Abstract
GABAA receptors (GABAARs) are the main inhibitory neurotransmitter receptors and have long been implicated in mediating at least part of the acute actions of ethanol. For example, ethanol and GABAergic drugs including barbiturates and benzodiazepines share many pharmacological properties. Besides the prototypical synaptic GABAAR subtypes, nonsynaptic GABAARs have recently emerged as important regulators of neuronal excitability. While high doses (≥100 mM) of ethanol have been reported to enhance activity of most GABAAR subtypes, most abundant synaptic GABAARs are essentially insensitive to ethanol concentrations that occur during social ethanol consumption (<30 mM). However, extrasynaptic δ and β3 subunit-containing GABAARs, associated in the brain with α4or α6 subunits, are sensitive to low millimolar ethanol concentrations, as produced by drinking half a glass of wine. Additionally, we found that a mutation in the cerebellar α6 subunit (α6R100Q), initially reported in rats selectively bred for increased alcohol sensitivity, is sufficient to produce increased alcohol-induced motor impairment and further increases of alcohol sensitivity in recombinant α6β3δ receptors. Furthermore, the behavioral alcohol antagonist Ro15-4513 blocks the low dose alcohol enhancement on α4/6/β3δ receptors, without reducing GABA-induced currents. In binding assays α4β3δ GABAARs bind [3H] Ro15-4513 with high affinity, and this binding is inhibited, in an apparently competitive fashion, by low ethanol concentrations, as well as analogs of Ro15-4513 that are active to antagonize ethanol or Ro15-4513’s block of ethanol. We conclude that most low to moderate dose alcohol effects are mediated by alcohol actions on alcohol/Ro15-4513 binding sites on GABAAR subtypes.
Keywords: Alcohol, GABAA receptor, Tonic current, Ion channel, Benzodiazepine, Barbiturate, Anesthetics
1. Introduction: attempts to identify the receptors for low dose alcohol action
Ethanol (EtOH) is by far the most widely used and abused drug and EtOH-related accidents and other problems caused by alcohol abuse and addiction cause tremendous human suffering. On the other hand, there is increasing evidence that controlled low to moderate EtOH consumption has a number of long-term positive health implications. These potential beneficial alcohol effects include lower rates of myocardial infarction, reduced heart failure rate, lower risk for dementia, decreased risk of diabetes and reduced risk of osteoporosis (Pinder & Sandler, 2004; Standridge et al., 2004). Despite these important implications for human health and well-being, and despite a large effort of biomedical research dedicated to alcohol-related topics, our understanding of the molecular mechanisms of ethanol effects remains unclear (best illustrated by recent reviews: Criswell & Breese, 2005; Siggins et al., 2005; Weiner & Valenzuela, in press).
The view that EtOH and anesthetics act via modulation of fluidity of the lipid membrane of nerve cells with little or no specificity (Franks & Lieb, 1981; Suzdak et al., 1986a) has shifted to a protein/receptor based target with much emphasis on the GABA-mimetic actions of alcohol (Criswell & Breese, 2005) and direct actions of ethanol on GABAA receptors (GABAARs) (Korpi et al., 1998; Aguayo et al., 2002).
Because alcohol affects the brain only at grotesquely enormous concentrations (mM) compared to other drugs which usually act at much lower concentrations (nM–μM range) and due the inability to find receptors that respond to alcohol doses experienced during low to moderate drinking (around 1–20 mM blood alcohol level: the legal blood alcohol driving limit in most US states is 17.4 mM), it was assumed that effects of EtOH are caused by a summation of effects on a number of different receptors that are affected at doses much higher than those needed for light intoxication (e.g., after drinking half a glass of wine). Such high dose effects of alcohol have been studied extensively on a number of membrane proteins (and also soluble proteins like luciferase; Ueda & Suzuki, 1998) in recombinant systems and with electrophysiological studies in neurons and brain slices (for recent reviews, see Criswell & Breese, 2005; Siggins et al., 2005; Weiner & Valenzuela, in press).
It is not surprising that at doses between 30 mM and 300 mM, EtOH leads to measurable alterations in the functional properties of many proteins, including EtOH-induced changes in membrane properties that might indirectly alter the function of membrane receptors and channels. Furthermore, it is a leap to imply from the observation that EtOH affects some measurable parameter in an in vitro preparation and that these receptors must be important for in vivo EtOH effects. This is particularly true in many cases where anesthetic concentrations (≥50 mM) or even doses that are fatal in most humans (≥ 100 mM) were studied. Furthermore, in many studies of alcohol effects (in isolated neurons, brain slices, and in vivo in animals), the target proteins (alcohol receptors) that cause these phenomena are usually unknown; and because these systems are complex, data tend to be difficult to interpret.
Several systems have been proposed as final common pathways for alcohol effects that lead to alcohol intoxication and the hedonistic effects that may lead to alcohol addiction, but these must be triggered by the initial action at the actual (often unknown) alcohol target and so will not be considered further. These systems might include increased GABA release from nerve endings in appropriate circuits (Siggins et al., 2005) and G-protein coupled receptors pathways, in particular adenosine, β-adrenergic and GABAB receptors that have all been proposed to lead to changes in alcohol sensitivity (Proctor & Dunwiddie, 1984; Allan et al., 1991; Lee et al., 1995; Dar & Meng, 2004). The downstream effectors of these G-protein-coupled receptors could be related in function to effects on a common reward circuit that is activated by all drugs of abuse which seems to involve the cyclic AMP-mediated signaling pathway, in particular cAMP-activated protein kinase (Diamond & Gordon, 1997; Yao et al., 2003; Newton & Messing, 2006) and potential downstream effectors like CREB (Pandey et al., 2005) and DARPP-32 (Svenningsson et al., 2005).
Receptors and channels that have been shown to respond directly to alcohol include ionotropic glutamate receptors (GluR), potassium channels, calcium channels, adenosine receptors, as well as several members of the cys-loop superfamily of ligand-gated ion channels. One must ask if these candidate targets can qualify as relevant receptors for alcohol action by showing modulation in vitro in recombinant expression systems and in cells at concentrations achieved in brain after 1–2 glasses of wine (≪30 mM). Of course, many possible explanations exist for failure to show in vitro effects, such as lack of necessary accessory gene products in recombinant expression systems, for example, trafficking factors, scaffolding proteins, kinases (phosphorylation state), small molecule cofactors, etc. Plus, not all subtypes of these potential target proteins have been studied carefully.
Potassium channels represent legitimate candidates for alcohol receptors. The BK (big, Ca2+-dependent) K+ channels are enhanced by high concentrations of EtOH. In nematodes (C. elegans), alcohol is toxic and impairs locomotion; worms carrying a non-functional BK channel gene become resistant to this EtOH action, which occurs at a medium concentration between 100 mM and 500 mM, but an internal concentration claimed to be 20–40 mM (Davies et al., 2003). The G protein-coupled inwardly rectifying K+ channels, GIRK (Kobayashi et al., 1999; Lewohl et al., 1999), are enhanced by >10 mM EtOH. GIRK channel knock-out mice were shown to have diminished analgesic actions of EtOH (Blednov et al., 2003).
Ionotropic GluR, both NMDA (Lovinger et al., 1989) and non-NMDA (Woodward, 2000; Carta et al., 2003), have been reported to be inhibited by EtOH at relevant low concentrations. The partial NMDA receptor antagonism observed with ethanol in vitro is consistent with the action of the anesthetic ketamine on NMDA receptors at relevant anesthetic concentrations (Liu et al., 2006). However, the significance of NMDA and other GluR in mediating low dose EtOH effects remains to be established, particularly given that ethanol and ketamine have very different psychopharmacological actions (Morgan et al., 2004).
Virtually all the cys-loop ligand-gated ion channel receptors (LGIC) are modulated by ≥100 mM EtOH. These include receptors for serotonin, 5HT3 (enhanced (Lovinger & White, 1991), glycine (enhanced; Mihic et al., 1997; Lei et al., 2000; Aguayo et al., 2002; Roberto et al., 2003), nicotinic acetylcholine (nACh, inhibited; Forman & Miller, 1989) and GABAA (enhanced; Grobin et al., 1998; Aguayo et al., 2002). Because reconstituted recombinant receptors might not show the same alcohol sensitivity as native receptors in native neurons (due to, e.g., the lack of accessory proteins), it remains possible that these channels might mediate acute low dose EtOH effects. It is also possible that the cys-loop receptor family of ion channel proteins (which includes GABAA, glycine and nACh receptors) share common structural features, such as the water-filled pocket proposed for the low affinity alcohol/anesthetic sites at the extracellular end of the transmembrane domain (Yamakura et al., 2001), within one subunit or between subunits. Such a pocket could allosterically modulate channel kinetics, but due to the low energy involved in drug binding in this pocket, there is low potency and limited specificity for the modulators. This could be involved in the action of anesthetic levels (≥100 mM) but would not explain the action of ‘one glass of wine’ levels of alcohol.
While GABAARs have long been implicated in mediating alcohol actions in behavioral studies, the lack of direct alcohol effects at relevant low doses in many studies on synaptic GABAARs led to the view that alcohol must somehow indirectly increase GABAAR function in a GABA-mimetic manner, possibly involving increased GABA release (Breese et al., 2006), including mediatory and modulatory actions of neuroactive steroids (Morrow et al., 2001). However, we found in recombinant expression studies that some GABAARs possibly could account for the ‘one glass of wine’ receptor: only certain subtypes of GABAARs, those containing the β3 and δ subunits, were found to respond to EtOH with a threshold response of 3 mM when expressed in oocytes (Wallner et al., 2003). These δ subunit-containing GABAAR subtypes are present in the brain and show an extrasynaptic location. These GABAARs contribute to a persistent or tonic GABAergic inhibitory influence on neurons (Brickley et al., 1996) whose importance in setting the “overall” neuronal excitability has only fairly recently been recognized (Farrant & Nusser, 2005).
While it has been widely assumed that some GABAAR that mediate GABAergic synaptic transmission are sensitive to EtOH enhancement, this review focuses on the increasing evidence that the direct enhancement of nonsynaptic δ subunit-containing GABAAR may explain many of the acute low dose alcohol effects in our brain. The EtOH relevance has probably gone unnoticed because these types of receptors have not been much studied and are not very abundant. The development of the awareness of the physiological importance of these extrasynaptic receptors has coincided with the findings of their unique pharmacological properties, in particular their unique sensitivity to modulatory drugs, possibly due to a low GABA efficacy (despite high potency) (Bianchi & Macdonald, 2003; Wallner et al., 2003; Feng et al., 2004).
2. GABAA receptors as alcohol targets
2.1. Early pharmacological evidence for the involvement of GABAA receptors in alcohol actions
GABAARs have long been implicated in the intoxicating actions of alcohol because positive modulators of GABAARs such as barbiturates show striking similarities to alcohol in their effects on human behavior (Isbell et al., 1950). In addition, it was demonstrated that the GABAAR agonists (e.g., muscimol) potentiated the sedative properties of EtOH, while the opposite effect, a reduction of EtOH-produced sedation or motor-incoordinating effects, was seen upon administration of the GABAAR blocking agents picrotoxin and bicuculline (Hakkinen & Kulonen, 1976; Frye & Breese, 1982; Liljequist & Engel, 1982; Martz et al., 1983). Picrotoxin and bicuculline are not practical clinically as alcohol antagonists, because they lead to life-threatening convulsions. In addition, benzodiazepines also share similarities with EtOH action, and have additive, possibly even synergistic (super additive) effects, when taken together with EtOH (Hu et al., 1987; Tauber et al., 2003). Other GABAergic drugs show cross-tolerance and cross-dependence with EtOH, suggesting similar mechanisms (Khanna et al., 1998). Different strains of laboratory animals show variable sensitivity to EtOH and this correlates somewhat with barbiturates but very well with benzodiazepine sensitivity (Harris & Allan, 1989). This pharmacogenomic connection suggests a common brain pathway, if not receptor, for the actions of these drugs. In vitro studies supporting a GABA-mimetic action of EtOH, direct or indirect, are consistent with this notion (Breese et al., 2006).
