Abstract
Backgrounds and Purpose
Superoxide is associated with spontaneous intracerebral hemorrhage (ICH) during hypertension. The goal of this study was to test the hypothesis that changes in superoxide, in genetically altered mice with deletion and overexpression of CuZn-superoxide dismutase (SOD1), modulate susceptibility to ICH.
Methods
Chronic hypertension was produced by infusion of angiotensin II (AngII) and an inhibitor of nitric oxide synthase in drinking water in SOD1 transgenic (SOD1Tg) mice, SOD1 deficient (SOD1−/−) mice, and their respective wild-type (WT)-littermates. Acute hypertension was produced by daily injections of AngII in some mice with chronic hypertension to produce ICH. We evaluated susceptibility to ICH, oxidative stress [superoxide, NAD(P)H oxidase activity, SOD activity], gene expression, and activity of matrix metalloproteinases (MMPs).
Results
Incidence, size, and number of ICH were reduced in SOD1Tg mice, and were increased in SOD1−/− mice compared to their WT-littermates. Levels of superoxide increased in the brain even before developing ICH in WT-littermates, while levels of superoxide remained low in SOD1Tg mice. Changes in level of MMP-9 paralleled oxidative stress in SOD1Tg mice and WT-littermates. Moreover, levels of superoxide and MMP-9 were greater in SOD1−/− mice than WT-littermates after induction of ICH. Active MMPs colocalized on cerebral vessels that appeared to lead toward regions with ICH.
Conclusions
These results suggest that superoxide contributes to the pathogenesis of spontaneous ICH, possibly through activation of MMP-9, and that SOD1 protects against spontaneous ICH during hypertension.
Keywords: Oxidative stress, SOD1, MMP-9, brain hemorrhage
Introduction
Several years ago, we developed the first model of spontaneous intracranial hemorrhage (ICH) in chronically hypertensive mice.1 A limitation of the model is that it is difficult to breed the mice with other genetically altered mice, because the mice are double transgenic. Therefore, we recently developed another experimental model of ICH in hypertensive C57BL/6 mice.2
Mice with acute hypertension, induced by daily injection of angiotensin II (AngII), superimposed on chronic hypertension, have a high incidence of spontaneous ICH.2 There was an association of increases in AngII-mediated oxidative stress with spontaneous ICH during hypertension. An association of oxidative stress and ICH, however, clearly does not provide direct evidence for a causal relationship.
Copper/zinc-superoxide dismutase (SOD1) is a crucial antioxidant enzyme. Deficiency of SOD1 increases superoxide and produces vascular dysfunction in large arteries and microvessels, augments vascular dysfunction produced by AngII, and increases expression and activation of matrix metalloproteinase-9 (MMP-9).3,4,5 Overexpression of SOD1 decreases oxidative stress, attenuates induction and activation of MMP-9, and protects against vascular dysfunction.5,6 In this study, we tested the hypothesis that decreases in superoxide by overexpression of SOD1 protect against development of spontaneous ICH, and that increases in superoxide by deficiency in SOD1 increase susceptibility to ICH.
Materials and Methods
Experimental Animals
Studies were conducted in 8-month-old male hemizygous CuZnSOD-transgenic (SOD1Tg) mice (n=40) and wild-type (WT)-littermates (n=44), and homozygous CuZnSOD-deficient (SOD1−/−) mice (n=15) and WT-littermates (n=14). SOD1Tg mice, SOD1−/− mice, and their WT-littermates were produced as described previously.5 Breeding and genotyping were performed in a virus- and pathogen-free barrier facility at the University of Iowa. The genotype of each mouse was ascertained by polymerase chain reaction of DNA isolated from tail biopsy samples as described previously.4 All experimental protocols and procedures conform to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
There were two cohorts of mice in studies using SOD1Tg mice and WT-littermates. The first cohort was used to measure systolic blood pressure (SBP), incidence of stroke, and size of ICH. Mice in this cohort were euthanized with an injection of Nembutal (150 mg/kg, ip) when a mouse developed signs of stroke, or at day 28 if the mouse did not have neurological signs. The second cohort was used to evaluate oxidative stress, expression of mRNA, and MMPs. In the second cohort, when a mouse developed signs of stroke, we euthanized the mouse with signs of stroke and one mouse without neurological signs from each of the other groups, to collect tissue samples after the same duration of treatment.
