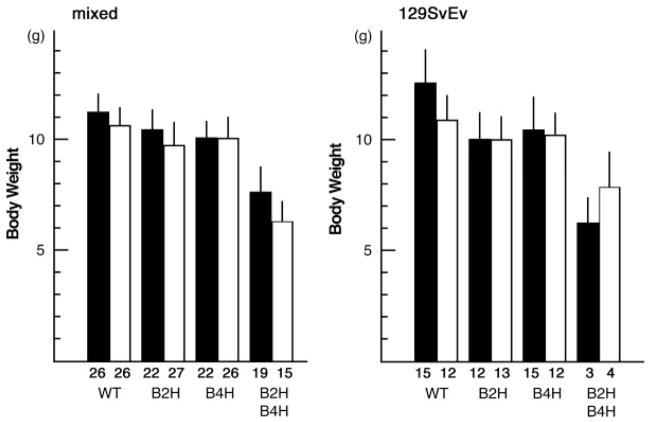
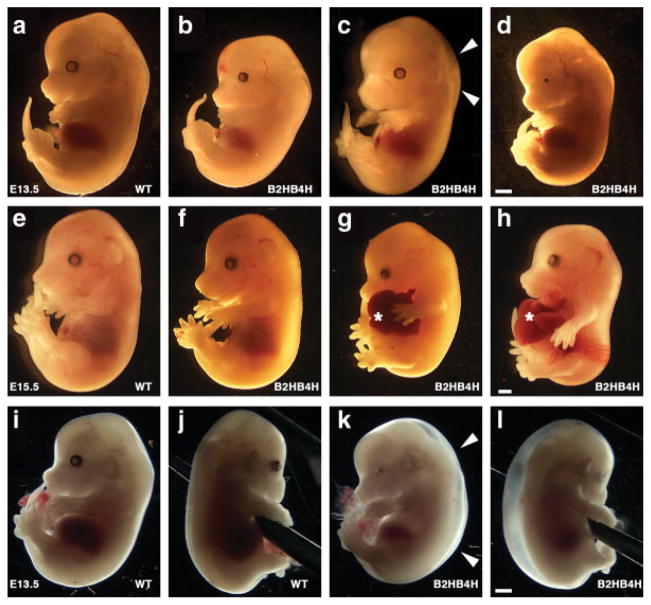
First, we set up matings between Bmp2 heterozygous and Bmp4 heterozygous mice, which had a mixed genetic background of 129SvEv and C57BL6. In this combination, 25% of pups should be compound heterozygotes for Bmp2 and Bmp4 (Bmp2+/−;Bmp4+/−). However, genotyping after the weaning stage revealed that only 10.6% of pups out of 415 mice were compound heterozygotes, whereas the other genotypes were in reasonable numbers (36.3% for wild type, 29.2% for Bmp4 single het, 23.9% for Bmp2 single het). These survived compound heterozygotes were smaller than littermates in both sexes at 3 weeks (Fig. 1, total 89 males and 94 females). The relative body weight of the survived compound heterozygous males and females to wild-type males and females were 68% and 59%, respectively (see Fig. 1). Swapping the genotypes of parents did not cause any overt changes in the ratio and body weight of compound heterozygotes (data not shown). Since both Bmp4 null and Bmp2 null embryos die in early stages of development, we speculated that compound heterozygotes might demonstrate an embryonic phenotype either similar to these nulls or something unique. Results of timed mating revealed that we recovered expected ratios of compound heterozygotes during mid to late gestation, suggesting that compound heterozygotes were able to survive much longer than homozygous null embryos for each gene. Some of the compound heterozygotes developed interesting phenotypes depicted in Figure 2. They showed severe edema as early as E13.5 (2 out of 36 at E13.5, Fig. 2c,k,l) suggesting defects in heart function. Some others showed eye defects (7 out of 73 at E13.5-E15.5, Fig. 2d,k) or body wall closure defects that lead to hernia (7 out of 73 at E13.5-E15.5, Fig. 2g,h, asterisk). However, the majority of compound heterozygotes in these stages were grossly normal (55 out of 73 at E13.5-E15.5, depicted in Fig. 2b,f), as well as all of the wild type or single heterozygous mutant embryos for each gene (except small portion of Bmp2 heterozygous embryos, see below). These results indicated that severe edema or body wall closure defects were not the only cause of the shortage of compound heterozygotes by the time of weaning. Ratios of compound heterozygotes were relatively normal in mid/late gestation (Table 1), indicating that the majority of compound heterozygotes can survive to late gestation, which excludes the possibility that the majority of them die earlier. Since previous studies have indicated the importance of BMP signaling in heart development, and a small number of the embryos showed edema as shown in Figure 2, we hypothesized that abnormalities that develop in the heart of compound heterozygous embryos would compromise their survivability during the perinatal period.
FIG. 1.
Body weight of 3-week-old mice. Mixed background (left) and 129SvEV background (right). Black bar, male; white bar, female; WT; wild type, B2H, Bmp2 heterozygous mice, B4H, Bmp4 heterozygous mice, B2HB4H, compound heterozygous mice for Bmp2 and Bmp4. Numbers of animals measured are shown underneath of each bar. B2HB4H mice are smaller in their body weight than other genotype in both sexes (P < 0.0001).
FIG. 2.
Abnormalities developed in the compound heterozygous embryos. Whole view of mouse embryos at E13.5 (a–d and i–l), E15.5 (e–h), wild type (a, e, i, and j), and compound heterozygous (b–d, f–h, and k, l) embryos. Compound heterozygous embryos showed no significant abnormality (b and f), edema (c and k, l, white arrowheads), small eye (d and k, l), and hernia (g and h, asterisk). i and j, and k and l are the same embryo, respectively. Scale bar, 1 mm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table 1.