Neuropharmacologists, early on, examined EtOH for effects on neurons in anesthetized animals or acute slices, and reported variable effects on nerve firing, excitatory or inhibitory transmission (Durand et al., 1981; Siggins et al., 1987; Deitrich et al., 1989; Lovinger et al., 1989; White et al., 1990; Narahashi et al., 1991; Morrisett & Swartzwelder, 1993). In some cases it was shown that GABA-mediated inhibitory currents in neurons are enhanced by relevant alcohol doses (Aguayo, 1990), or that there was at least a GABA-mimetic effect, which could be due to postsynaptic enhancement or increased presynaptic activity and/or release (Criswell & Breese, 2005; Siggins et al., 2005).
2.2. Low concentration ethanol enhancement on GABAA receptor-mediated 36Cl-flux assays in cell-free synaptoneurosomes and neurons
Using 36Cl− flux assays (Harris & Allan, 1985), a number of groups showed that EtOH at low millimolar concentrations increased Cl− flux in cell-free brain membrane homogenates (Suzdak et al., 1986b; Suzdak & Paul, 1987; Morrow et al., 1988).
What are the properties of these flux assays? The preparations were low-speed pellets, containing at least some synaptoneurosomes: these are pinched-off and resealed presynaptic nerve endings containing organelles and release apparatus, attached to postsynaptic cell membrane fragments in closed vesicular form that are capable of postsynaptic receptor-mediated ion flux. These preparations also include some synaptosomes (pinched off and resealed presynaptic nerve endings in closed vesicular form), which are unlikely to contribute to the flux signal through synaptic receptors because these receptors, in adhering fragments of postsynaptic membrane, are not attached to membrane-bound closed vesicular structures (Titulaer & Ghijsen, 1997). Membrane preparations used for 36Cl− flux assays also include nonsynaptic (resealed vesicular) plasma membranes containing extrasynaptic receptors that contribute to the flux signal. This 36Cl− flux is mediated by GABAARs as it can be increased by application of GABA or muscimol, and is inhibited by general GABAAR blockers like bicuculline and picrotoxin.
Particularly interesting is that the behavioral alcohol antagonist Ro15-4513 (a benzodiazepine) specifically blocked ethanol-induced synaptoneurosomal GABA/Cl− current increases (Suzdak et al., 1986a). Importantly, a parallel approach reported EtOH enhancement of GABAAR-mediated 36Cl− flux in intact cultured neuronal cells (Ticku et al., 1986), and this EtOH enhancement was inhibited by the benzodiazepine Ro15-4513 (Mehta & Ticku, 1988). These reports showed that GABAARs were targets of low dose EtOH, demonstrated in 2 different in vitro assays available at that time, despite controversial results from electrophysiology.
These cell-free neurochemical studies using 36Cl− flux assays in synaptoneurosome preparations provided strong evidence that EtOH at low intoxicating doses (3–30 mM) can stimulate Cl− flux through GABAARs. The fact that in some of these studies Cl− flux is detectable and can be stimulated by EtOH even without the addition of exogenous GABA to synaptoneurosomes (Suzdak et al., 1986a), suggests that this flux might be due to highly GABA-sensitive receptors that can respond to low endogenous GABA concentrations present in these preparations (DeLorey & Brown, 1992). In addition, these 36Cl− flux measurements have a fairly slow time resolution when compared to the ultrafast synaptic GABA events, suggesting that these synaptoneurosomal Cl− flux measurements might be preferentially due to the activity of highly ethanol-sensitive (possibly δ subunit-containing) GABAARs. Even without added GABA, synaptoneurosomal preparations may have contained at least 100 nM endogenous GABA, a GABA concentration sufficient to activate δ subunit-containing receptors, whereas the much less alcohol-sensitive γ2-containing receptors are likely to be functionally silent under these conditions because of their much lower GABA sensitivity and fast desensitization. It is perhaps not surprising that the results of such Cl− flux measurements vary, depending on the brain regions they are derived from, the age of the animals used, the exact protocol used to isolate the membrane fractions, the content of synaptoneurosomes, and the amount of concentrations of GABA or GABA agonists like muscimol added to measure EtOH stimulation. Difficulties in the reproducibility of low dose ethanol enhancement in these Cl− flux assays in different laboratories (Uusi-Oukari & Korpi, 1989; Mihic et al., 1992) might have raised skepticism concerning the validity of these data to explain low dose alcohol effects on GABAARs. In addition, synaptic physiologists might have considered the conditions under which 36Cl− flux assays are performed non-physiologically, but they do make sense when considering that continuously active, highly GABA-sensitive GABAARs (like those containing the δ subunit) were measured under these experimental conditions.
2.3. Evidence for low concentration alcohol enhancement of GABAA receptors from electrophysiological recordings from neurons in cultures and slices
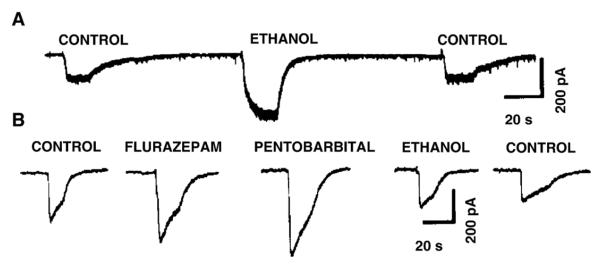
There are reports that showed that under certain conditions GABAARs can be activated by low (<30 mM) relevant EtOH concentrations in isolated and cultured neurons. For example, Aguayo (1990) showed that low concentrations of EtOH can increase the GABA current evoked by 2.5 μM GABA, a minimally desensitizing concentration in cultured hippocampal as well as cerebral cortical neurons. However, this effect was seen only in a fraction (up to 50%) of neurons and it was reported that the increase in EtOH enhancement diminished when the GABA concentration was raised. This is consistent with the notion that the activation of abundant, but EtOH-insensitive, synaptic GABAARs obscures or dilutes the EtOH enhancement when higher GABA concentrations (>3 μM) are applied to these neurons. In addition, the age of the culture could be important, consistent with the finding that GABAAR δ subunits are only expressed at the latter stages of development (Aguayo et al., 2002). Fig. 1 shows GABA current recordings (evoked by application of 2.5 μM GABA) from an EtOH-sensitive and an EtOH-insensitive neuron (reproduced from Aguayo, 1990). Similar low dose alcohol effects on isolated neurons from several brain regions and species were also reported by Reynolds et al. (1992) who showed that low (1–50 mM) EtOH concentrations produced an enhancement of I-GABA, evoked by local low concentration GABA application to the cell soma by brief pressure pulses, in a large fraction of neurons (~50% in rat, mouse and chick cerebral cortex).
Fig. 1.
A fraction of cultured neurons are enhanced by low doses of ethanol. (A) Reversible enhancement of GABA currents, evoked by application of 2.5 μM GABA, in whole cell voltage clamped (holding potential, 50 mV) cerebral cortical neurons by 20 mM ethanol. (B) Shows a neuron with EtOH-insensitive (20 mM) GABA currents that are enhanced by flurazepam (20 μM) and pentobarbital (20 μM). (Figure from Aguayo, 1990; with permission from the author and the publisher.)
Despite the fact that there was considerable variability and not all neurons are responsive to low dose EtOH, the abovementioned findings clearly implicated GABAARs as potential sites for EtOH actions. However, as electrophysiological methods became more sophisticated and researchers gained the ability to record synaptic events of connected neurons in brain slice preparations, most studies showed that individual GABAergic synaptic events are not enhanced by relevant EtOH concentrations, which eventually lead researchers to question the role of postsynaptic GABAAR as direct EtOH targets (Breese et al., 2006; Weiner & Valenzuela, in press). However, given that there are many different synapses, the situation is almost certainly complex; and it has been shown that under certain conditions and in certain locations synaptic GABAARs can be sensitive to low relevant EtOH concentrations (Wan et al., 1996; Weiner et al., 1997; reviewed by Weiner & Valenzuela, in press). Particularly interesting in this context is a recent report that chronic intermittent EtOH treatment leads to a decrease in extrasynaptic GABAAR responsiveness to ETOH, whereas synaptic receptors become more sensitive to EtOH (Liang et al., 2006).
In some brain regions, for example cerebellar Purkinje cells, EtOH at relevant pharmacological doses decreases excitability, which was demonstrated to be related to an increase in GABA-mediated inhibition, leading to reduced firing rates of cerebellar Purkinje cells (Palmer & Hoffer, 1990; Weiner & Valenzuela, in press). A popular candidate of EtOH target was the β-adrenergic signaling pathway, because EtOH enhanced GABAAR-mediated inhibition of cell firing by norepinephrine (Smith et al., 1987). Potentiation by EtOH of inhibitory synapses in brain slices was observed, but only under certain prescribed conditions, conditions which varied from one laboratory to another, such as how the slices were treated or what drugs were utilized (Wan et al., 1996; Weiner et al., 1997; Roberto et al., 2003). Numerous negative reports of EtOH–GABA interactions complicated the picture, and most of the positive effects could be interpreted as indirect.
The inability to observe reproducible low dose EtOH enhancement of GABAAR currents was suggested to involve either (1) a highly subunit-selective GABAARs that varied with cells and therefore assays and investigators; or (2) an indirect effect on GABAARs where the actual EtOH receptor was some other protein that directly or indirectly modulates GABAAR function (Newton & Messing, 2006). The indirect modulation might involve protein phosphorylation, either of GABAAR protein or another protein (Harris et al., 1995). The first possibility was supported by the ‘γ2L subunit’ hypothesis (Wafford et al., 1991), which is now considered unlikely (Sigel et al., 1993; Marszalec et al., 1994; Homanics et al., 1999; Wallner et al., 2003). The second hypothesis was also potentially related to the γ2L splice variant, since it differed from γ2S having added an 8-amino acid insert containing a protein kinase C (PKC) substrate site. Wafford and Whiting showed that the proposed greater ethanol sensitivity of the γ2L subunit was abolished (in α2β2γ2LS343A receptors) by the γ2LS343A mutation that removed this PKC phosphorylation site (Wafford & Whiting, 1992). Others suggested that PKC isoforms (Weiner et al., 1994; Harris et al., 1995; Hodge et al., 1999; Olive & Hodge, 2000; Proctor et al., 2003) modulated EtOH sensitivity of GABAARs, depending on the subtype of PKC (Boehm et al., 2004), but probably by acting on other proteins, possibly related to presynaptic effects on GABA release, or postsynaptic effects on receptor density and/or trafficking.
Nevertheless, as evidence mounted for general anesthetic enhancement of GABAAR function, long chain alcohol anesthetics like n-octanol were among the agents shown to be active (Wick et al., 1998). The potency was related to the chain length (and lipid solubility, cf. Meyer-Overton), that is, octanol was more potent than hexanol, and hexanol was more potent than butanol. It followed that ethanol would also be active, but only at very high doses, like 0.1–1.0 M, and probably is fatal to organisms (Nakahiro et al., 1991; Narahashi et al., 1992). The exact sensitivity could and probably did vary with the subunit composition of the GABAAR, but effects at relevant (≤ 30 mM) ethanol concentrations were highly controversial as they were not found in all neurons studied (Breese et al., 1993). It is interesting that almost all of these workers cited in this section (and many others) at one time or another took an anti-GABAAR view of alcohol acute actions, and later supported such a connection. However, many of these positive observations involved high (≥100 mM) doses. One difference between EtOH and general anesthetics was the lack of readily reproducible effect of EtOH on ligand (e.g., GABA, benzodiazepine, or convulsant) binding in vitro, as opposed to anesthetics, where agents that enhance function (Cl− flux or electrophysiology) also modulate binding (Olsen et al., 1991).
2.4. Tonic GABA currents and recombinant δ subunit-containing receptors as mediators of alcohol and anesthetic effects
Recombinant GABAARs are enhanced by EtOH concentrations ≥100 mM, mediated through a potential EtOH “site” within the membrane-spanning parts of the receptor (Mihic et al., 1997) which is also important for anesthetic effects of etomidate and propofol. However, recombinant GABAARs sensitive to relevant intoxicating concentrations of EtOH (3–30 mM) have been identified only very recently (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), and both of these studies show that the presence of the δ subunit (presumably in place of the γ2 subunit) is necessary for highly EtOH-sensitive receptors. In our hands both δ and β3 subunits are necessary to produce GABAAR subtypes that are sensitive to low millimolar EtOH (Fig. 2A)(Wallner et al., 2003; Hanchar et al., 2004).