Only one cohort of SOD1−/− mice and WT-littermates was studied, because of limited availability of these mice: SBP and incidence of sign of stroke were estimated and mice were euthanized when a mouse developed signs of stroke. Brains were used to evaluate size and number of ICH, oxidative stress, and MMPs.
Model of spontaneous intracerebral hemorrhage
The methods to produce spontaneous ICH and to assess signs of stroke in mice were described recently in detail.2 Briefly, mice were treated with AngII-infusion (1,000 ng/kg/min; Sigma-Aldrich, St Louis, MO) and L-NAME (100 mg/kg/day; Sigma-Aldrich) in drinking water to produce chronic hypertension (HT) (chronically hypertensive group). One week later, transient acute hypertension was produced by daily AngII-injection (0.5 μg/g, twice/day, sc) (chronic/acute HT group). SBP was measured daily in conscious mice using tail cuff plethysmography. Clinical signs of stroke were assessed by daily neurological examinations at least three times per day, including contralateral forelimb extension, circling behavior, or other motor dysfunction, as described previously.2,7 Mice without any treatments were used as control mice.
Mice were euthanized and perfused transcardially with phosphate-buffered saline (PBS). Paraffin-embedded brain was serially sectioned at 5 μm, resulting in about 2,000–2,400 sections of the entire brain of each mouse, for determination of size and number of ICH. The first and second sections of every set of five serial sections were stained with hematoxylin and eosin (H&E) and with diaminobenzidine (DAB), respectively.2,7 Diaminobenzidine reacts with peroxidases in red blood cells, and facilitates precise identification of ICH, because DAB highlights hemorrhages and nonhemorrhagic areas unstained.2,7 All section stained with H&E and DAB were screened, and images of ICH were captured and analyzed with Image J software (NIH) to quantify the size and number of hemorrhages. The size of ICH was estimated as follow: [area (mm2) of ICH on each section] × [25×10−3 (mm): distance between successive DAB-stained sections].
Brain preparation for oxidative stress, gene expression, and MMPs
Brains of the second cohort of SOD1Tg mice and WT-littermates were collected 12±1 (mean±SE) days after start of treatment. During this period, mice in the chronic/acute HT group of WT-littermates developed signs of stroke, and SOD1Tg mice did not develop any neurological signs.
In SOD1−/− mice and WT-littermates, brains were collected when the mice developed neurological signs, at 10±1 days in SOD1−/− mice and 14±2 days in WT-littermates.
Brains were perfused transcardially with PBS, and cut sagittally in half. Half of each mouse brain was fixed and used for histological detection of ICH. The middle third of the other half of the brain (4 mm thick) was not fixed, and was used to evaluate oxidative stress [superoxide and NAD(P)H oxidase activity, SOD activity], expression of mRNA, and MMPs. This slice of brain contains cerebral cortex, basal ganglia, thalamus, hippocampus, and upper part of brainstem.2
Oxidative Stress
Superoxide and NAD(P)H oxidase activity in brain homogenates were quantified with lucigenin-enhanced chemiluminescence.2,7 NAD(P)H oxidase activity was estimated as levels of superoxide after adding NADPH (100 μmol/L) to brain homogenates.
Total superoxide dismutase (SOD) activity of brain homogenates was determined using the WST SOD assay kit (Sigma-Aldrich) as reported previously.2
Expression levels of pro-oxidant/NAD(P)H oxidase subunits (Nox1, Nox2, Nox4, and p47phox), antioxidants (SOD1, SOD2, SOD3, and catalase), and Nrf2 were measured by quantitative real-time RT-PCR using the TaqMan method as described previously.2,8
Matrix Metalloproteinases
Levels of MMP-2 and MMP-9 in brain homogenates were evaluated using gelatin zymography as described previously.2 The organomercurial compound 4-aminophenylmercuric acetate (APMA; Sigma-Aldrich) was used to activate murine MMP-9 standard.
In situ gelatinolytic activity was assessed on frozen sections of half brains of three SOD−/−mice, as described previously.2 Briefly, coronal sections (10 μm thick) were cut for the entire brain of each mouse. One of every five serial sections was stained with H&E and DAB for detection of ICH. The remaining sections were used for MMP in situ zymography and immunohistochemistry as described previously.2 We also examined vessels that appeared to lead to ICH on serial sections of entire brains. Despite intensive examination of the tissue around ICH in more than 1,200 specimens/mouse in three SOD1−/− mice, we detected only a small number of vessels that appeared to lead to ICH, and thus may be the ‘culprit’ vessels.
