Abstract
Recent reports support the long-standing hypothesis that acetyl phosphate, a physiologically relevant small molecule, can serve as a phosphoryl donor to a subset of two-component response regulators that regulate diverse cellular processes. Since acetyl phosphate is a central metabolite, this ability would link nutritional status to global signaling. This review will first introduce acetyl phosphate and its pathway. It will then summarize the most compelling evidence supporting the hypothesis and list predicted properties of an acetyl phosphate-sensitive pathway. Next, it will describe emerging evidence that acetyl phosphate and/or its pathway can influence diverse cellular processes across a broad spectrum of bacteria. Finally, the review will explore the possibility that other metabolites can function in a capacity similar to acetyl phosphate.
Introduction
Acetyl phosphate (acP) is the intermediate of the phosphotransacetylase (Pta) – acetate kinase (AckA) pathway (Fig. 1A). Reversible in vivo, the Pta-AckA pathway interconverts Coenzyme A (CoASH), ATP and acetate with acetyl-Coenzyme A (acCoA), ADP and inorganic phosphate (Pi). This reversibility permits both acCoA synthesis (acetate activation) and acetate evolution (acetogenesis). During acetogenesis, Pta synthesizes acP and CoASH from acCoA and Pi, while AckA generates ATP from acP and ADP. Simultaneously, AckA produces acetate, which cells excrete into the environment. Thus, the steady state concentration of acP depends upon the rate of its formation catalyzed by Pta and the rate of its degradation catalyzed by AckA [1,2].
Figure 1.
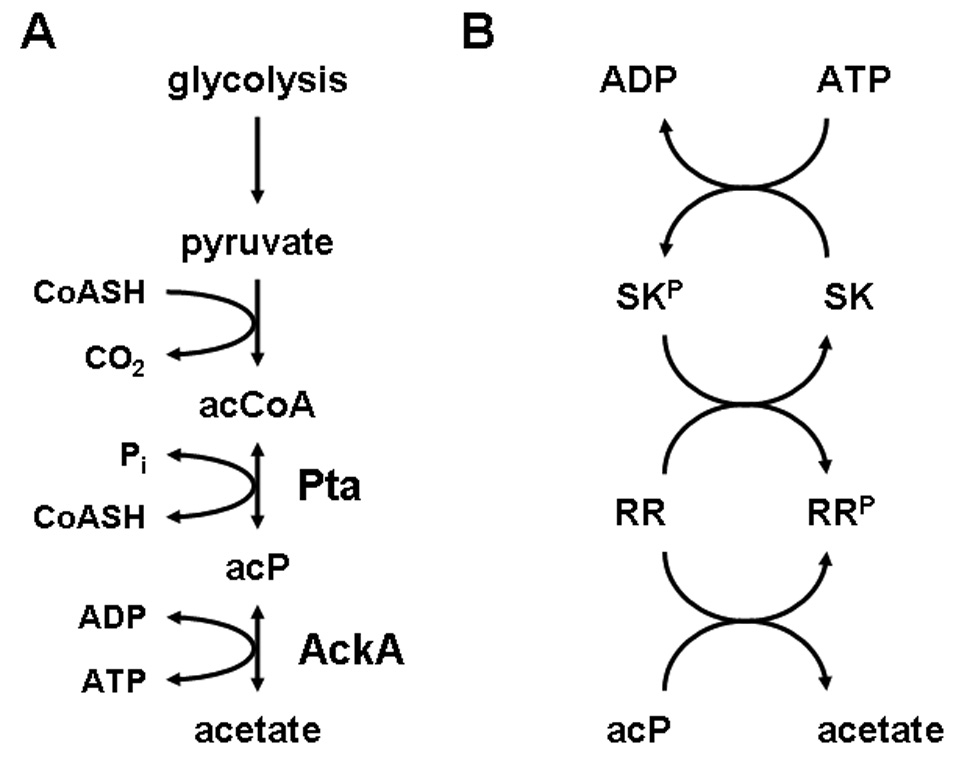
(A) Schematic of the Pta-AckA pathway. CoASH, coenzyme A; acCoA, acetyl-CoA; Pi, inorganic phosphate; Pta, phosphotransacetylase; acP, acetyl phosphate; AckA, acetate kinase. (B) Schematic of a two-component pathway in which the response regulator (RR) possesses two distinct sources for its phosphoryl group: its cognate sensor kinase (SK) and acP. Note that some SKs also can function as RR phospho-aspartyl phosphatases, removing the phosphoryl group from the phosphorylated form of their cognate RR, and leading to formation of the unphosphorylated RR and Pi.