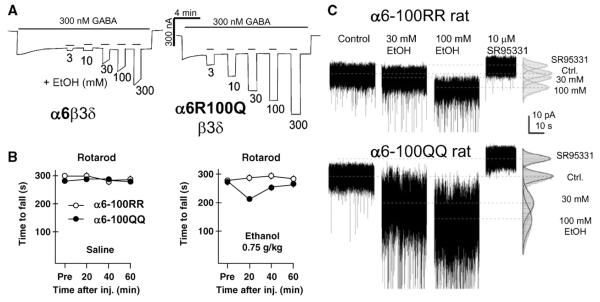
Fig. 2.
A point mutation in the cerebellar α6 subunit leads to increased alcohol sensitivity on (A) recombinant receptors expressed in oocytes, (B) in animals carrying this mutation and (C) in tonic currents from cerebellar granule cells. (A) Currents were recorded from oocytes with either α6β3δ or α6R100Qβ3δ mutant receptors. Ethanol, (concentrations in mM) co-applied with 300 nM GABA, leads to a dose-dependent and reversible increase in holding current. Receptors containing the α6R100Q mutation show a dramatically increased EtOH response. (B) Rats homozygous for either the α6-100R or α6-100Q allele were tested on an accelerating rotarod (4–40 rpm within 5 min) before, and 20, 40 and 60 min after intraperitoneal ethanol (or saline) injection. Under control conditions (saline injection) these groups of rats do not differ. However, after a low dose of EtOH (0.75 g/kg i.p.) where rats carrying the α6-100R allele (n = 7) show no signs of motor impairment, α6-100QQ (n = 8) rats are already significantly impaired. Error bars (SEM) do not, or only slightly, exceed the symbol sizes. (C) Tonic GABA current recorded from cerebellar granules cells in slices from α6-100RR (upper traces) and α6-100QQ (lower traces) rats show that the α6-100Q mutation leads to a dramatic increase in ethanol (30 and 100 mM) enhancement of the tonic current. (Figure reproduced from Hanchar et al., 2005).
δ subunit-containing GABAARs have been shown to be excluded from GABAergic synapses (Nusser et al., 1998; Wei et al., 2003) and are activated by ambient extracellular GABA concentrations (Brickley et al., 1996; Mody, 2001) in the order of 0.3–1 μM (DeLorey & Brown, 1992; Mody, 2001). Recombinant receptors containing the δ subunit and tonic currents mediated by these GABAAR are also highly sensitive to allosteric modulators (Brown et al., 2002; Wohlfarth et al., 2002) including steroids, anesthetics, and EtOH. Recordings of tonic currents in dentate gyrus granule cells (with a likely subunit composition of α4β3δ subunits) and in cerebellar granule cells (α6β3δ) confirm the high neurosteroid and EtOH sensitivity seen with recombinant receptors (Stell et al., 2003; Carta et al., 2004; Wei et al., 2004; Hanchar et al., 2005; Liang et al., 2006).
The failure to see low (relevant concentration, <30 mM) EtOH effects on GABAARs in more recent studies is probably due to the fact that these studies focused on recombinant receptors containing γ2 subunits and the more abundant synaptic GABAARs in slices and cultured neurons, although as discussed above, it remains possible that under certain conditions synaptic GABAAR subtypes are sensitive to relevant EtOH concentrations and might contribute to behavioral EtOH effects.
Besides showing increased GABA sensitivity and slower desensitization, δ subunit-containing GABAARs have another striking feature that distinguishes these receptors from the synaptic γ2-containing receptors. In recombinant systems these receptors (even at saturating GABA concentrations) can be dramatically enhanced by drugs like barbiturates, neurosteroids and etomidate and propofol, suggesting that the open probability of these receptors even at receptors fully occupied by GABA is low: in other words, GABA is only a partial agonist at these receptors (Wohlfarth et al., 2002; Bianchi & Macdonald, 2003; Wallner et al., 2003; Feng et al., 2004). It is tempting to suggest that these anesthetic drugs might act by converting GABA from a partial agonist into a full agonist and it is this action that leads to a dramatic decrease of neuronal activity leading to anesthesia. Fully consistent with this notion is our finding that tonic currents in cerebellar granule cells are highly sensitive to EtOH and therefore must contain the β3 subunit, and that the β3 subunit-containing receptors mediate the in vivo anesthetic effects of etomidate and propofol because a point mutation in the β3 subunit (N265M) that eliminates etomidate and propofol effects on recombinant receptors in knock-in mice essentially eliminates etomidate and propofol anesthetic effects (Jurd et al., 2003). In contrast, a similar mutation in the β2 subunit (β2N265S) only eliminates the sedative “side” effects of etomidate and propofol but not the anesthetic effects (Reynolds et al., 2003). Therefore it is tempting to speculate that the β3 selectivity of these GABA-anesthetic drugs could be due to the fact that in many brain regions δ subunit-containing receptors are associated with the β3 subunit (although there are exceptions, e.g., the thalamus expresses only low amounts of the β3 subunit and the abundant δ likely is paired with α4β2; Sieghart & Sperk, 2002). Mutations at the β3N265M homologous residues in transmembrane region 2 of GABAR subunits have been shown to eliminate the high dose (≥100 mM) “anesthetic” ethanol effects on certain types of GABA receptors (Mihic, 1999). It therefore will be interesting to study if and to what extent existing β3N265M and the β2N265S knock-in mice would differ in their alcohol sensitivity at high and low doses of alcohol.
While many of the details on the molecular mechanisms of how EtOH modulates, how much and at what EtOH concentrations these specialized type of GABAAR receptors contribute to acute alcohol effects, and how tolerance and addiction affect these receptors remain open; the identification of α4/6β3δ subunit-containing receptors together with reports that at the same concentrations EtOH enhances tonic currents suggest that these functionally unique types of GABA receptors are important direct alcohol targets.
2.5. A polymorphism in the cerebellar GABAAR α6(α6R100Q) subunit protein leads to increased alcohol sensitivity in vitro and in vivo
Researchers discovered more evidence for GABAAR involvement in the intoxicating actions of EtOH when selectively outbred rat lines were shown to differ in their motor-impairment response to both benzodiazepines and EtOH (Hellevuo et al., 1989). These rat lines were segregated (by selective breeding over many generations) into 2 groups, EtOH-sensitive (alcohol non-tolerant, ANT) and EtOH-insensitive (alcohol tolerant, AT) (Eriksson, 1990). They were selected in a way that was not based on any differences in alcohol metabolism, largely determined by levels and isoforms of the alcohol dehydrogenase enzymes (for reviews, see Ehrig et al., 1990; Ramchandani et al., 2001). These AT and ANT animals were selected (in about 30 generations) on a tilting plane, a task that tested the motor performance of these animals to rapidly adjust their body posture to resist sliding down on a rough surfaced plane, raised at a constant speed (Eriksson, 1990).
It was reported that the ANT rats lost most of the diazepam-insensitive cerebellar [3H]Ro15-4513 binding, that is, [3H] Ro15-4513 binding in the presence of ≥10 μM cold diazepam (Uusi-Oukari & Korpi, 1990; Uusi-Oukari & Korpi, 1991). The reason for this pharmacological change was shown to be a ‘point mutation’ (polymorphism) in the “benzodiazepine site” α6(R100Q) of the cerebellar GABAAR α6 subunit (Korpi et al., 1993; Korpi & Seeburg, 1993). The α6R100Q point mutation makes α6-containing receptors, when expressed with β2 and γ2 subunits, diazepam-sensitive (wild-type α6β2γ2-containing receptors are insensitive to classical BZ agonists like diazepam), thus providing an explanation not only for the changes in Ro15-4513 pharmacology but also for the increased behavioral benzodiazepine sensitivity of these ANT rats. However, because of the lack of EtOH effects with the α6(R100Q) mutant in recombinant systems (when expressed with β2 and γ2), it was speculated that alterations in genes other than α6 (but cosegregating with it) might be responsible for the high EtOH sensitivity of these animals (Farrant & Cull-Candy, 1993; Korpi et al., 1993).
The same α6(R100Q) polymorphism has also been found enriched in the independently derived “Sardinian alcohol non-preferring sNP” rats (Saba et al., 2001; Sanna et al., 2003), a rat line that was segregated from Sardinian alcohol preferring rats (sP) based on their voluntary alcohol consumption. The identification of the same “mutation” occurring in 2 different rat lines is likely due to the selection of a fairly frequent naturally occurring α6(100Q) allele. The fact that the published rat genomic sequence is the α6-100Q allele is fully consistent with this notion. However, the α6-100Q allele has not been found in certain rat strains, consistent with the observation that rat lines selected from these strains for differences in alcohol preference only carry the α6-100R WT allele (Carr et al., 2003) and most rat and mouse lines selectively bred for differences in alcohol sensitivity also do not show changes in diazepam-insensitive [3H]Ro15-4513 binding in the cerebellum (Uusi-Oukari & Korpi, 1991).
The selective breeding in the ANT/AT animals led to an almost complete separation of WT (α6-100R) and (α6-100Q) alleles, supporting the notion that the α6-100Q allele makes an important contribution to the increased alcohol-induced motor incoordination in ANT rats. In contrast, the α6-100Q allele is only enriched in the alcohol non-preferring animals (Saba et al., 2001), indicating that the α6-100Q allele might contribute to the alcohol non-preference, but its relative importance is lower when compared to other genes, yet to be identified, which contribute to alcohol preference in these animals.
It was found that cerebellar granule cells, the only abundant neuron that expresses the α6 subunit, also has high levels of δ and β3 subunit protein (Pirker et al., 2000) (see Fig. 5). Therefore cerebellar granule cells express all subunits required to make highly alcohol-sensitive receptors in recombinant expression (Wallner et al., 2003). We showed that the α6-R100Q when expressed with β3 and δ subunits led to a further increase of the already high alcohol sensitivity of recombinant α6β3δ receptors (Hanchar et al., 2005), thereby providing the molecular basis of the increased alcohol sensitivity of ANT animals (Fig. 2A). In addition, we found, consistent with the notion that the α6-R100Q allele is frequent in certain rat lines, that in Sprague-Dawley laboratory rats (supplied by Charles Rivers) the α6R100Q “mutation” is a frequently-occurring allele (a naturally occurring knock-in point mutation). We used these animals to show that the α6-100Q allele is sufficient for increased sensitivity to the motor-incoordinating effects of EtOH. The increased EtOH-impairment in α6-100QQ rats is particularly pronounced at the lowest alcohol dose (0.75 g/kg) where only the α6-100Q mutant animals show a decrease in motor performance on the rotarod (Fig. 2B). In addition, we showed that tonic currents in cerebellar granule cells in slices from homozygous α6-100QQ animals show increased EtOH sensitivity relative to α6-100R/R animals (Hanchar et al., 2005) (Fig. 2C).
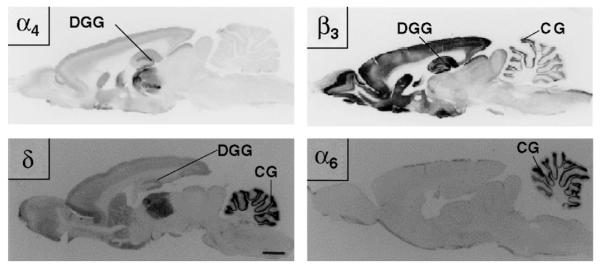
Fig. 5.