Statistics
Results are expressed as mean±SE. Analysis of variance followed by Bonferroni test was used for comparison of multiple groups. Mann-Whitney’s U test was used for comparison of two groups. A probability value of P<0.05 was considered significant. Cumulative incidence of signs of stroke was evaluated using a Kaplan-Meier test and the difference among groups was analyzed by log rank test.
Results
CuZnSOD-transgenic mice and wild-type littermates
Systolic blood pressure (SBP)
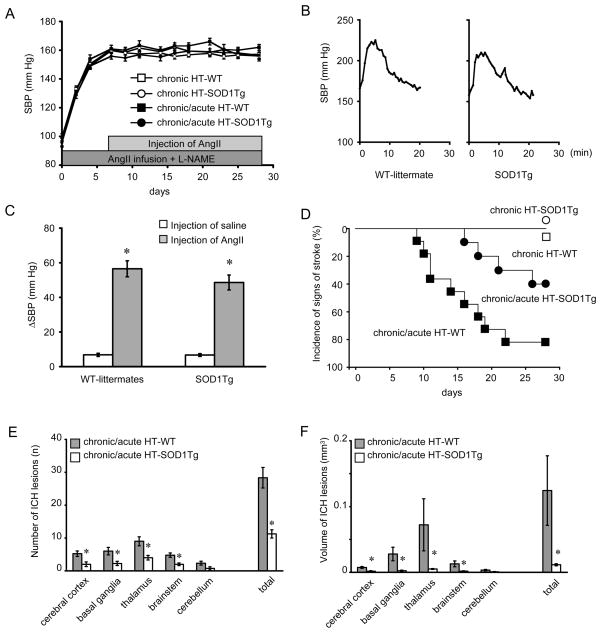
SBP increased from 96±3 (mean±SE) to 160±2 mm Hg and from 99±3 to 160±3 mm Hg in SOD1Tg mice and WT-littermates, respectively, 7 days after initiation of chronic hypertension (Fig. 1A). Basal levels of SBP (Fig. 1A) were similar in SOD1Tg mice and WT-littermates during chronic hypertension. SBP increased for about 20 min after injection of AngII, and was similar in chronic/acute HT group of SOD1Tg mice and WT-littermates (Fig. 1B, 1C).
Figure 1.
(A) Systolic blood pressure (SBP) in chronically hypertensive (chronic HT) mice and in mice with chronic and acute hypertension (chronic/acute HT). n=10–11 in each group. (B) SBP after AngII-injection in chronically hypertensive WT-littermate and SOD1Tg mouse. (C) SBP after injection of saline or AngII in WT-littermates (n=11) and SOD1Tg mice (n=10) with chronic hypertension. *p<0.05 vs respective saline-injected mice. (D) Kaplan-Meier plot of incidence of signs of stroke. (E) Number and (F) volume of spontaneous ICH per mouse in chronic/acute HT-SOD1Tg mice (n=4) and in chronic/acute HT-WT (n=9). *p<0.05 vs chronic/acute HT-WT.
Signs of stroke
In the chronic/acute HT group, SOD1Tg mice had a lower incidence (40 vs 82%), and later onset (20±2 vs 14±2 days after start of AngII-infusion and L-NAME) of signs of stroke compared to WT-littermates (p<0.05) (Fig. 1D). No SOD1Tg mice and WT-littermates in the chronically hypertensive group, without acute hypertension, showed signs of stroke (Fig. 1D).
Histological analysis
All SOD1Tg mice and WT-littermates that showed signs of stroke in the chronic/acute HT group had multiple ICHs, which were distributed widely in the brain (Fig. 1E, 1F). The number and volume of ICH in cerebral cortex, basal ganglia, thalamus, and brainstem were less in SOD1Tg mice than WT-littermates (Fig. 1E, 1F). No mice without neurological signs had histologically detectable ICH.