Ratio of Genotyping of Offspring From the Breeding Between Bmp2 het and Bmp4 het
| Stage | Wild type (%) | Bmp2 het (%) | Bmp4 het (%) | Compound het (%) | Total | Resorption site |
|---|---|---|---|---|---|---|
| E13.5 | 18 (15.4) | 37 (31.6) | 25 (21.4) | 36 (30.8) | 117 | 6 |
| E14.5 | 9 (20.5) | 9 (20.5) | 12 (27.3) | 14 (31.8) | 44 | 21 |
| E15.5 | 26 (25.0) | 27 (26.0) | 28 (26.9) | 23 (22.1) | 104 | 25 |
| E18.5 | 23 (28.4) | 18 (22.2) | 19 (23.4) | 21 (35.9) | 81 | 9 |
| 3 wk | 129 (39.8) | 80 (24.7) | 86 (26.5) | 29 (9.0) | 324 | n/a |
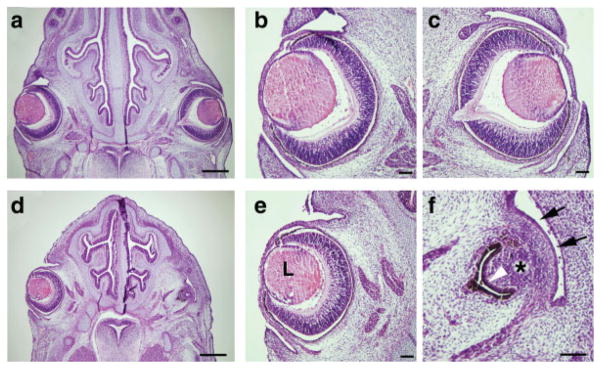
Histological observation revealed that some of the compound heterozygous mutant embryos developed microphthalmia (see Fig. 3). The particular compound embryo shown in Figure 3 had smaller a left eye than that of wild type (mild form of microphthalmia), whereas the right eye showed severe microphthalmia characterized by lens remnant, corneal-conjuntival epithelium, and double-layered retinal pigment epithelium. These results suggest that the retina started to degenerate soon after its formation and was subsequently replaced by the retinal pigment (inner layer of pigmented epithelium) (Fig. 3f).
FIG. 3.
Histological observation of eye abnormalities. H&E staining of head sections at E13.5. Wild type (a–c) and compound heterozygous (d–f) embryos are shown. The compound heterozygous mutant showed mild (e) and severe (f) forms of microphthalmia. L, lens; asterisk, remnant of lens; arrows, presumptive corneal-conjunctival epithelium; white arrowhead, degenerated retina replaced by pigment. Scale bar, 500 μm (a, d), 100 μm (b, c, e, f).
We previously noticed that a small portion of heterozygous embryos for Bmp2 develop neural tube closure defects especially in the cephalic region (Castranio and Mishina, 2008). The frequency of the neural tube defects was not increased by addition of Bmp4 heterozygosity to the Bmp2 heterozygous null background (6 out of 41 embryos for Bmp2 heterozygous embryos and 4 out of 42 embryos for Bmp2/Bmp4 compound heterozygous embryos) suggesting that dosage of BMP4 has less of an impact on developing the neural closure phenotype than that of BMP2.
Compound Heterozygotes Show Developmental Abnormalities in Heart
Histological observation of the E13.5 heart revealed that wild-type embryos are about to fuse the interventricular septum to the top portion of cardiac wall (2/4 are fused, 2/4 are not, Fig. 4a–d). In the case of compound heterozygous mice, 6 out of 6 showed ventricular septal defects (VSD) (Fig. 4e–h). To distinguish if this as a persistent defect or a delay of fusion, we next made histological observations at E15.5. At this stage, the septum in nearly all of wild type (13/14) and in all of both single heterozygous embryos examined were fused, whereas the majority of the compound heterozygous embryos still showed VSD (12/16) (Fig. 4m–t).
FIG. 4.
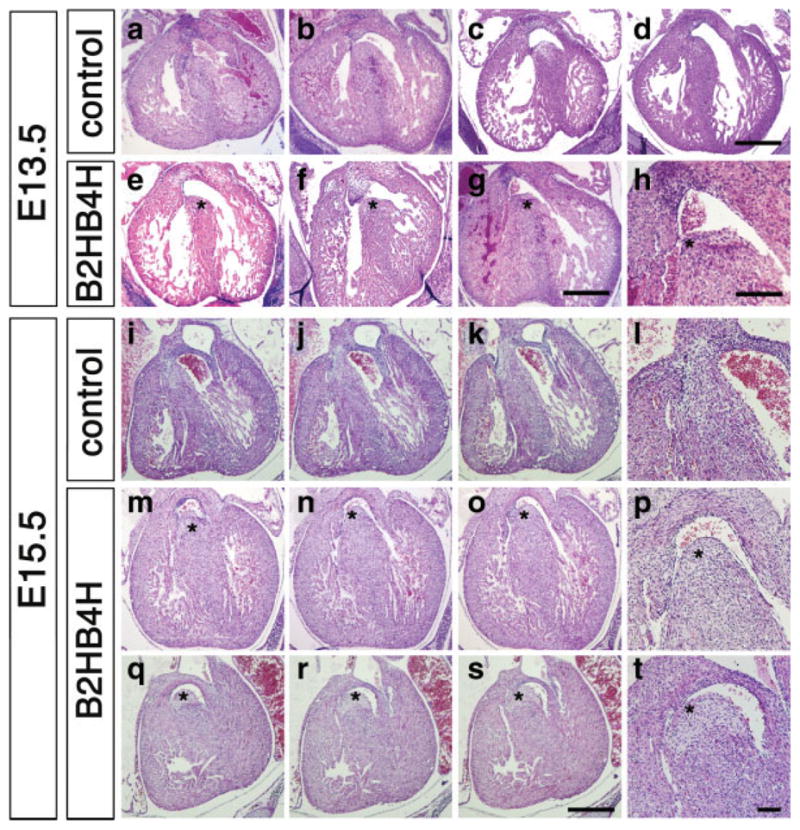
Heart abnormalities developed in compound heterozygous embryos. H&E staining of heart sections at E13.5 (a–h) and E15.5 (i–t). At E13.5, ventricular septum started to close in wild type (a and b), Bmp2 heterozygous (c), Bmp4 heterozygous (d) embryos, but in the compound heterozygous embryos, all of ventricular septum have not closed yet (e–h, asterisks). Two examples for WT and three examples for the compound heterozygotes are shown. At E15.5, all ventricular septum are completely closed in wild type (i–k; sequential with 35 μm interval, l; magnified image of j). In the compound heterozygous embryos the ventricular septum has not closed (asterisks, m–o; sequential images with 35 μm interval, p; magnified image of a region in n, q–s; sequential images with 35 μm interval, t; magnified image of a region in r). Two examples are shown. Scale bar, 500 μm (a–g); 200 μm (h); 250 μm (j–k, m–o, q–r); 100 μm (l, p, and t).