Immunolocalization of the GABAAR subunits (α4, α6, β3, δ) that form highly alcohol-sensitive GABAAR subtypes. The α6 subunit is exclusively expressed in the cerebellar granule cells (CG), the most numerous neuron in the brain that also has high levels of β3 and δ subunit protein. Receptors formed by α6βδ subunits are known to mediate tonic currents in cerebellar granule cells and alcohol effects on these receptors make an important contribution to alcohol-induced motor impairment (Hanchar et al., 2005). Receptors formed by α4 and δ subunits are more widespread in the brain and are responsible for tonic currents, for example, in dentate gyrus granule (DGG) neurons in the hippocampus. Like α4β3δ recombinant receptors expressed in oocytes, tonic currents in DGG cells are highly sensitive to ethanol (Wei et al., 2004). Both CG and DGG neurons show high sensitivity to the neuroactive steroid THDOC (Stell et al., 2003). (Figure reproduced from Pirker et al., 2000, with permission from Elsevier.)
One report that tonic GABA currents in cerebellar granule cells can be enhanced by low concentrations of EtOH has been interpreted as being caused by an increase in presynaptic GABA release, because the increase in tonic GABA currents produced by EtOH is accompanied by an increase in firing frequency (Carta et al., 2004). In the Carta et al. study, ethanol-induced increases in tonic GABA current are abolished by the sodium channel blocker tetrodotoxin (TTX) that also blocks all GABA releases (except for rare spontaneous release events). We showed that if GABA is replenished in the slice preparation by adding 300 nM GABA, TTX (1 μM) does not block ethanol enhancement (Hanchar et al., 2005). In addition, α6-100QQ homozygous rats show not only dramatically increased EtOH sensitivity of tonic currents but also show a higher EtOH-induced increase in firing frequency of cerebellar granule cells. Because the α6 subunit is exclusively expressed in cerebellar granule cells, and the changes are associated with the genotype (increased firing frequency in the alcohol hypersensitive α6-100QQ rats), we suggested that alcohol effects on extrasynaptic α6-containing GABAARs cause the increased firing frequency. In other words, the increased firing of Golgi interneurons observed with ethanol is likely due to network properties, involving circuits preserved in the cerebellar slice preparation (Hanchar et al., 2005). The same group that reported that TTX blocked EtOH enhancement of tonic currents in cerebellar granule cells (to support their hypothesis of a presynaptic EtOH effect) also did not find increased EtOH sensitivity of tonic currents using inbred ANT and AT rats, homozygous for the α6-100Q and α6-100R alleles, respectively (Valenzuela et al., 2005; see response by Otis et al., 2005).
While we think that positive results by our group and others demonstrate the importance of tonic GABA receptors in EtOH sensitivity, there have been negative results that are in apparent contradiction to the finding that the α6-100Q allele leads to alcohol hypersensitivity. One of these is the observation that α6 knock-out animals do not show changes in EtOH sensitivity (Homanics et al., 1997b), and the other is that backcrosses of ANT/AT animals did not show a segregation of the EtOH-sensitive phenotype with the mutant α6 alleles (Korpi et al., 1992; Radcliffe et al., 2004). We think that a possible, maybe even likely, explanation is differences in the EtOH doses at which behavioral assays for alcohol intoxications are performed. With the α6-100Q naturally occurring knock-in point mutation, the most dramatic difference was at the lowest alcohol dose of 0.75 g/kg (where only the α6-100QQ animals show alcohol-induced motor impairment, Fig. 3B), no significant differences were detected at doses ≥1.75 g/kg EtOH i.p. This is unexpected because the initial motor-incoordination selection to generate the ANT/AT animals was done at an EtOH dose of 2 g/kg i.p. (Eriksson, 1990). During the 30 generations of selection to generate the AT/ANT lines, other changes might have been selected that allowed the penetrance of the α6-100Q EtOH-supersensitive phenotype. Such additional factors that allowed the penetrance of the α6 phenotype at the 2 g/kg dose might have been lost during backcrossing. Similarly, α6 total knock-out animals have only been tested at fairly high EtOH doses (Homanics et al., 1997b), where no difference in the α6-100Q natural knock-in was seen, and it remains to be determined if a careful study of lower alcohol doses might reveal a slightly diminished alcohol sensitivity in motor-coordination tests in the α6 total knock-outs.
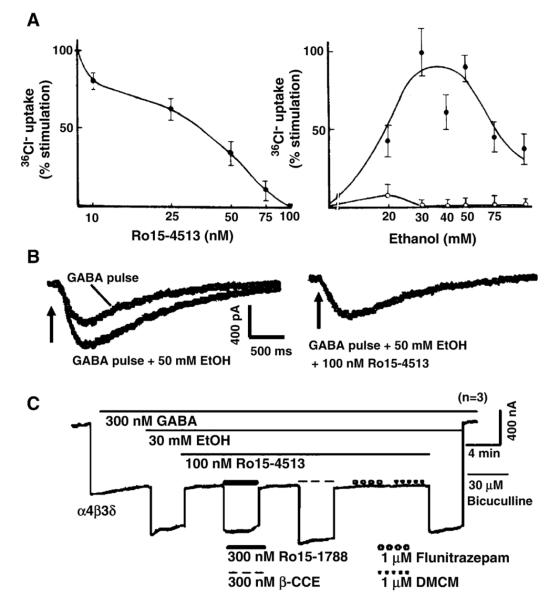
Fig. 3.
Alcohol enhancement of GABAARs by 50 mM (or 30) EtOH in (A)Cl− flux assays, (B) in neurons, and (C) in recombinant α4β3δ receptors is blocked by the behavioral alcohol antagonist Ro15-4513. (A) Left panel shows that the dose-dependent block of 50 mM ethanol enhancement of 36Cl− uptake into rat cortical synaptoneurosomes by Ro15-4513, with complete block at 100 nM. The right panel shows percent stimulation of 36Cl− flux by EtOH alone (●) or in the presence of 100 nM Ro15-4513 (○). (Figure from Suzdak et al., 1986a.) (B) GABA currents were evoked by brief pressure pulses of GABA (25 μM GABA in the pipette) to the soma of a cultured rat cerebral cortical neurons. The resulting GABA current is enhanced by bath application of 50 mM ethanol. In the same cell the EtOH enhancement is blocked by bath co-application 100 nM Ro15-4513 together with 50 mM ethanol (right panel). (Figure redrawn from Reynolds et al., 1992, with permission from Elsevier.) (C) Recombinant α4β3δ GABAAR are enhanced by 30 mM ethanol and this enhancement is blocked by 100 nM Ro15-4514. The ethanol blocking effects are reversed by flumazenil (Ro15-1788) as well as β-CCE (each at 300 nM), but not by the classical benzodiazepine flunitrazepam or the β-carboline DMCM (each at 1 μM). Note that both flumazenil as well as β-CCE have been reported to reverse the alcohol antagonism observed with Ro15-4513 rats. (Figure modified from Wallner et al., 2006.)
In summary, these findings on the α6-R100Q alcohol-supersensitive mutation provide the first evidence that links EtOH-sensitive extrasynaptic δ subunit-containing GABA receptors and tonic GABA currents to the behavioral actions of EtOH.
2.6. A GABAA receptor ligand, the benzodiazepine Ro15-4513, is a specific antagonist of ethanol actions on extrasynaptic GABAA receptors
Because the pharmacological profile of EtOH bears many similarities to that of benzodiazepines and barbiturates (discussed in Section 2.1), Hoffmann-LaRoche scientists routinely tested newly synthesized benzodiazepines for their interaction with EtOH and barbiturates. While most classical BZ agonists enhance EtOH effects (in an additive or maybe even synergistic manner), the imidazobenzodiazepine Ro15-4513 blocked behavioral actions of EtOH without affecting EtOH metabolism or blood EtOH levels, without obvious behavioral effects in rats by itself (up to 10 mg/kg) (Bonetti et al., 1985; Polc, 1985).
The EtOH antagonist effect of Ro15-4513 is stunning: at a dose of 2 g/kg EtOH that leads to severe intoxication with ataxia and heavy sedation in rats, most animals are rescued from any signs of visible intoxication by 3 mg/kg Ro15-4513 (Suzdak et al., 1986a). A number of detailed studies by different groups showed that Ro15-4513 prevented the increased exploration and locomotion at very low EtOH doses (0.25, 0.5 and 0.75 g/kg in rats) (June & Lewis, 1994), the anxiolytic effects at low doses (1 g/kg in rats) (Suzdak et al., 1986a; Glowa et al., 1988; Becker & Hale, 1991) the sedative, motor impairing, as well as amnestic effects at moderate EtOH doses (2 g/kg rats or mice) (Suzdak et al., 1986a; Bonetti et al., 1988; Nabeshima et al., 1988; Dar, 1995), as well as the anticonvulsant (seizure protective) effects of EtOH (2 g/kg) against bicuculline and picrotoxin-induced convulsions (Kulkarni & Ticku, 1989). In addition, the observation that Ro15-4513 reduces EtOH self-administration (June et al., 1991; Rassnick et al., 1993; Petry, 1995) suggests that the rewarding effects of EtOH might be mediated by EtOH/Ro15-4513-sensitive GABAAR. However, Ro15-4513 does not prevent all EtOH effects: at higher EtOH doses (≥2 g/kg in rats), Ro15-4513 significantly reduces, but does not prevent the anesthetic (“sleep”-inducing) effects of EtOH (Marrosu et al., 1989), and Ro15-4513 does not prevent the hypothermic effects of EtOH (Hoffman et al., 1987; Syapin et al., 1987), consistent with the notion that part of the hypothermic (and analgesic) EtOH actions are mediated by other targets, possibly GIRK K+ channels (Blednov et al., 2003; Costa et al., 2005). In addition, it was shown that Ro15-4513 provided only limited protection to lethal effects at massive EtOH doses (Kolata, 1986; Lister & Nutt, 1987; Marrosu et al., 1989). Because of Ro15-4513’s limited ability to reverse lethal EtOH effects and because of the legal implications of having a drug blocking EtOH actions without actually lowering blood EtOH levels, Hoffmann-LaRoche decided not to pursue EtOH antagonist benzodiazepines for commercialization (Kolata, 1986). In addition, there were studies that showed that Ro15-4513 can trigger tremors in squirrel monkeys (Miczek & Weerts, 1987) and that it has proconvulsive properties in rodents, in particular at high doses; these effects are probably mediated by the weak inverse agonist activity on certain types of GABAAR (Lister & Nutt, 1987).
Ro15-4513 is the azido analog of the benzodiazepine receptor antagonist flumazenil (a.k.a. Ro15-1788), now used in the clinic to reverse benzodiazepine effects and overdoses. Despite the structural similarity with Ro15-4513, flumazenil is completely ineffective as an acute EtOH antagonist and when given together with Ro15-4513 prevents EtOH antagonism. The feature of flumazenil to antagonize Ro15-4513’s EtOH antagonistic effects is shared by the BZ-site ligands β-CCE, FG7142 and CGS-8216 (Suzdak et al., 1986a; Glowa et al., 1988). The observation that the more efficacious inverse agonists β-CCE and FG7142 at low doses did not only lead to EtOH antagonism but were shown to prevent the EtOH antagonist action of Ro15-4513 argued against the notion that it is the weak inverse agonist activity of Ro15-4513 on certain types of GABAARs that explains the behavioral EtOH antagonism. However, the inverse agonist action of Ro15-4513 may explain why in some studies Ro15-4513 was reported to diminish barbiturate effects (for review, see Lister & Nutt, 1987), although the barbiturate antagonism was not seen in all studies (Suzdak et al., 1986a). In some behavioral studies FG7142 was also reported to reverse EtOH effects, although only at doses far higher than those required for Ro15-4513 (Lister, 1987; Ticku & Kulkarni, 1988; Kulkarni & Ticku, 1989).
As discussed above (see Sections 2.3 and 2.4), under conditions that favor the detection of highly GABA sensitive receptors, the activity of GABAAR was shown to be enhanced by low EtOH concentrations. Suzdak et al. (1986a) were the first to show that this low dose of EtOH enhancement observed in 36Cl− flux assays in synaptoneurosomes is abolished by the behavioral alcohol antagonist Ro15-4513 in a dose-dependent manner, with a complete block of 50 mM EtOH effects with 100 nM Ro15-4513 (Fig. 3A). Furthermore, it was demonstrated that the low dose ethanol enhancement of GABA currents in neurons, seen under experimental conditions that preferentially activated highly GABA-sensitive tonic currents (discussed above), are reversed by 100 nM Ro15-4513 (Reynolds et al., 1992) (see Fig. 3B). In addition, other reports found that the decrease in spontaneous neuronal firing frequency by relevant doses of EtOH, seen in many neuronal cell types, is reversed by Ro15-4513 (Palmer et al., 1988; Marrosu et al., 1989).