Oxidative stress
In WT-littermates, the level of superoxide in the brain was low in control mice, tended to increase [not significant (N.S.)] in chronically hypertensive mice, increased significantly in chronic/acute HT group without ICH, and was highest in chronic/acute HT group with ICH (Fig. 2A). In contrast, the level of superoxide did not differ among groups in SOD1Tg mice, and was less in SOD1Tg mice than WT-littermates in chronic/acute HT group without ICH (Fig. 2A). NAD(P)H oxidase activity increased in parallel with increases in basal levels of superoxide in WT-littermates (Fig. 2B). In SOD1Tg mice with chronic/acute HT, without ICH, NAD(P)H oxidase activity also was higher than the control group (Fig. 2B). Total SOD activity was higher in SOD1Tg mice than in WT-littermates (Fig. 2C).
Figure 2.
(A) Basal level of superoxide, (B) NAD(P)H oxidase activity, and (C) total SOD activity in brain homogenates. n=6 in each group (A,B) and 16 in C. *p<0.05 vs respective control mice. †p<0.05 vs respective chronic HT mice. §p<0.05 vs respective chronic/acute HT mice without ICH. #p<0.05.
Gene expression (mRNA)
In WT-littermates, expression of subunits of NAD(P)H oxidase did not increase in chronically hypertensive mice (Fig. 3A). Expression of Nox1 and Nox4 increased significantly in the chronic/acute HT group without ICH, and increased further in the chronic/acute HT group with ICH. Expression of Nox2 and p47phox increased only in chronic/acute HT group with ICH. In SOD1Tg mice of the chronic/acute HT group without ICH, expression of Nox2 and Nox4 also increased.
Figure 3.
Relative expression of mRNA of (A) NAD(P)H oxidase subunits, (B) antioxidant (SOD1, SOD2, SOD3, and catalase), and (C) Nrf2 in brain. n=6 in each group. *p<0.05 vs respective control mice. †p<0.05 vs respective chronic HT mice. §p<0.05 vs respective chronic/acute HT mice without ICH. #p<0.05. N.S.: not significant.
As expected, expression of SOD1 increased greatly in SOD1Tg mice (Fig. 3B). Expression of SOD2 and catalase decreased significantly in WT-littermates in chronic/acute HT group, without and with ICH (Fig. 3B). Expression of other antioxidant enzymes was not different among groups in SOD1Tg mice. In WT-littermates, expression of nuclear factor-erythroid factor 2 (Nrf2) (Fig. 3C), a redox-sensitive transcription factor that appears to upregulate antioxidant genes, was decreased significantly in mice with chronic/acute HT, without and with ICH; in SOD1Tg mice, expression of Nrf2 was not different among groups.
Matrix metalloproteinases
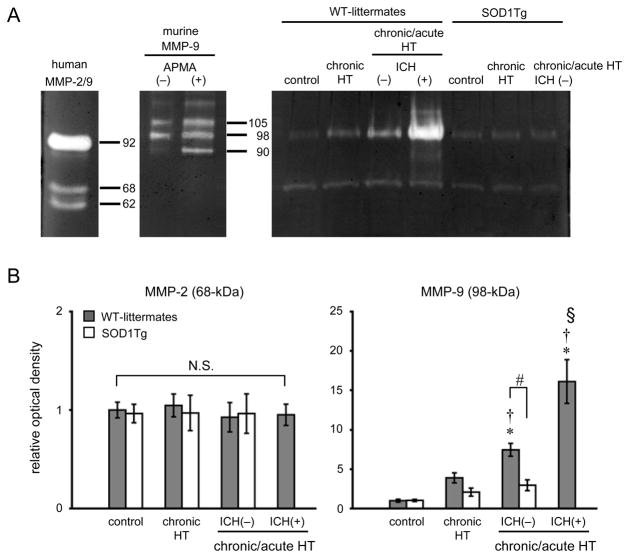
In WT-littermates, gelatinolytic activity of MMP-9 was barely detectable in the control group (Fig. 4A, 4B). Levels of MMP-9 (98-kDa) tended to increase (N.S.) in chronically hypertensive mice, increased significantly in chronic/acute HT mice without ICH, and increased further in chronic/acute HT mice with ICH (Fig. 4B). In contrast to WT-littermates, levels of MMP-9 (98-kDa) in SOD1Tg mice remained low in all groups; levels of MMP-9 (98-kDa) in chronic/acute HT mice without ICH were significantly less in SOD1Tg mice than in WT-littermates. Levels of MMP-2 (68-kDa) were detectable, but were not different among SOD1Tg mice and WT-littermates. No detectable levels of the cleaved form of MMP-9 and MMP-2 were observed in any group, except in WT-littermates with ICH.