Since the ratio of the compound heterozygotes is relatively normal in mid/late gestation (Table 1), and we observed little death of pups during lactation period, we speculated that some of the compound heterozygotes die immediately after birth. E18.5 embryos were recovered from pregnant females by C-section and were evaluated for their ability to survive. All pups started to breathe, however, about half of compound heterozygotes showed respiratory difficulties, and died within 30 minutes (Table 2). Compound heterozygotes were smaller than other types of pups (P < 0.001). Among compound heterozygotes, the ones that died had lower body weights than survivors in body weight (949 mg ± 10.6 vs. 1042 mg ± 9.6 mg, respectively, P < 0.005). Examination of heart histology revealed a very clear contrast. The majority of compound heterozygotes that survived after C-section showed a relatively normal heart histology (4/5), including complete formation of the interventricular septum (Fig. 5c,d). Interestingly, all of the compound heterozygotes that died within 30 minutes after C-section showed VSD (Fig. 5e,f). This result suggests that perinatal death is probably due to heart malfunction caused by VSD because we did not see any overt changes in the lung (see below). Other types of abnormalities including atrial septal defects (ASD), double outlet right ventricle (DORV), and persistent truncus arteriosus (PTA) were not observed in the compound heterozygous mutant pups (Supporting Information Fig. 1 and data not shown).
Table 2.
Results of C-Section
| Genotype | Total | Infrequent breath (%) | Dead (%) | Body weight (mg) average (STD) |
|---|---|---|---|---|
| Wild type | 23 | 2 (8.7) | 0 (0) | 1,143.0 (120.5) |
| Bmp2 Het | 18 | 1 (5.6) | 1 (5.6) | 1,162.5 (87.7) |
| Bmp4 Het | 19 | 1 (5.3) | 1 (5.3) | 1,137.2 (191.4) |
| Compound Het | 21 | 12 (57.1)** | 11 (52.3)** | 993.1 (111.0)* |
| Total | 81 | 16 (19.8) | 15 (18.5) | 1,107.1 (147.1) |
P < 0.001, Student t-test.
P < 0.001 to Bmp2 Het or Bmp4 Het, Fisher’s exact test.
FIG. 5.
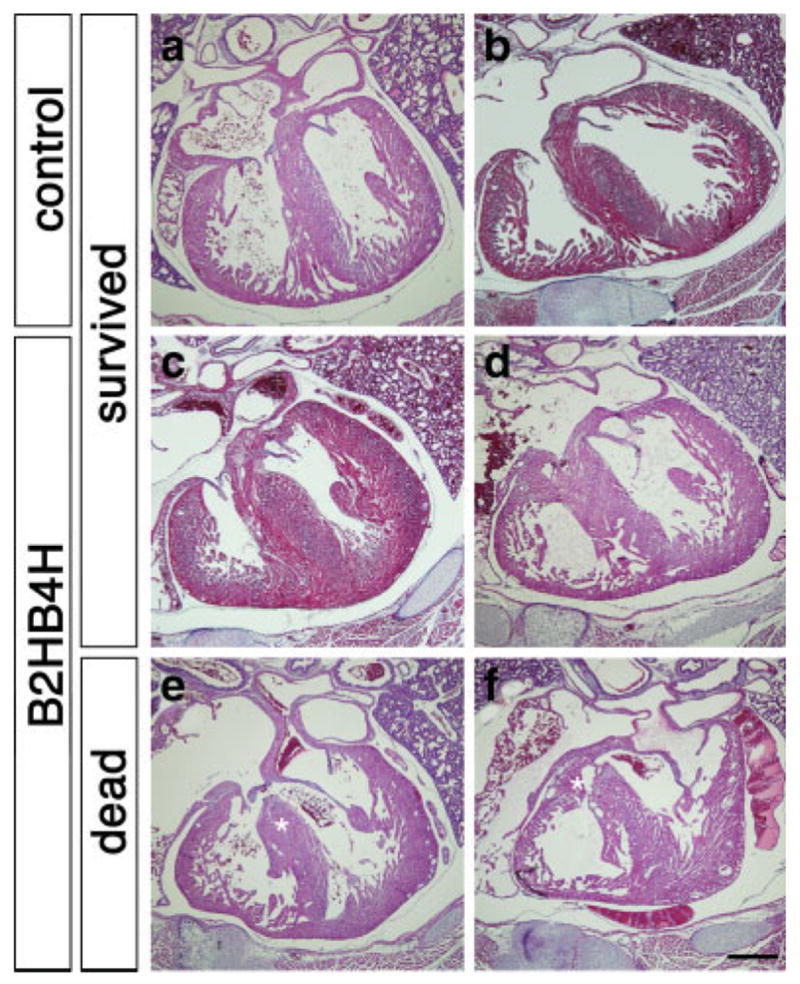
Defects in heart septation in the compound heterozygous mutants at E18.5. Fetuses were recovered at E18.5 by C-section and breathing was initiated by mild stimuli. Histological sections of heart were stained with H&E. Wild type (a and b) and majority of the compound heterozygous pups that revived after C-section (c and d, four of five) showed no evidence of VSD. Compound heterozygous mutants that did not revive showed VSD (e and f, asterisks, six of eight). Two examples for each case are shown. Scale bar, 500 μm.