Acetogenesis serves several key functions. It recycles CoASH, facilitating glycolytic flux and, hence, rapid growth in the presence of excess carbon, e.g. glucose [1,2]. This function also can re-initiate stalled TCA cycle function, providing CoASH to convert α-ketoglutarate to succinyl-CoA [3]. Critically, acetogenesis provides the majority of ATP in the absence of robust TCA cycle activity [1,2]. Thus, acetogenesis may be especially critical for pathogens that do not possess a complete TCA cycle, e.g. Yersinia pestis, Streptococcus pneumoniae and certain isolates of Staphylococcus aureus [4].
However, the conversion of acCoA to acetate and ATP often does not go to completion: for example, Escherichia coli cells maintain a significant pool of acP [5,6], which serves two distinct and equally important functions. First, acP serves as a storage molecule: of carbon (C), of phosphate (OPO3), and of energy in the form of its high-energy C-OPO3 bond [7]. This bond possesses a larger ΔG0 of hydrolysis (−43.3 kJ/mol) than ATP; i.e. acP stores more energy than ATP [8,9]. The ability to store energy forms the basis for acP's second role as a global signal.
Evidence that acP is a global signal
AcP has been proposed to act as a global signal by donating its phosphoryl group to a subset of response regulators of the family of two-component signal transduction (2CST) pathways [10,11]. The most fundamental of 2CST pathways consists of a sensor kinase (SK) and a response regulator (RR) (Fig. 1B). The SK autophosphorylates a conserved histidine residue using ATP (but not acP) as its phosphoryl donor. The RR autophosphorylates on a conserved aspartate residue, using its phosphorylated cognate SK as phosphoryl donor. Some SKs also possess RR phospho-aspartyl phosphatase activity [12].
A wealth of data supports the proposal that acP can serve as a phosphoryl donor to RRs. First, many purified RRs autophosphorylate using acP as their phosphoryl donor [1]. Second, the acP concentration varies several orders of magnitude in response to environmental conditions [5,6]. Third, the acP concentration in wild-type E. coli cells can reach at least 3 mM [5], which suffices for efficient autophosphorylation in vitro. The rate of phosphotransfer from acP to the canonical RR CheY increases linearly with acP concentration and does not saturate [13]. Thus, one would expect that the in vivo CheY-P concentration due to acP-dependent autophosphorylation would be directly proportional to acP concentration over the entire range of acP concentrations experienced by the cell. Fourth, acP can regulate about one hundred E. coli genes, inhibiting genes for flagellation, while activating genes for encapsulation and piliation [14]. This impact by acP on flagellar and capsule genes depends on RcsB [15], a well-characterized RR known to control hundreds of genes in E. coli [16] and Salmonella enterica [17]. Importantly, this dependence on RcsB does not extend to the cognate SK, RcsC [15]. Together, these reports support the hypothesis that acP can act as a global signal in vivo and that acP does so by donating its phosphoryl group directly to certain RRs.
Evolution of two-component signaling: a hypothesis
The ability of acP to activate RRs may represent an ancient mechanism [10]. Because it is much simpler than ATP and possesses more energy, acP may have been the primordial (pre-ATP) source of metabolic energy [18]. If so, then it is easy to imagine evolution of the original acP-sensitive RR, permitting primordial cells to monitor and respond to their energy status. Fine-tuning of this primitive signal pathway would have required regulation and thus favored evolution of a cognate phosphatase. Later, this phospho-RR phosphatase could have evolved kinase activity and, in many cases but not all, lost its original activity.
Specifications of an acP-sensitive 2CST pathway
RRs that respond to acP under physiologically relevant conditions seem to possess the following properties: 1) their cognate SK functions primarily as a net phosphatase, 2) they exist in excess over their cognate SK, or 3) they are orphans that lack a cognate SK. Class 1 includes RcsB and NtrC/NRI. Certain environmental conditions favor the phosphatase activity of their cognate SKs (RcsC and NtrB/NRII, respectively). Under such conditions, activation of the respective RRs (RcsB and NtrC/NRI) depends on the acP pool [15,19]. Class 2 includes OmpR, which is synthesized in excess over its cognate SK EnvZ [20]. OmpR activation often depends on EnvZ kinase activity; however, under conditions that do not favor kinase activity, activation depends strictly on acP [21–23]. Class 3 includes RssB of E. coli. In the absence of a cognate SK, activity of the orphan RR RssB depends in part upon acP [24].