Figure 4.
(A) Gelatin zymography showing gelatinolytic activity of MMP-2 (68-kDa: latent form; 62-kDa: cleaved form) and MMP-9 (98- and 105-kDa: latent form; 90-kDa: cleaved form). APMA: chemical activator for MMP-9. (B) Relative optical density of MMP-2 (68-kDa) and MMP-9 (98-kDa) against respective standard. n=6 in each group. *p<0.05 vs respective control mice. †p<0.05 vs respective chronic HT mice. §p<0.05 vs respective chronic/acute HT mice without ICH. #p<0.05. N.S.: not significant.
CuZnSOD-deficient mice and wild-type littermates
Systolic blood pressure
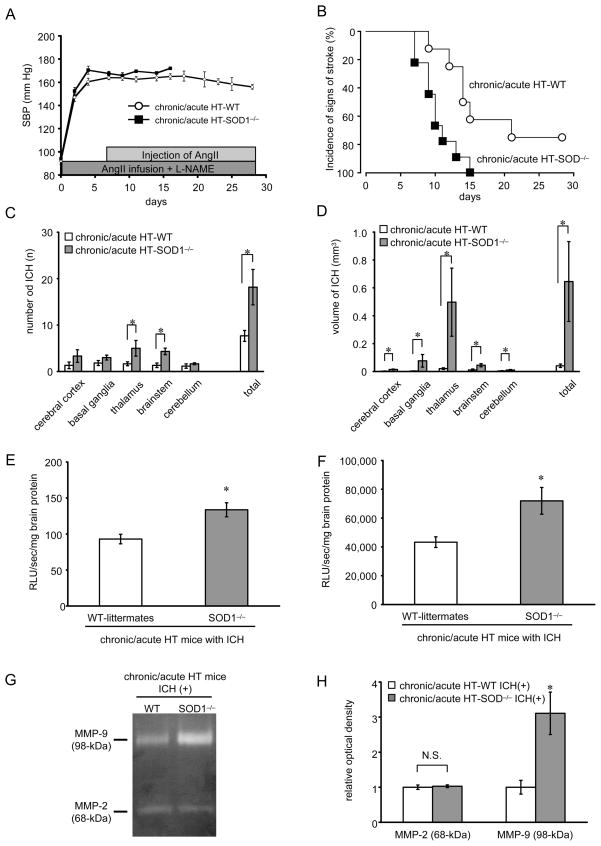
Basal levels of SBP were not different in SOD1−/− and WT-littermates (Fig. 5A). Acute increases in SBP after injection of AngII were not different (p=0.08) between chronically hypertensive SOD1−/− mice (65±3 mmHg) and WT-littermates (57±3).
Figure 5.
(A) Basal systolic blood pressure (SBP) in chronic/acute HT group; SOD1−/− mice (chronic/acute HT-SOD−/−, n=9) and WT-littermates (chronic/acute HT-WT, n=8). (B) Kaplan-Meier plot of incidence of signs of stroke. (C) Number and (D) volume of ICH per mouse in SOD1−/− mice (n=9) and in WT-littermates (n=6). *p<0.05. (E) Basal level of superoxide and (F) NAD(P)H oxidase activity in brain homogenates of chronic/acute HT group with ICH in SOD1−/− mice and in WT-littermates. (G) Gelatin zymography showing gelatinolytic activity of MMP-2 (68-kDa: latent form) and MMP-9 (98-kDa: latent form). (H) Relative optical density of MMP-2 (68-kDa) and MMP-9 (98-kDa) in chronic/acute HT group with ICH; SOD1−/− mice [chronic/acute HT-SOD−/− ICH(+), n=6] and WT-littermates [chronic/acute HT-WT ICH(+), n=6]. *p<0.05 vs chronic/acute HT-WT mice with ICH.
Signs of stroke
All SOD1−/− mice and 6 of 8 WT-littermates (75%) with acute, superimposed on chronic, hypertension developed signs of stroke 10±1 days and 14±2 days after start of AngII-infusion and L-NAME, respectively (log rank test, p<0.01) (Fig. 5B).