We examined levels of several markers including MSX2, a known downstream target of BMP signals (Brugger et al., 2004) and SOX9, a marker for neural crest derivatives that populate atrioventricular cushions (Nie et al., 2008), but no overt changes were observed between wild type and the compound heterozygous heart (Supporting Information Fig. 2).
To understand the effects of lowered BMP signaling on heart development after birth, we made histological observations on 3-week-old hearts. The heart wall seemed normal (Fig. 6a,d,g), but leaflets in the tricuspid valve and mitral valve in hearts of compound heterozygotes showed abnormal morphology (Fig. 6e,h, and Fig. 6f,i, respectively). They were prolonged and more prominent in particular when body weight was low. These defects were not obvious in C-sectioned samples at E18.5 (Fig. 5 and data not shown) suggesting that these defects are developed postnatally. Histological observation of 2-month and 4-month-old hearts showed more dramatic changes in valve form. They were elongated and branched (Fig. 6o,q,r, n = 4 for control and compound heterozygous mutants). No abnormalities were found in single heterozygous mutant mice for either for Bmp2 or Bmp4.
FIG. 6.
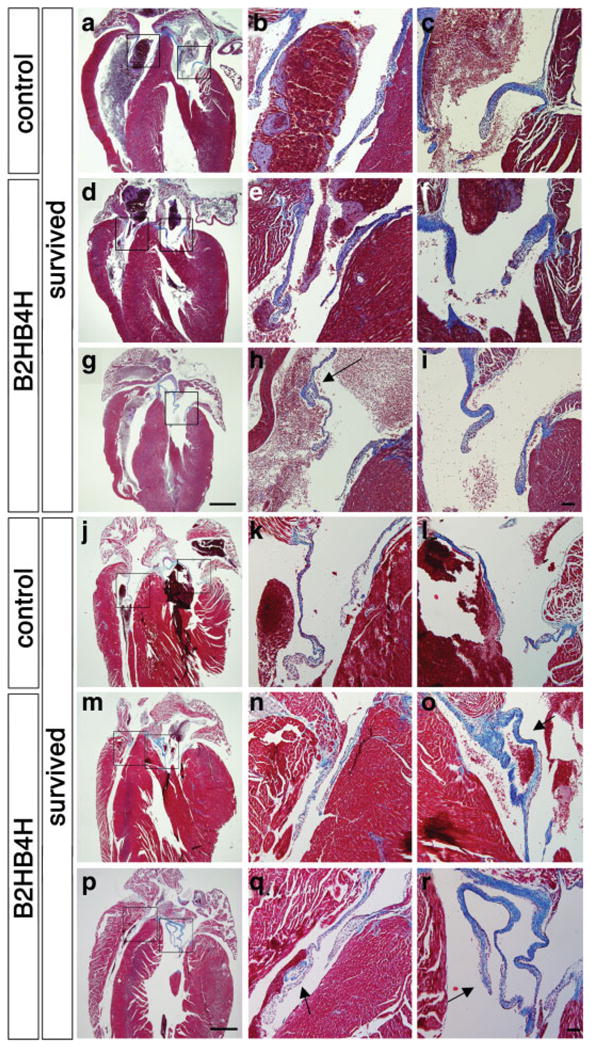
Valve abnormalities in the compound heterozygous mutant pups. Heart sections including atrioventricular valves from 3-week-old pups (a–i) and from adults (4 months for j–o and 2 months for p–r) were stained with Masson’s trichrome. Wild type (a–c, j–k) and two examples of compound heterozygous pups (d–f, g–i, m–o, p–r) are shown for each stage. Magnified images of right (tricuspid) and left (mitral) atrioventricular valves are shown in b, e, h, k, n and q and c, f, i, l, o, and r, respectively. h is a magnified image from an adjacent of g. Blue staining indicates atrioventricular valves. An arrow indicates branched atrioventricular valve (h, o, q, r). Scale bar, 1 mm (a, d, g, j, m, and p); 100 μm (b, c, e, f, h, i, k, l, n, o, q, and r).
No Overt Abnormalities are Found in Lung
Since half of compound heterozygotes fail to initiate continuous breathing, we also examined the histology of the lungs. At E15.5, there were no overt morphological changes (Supporting Information Fig. 3a,b). However, at 3 weeks, the color of lungs in compound heterozygotes was pale compared with that in control (Supporting Information Fig. 3c,d). The histology of lungs at the time of C-section revealed less alveolar spaces in compound heterozygotes (Supporting Information Fig. 3e–l), however, the changes were minimal. Three-week-old samples showed the similar tendency, lower numbers of alveolar vesicles (Supporting Information Fig. 3m–p); however, the thickness of the epithelial layer of bronchioles was comparable. SP-C and CC10, markers for alveoli epithelium, were produced in both control and compound heterozygous mutant lung in comparable manners (Supporting Information Fig. 4).