We found that 100 nM Ro15-4513 also blocks in the EtOH augmentation on recombinant α4/6β3δ GABAAR. Fig. 3C shows a representative recording demonstrating that the enhancement of recombinant α4β3δ GABAAR by 30 mM EtOH is blocked by 100 nM Ro15-4513. At this Ro15-4513 concentration, Ro15-4513 has no effect on the GABA-induced current; that is, it specifically blocks the low concentration alcohol enhancement. The alcohol antagonist action of Ro15-4513 on α4β3δ GABAARs is reversed by flumazenil and β-CCE, 2 compounds that have been previously reported to also reverse Ro15-4513’s alcohol antagonism in behavioral studies (Suzdak et al., 1986a; Glowa et al., 1988). Therefore we can mirror the behavioral alcohol antagonist effects of Ro15-4513, including the reversal of Ro15-4513’s alcohol antagonism by flumazenil and β-CCE on recombinant α4/6β3δ subunit-containing receptors (Fig. 3C).
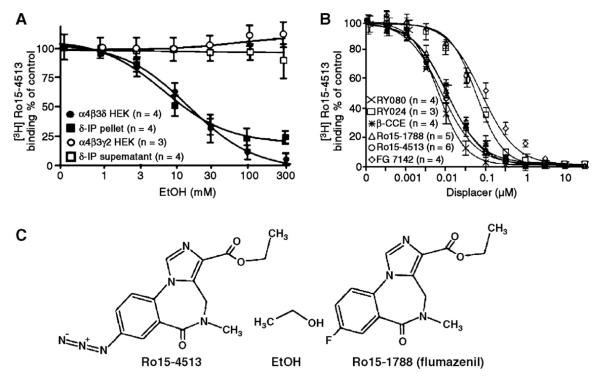
As expected from the alcohol antagonism of Ro15-4513 on δ subunit-containing receptors, we showed that native and recombinant α4/6β3δ GABAAR have a high affinity [3H]Ro15-4513 binding site (Hanchar et al., 2006). [3H]Ro15-4513 (but not [3H]flumazenil) binding to native (immunopurified from cerebellum) and recombinant δ subunit-containing (α4β3δ) GABAAR (not the binding of [3H]Ro15-4513 to the classical BZ site) is inhibited by low millimolar concentrations of EtOH (Fig. 4A) in an apparently competitive manner. It seems likely that the unique azido group of Ro15-4513 (fluorine in flumazenil) is the moiety that competes with EtOH for the same binding pocket. Consistent with this view are 2 recent reports of behavioral EtOH antagonist activity of Ro15-4513/flumazenil congeners named RY024 and RY023, that have a cyano- or an acetylene – instead of an azido – group at the C7 position of the BZ ring (McKay et al., 2004; Cook et al., 2005). In addition, it has been shown that the C7 position in classical benzodiazepines is close to the a critical histidine residue in GABAAR α1,2,3,5 subunits (α1-H101) (Berezhnoy et al., 2004) that is required for sensitivity of GABAAR to classical benzodiazepines in vitro (Wieland et al., 1992; Benson et al., 1998) and in vivo (Rudolph et al., 1999) and photolabeled by [3H]flunitrazepam (Duncalfe et al., 1996). The residue homologous to this histidine is an arginine (R100) in α4 and α6 GABAAR subunits, and as described above in Section 2.5, leads to increased EtOH sensitivity of β3 and δ subunit-containing receptors in vivo and in vitro (Hanchar et al., 2005). It therefore seems likely that the α4/6-100R/Q residue is critical for alcohol sensitivity because it is close to, and might even directly line, the EtOH binding site on these subtypes of GABAARs.
Fig. 4.
Native immuno-purified and recombinant δ subunit-containing receptors contain a high affinity [3H]Ro15-4513 binding site that is sensitive to alcohol displacement. (A) Left panel: [3H]Ro15-4513 binding (10 nM) to δ subunit-antibody immuno purified cerebellar and recombinant α4β3δ GABAAR is inhibited by low ethanol concentrations. In contrast, [3H]Ro15-4513 binding to classical γ2 subunit-containing receptors is not altered in the presence of ethanol. Right panel: Pharmacological characterization of the [3H]Ro15-4513 binding site on α4β3δ receptors. Compounds that have been previously shown to prevent Ro15-4513 antagonism (Ro15-1788, β-CCE, FG7142) or that show behavioral alcohol antagonism (RY024, RY080) displace [3H]Ro15-4513 from its binding site on α4β3δ receptors. Classical benzodiazepine agonists like diazepam, midazolam or flunitrazepam do not displace [3H]Ro15-4513 from α4β3δ GABAARs at concentrations <100 μM. (B) Chemical structures of ethanol and the imidazobenzodiazepines Ro15-4513 and flumazenil. Compounds RY080 and RY024 are congeners of Ro15- 4513 that carry an acetylene group in place of the azido group in Ro15-4513. (Figure reproduced from Hanchar et al., 2006.)
Furthermore, we show that binding of the EtOH antagonist [3H]Ro15-4513 is also inhibited by BZ site ligands (FG7142, β-CCE) that have been previously shown to inhibit Ro15-4513’s EtOH antagonist action (Hanchar et al., 2006) (see Fig. 4B). This [3H]Ro15-4513 site in δ subunit-containing GABAARs has not been found previously, likely because it is insensitive to the classical BZ site ligands like diazepam and flunitrazepam. In addition, α4/6β3δ receptors are of relatively low abundance and binding of the high affinity ligands Ro15-4513 and flumazenil to these GABAAR subtypes is functionally silent, except under special circumstances, that is, in the presence of EtOH enhanced receptors, and therefore remain usually undetected in functional assays. Consistent with the low abundance of δ subunit-containing receptors we show that only a small fraction, ~6% of total [3H]Ro15-4513 binding in the cerebellum, is inhibited by low doses of EtOH (Hanchar et al., 2006).
At EtOH concentrations exceeding 30 mM there is a component of the EtOH enhancement in α4β3δ GABAARs that is resistant to Ro15-4513 block, but this is eliminated by a mutation in the β3 subunit (β3-N265M) (Wallner et al., 2006). Receptors composed of α4β3N265Mδ have a saturable EtOH dose–response curve, with a half maximal response at 16 mM (Wallner et al., 2006). The β3N265M mutation has been previously shown to eliminate high dose EtOH (200 mM) effects on recombinant GABAAR (Mihic et al., 1997). Therefore, GABAARs have 2 distinct EtOH sites, a site in the transmembrane region that might contribute to the very high dose anesthetic alcohol effects and a “high affinity” site at certain subtypes of GABAARs that is blocked by the behavioral alcohol antagonist Ro15-4513.
We conclude that GABAARs that carry EtOHl/Ro15-4513 binding sites are important mediators of behavioral effects in mammalian brains.
3. Summary and future directions
GABAAR subtypes containing the δ subunit, in vivo most frequently associated with α4or α6 subunits, show an extra- or perisynaptic location and unique physiology and pharmacology. These receptors mediate tonic currents in vivo, and in recombinant systems when expressed with the β3 subunit are sensitive to EtOH concentrations that mirror the blood alcohol concentrations achieved during low and moderate ethanol consumption. These highly EtOH-sensitive receptors are positioned in the CNS appropriately to account for many of the acute effects of EtOH achieved by blood levels during social drinking. Fig. 5 shows the distribution of GABAAR subunits in rat brain that are required to make the ‘one glass of wine’ receptors in the brain (Pirker et al., 2000; Peng et al., 2002). Note a significant level of all 3 subunits, α4, β3, and δ, in the dentate gyrus, frontal cortex, parts of the neostriatum, and other forebrain nuclei.
If these GABAARs mediate low dose ethanol effects, the behaviors affected would involve circuitry in which these GABAARs are major participants, a testable hypothesis. In fact we show that a mutation in the α6 subunit, highly and almost exclusively expressed in cerebellar granule cells, is responsible for increased alcohol-induced motor impairment in animals that carry this mutation. Receptors formed by α4, β3 and δ subunits are found in regions of the brain where they are likely to mediate the amnestic (hippocampus), the sedative, anxiolytic and possibly also the rewarding effects of EtOH. Sedation, relief of anxiety, amnesia, motor impairment and rewarding effects are modalities of ethanol intoxication that have been reported to be reversed by the alcohol antagonist Ro15-4513 in behavioral studies.
The rat single nucleotide genetic variant in the GABAAR α6 subunit (α6100Q), showing supersensitivity to EtOH, leads to 2 important implications: (a) it demonstrates the in vivo relevance of the newly discovered low dose EtOH-sensitive GABAAR: extrasynaptic GABAAR can serve as the ‘one glass of wine receptors’ in brain; and (b) it provides a possible biochemical insight into the actual EtOH binding site: a ‘benzodiazepine site’ in the extracellular domain of the GABAAR protein might be involved in EtOH modulation. This led to our discovery that the in vivo EtOH antagonist action of the benzodiazepine Ro15-4513 is due to its specific block of the EtOH enhancement on subtypes of GABAARs (like those containing the δ subunit), consistent with their role in low dose EtOH effects. These GABAAR subtype proteins appear to have a specific site of EtOH interaction at subunit interfaces in the extracellular domain that allow a potent and efficacious modulation by EtOH. The sites most sensitive to the modulation by EtOH are not, like those of most general anesthetics, in the trans-membrane domains, but like BZ, in the extracellular domain in modified GABA agonist sites, but only on certain subtypes of GABAARs. It remains possible that similar EtOH modulation sites of high potency or efficacy will be found on other types of GABAARs or other receptor channels. For the moment, several observations are consistent with the new idea that certain subtypes of GABAARs are indeed acting as the pharmacologically relevant EtOH receptors.
To confirm that subtypes of GABAARs do indeed mediate EtOH effects, there is a need for either knock-out animals or knock-in point mutations. However, results obtained from knock-out animals can be complicated (and sometimes even misleading) as these animals lack gene products of interest during their ontogenesis, and this often leads to compensatory changes. Probably the most impressive example is the comparison of β3 knock-out mice with mice that carry a single point mutation (GABAAR-β3N265M). It is difficult to understand how the total knock-out could show a much less pronounced phenotype (Homanics et al., 1997a) in terms of reduced etomidate/propofol sensitivity than a mouse that carries only a single β3N265M point mutation in the second transmembrane region (Jurd et al., 2003). Another example is our finding that the α6-R100Q polymorphism increases the EtOH-induced motor coordination in rats, whereas no changes in EtOH sensitivity have been reported for the α6 total knock-out mouse, with the assays and EtOH doses employed to date (Homanics et al., 1999). It remains possible that effects of lower doses of EtOH might be more likely to show changes in behavior.
However, we do not think that knock-out data are not valuable (Olsen & Homanics, 2000). Hopefully, with a better understanding of physiology and pharmacology of EtOH effects on GABAARs we should be able to eventually understand why δ knock-out animals differ in certain, but not all aspects of EtOH sensitivity (Mihalek et al., 2001). The seizure protective effect of EtOH at a moderately intoxicating dose (2 g/kg IP) is completely missing in the δ subunit knock-out animals (Mihalek et al., 2001, Fig. 3)(δ−/− animals show increased bicuculline-induced seizure threshold vs. wild type, and this is not further altered by 2 g/kg EtOH). Also, δ−/− animals show reduced voluntary EtOH consumption, consistent with the observation that Ro15-4513 reduces EtOH self-administration. Also consistent with the finding that Ro15-4513 does not reverse the hypothermic EtOH effect is that δ subunit ablation has no effect on hypothermic EtOH effects. In addition, consistent with the notion that “anesthetic” EtOH effects are primarily mediated by Ro15-4513-insensitive EtOH actions, there is little or no genotype effect on “sleep time” at a dose of 3.5 g/kg in the δ subunit knock-out mice or the α6-100Q (natural polymorphism) rat mutants.