Histological analysis
All SOD1−/− mice and WT-littermates that showed signs of stroke had multiple ICHs, which were distributed widely in the brain (Fig. 5C, 5D). There were more ICH in thalamus and brainstem in SOD1−/− mice than in WT-littermates (Fig. 5C). Volume of ICH in all regions of brain of SOD1−/− mice was larger than that of WT-littermates (Fig. 5D). WT-littermates without neurological signs did not have histologically detectable ICH.
Oxidative stress
Total SOD activity in normal SOD1−/− mice (15±1 U/mg brain protein) was significantly less than in WT-littermates (110±9) (p<0.05). After development of ICH, basal levels of superoxide (Fig. 5E) and NAD(P)H oxidase activity (Fig. 5F) in the brain were significantly higher in SOD1−/− mice than WT-littermates.
Matrix metalloproteinases
Levels of MMP-9 (98-kDa) were higher in SOD1−/− mice with ICH than WT-littermates with ICH (Fig. 5G, 5H). Levels of MMP-2 (68-kDa) were not different between the 2 groups (Fig. 5G, 5H).
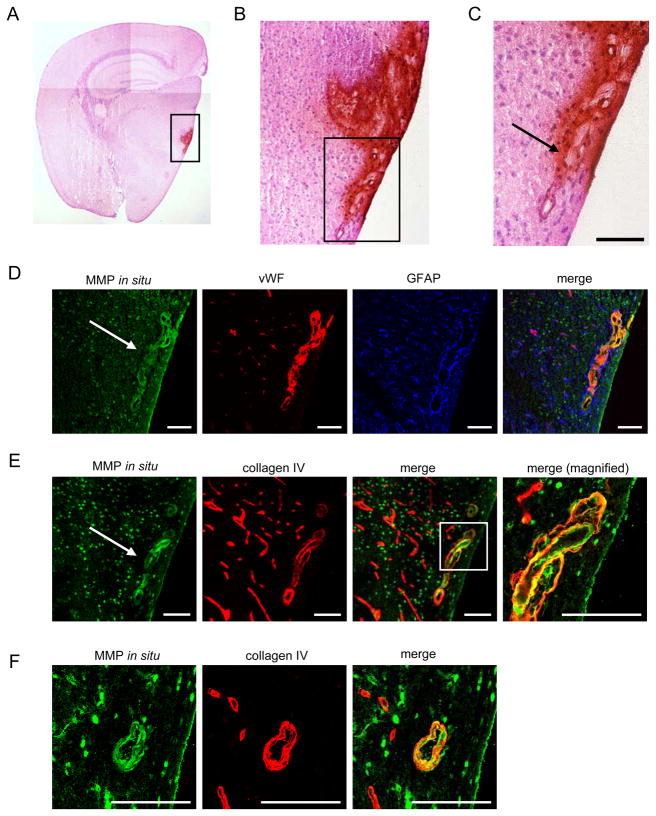
Intense in situ gelatinolytic activity on cerebral vessels was found diffusely on endothelial cells and extracellular matrix of vessels that led toward regions with ICH (Fig. 6A–E). In situ gelatinolytic activity was also occasionally detected on cerebral vessels that were not close to an ICH lesion (Fig. 6F).
Figure 6.
ICH in thalamus of SOD1−/− mouse in chronic/acute HT group. (A–C) DAB staining followed by H&E staining showing ICH lesion in thalamus. Boxes in panels (A) and (B) were magnified in panels (B) and (C), respectively. Arrow indicates a cerebral vessel that appeared to lead toward a region with ICH. (D–F) In situ zymography combined with immunohistochemistry on cerebral vessel in thalamus. Box in panel (E merge) was magnified in the panel at far right (merge, magnified). (F) Vessel that is distant from an ICH region in the thalamus. vWF: endothelial cell. GFAP: astrocytes. Collagen type IV: extracellular matrix. Images of (C) to (E) were derived from serial sections. Bars: 100 μm.
Discussion
There are two major new findings in this study. First, overexpression of SOD1 protects against increases in levels of superoxide and MMP-9, and prevents the development of spontaneous ICH during hypertension. Increases in levels of superoxide and MMP-9 preceded the development of ICH in wild-type mice, and these levels remained low in SOD1Tg mice. Second, deficiency of SOD1 increased susceptibility to ICH. ICH occurred earlier in SOD1 deficient mice than in WT-littermates, and the number and size of ICH were greater in SOD1 deficient mice than in WT-littermates. These findings provide strong evidence that oxidative stress, and perhaps activation of MMP-9, contribute to spontaneous ICH.