Genetic Background Affects Severity of the Phenotypes
Because the genetic background of the mice used were a mixture of 129SvEv and C57BL6, variability of the phenotypes might be explained by variability of genetic background. In fact, it is reported that phenotypes of Bmp4 null embryos change in different backgrounds. In an outbred background, homozygous null embryos can survive longer and reach the organogenesis stages whereas in a C57BL6 background, their phenotype resembles Bmpr1a or Bmpr2 null mutant embryos (Furuta and Hogan, 1998; Winnier et al., 1995). Phenotypes of Bmp4 heterozygous mutants are also affected by genetic background. In a C57BL6 background, small proportions of heterozygous mutant mice show minor abnormalities including an extra digit and circling behavior (Dunn et al., 1997). We previously noticed that Bmp2 heterozygous mutants in a C57BL6 background showed closure defects in the cephalic neural tube more frequently than heterozygous mutants in a mixed background of 129SvEv and C57BL6 (Castranio and Mishina, 2008). These facts suggest that the genetic background influences the severity of the phenotypes. To address this issue, we generated compound heterozygous mutants in a 129SvEv background and in a C57BL6 congenic background. For the latter case, we back-crossed heterozygous mice to C57BL6 more than seven times (expected ratio of C57 is over 99%). In mid-to-late gestation, a ratio of the compound heterozygotes was the same as expected (approximately 25%) (Table 3). A total of 307 pups in a 129SvEv background were genotyped at 3 weeks after birth and an under representation of compound heterozygotes were observed (Table 3). Like in mixed background, compound heterozygotes were smaller than wild type or heterozygotes for each gene [Fig. 1, the relative body weight of the survived compound heterozygotes to wild type was 53% (male) or 62% (female)]. In contrast, at 3 weeks none of the over 80 pups in a C57BL6 background were compound heterozygotes, suggesting that they died during the perinatal period due to severe defects caused by the genetic background (Table 3). These results also may suggest that genetic modifiers in the C57BL6 background may account, at least in part, for the variable phenotypes of compound heterozygous mutants found in the mixed genetic background.
Table 3.
Ratio of Genotyping of Offspring in Different Genetic Background
| Strain | Stage | Wild type (%) | Bmp2 het (%) | Bmp4 het (%) | Compound het (%) | Total |
|---|---|---|---|---|---|---|
| Mixture | E15.5 | 26 (25.0) | 27 (26.0) | 28 (26.9) | 23 (22.1) | 104 |
| 129SvEv | E15–16 | 12 (25.1) | 16 (28.5) | 14 (25.0) | 14 (25.0) | 56 |
| C57BL6 | E15–16 | 24 (40.0) | 13 (21.7) | 13 (21.7) | 10 (16.7) | 60 |
| Mixture | 3 wk | 129 (39.8) | 80 (24.7) | 86 (26.5) | 29 (9.0) | 324 |
| 129SvEv | 3 wk | 98 (31.9) | 92 (30.0) | 99 (32.2) | 18 (5.9) | 307 |
| C57BL6 | 3 wk | 36 (42.4) | 25 (29.4) | 24 (28.2) | 0 (0.0) | 85 |
Results from E15.5 were shown for mixed background. Results from E15.5 and E16.5 were combined for 129SvEv and C57BL6.
DISCUSSION
Dosage of BMP Signaling and Related Phenotype
We used a genetic approach to partially reduce the levels of BMP signaling and found that a proper dosage of BMP2 and BMP4 is critical for several aspects of normal development including functional heart formation. Most of the compound heterozygous individuals that died during the perinatal period showed ventricular septal defects, which might affect the initiation and continuation of breathing. Several reports using conditional and hypomorphic alleles of BMP ligands, receptors, and signaling molecules have demonstrated that BMPs play critical roles during heart development, including septation in the outflow tracts, proliferation of the heart wall, and EMT in the atrialventricular cushion (Delot et al., 2003; Gaussin et al., 2002, 2005, Jiao et al., 2003; Kaartinen et al., 2004; Kim et al., 2001; Liu et al., 2004; Ma et al., 2005; Nie et al., 2008; Park et al., 2006; Rivera-Feliciano and Tabin, 2006; Stottmann et al., 2004; Stroud et al., 2007; Wang et al., 2005). Histological observations of the compound heterozygous mutants described here suggest that proper formation of the interventricular septum may be the most sensitive process affected by the reduced levels of BMP signaling. Bmp2 is highly expressed in the atrioventricular canal myocardium between E11.5 and E14.5, and also expressed in endocardial cushion from E12.5 (Abdelwahid et al., 2001). Bmp4 is highly expressed in outflow tract myocardium and endocardial cushion (Abdelwahid et al., 2001). Bmpr1a is expressed nearly ubiquitously in the developing stage of the heart whereas Bmpr1b expression in the heart is very low (Dewulf et al., 1995). These expression patterns suggest that BMP2 and BMP4 produced in endocardial cushion signals through BMPRIA to play a role for fusion of interventricular septum to endocardial cushion. These facts also suggest that BMP2 and BMP4 play roles for morphological integrity of the tricuspid and mitral valves that are developed from atrioventricular canal.