While it was reported that δ knock-out animals do not differ in rotarod performance from WT mice, the effects of ethanol on δ−/− and δ+/+ mice were not reported. However, it was reported that there is no change in anxiolytic activity of EtOH in the elevated plus-maze test (Mihalek et al., 2001). Because it has been found that anxiolytic EtOH effects can be reversed by Ro15-4513 (e.g.,Suzdak et al., 1986a), this could be an indication that there may be additional, possibly non-δ-containing subtypes of GABAARthat may carry Ro15-4513-sensitive EtOH sites. Particularly α2 subunit-containing GABAARs may be good candidates for mediating anxiolytic EtOH effects (Low et al., 2000).
To confirm that subtypes of GABAARs are mediators of ethanol effects it will be important in the future to investigate mice that either have a GABAAR α4 subunit total knock-out or knock-in point mutations (e.g., α4-R100Q) that are expected to change the alcohol sensitivity of abundant Ro15-4513/ethanol sensitive GABAAR subtypes. Behavioral alcohol antagonists like Ro15-4513, in particular alcohol antagonists that lack the intrinsic inverse agonist activity of Ro15-4513, would be valuable tools to dissect alcohol effects that are mediated by the EtOH/Ro15-4513 site and clearly distinguish them from effects on other acute alcohol targets.
In addition, targeting of the alcohol site by certain benzodiazepine site ligands not only opens the possibility to identify the ethanol/Ro15-4513 binding site, but also to develop drugs that target the EtOH/Ro15-4513 site on GABAARs. Such drugs might be useful as alcohol antidotes as well as alcohol mimetics in the clinic. “Alcohol mimetics” (synthetic alcohol-site agonists) may be able to harness certain aspects of acute as well as long-term beneficial alcohol actions for therapeutic purposes.
Acknowledgments
This work was supported by a NIH pre-doctoral fellowship AA015460 to H.J.H., an Alcoholic Beverage Medical Research Foundation grant to M.W., NIH grants NS35985 and AA07680 and funds provided by the State of California for medical research on alcohol and substance abuse to R.W.O.
References
- Aguayo LG. Ethanol potentiates the GABAA-activated Cl− current in mouse hippocampal and cortical neurons. Eur J Pharmacol. 1990;187:127–130. doi: 10.1016/0014-2999(90)90349-b. [DOI] [PubMed] [Google Scholar]
- Aguayo LG, Peoples RW, Yeh HH, Yevenes GE. GABAA receptors as molecular sites of ethanol action. Direct or indirect actions? Curr Top Med Chem. 2002;2:869–885. doi: 10.2174/1568026023393426. [DOI] [PubMed] [Google Scholar]
- Allan AM, Burnett D, Harris RA. Ethanol-induced changes in chloride flux are mediated by both GABAA and GABAB receptors. Alcohol Clin Exp Res. 1991;15:233–237. doi: 10.1111/j.1530-0277.1991.tb01862.x. [DOI] [PubMed] [Google Scholar]
- Becker HC, Hale RL. Ro15-4513 antagonizes the anxiolytic effects of ethanol in a nonshock conflict task at doses devoid of anxiogenic activity. Pharmacol Biochem Behav. 1991;39:803–807. doi: 10.1016/0091-3057(91)90169-3. [DOI] [PubMed] [Google Scholar]
- Benson JA, Low K, Keist R, Möhler H, Rudolph U. Pharmacology of recombinant GABAA receptors rendered diazepam-insensitive by point-mutated α-subunits. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- Berezhnoy D, Nyfeler Y, Gonthier A, Schwob H, Goeldner M, Sigel E. On the benzodiazepine binding pocket in GABAA receptors. J Biol Chem. 2004;279:3160–3168. doi: 10.1074/jbc.M311371200. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABAA receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–10943. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Alva H, Harris RA. A pervasive mechanism for analgesia: activation of GIRK2 channels. Proc Natl Acad Sci U S A. 2003;100:277–282. doi: 10.1073/pnas.012682399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm SL, II, Ponomarev I, Jennings AW, Whiting PJ, Rosahl TW, Garrett EM, et al. GABAA receptor subunit mutant mice: new perspectives on alcohol actions. Biochem Pharmacol. 2004;68:1581–1602. doi: 10.1016/j.bcp.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Bonetti EP, Burkhard WP, Gabl M, Möhler H. A partial inverse benzodiazepine agonist Ro15-4513 antagonizes acute ethanol effects in mice and rats. Br J Pharmacol. 1985;86:463P. [Google Scholar]
- Bonetti EP, Burkard WP, Gabl M, Hunkeler W, Lorez HP, Martin JR, et al. Ro15-4513: partial inverse agonism at the BZR and interaction with ethanol. Pharmacol Biochem Behav. 1988;31:733–749. doi: 10.1016/0091-3057(88)90259-6. [DOI] [PubMed] [Google Scholar]
- Breese GR, Morrow AL, Simson PE, Criswell HE, McCown TJ, Duncan GE, et al. The neuroanatomical specificity of ethanol action on ligand-gated ion channels: a hypothesis. Alcohol Alcohol Suppl. 1993;2:309–313. [PubMed] [Google Scholar]
- Breese G, Criswell H, Carta M, Dodson PD, Hanchar HJ, Khisti RT, et al. Basis of the GABA-mimetic profile of ethanol. Alcohol Clin Exp Res. 2006;30:731–744. doi: 10.1111/j.0145-6008.2006.00086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr LG, Spence JP, Peter Eriksson CJ, Lumeng L, Li TK. AA and ANA rats exhibit the R100Q mutation in the GABAA receptor α6 subunit. Alcohol. 2003;31:93–97. doi: 10.1016/j.alcohol.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci U S A. 2003;100:6813–6818. doi: 10.1073/pnas.1137276100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci. 2004;24:3746–3751. doi: 10.1523/JNEUROSCI.0067-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JB, Foster KL, Eiler WJ, II, McKay PF, Woods J, II, Harvey SC, et al. Selective GABAA α5 benzodiazepine inverse agonist antagonizes the neurobehavioral actions of alcohol. Alcohol Clin Exp Res. 2005;29:1390–1401. doi: 10.1097/01.alc.0000175073.94575.86. [DOI] [PubMed] [Google Scholar]
- Costa AC, Stasko MR, Stoffel M, Scott-McKean JJ. G-protein-gated potassium (GIRK) channels containing the GIRK2 subunit are control hubs for pharmacologically induced hypothermic responses. J Neurosci. 2005;25:7801–7804. doi: 10.1523/JNEUROSCI.1699-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Breese GR. A conceptualization of integrated actions of ethanol contributing to its GABAmimetic profile: a commentary. Neuropsychopharmacology. 2005;30:1407–1425. doi: 10.1038/sj.npp.1300750. [DOI] [PubMed] [Google Scholar]
- Dar MS. Antagonism by intracerebellar Ro15-4513 of acute ethanol-induced motor incoordination in mice. Pharmacol Biochem Behav. 1995;52:217–223. doi: 10.1016/0091-3057(95)00107-8. [DOI] [PubMed] [Google Scholar]
- Dar MS, Meng ZH. Acute ethanol-induced adenosine diphosphate ribosylation regulates the functional activity of rat striatal pertussis toxin-sensitive g proteins. Alcohol Clin Exp Res. 2004;28:1299–1307. doi: 10.1097/01.alc.0000139817.53197.41. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Deitrich RA, Dunwiddie TV, Harris RA, Erwin VG. Mechanism of action of ethanol: initial central nervous system actions. Pharmacol Rev. 1989;41:489–537. [PubMed] [Google Scholar]
- DeLorey TM, Brown GB. Gamma-Aminobutyric acidA receptor pharmacology in rat cerebral cortical synaptoneurosomes. J Neurochem. 1992:2162–2169. doi: 10.1111/j.1471-4159.1992.tb10959.x. [DOI] [PubMed] [Google Scholar]
- Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol Rev. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- Duncalfe LL, Carpenter MR, Smillie LB, Martin IL, Dunn SM. The major site of photoaffinity labeling of the GABAA receptor by [3H]flunitrazepam is histidine 102 of the alpha subunit. J Biol Chem. 1996;271:9209–9214. doi: 10.1074/jbc.271.16.9209. [DOI] [PubMed] [Google Scholar]
- Durand D, Corrigall WA, Kujtan P, Carlen PL. Effect of low concentrations of ethanol on CA1 hippocampal neurons in vitro. Can J Physiol Pharmacol. 1981;59:979–984. doi: 10.1139/y81-149. [DOI] [PubMed] [Google Scholar]
- Ehrig T, Bosron WF, Li TK. Alcohol and aldehyde dehydrogenase. Alcohol Alcohol. 1990;25:105–116. doi: 10.1093/oxfordjournals.alcalc.a044985. [DOI] [PubMed] [Google Scholar]
- Eriksson CJP. Deitrich R, Pawlowski AA, editors. Finnish selective breeding studies for initial sensitivity to ethanol: update 1988 on the AT and ANT rat lines. U.S. Department of Health and Human Services, NIAAA; Rockville, MD: Initial Sensitivity to Alcohol. Research Monograph. 1990;vol. 20:61–86.