Oxidative stress, MMP-9, and spontaneous intracerebral hemorrhage
We recently developed a new experimental model of spontaneous ICH in C57BL/6 mice, based on the hypothesis that acute, superimposed on chronic, hypertension may increase oxidative stress and levels of MMPs, and lead to spontaneous ICH.2 We suggested that AngII-mediated oxidative stress increased, even before development of ICH, in endothelial cells and extracellular matrix of cerebral vessels, and in neurons.2 In this study, we confirmed that this model produces ICH in WT-littermates of SOD1Tg mice and of SOD1−/− mice, whose backgrounds are different from C57BL/6 mice that we studied previously.2 We also confirmed that superoxide and NAD(P)H oxidase activity increased in the brain of WT-littermates of SOD1Tg mice during acute and chronic hypertension, even before development of spontaneous ICH.
AngII-mediated oxidative stress was associated with concomitant upregulation of Nox1 and Nox4, and downregulation of antioxidant enzymes, as was in the previous study.2 Nox1 and Nox4 may have a pivotal role in increasing superoxide levels in vessels and brain of mice during increases in activity of the renin-angiotensin system.2 Downregulation of SODs may be explained, in part, by impairment of mechanisms that upregulate SOD expression through Nrf2.2 We also found that Nox2 expression was increased in WT-littermates of SOD1Tg mice with ICH, which was compatible with our previous finding that intraparenchymal blood per se produces an increase in superoxide due, at least in part, to upregulation of Nox2.7 Furthermore, changes in levels of MMP-9 paralleled levels of superoxide in the brain of WT-littermates of SOD1Tg mice. These findings support our previous observation in C57BL/6 mice, and demonstrate an association of increases in oxidative stress and activation of MMP-9 with spontaneous ICH in mice.
CuZnSOD and spontaneous intracerebral hemorrhage
To more directly determine whether there is a causal relationship between superoxide and spontaneous ICH, we studied SOD1-transgenic and -deficient mice. SOD1 is the most abundant of the three isoforms of SOD, in terms of total SOD expression and activity, within the vascular wall, and SOD1 reduces superoxide levels in cerebral blood vessels.4,9,10
We now report that overexpression of SOD1 protects against ICH, as the incidence, number, and size of ICH were decreased in SOD1Tg mice compared to WT-littermates. We also found that deficiency of SOD1 increases the incidence, number, and size of ICH. These changes in susceptibility to spontaneous ICH were concordant with changes in levels of superoxide and MMP-9.
Changes in blood pressure do not account for effects of SOD1 on susceptibility to ICH. Basal SBP and changes in SBP after induction of acute hypertension were not different in SOD1Tg mice, or SOD1−/− mice, and their WT-littermates. These findings are concordant with previous findings that blood pressure of SOD1-deficient and -transgenic mice was similar to blood pressure in WT-littermate.3,11
Instead, it is likely that effects of SOD1 on susceptibility to ICH are mediated by direct effects of superoxide on blood vessels. Overexpression of SOD1 in transgenic rats and mice decreases vascular oxidative stress, and attenuates induction and activation of MMP-9 mediated disruption of the blood-brain barrier after focal cerebral ischemia.6,12 Overexpression of SOD1 also protects against increases in superoxide and endothelial dysfunction in response to AngII.5 In contrast, deficiency of SOD1 increases superoxide levels in blood vessels, and enhances AngII-induced vascular dysfunction.4,5 SOD1 deficient mice also are susceptible to focal cerebral ischemia-reperfusion, with up-regulation of MMP-9, disruption of the blood-brain barrier, and a higher mortality than WT-littermates.3,13
We found in situ gelatinolytic activity on vessels that lead toward ICH, and occasionally on vessels that were not close to an ICH lesion. This finding corresponds to our previous finding that in situ gelatinolytic activity on cerebral vessels increased even before development of ICH.2
Thus, our findings in toto provide direct support for the hypothesis that increases in oxidative stress contribute to spontaneous ICH, possibly through activation of MMP-9, in hypertensive mice, and that SOD1 plays a critical role in protection against spontaneous ICH during hypertension.
Limitations
In this study, we have not provided direct evidence for a role of MMPs in spontaneous ICH. We speculate, however, that because induction and activation of MMPs are redox-sensitive,3 SOD1 may contribute to development of spontaneous ICH by modulating the activation of MMPs and thereby affecting degradation of cerebral vessels.