A closure defect in the posterior ventral body wall is another dosage sensitive process. It has a nearly identical phenotype of that found in the conditional mutant embryos of Bmpr1a induced by Dermo1-Cre (Sun et al., 2007). Double homozygous mutations of Msx1 and Msx2 show a similar omphalecele phenotype (Ogi et al., 2005) suggesting that during mid-gestation, both BMP2 and BMP4 work together to maintain a sufficient level of BMP signaling through BMPRIA to regulate levels of Msx1 and Msx2. Some of the compound heterozygous embryos for Bmp2 and Bmp4 developed hypomorphic eyes, which would be a similar phenotype to that caused by a retina-specific disruption of Bmpr1a in Bmpr1b null background (Murali et al., 2005). In this case, proliferation of the cells in the retinal neuroectoderm is drastically reduced at E11.5. Interestingly, no overt phenotype is observed if Bmpr1b allele is wild type (Murali et al., 2005). These suggest that BMP2 and BMP4 work together to send signaling through both BMPRIA and BMPRIB for proper retinal development during mid-gestation.
Some of the Bmp2 heterozygous mutant embryos develop closure defects in the cephalic neural tube leading to perinatal death (Castranio and Mishina, 2008). Interestingly, the ratio of heterozygous mutant embryos demonstrating the neural tube defects is altered depending on their genetic background. We recently generated a hypomorphic allele for Bmp2 by knocking in a polII-neo cassette, of which expression level was 10% compared with a wild-type allele, and embryos having the hypomorphic allele over the null allele showed higher frequency of the neural tube defects (Singh et al., 2008). However, as described in the Result section, the frequency of the neural tube defects in the compound heterozygous embryos for Bmp2 and Bmp4 was not increased compared with that in Bmp2 heterozygous embryos. Although small proportions of Bmp4 heterozygous mutant mice show abnormalities, no perinatal death is reported in both inbred (congenic) and mixed background (Dunn et al., 1997) (unpublished observation). We have dissected over 500 heterozygous embryos for Bmp4 in various genetic backgrounds but never found neural tube closure defects in this genotype (data not shown). Together, the data suggest that dosage of BMP2, but not BMP4, would be of primary importance for development of neural tube closure defects in the cephalic region. Generation of a hypomorphic allele for Bmp4, by introducing a mutation in the cleavage site for maturation is previously reported (Goldman et al., 2006). It would be an interesting future experiment to further reduce the amount of BMP2 and BMP4 by combining these hypomorphic alleles to uncover relatively less sensitive biological processes, which may include limb bud formation and subsequent digit specification.
Potential Interactions of BMP Ligands for Their Functions
Several reports demonstrate that compound homozygous mutants reveal overlapping functions of BMP ligands. For example, double homozygous null embryos for Bmp5 and Bmp7 die by E10.5 even though the impact of the loss of one of these genes for proper embryogenesis is negligible (Solloway and Robertson, 1999). Double homozygous null embryos for Bmp6 and Bmp7 show similar defects in interventricular septation as we have shown in this article (Kim et al., 2001). These results suggest that some of the BMPs share redundant functions especially in the tissues where they are co-expressed. Compound heterozygous mutant mice for Bmp4 and Bmp7 showed an abnormal skeletal phenotype, which also suggests that dosage of BMP signaling may play a role in the morphology of bones (Katagiri et al., 1998).
It is reported that heterodimer molecules for BMPs may have functions distinct from their homodimers. In frog, purified BMP4/7 heterodimer has a potent mesoderm inductive ability compared with a mixture of BMP4 homodimer and BMP7 homodimer (Suzuki et al., 1997). In cell culture and in vivo bone defect models, BMP2/7 heterodimer shows a more potent ability for induction of alkaline phosphatase and differentiation toward osteoblast lineages (Koh et al., 2008; Tsuji et al., 1998; Zhu et al., 2006). In Drosophila, Screw-Dpp heterodimers shows potent activity for specification of dorsal tissues (Shimmi et al., 2005). Although these are the examples of heterodimers between the Dpp subgroup (BMP2, BMP4) and the 60A subgroup (BMP5, BMP6, BMP7), these facts suggest BMP2/4 heterodimer may also have unique functions. It is an alternative possibility that an alteration of the amount of heterodimers for BMP2and BMP4 may be a cause of the abnormalities reported here. A knock-in experiment of Bmp2 gene into the Bmp4 locus (or vice versa) may shed light on this possibility.
BMP Dose and Heart Functions
Most of the surviving compound heterozygous mutants can live for over a year, but the majority are smaller in body size (weight and statue). One of the interesting possibilities for smaller body size would be insufficient circulation. Since wall thickness of heart chambers in the compound heterozygous mutants are seemingly thinner than that of control at neonatal stage (see Fig. 5), it is possible to speculate that ejection volume in the mutants could be smaller. An alternative possibility is that insufficient circulation is caused by the abnormal morphology of valve leaflets, as they age (see Fig. 6). Atrioventricular cushion-specific disruption of Bmpr1a causes defects in leaflet formation in both mistral and tricaspid valves, which in turn leads to higher blood pressure in the atrium (Gaussin et al., 2005; Stroud et al., 2007). Thus, we speculate that reduction of BMP2 and/or BMP4 signaling through BMPRIA in the valves would be one of the causes of morphological abnormalities found in human cases. It is reported that cell proliferation in heart valves stops 1 week after birth (Kruithof et al., 2007). There is no overt difference in proliferative ability of cells in valves at E18.5 and 1 week after birth (data not shown), suggesting BMP signaling may be important for morphological changes of the cells in the valves during postnatal development. It will be of interest in the future to assess functionality of the heart of survived compound heterozygotes because it is expected that abnormal valve structure may interfere with blood flow from atriums to ventricles.