- Farrant M, Cull-Candy S. GABA receptors, granule cells and genes. Nature. 1993;361:302–303. doi: 10.1038/361302a0. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Feng HJ, Bianchi MT, MacDonald RL. Pentobarbital differentially modulates α1β3δ and α1β3γ2L GABAA receptor currents. Mol Pharmacol. 2004;66:988–1003. doi: 10.1124/mol.104.002543. [DOI] [PubMed] [Google Scholar]
- Forman SA, Miller KW. Molecular sites of anesthetic action in postsynaptic nicotinic membranes. Trends Pharmacol Sci. 1989;10:447–452. doi: 10.1016/S0165-6147(89)80009-4. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Is membrane expansion relevant to anaesthesia? Nature. 1981;292:248–251. doi: 10.1038/292248a0. [DOI] [PubMed] [Google Scholar]
- Frye GD, Breese GR. GABAergic modulation of ethanol-induced motor impairment. J Pharmacol Exp Ther. 1982;223:750–756. [PMC free article] [PubMed] [Google Scholar]
- Glowa JR, Crawley J, Suzdak PD, Paul SM. Ethanol and the GABA receptor complex: studies with the partial inverse benzodiazepine receptor agonist Ro15-4513. Pharmacol Biochem Behav. 1988;31:767–772. doi: 10.1016/0091-3057(88)90263-8. [DOI] [PubMed] [Google Scholar]
- Grobin AC, Matthews DB, Devaud LL, Morrow AL. The role of GABAA receptors in the acute and chronic effects of ethanol. Psychopharmacology (Berl) 1998;139:2–19. doi: 10.1007/s002130050685. [DOI] [PubMed] [Google Scholar]
- Hakkinen HM, Kulonen E. Ethanol intoxication and gamma-aminobutyric acid. J Neurochem. 1976;27:631–633. doi: 10.1111/j.1471-4159.1976.tb12295.x. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on GABAA receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABAA receptor activity. Nat Neurosci. 2005;8:339–345. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkin P, Meera P, Supavilai P, Sieghart W, Wallner M, et al. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δ GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8550. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Allan AM. Functional coupling of gamma-aminobutyric acid receptors to chloride channels in brain membranes. Science. 1985;228:1108–1110. doi: 10.1126/science.2581319. [DOI] [PubMed] [Google Scholar]
- Harris RA, Allan AM. Alcohol intoxication: ion channels and genetics. FASEB J. 1989;3:1689–1695. doi: 10.1096/fasebj.3.6.2467834. [DOI] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of GABAA receptors. Proc Natl Acad Sci U S A. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellevuo K, Kiianmaa K, Korpi ER. Effect of GABAergic drugs on motor impairment from ethanol, barbital and lorazepam in rat lines selected for differential sensitivity to ethanol. Pharmacol Biochem Behav. 1989;34:399–404. doi: 10.1016/0091-3057(89)90333-x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, et al. Supersensitivity to allosteric GABAA receptor modulators and alcohol in mice lacking PKCepsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Tabakoff B, Szabo G, Suzdak PD, Paul SM. Effect of an imidazobenzodiazepine, Ro15-4513, on the incoordination and hypothermia produced by ethanol and pentobarbital. Life Sci. 1987;41:611–619. doi: 10.1016/0024-3205(87)90415-2. [DOI] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, et al. Mice devoid of GABAA receptor β3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci USA. 1997a;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, Ferguson C, Quinlan JJ, Daggett J, Snyder K, Lagenaur C, et al. Gene knockout of the α6 subunit of the GABAA receptor: lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol Pharmacol. 1997b;51:588–596. doi: 10.1124/mol.51.4.588. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Harrison NL, Quinlan JJ, Krasowski MD, Rick CE, de Blas AL, et al. Normal electrophysiological and behavioral responses to ethanol in mice lacking the long splice variant of the γ2 subunit of the GABAA receptor. Neuropharmacology. 1999;38:253–265. doi: 10.1016/s0028-3908(98)00177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WY, Reiffenstein RJ, Wong L. Interaction between flurazepam and ethanol. Alcohol Drug Res. 1987;7:107–117. [PubMed] [Google Scholar]
- Isbell H, Altschul S, Kornetsky CH, Eisenman AJ, Flanary HG, Fraser HF. Chronic barbiturate intoxication. AMA Arch Neurol Psychiatry. 1950:1–28. doi: 10.1001/archneurpsyc.1950.02310250007001. [DOI] [PubMed] [Google Scholar]
- June HL, Lewis MJ. Interactions of Ro15-4513, Ro15-1788 (flumazenil) and ethanol on measures of exploration and locomotion in rats. Psychopharmacology (Berl) 1994;116:309–316. doi: 10.1007/BF02245334. [DOI] [PubMed] [Google Scholar]
- June HL, Lummis GH, Colker RE, Moore TO, Lewis MJ. Ro15-4513 attenuates the consumption of ethanol in deprived rats. Alcohol Clin Exp Res. 1991;15:406–411. doi: 10.1111/j.1530-0277.1991.tb00538.x. [DOI] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABAA receptor β3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- Khanna JM, Kalant H, Chau A, Shah G. Rapid tolerance and crosstolerance to motor impairment effects of benzodiazepines, barbiturates, and ethanol. Pharmacol Biochem Behav. 1998;59:511–519. doi: 10.1016/s0091-3057(97)00477-2. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ikeda K, Kojima H, Niki H, Yano R, Yoshioka T, et al. Ethanol opens G-protein-activated inwardly rectifying K+ channels. Nat Neurosci. 1999;2:1091–1097. doi: 10.1038/16019. [DOI] [PubMed] [Google Scholar]
- Kolata G. New drug counters alcohol intoxication. Science. 1986;234:1198–1199. doi: 10.1126/science.3775379. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Seeburg PH. Natural mutation of GABAA receptor alpha 6 subunit alters benzodiazepine affinity but not allosteric GABA effects. Eur J Pharmacol. 1993;247:23–27. doi: 10.1016/0922-4106(93)90133-t. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Uusi-Oukari M, Castren E, Suzdak PD, Seppala T, Sarviharju M, et al. Cerebellar GABAA receptors in two rat lines selected for high and low sensitivity to moderate alcohol doses: pharmacological and genetic studies. Alcohol. 1992;9:225–231. doi: 10.1016/0741-8329(92)90058-i. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Kleingoor C, Kettenmann H, Seeburg PH. Benzodiazepine-induced motor impairment linked to point mutation in cerebellar GABAA receptor. Nature. 1993;361:356–359. doi: 10.1038/361356a0. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Makela R, Uusi-Oukari M. Ethanol: novel actions on nerve cell physiology explain impaired functions. News Physiol Sci. 1998;13:164–170. doi: 10.1152/physiologyonline.1998.13.4.164. [DOI] [PubMed] [Google Scholar]
- Kulkarni SK, Ticku MK. Ro15-4513 but not FG7142 reverses anticonvulsant effects of ethanol against bicuculline- and picrotoxin-induced convulsions in rats. Pharmacol Biochem Behav. 1989;32:233–240. doi: 10.1016/0091-3057(89)90239-6. [DOI] [PubMed] [Google Scholar]
- Lee RS, Smith SS, Chapin JK, Shimizu N, Waterhouse BD, Maddus BN, et al. Effects of systemic and local ethanol on responses of rat cerebellar Purkinje neurons to iontophoretically applied norepinephrine and gamma-aminobutyric acid. Brain Res. 1995;687:12–21. doi: 10.1016/0006-8993(95)00286-y. [DOI] [PubMed] [Google Scholar]
- Lei Q, Jones MB, Talley EM, Schrier AD, McIntire WE, Garrison JC, et al. Activation and inhibition of G protein-coupled inwardly rectifying potassium (Kir3) channels by G protein beta gamma subunits. Proc Natl Acad Sci U S A. 2000;97:9771–9776. doi: 10.1073/pnas.97.17.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewohl JM, Wilson WR, Mayfield RD, Brozowski SJ, Morrisett RA, Harris RA. G-protein-coupled inwardly rectifying potassium channels are targets of alcohol action. Nat Neurosci. 1999;2:1084–1090. doi: 10.1038/16012. [DOI] [PubMed] [Google Scholar]
- Liang J, Zhang N, Cagetti E, Houser CR, Olsen RW, Spigelman I. Chronic intermittent ethanol-induced switch of ethanol actions from extrasynaptic to synaptic hippocampal GABAA receptors. J Neurosci. 2006;26:1749–1758. doi: 10.1523/JNEUROSCI.4702-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljequist S, Engel J. Effects of GABAergic agonists and antagonists on various ethanol-induced behavioral changes. Psychopharmacology (Berl) 1982:71–75. doi: 10.1007/BF00470592. [DOI] [PubMed] [Google Scholar]
- Lister RG. The benzodiazepine receptor inverse agonists FG 7142 and Ro15-4513 both reverse some of the behavioral effects of ethanol in a holeboard test. Life Sci. 1987;41:1481–1489. doi: 10.1016/0024-3205(87)90713-2. [DOI] [PubMed] [Google Scholar]
- Lister RG, Nutt DJ. Is Ro15-4513 a specific alcohol antagonist? Trends Neurosci. 1987;10:223–225. [Google Scholar]
- Liu J, Ji XQ, Zhu XZ. Comparison of psychic emergence reactions after (+/−)-ketamine and (+)-ketamine in mice. Life Sci. 2006;78:1839–1844. doi: 10.1016/j.lfs.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991;40:263–270. [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. Ethanol inhibits NMDA-activated ion current in hippocampal neurons. Science. 1989;243:1721–1724. doi: 10.1126/science.2467382. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Marrosu F, Carcangiu G, Passino N, Aramo S, Mereu G. Antagonism of ethanol effects by Ro15-4513: an electrophysiological analysis. Synapse. 1989;3:117–128. doi: 10.1002/syn.890030203. [DOI] [PubMed] [Google Scholar]
- Marszalec W, Kurata Y, Hamilton BJ, Carter DB, Narahashi T. Selective effects of alcohols on GABAA receptor subunits expressed in human embryonic kidney cells. J Pharmacol Exp Ther. 1994;269:157–163. [PubMed] [Google Scholar]
- Martz A, Deitrich RA, Harris RA. Behavioral evidence for the involvement of gamma-aminobutyric acid in the actions of ethanol. Eur J Pharmacol. 1983;89:53–62. doi: 10.1016/0014-2999(83)90607-6. [DOI] [PubMed] [Google Scholar]
- McKay PF, Foster KL, Mason D, Cummings R, Garcia M, Williams LS, et al. A high affinity ligand for GABAA-receptor containing a5 subunit antagonizes ethanol’s neurobehavioral effects in Long-Evans rats. Psychopharmacology (Berl) 2004;172:455–462. doi: 10.1007/s00213-003-1671-z. [DOI] [PubMed] [Google Scholar]
- Mehta AK, Ticku MK. Ethanol potentiation of GABAergic transmission in cultured spinal cord neurons involves GABAA-gated chloride channels. J Pharmacol Exp Ther. 1988;246:558–564. [PubMed] [Google Scholar]
- Miczek KA, Weerts EM. Seizures in drug-treated animals. Science. 1987;235:1127–1128. [PubMed] [Google Scholar]
- Mihalek RM, Bowers BJ, Wehner JM, Kralic JE, VanDoren MJ, Morrow AL, et al. GABAA-receptor d subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol Clin Exp Res. 2001;25:1708–1718. [PubMed] [Google Scholar]
- Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Wu PH, Kalant H. Potentiation of gamma-aminobutyric acid-mediated chloride flux by pentobarbital and diazepam but not ethanol. J Neurochem. 1992;58:745–751. doi: 10.1111/j.1471-4159.1992.tb09781.x. [DOI] [PubMed] [Google Scholar]
- Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, et al. Sites of alcohol and volatile anaesthetic action on GABAA and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Mofeez A, Brandner B, Bromley L, Curran HV. Acute effects of ketamine on memory systems and psychotic symptoms in healthy volunteers. Neuropsychopharmacology. 2004;29:208–218. doi: 10.1038/sj.npp.1300342. [DOI] [PubMed] [Google Scholar]
- Morrisett RA, Swartzwelder HS. Attenuation of hippocampal long-term potentiation by ethanol: a patch-clamp analysis of glutamatergic and GABAergic mechanisms. J Neurosci. 1993;13:2264–2272. doi: 10.1523/JNEUROSCI.13-05-02264.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Benzodiazepine, barbiturate, ethanol and hypnotic steroid hormone modulation of GABA-mediated chloride ion transport in rat brain synaptoneurosomes. Adv Biochem Psychopharmacol. 1988;45:247–261. [PubMed] [Google Scholar]
- Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Tohyama K, Kameyama T. Reversal of alcohol-induced amnesia by the benzodiazepine inverse agonist Ro15-4513. Eur J Pharmacol. 1988;155:211–217. doi: 10.1016/0014-2999(88)90506-7. [DOI] [PubMed] [Google Scholar]
- Nakahiro M, Arakawa O, Narahashi T. Modulation of gamma-aminobutyric acid receptor-channel complex by alcohols. J Pharmacol Exp Ther. 1991;259:235–240. [PubMed] [Google Scholar]
- Narahashi T, Arakawa O, Nakahiro M, Twombly DA. Effects of alcohols on ion channels of cultured neurons. Ann N Y Acad Sci. 1991;625:26–36. doi: 10.1111/j.1749-6632.1991.tb33827.x. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Arakawa O, Brunner EA, Nakahiro M, Nishio M, Ogata N, et al. Modulation of GABA receptor-channel complex by alcohols and general anesthetics. Adv Biochem Psychopharmacol. 1992;47:325–334. [PubMed] [Google Scholar]
- Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF, Hodge CW. Co-localization of PKCepsilon with various GABA(A) receptor subunits in the mouse limbic system. Neuro-Report. 2000;11:683–687. doi: 10.1097/00001756-200003200-00006. [DOI] [PubMed] [Google Scholar]
- Olsen RW, Homanics GE. Function of GABAA receptors: insights from mutant and knockout mice. In: Martin DL, Olsen RW, editors. GABA in the Nervous System: The View at Fifty Years. 2000. pp. 81–96. [Google Scholar]
- Olsen RW, Sapp DM, Bureau MH, Turner DM, Kokka N. Allosteric actions of central nervous system depressants including anesthetics on subtypes of the inhibitory GABAA receptor-chloride channel complex. Ann N Y Acad Sci. 1991;625:145–154. doi: 10.1111/j.1749-6632.1991.tb33838.x. [DOI] [PubMed] [Google Scholar]
- Otis TS, Hanchar HJ, Dodson PD, Olsen RW, Wallner M. Response to Valenzuela et al. Alcohol Clin Exp Res. 2005;29:1358. [Google Scholar]
- Palmer MR, Hoffer BJ. GABAergic mechanisms in the electrophysiological actions of ethanol on cerebellar neurons. Neurochem Res. 1990;15:145–151. doi: 10.1007/BF00972204. [DOI] [PubMed] [Google Scholar]
- Palmer MR, van Horne CG, Harlan JT, Moore EA. Antagonism of ethanol effects on cerebellar Purkinje neurons by the benzodiazepine inverse agonists Ro15-4513 and FG7142: electrophysiological studies. J Pharmacol Exp Ther. 1988;247:1018–1024. [PubMed] [Google Scholar]
- Pandey SC, Chartoff EH, Carlezon WA, Jr., Zou J, Zhang H, Kreibich AS, et al. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin Exp Res. 2005;29:176–184. doi: 10.1097/01.alc.0000153550.31168.1d. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, et al. GABAA receptor changes in d subunit-deficient mice: altered expression of α4 and γ2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Petry NM. Ro15-4513 selectively attenuates ethanol, but not sucrose, reinforced responding in a concurrent access procedure; comparison to other drugs. Psychopharmacology (Berl) 1995;121:192–203. doi: 10.1007/BF02245630. [DOI] [PubMed] [Google Scholar]
- Pinder RM, Sandler M. Alcohol, wine and mental health: focus on dementia and stroke. J Psychopharmacol. 2004;18:449–456. doi: 10.1177/0269881104047272. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Polc P. Interactions of partial inverse benzodiazepine agonists Ro15-4513 and FG7142 with ethanol in rats and cats. Br J Pharmacol. 1985;86:465P. [Google Scholar]
- Proctor WR, Dunwiddie TV. Behavioral sensitivity to purinergic drugs parallels ethanol sensitivity in selectively bred mice. Science. 1984;224:519–521. doi: 10.1126/science.6324348. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Poelchen W, Bowers BJ, Wehner JM, Messing RO, Dunwiddie TV. Ethanol differentially enhances hippocampal GABAA receptor-mediated responses in protein kinase C gamma (PKC gamma) and PKC epsilon null mice. J Pharmacol Exp Ther. 2003;305:264–270. doi: 10.1124/jpet.102.045450. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Hoffmann SE, Deng XS, Asperi W, Fay T, Bludeau P, et al. Behavioral characterization of alcohol-tolerant and alcohol non-tolerant rat lines and an f(2) generation. Behav Genet. 2004;34:453–463. doi: 10.1023/B:BEGE.0000023650.32243.39. [DOI] [PubMed] [Google Scholar]
- Ramchandani VA, Bosron WF, Li TK. Research advances in ethanol metabolism. Pathol Biol (Paris) 2001;49:676–682. doi: 10.1016/s0369-8114(01)00232-2. [DOI] [PubMed] [Google Scholar]
- Rassnick S, D’Amico E, Riley E, Koob GF. GABA antagonist and benzodiazepine partial inverse agonist reduce motivated responding for ethanol. Alcohol Clin Exp Res. 1993;17:124–130. doi: 10.1111/j.1530-0277.1993.tb00736.x. [DOI] [PubMed] [Google Scholar]
- Reynolds JN, Prasad A, MacDonald JF. Ethanol modulation of GABA receptor-activated Cl− currents in neurons of the chick, rat and mouse central nervous system. Eur J Pharmacol. 1992;224:173–181. doi: 10.1016/0014-2999(92)90802-b. [DOI] [PubMed] [Google Scholar]
- Reynolds DS, Rosahl TW, Cirone J, O’Meara GF, Haythornthwaite A, Newman RJ, et al. Sedation and anesthesia mediated by distinct GABAA receptor isoforms. J Neurosci. 2003;23:8608–8617. doi: 10.1523/JNEUROSCI.23-24-08608.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci U S A. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, et al. Benzodiazepine actions mediated by specific GABAA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Saba L, Porcella A, Congeddu E, Colombo G, Peis M, Pistis M, et al. The R100Q mutation of the GABAA α6 receptor subunit may contribute to voluntary aversion to ethanol in the sNP rat line. Brain Res Mol Brain Res. 2001;87:263–270. doi: 10.1016/s0169-328x(01)00003-1. [DOI] [PubMed] [Google Scholar]
- Sanna A, Congeddu E, Porcella A, Saba L, Pistis M, Peis M, et al. Characterization of wild-type (R100R) and mutated (Q100Q) GABAA α6 subunit in Sardinian alcohol non-preferring rats (sNP) Brain Res. 2003;967:98–105. doi: 10.1016/s0006-8993(02)04230-0. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Sigel E, Baur R, Malherbe P. Recombinant GABAA receptor function and ethanol. FEBS Lett. 1993;324:140–142. doi: 10.1016/0014-5793(93)81380-i. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Bloom FE, French ED, Madamba SG, Mancillas J, Pittman QJ, et al. Electrophysiology of ethanol on central neurons. Ann N Y Acad Sci. 1987;492:350–366. doi: 10.1111/j.1749-6632.1987.tb48692.x. [DOI] [PubMed] [Google Scholar]
- Siggins GR, Roberto M, Nie Z. The tipsy terminal: presynaptic effects of ethanol. Pharmacol Ther. 2005;107:80–98. doi: 10.1016/j.pharmthera.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Smith SS, Waterhouse BD, Woodward DJ. Locally applied progesterone metabolites alter neuronal responsiveness in the cerebellum. Brain Res Bull. 1987;18:739–747. doi: 10.1016/0361-9230(87)90209-7. [DOI] [PubMed] [Google Scholar]
- Standridge JB, Zylstra RG, Adams SM. Alcohol consumption: an overview of benefits and risks. South Med J. 2004;97:664–672. doi: 10.1097/00007611-200407000-00012. [DOI] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by d subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, et al. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzdak PD, Paul SM. Ethanol stimulates GABA receptor-mediated Cl− ion flux in vitro: possible relationship to the anxiolytic and intoxicating actions of alcohol. Psychopharmacol Bull. 1987;23:445–451. [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science. 1986a;234:1243–1247. doi: 10.1126/science.3022383. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci U S A. 1986b;83:4071–4075. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAS. 2005;7:E353–E360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syapin PJ, Gee KW, Alkana RL. Ro15-4513 differentially affects ethanol-induced hypnosis and hypothermia. Brain Res Bull. 1987;19:603–605. doi: 10.1016/0361-9230(87)90078-5. [DOI] [PubMed] [Google Scholar]
- Tauber M, Calame-Droz E, Prut L, Rudolph U, Crestani F. Alpha2-GABAA receptors are the molecular substrates mediating precipitation of narcosis but not of sedation by the combined use of diazepam and alcohol in vivo. Eur J Neurosci. 2003;18:2599–2604. doi: 10.1046/j.1460-9568.2003.02988.x. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Kulkarni SK. Molecular interactions of ethanol with GABAergic system and potential of Ro15-4513 as an ethanol antagonist. Pharmacol Biochem Behav. 1988;30:501–510. doi: 10.1016/0091-3057(88)90487-x. [DOI] [PubMed] [Google Scholar]
- Ticku MK, Lowrimore P, Lehoullier P. Ethanol enhances GABA-induced 36Cl-influx in primary spinal cord cultured neurons. Brain Res Bull. 1986;17:123–126. doi: 10.1016/0361-9230(86)90168-1. [DOI] [PubMed] [Google Scholar]
- Titulaer MN, Ghijsen WE. Synaptoneurosomes. A preparation for studying subhippocampal GABAA receptor activity. Methods Mol Biol. 1997;72:49–59. doi: 10.1385/0-89603-394-5:49. [DOI] [PubMed] [Google Scholar]
- Ueda I, Suzuki A. Is there a specific receptor for anesthetics? Contrary effects of alcohols and fatty acids on phase transition and bioluminescence of firefly luciferase. Biophys J. 1998;75:1052–1057. doi: 10.1016/S0006-3495(98)77594-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Cerebellar GABAA receptor binding and function in vitro in two rat lines developed for high and low alcohol sensitivity. Neurochem Res. 1989;14:733–739. doi: 10.1007/BF00964950. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Diazepam sensitivity of the binding of an imidazobenzodiazepine, [3H]Ro 15-4513, in cerebellar membranes from two rat lines developed for high and low alcohol sensitivity. J Neurochem. 1990;54:1980–1987. doi: 10.1111/j.1471-4159.1990.tb04901.x. [DOI] [PubMed] [Google Scholar]
- Uusi-Oukari M, Korpi ER. Specific alterations in the cerebellar GABAA receptors of an alcohol-sensitive ANT rat line. Alcohol Clin Exp Res. 1991;15:241–248. doi: 10.1111/j.1530-0277.1991.tb01864.x. [DOI] [PubMed] [Google Scholar]
- Valenzuela CF, Mameli M, Carta M. Letter to the editor. Alcohol Clin Exp Res. 2005;29:1356–1357. doi: 10.1097/01.alc.0000171926.66397.94. (author reply 1358) [DOI] [PubMed] [Google Scholar]
- Wafford KA, Whiting PJ. Ethanol potentiation of GABAA receptors requires phosphorylation of the alternatively spliced variant of the γ2 subunit. FEBS Lett. 1992;313:113–117. doi: 10.1016/0014-5793(92)81424-k. [DOI] [PubMed] [Google Scholar]
- Wafford KA, Burnett DM, Leidenheimer NJ, Burt DR, Wang JB, Kofuji P, et al. Ethanol sensitivity of the GABAA receptor expressed in Xenopus oocytes requires 8 amino acids contained in the γ2L subunit. Neuron. 1991;7:27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances α4β3δ and α6β3δ GABAA receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Low dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc Natl Acad Sci U S A. 2006;103:8540–8545. doi: 10.1073/pnas.0600194103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Berton F, Madamba SG, Francesconi W, Siggins GR. Low ethanol concentrations enhance GABAergic inhibitory postsynaptic potentials in hippocampal pyramidal neurons only after block of GABAB receptors. Proc Natl Acad Sci U S A. 1996;93:5049–5054. doi: 10.1073/pnas.93.10.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of d subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J Neurosci. 2004;24:8379–8382. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JL, Valenzuela CF. Ethanol modulation of GABAergic transmission: the view from the slice. Pharmacol Ther. doi: 10.1016/j.pharmthera.2005.11.002. (in press) [DOI] [PubMed] [Google Scholar]
- Weiner JL, Zhang L, Carlen PL. Potentiation of GABAA-mediated synaptic current by ethanol in hippocampal CA1 neurons: possible role of protein kinase C. J Pharmacol Exp Ther. 1994;268:1388–1395. [PubMed] [Google Scholar]
- Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 1997;77:1306–1312. doi: 10.1152/jn.1997.77.3.1306. [DOI] [PubMed] [Google Scholar]
- White G, Lovinger DM, Weight FF. Ethanol inhibits NMDA-activated current but does not alter GABA-activated current in an isolated adult mammalian neuron. Brain Res. 1990;507:332–336. doi: 10.1016/0006-8993(90)90292-j. [DOI] [PubMed] [Google Scholar]
- Wick MJ, Mihic SJ, Ueno S, Mascia MP, Trudell JR, Brozowski SJ, et al. Mutations of gamma-aminobutyric acid and glycine receptors change alcohol cutoff: evidence for an alcohol receptor? Proc Natl Acad Sci U S A. 1998;95:6504–6509. doi: 10.1073/pnas.95.11.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieland HA, Luddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABAA receptors containing the δ subunit. J Neurosci. 2002:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward JJ. Ethanol and NMDA receptor signaling. Crit Rev Neurobiol. 2000;14:69–89. doi: 10.1080/08913810008443548. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Bertaccini E, Trudell JR, Harris RA. Anesthetics and ion channels: molecular models and sites of action. Annu Rev Pharmacol Toxicol. 2001;41:23–51. doi: 10.1146/annurev.pharmtox.41.1.23. [DOI] [PubMed] [Google Scholar]
- Yao L, Fan P, Jiang Z, Mailliard WS, Gordon AS, Diamond I. Addicting drugs utilize a synergistic molecular mechanism in common requiring adenosine and Gi-beta gamma dimers. Proc Natl Acad Sci U S A. 2003;100:14379–14384. doi: 10.1073/pnas.2336093100. [DOI] [PMC free article] [PubMed] [Google Scholar]