Conclusions
Overexpression of SOD1 decreased basal levels of superoxide and the incidence, size, and number of spontaneous ICH, while deficiency of SOD1 increased the susceptibility to ICH. Furthermore, spontaneous ICH occurred later in mice with overexpression of SOD1 and earlier in mice with deficiency in SOD1 than respective WT-littermates. Changes in level of MMP-9 paralleled the basal levels of superoxide. These findings suggest that SOD1 may protect against spontaneous ICH during hypertension by reducing levels of superoxide.
Acknowledgments
We thank Drs. Frank M. Faraci and Gary L. Baumbach for discussions regarding the data and study design and Dr. Yi Yang for technical assistance. This study was supported by NIH Grants NS24621 and HL62984, and funds from a Carver College of Medicine Program of Excellence.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Iida S, Baumbach GL, Lavoie JL, Faraci FM, Sigmund CD, Heistad DD. Spontaneous stroke in a genetic model of hypertension in mice. Stroke. 2005;36:1253–1258. doi: 10.1161/01.str.0000167694.58419.a2. [DOI] [PubMed] [Google Scholar]
- 2.Wakisaka Y, Chu Y, Miller JD, Rosenberg GA, Heistad DD. Spontaneous intracerebral hemorrhage during acute and chronic hypertension in mice. J Cereb Blood Flow Metab. doi: 10.1038/jcbfm.2009.183. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZnSOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- 5.Didion SP, Kinzenbaw DA, Faraci FM. Critical role for CuZn-superoxide dismutase in preventing angiotensin-II-induced endothelial dysfunction. Hypertension. 2005;46:1147–1153. doi: 10.1161/01.HYP.0000187532.80697.15. [DOI] [PubMed] [Google Scholar]
- 6.Morita-Fujimura Y, Fujimura M, Gasche Y, Copin JC, Chan PH. Overexpression of copper and zinc superoxide dismutase in transgenic mice prevents the induction and activation of matrix metalloproteinases after cold injury-induced brain trauma. J Cereb Blood Flow Metab. 2000;20:130–138. doi: 10.1097/00004647-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 7.Wakisaka Y, Miller JD, Chu Y, Baumbach GL, Wilson S, Faraci FM, Sigmund CD, Heistad DD. Oxidative stress through activation of NAD(P)H oxidase in hypertensive mice with spontaneous intracranial hemorrhage. J Cereb Blood Flow Metab. 2008;28:1175–1185. doi: 10.1038/jcbfm.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu Y, Heistad DD, Knudtson KL, Lamping KG, Faraci FM. Quantification of mRNA for endothelial NO synthase in mouse blood vessels by real-time polymerase chain reaction. Arterioscler Thromb Vasc Biol. 2002;22:611–616. doi: 10.1161/01.atv.0000012663.85364.fa. [DOI] [PubMed] [Google Scholar]
- 9.Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 10.Baumbach GL, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for CuZn superoxide dismutase. Stroke. 2006;37:1850–1855. doi: 10.1161/01.STR.0000227236.84546.5a. [DOI] [PubMed] [Google Scholar]
- 11.Fujimura M, Morita-Fujimura Y, Narasimhan O, Copin JC, Kawase M, Chan PH. Copper-Zinc superoxide dismutase prevents the early decrease of apurinic/apyriminic endonuclease and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 1999;30:2408–2415. doi: 10.1161/01.str.30.11.2408. [DOI] [PubMed] [Google Scholar]
- 12.Kamada H, Yu F, Nito C, Chan PH. Influence of hyperglycemia on oxidative stress and matrix metalloproteinase-9 activation after focal cerebral ischemia/reperfusion in rats. Relation to blood-brain barrier dysfunction. Stroke. 2007;38:1044–1049. doi: 10.1161/01.STR.0000258041.75739.cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo T, Reaume AG, Huang TT, Carlson E, Murakami K, Chen SF, Hoffman EK, Scott RW, Epstein CJ, Chan PH. Reduction of CuZn-superoxide dismutase activity exacerbates neuronal cell injury and edema formation after transient focal cerebral ischemia. J Neurosci. 1997;17:4180–4189. doi: 10.1523/JNEUROSCI.17-11-04180.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]