MATERIALS AND METHODS
Mouse
Generation and characterization of mutant mouse strains were described elsewhere (Winnier et al., 1995; Zhang and Bradley, 1996). All mouse experiments were performed in accordance with NIEHS/NIH guidelines covering the humane care and use of animals in research.
PCR Genotyping
Mouse genotypes were initially determined by Southern blot described elsewhere (Winnier et al., 1995; Zhang and Bradley, 1996). Subsequently, each allele was identified by PCR using the following primers. For Bmp2, primer 1, 5′-AGCATGAACCCTCATGTGTTGG-3′; primer 2, 5′-GTGACATTAGGCTGCTGTAGCA-3′; primer 3, 5′-GAGACTAGTGAGACGTGCTACT-3′. For Bmp4, primer 4, 5′-TTGATCTTTCGGACCTGTTTTA-3′; primer 5, 5′-ACGACCATCAGCATTCGG-3′; primer 6, 5′-GAATTCGCCAATGACAAGAC-3′.
Histology
Embryos were fixed in 4% paraformaldehyde, embedded in paraffin, and stained with Hematoxylin and Eosin (H&E). Some sections from adult heart were stained with Masson’s trichrome to visualize collagen fibers.
Immunohistochemistry
Immunohistochemistry was performed by standard procedures. Briefly, 6 μm paraffin sections were subjected to antigen retrieval (10 mM citrate buffer, pH 6.0 with microwave treatment for 10 minutes) and subsequently incubated with anti-SOX9 (H-90, Santa Cruz), anti-MSX2 (M1069, Sigma), anti-SP-C (AB3428, Millipore), or anti-CC10 (T-18, Santa Cruz) with dilution of 1/10-1/100 at 4°C for overnight. Either HRP conjugated or Alexa fluor-488 labeled secondary antibodies were used for detection.
Supplementary Material
Acknowledgments
Contract grant sponsor: Intramural Research Program of the NIEHS/NIH; Contract grant sponsor: RIKEN Brain Science Institute (to Y.M.) Published online 23 April 2009 in Wiley InterScience (www.interscience.wiley.com).
The authors gratefully thank Drs. Hongbing Zhang and Allan Bradley for Bmp2 mice; Drs. Ray Dunn, Glenn Winnier, and Brigid Hogan for Bmp4 mice; Lee E. Davis, Ijemoma Nwosu, and Toni Ward for excellent technical support on experiments; Yas Furuta, Manas Ray, Shyamal Peddada and Greg Scott for valuable comments on the manuscript; Tonya Simmons for mouse husbandry; and Drs. Devorah Goldman and Jan Christian for exchanging unpublished results.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Abdelwahid E, Rice D, Pelliniemi LJ, Jokinen E. Overlapping and differential localization of Bmp-2. Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001;305:67–78. doi: 10.1007/s004410100399. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Bachiller D, Klingensmith J, Kemp C, Belo JA, Anderson RM, May SR, McMahon JA, McMahon AP, Harland RM, Rossant J, De Robertis EM. The organizer factors Chordin and Noggin are required for mouse forebrain development. Nature. 2000;403:658–661. doi: 10.1038/35001072. [DOI] [PubMed] [Google Scholar]
- Balemans W, Van Hul W. Extracellular regulation of BMP signaling in vertebrates: A cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Brugger SM, Merrill AE, Torres-Vazquez J, Wu N, Ting MC, Cho JY, Dobias SL, Yi SE, Lyons K, Bell JR, Arora K, Warrior R, Maxson R. A phylogenetically conserved cis-regulatory module in the Msx2 promoter is sufficient for BMP-dependent transcription in murine and Drosophila embryos. Development. 2004;131:5153–5165. doi: 10.1242/dev.01390. [DOI] [PubMed] [Google Scholar]
- Castranio T, Mishina Y. BMP2 is required for cephalic neural tube closure in the mouse. Dev Dyn. 2008;238:110–122. doi: 10.1002/dvdy.21829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia AC, Costa M, Moraes F, Bom J, Novoa A, Mallo M. Bmp2 is required for migration but not for induction of neural crest cells in the mouse. Dev Dyn. 2007;236:2493–2501. doi: 10.1002/dvdy.21256. [DOI] [PubMed] [Google Scholar]
- de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Reedy M, Oliver T, Mishina Y, Hogan BL. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev Biol. 2006;291:67–82. doi: 10.1016/j.ydbio.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Hogan BL. BMP4 is essential for lens induction in the mouse embryo. Genes Dev. 1998;12:3764–3775. doi: 10.1101/gad.12.23.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JB, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97:219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci USA. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman DC, Hackenmiller R, Nakayama T, Sopory S, Wong C, Kulessa H, Christian JL. Mutation of an upstream cleavage site in the BMP4 prodomain leads to tissue-specific loss of activity. Development. 2006;133:1933–1942. doi: 10.1242/dev.02368. [DOI] [PubMed] [Google Scholar]
- Gu Z, Reynolds EM, Song J, Lei H, Feijen A, Yu L, He W, MacLaughlin DT, van den Eijndenvan Raaij J, Donahoe PK, Li E. The type I serine/threonine kinase receptor ActRIA (ALK2) is required for gastrulation of the mouse embryo. Development. 1999;126:2551–2561. doi: 10.1242/dev.126.11.2551. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Boorla S, Frendo JL, Hogan BL, Karsenty G. Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet. 1998;22:340–348. doi: 10.1002/(SICI)1520-6408(1998)22:4<340::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev Biol. 2001;235:449–466. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Koh JT, Zhao Z, Wang Z, Lewis IS, Krebsbach PH, Franceschi RT. Combinatorial gene therapy with BMP2/7 enhances cranial bone regeneration. J Dent Res. 2008;87:845–849. doi: 10.1177/154405910808700906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Scott G, Nagy A, Kaartinen V, Mishina Y. BMP type I receptor ALK2 is essential for proper patterning at late gastrulation during mouse embryogenesis. Dev Dyn. 2007;236:512–517. doi: 10.1002/dvdy.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof BP, Krawitz SA, Gaussin V. Atrioventricular valve development during late embryonic and postnatal stages involves condensation and extracellular matrix remodeling. Dev Biol. 2007;302:208–217. doi: 10.1016/j.ydbio.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchialarch artery remodeling. Proc Natl Acad Sci USA. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Mishina Y. Function of bone morphogenetic protein signaling during mouse development. Front Biosci. 2003;8:d855–869. doi: 10.2741/1097. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Crombie R, Bradley A, Behringer RR. Multiple roles for activin-like kinase-2 signaling during mouse embryogenesis. Dev Biol. 1999;213:314–326. doi: 10.1006/dbio.1999.9378. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Murali D, Yoshikawa S, Corrigan RR, Plas DJ, Crair MC, Oliver G, Lyons KM, Mishina Y, Furuta Y. Distinct developmental programs require different levels of Bmp signaling during mouse retinal development. Development. 2005;132:913–923. doi: 10.1242/dev.01673. [DOI] [PubMed] [Google Scholar]
- Nie X, Deng CX, Wang Q, Jiao K. Disruption of Smad4 in neural crest cells leads to mid-gestation death with pharyngeal arch, craniofacial and cardiac defects. Dev Biol. 2008;316:417–430. doi: 10.1016/j.ydbio.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogi H, Suzuki K, Ogino Y, Kamimura M, Miyado M, Ying X, Zhang Z, Shinohara M, Chen Y, Yamada G. Ventral abdominal wall dysmorphogenesis of Msx1/Msx2 double-mutant mice. Anat Rec A Discov Mol Cell Evol Biol. 2005;284:424–430. doi: 10.1002/ar.a.20180. [DOI] [PubMed] [Google Scholar]
- Park C, Lavine K, Mishina Y, Deng CX, Ornitz DM, Choi K. Bone morphogenetic protein receptor 1A signaling is dispensable for hematopoietic development but essential for vessel and atrioventricular endocardial cushion formation. Development. 2006;133:3473–3484. doi: 10.1242/dev.02499. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol. 2006;295:580–588. doi: 10.1016/j.ydbio.2006.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmi O, Umulis D, Othmer H, O’Connor MB. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell. 2005;120:873–886. doi: 10.1016/j.cell.2005.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP, Castranio T, Scott G, Guo D, Harris MA, Ray M, Harris SE, Mishina Y. Influences of reduced expression of maternal bone morphogenetic protein 2 on mouse embryonic development. Sex Dev. 2008;2:134–141. doi: 10.1159/000143431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development. 1999;126:1753–1768. doi: 10.1242/dev.126.8.1753. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–2218. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud DM, Gaussin V, Burch JB, Yu C, Mishina Y, Schneider MD, Fishman GI, Morley GE. Abnormal conduction and morphology in the atrioventricular node of mice with atrioventricular canal targeted deletion of Alk3/Bmpr1a receptor. Circulation. 2007;116:2535–2543. doi: 10.1161/CIRCULATIONAHA.107.696583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Liu YH, Chen H, Nguyen MP, Mishina Y, Upperman JS, Ford HR, Shi W. Deficient Alk3-mediated BMP signaling causes prenatal omphalocele-like defect. Biochem Biophys Res Commun. 2007;360:238–243. doi: 10.1016/j.bbrc.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Kaneko E, Maeda J, Ueno N. Mesoderm induction by BMP-4 and -7 heterodimers. Biochem Biophys Res Commun. 1997;232:153–156. doi: 10.1006/bbrc.1997.6219. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Ito Y, Noda M. Expression of the PEBP2alphaA/AML3/CBFA1 gene is regulated by BMP4/7 heterodimer and its overexpression suppresses type I collagen and osteocalcin gene expression in osteoblastic and nonosteoblastic mesenchymal cells. Bone. 1998;22:87–92. doi: 10.1016/s8756-3282(97)00267-6. [DOI] [PubMed] [Google Scholar]
- Wang J, Sridurongrit S, Dudas M, Thomas P, Nagy A, Schneider MD, Epstein JA, Kaartinen V. Atrioventricular cushion transformation is mediated by ALK2 in the developing mouse heart. Dev Biol. 2005;286:299–310. doi: 10.1016/j.ydbio.2005.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yanagita M. BMP antagonists: their roles in development and involvement in pathophysiology. Cytokine Growth Factor Rev. 2005;16:309–317. doi: 10.1016/j.cytogfr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Ying Y, Qi X, Zhao GQ. Induction of primordial germ cells from murine epiblasts by synergistic action of BMP4 and BMP8B signaling pathways. Proc Natl Acad Sci USA. 2001;98:7858–7862. doi: 10.1073/pnas.151242798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Zhao GQ. Cooperation of endoderm-derived BMP2 and extraembryonic ectoderm-derived BMP4 in primordial germ cell generation in the mouse. Dev Biol. 2001;232:484–492. doi: 10.1006/dbio.2001.0173. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
- Zhu W, Kim J, Cheng C, Rawlins BA, Boachie-Adjei O, Crystal RG, Hidaka C. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone. 2006;39:61–71. doi: 10.1016/j.bone.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.