Emerging acP stories
Early studies implicating acP in biologically relevant activation of RRs, especially NtrC/NRI and OmpR, have been reviewed extensively [1]. Below, I will focus on more recent developments.
CpxR, carbon excess and an envelope stress response
The CpxAR 2CST system of E. coli and other pathogens senses diverse envelope stresses and promotes transcription of genes that remedy these stresses [25]. A key member of the CpxR regulon is cpxP, whose promoter responds to two classes of stimuli: those that depend on the SK CpxA (e.g. alkaline pH) and those that do not (e.g. exposure to excess carbon) [26,27]. Critically for an acP-sensitive pathway, CpxA functions as a net phosphatase under the latter conditions [26]. The existence of this CpxA-independent, CpxR-dependent response begs the question: Does the excess carbon response require CpxR phosphorylation? The answer appears to be yes; excess carbon-induced cpxP transcription requires the conserved residue D51. Moreover, acP seems to be the phosphoryl donor: cells that cannot synthesize acP (pta ackA) do not respond to excess carbon, while cells that accumulate acP (ackA) respond more strongly than the wild-type parent. Intriguingly, this behavior is only apparent upon mutation of a conserved lysine (K291) of the alpha subunit of RNA polymerase (Bruno Lima, Bozena Zemaitaitis, and Alan J. Wolfe, unpublished). Because K291 has been reported to be acetylated [28], these observations are consistent with a model in which the excess carbon response depends on acP [27] and some non-acP-dependent consequence of Pta-AckA pathway disruption - perhaps acetylation of RNAP [26]. This role for acP could potentially extend to type three secretion, attachment, and virulence because the CpxAR system of Y. pseudotuberculosis fundamentally behaves like that of E. coli [29].
VanR and antibiotic resistance
Exposure of Streptomyces coelicolor or Enterococcus faecium to the antibiotic vancomycin induces resistance. Regulation of resistance requires the 2CST pathway VanSR. Like CpxA, the SK VanS can function as a net kinase or a net phosphatase. Exposure to vancomycin favors kinase activity, phosphorylation of the RR VanR, and transcription of the vancomycin resistance genes (van) [30]. In the absence of vancomycin, however, VanS phosphatase activity is favored [30]. Because vanS mutants lack the cognate kinase and express van constitutively [31] and because resistance requires the conserved residue D51 of VanR, an alternative phosphoryl donor likely exists. The evidence overwhelmingly points to acP. First, mutants lacking both VanS and the Pta-AckA pathway are vancomycin sensitive and do not express van genes. Second, acP donates its phosphoryl group to purified VanR [30]. Third, heterologous expression of VanR and a VanR-dependent promoter in an E. coli ackA mutant, which accumulates acP [5], results in elevated transcription [32].
OmpR and antibiotic synthesis
Xenorhabdus nematophilus, which engages in a mutualistic association with its nematode host and a pathogenic association with insect hosts, produces the antibiotic xenocoumacin. Because the RR OmpR inhibits synthesis and the loss of its cognate SK EnvZ increases antibiotic synthesis, inhibition likely requires phospho-OmpR [33]. EnvZ, however, is unlikely to be the only phosphoryl donor: like cells that lack EnvZ, those that retain EnvZ but lack acP (pta ackA) produce more antibiotic than the wild-type parent, while envZ mutants that accumulate acP (envZ ackA) synthesize less [34]. Thus, both EnvZ and acP likely contribute to OmpR phosphorylation.
Rrp2 and Lyme disease
The RR Rrp2 of the Lyme disease spirochete B. burgdorferi controls expression of several major virulence genes. Phosphorylation of Rrp2 and thus its activation appears to depend on acP. Inactivation of the four B. burgdorferi SKs has no effect upon Rrp2-dependent expression. Multiple attempts to inactivate the Pta-AckA pathway failed, suggesting that this pathway may be essential to growth in vitro. This is not surprising, since B. burgdorferi lacks both the TCA cycle and all known enzymes that convert pyruvate to acCoA. However, exposure to exogenous acetate activates Rrp2-dependent genes in a dose-dependent manner, while overexpression of Pta virtually eliminates this acetate response. Together, these observations argue for a model in which acP plays a major role in Borrelia pathogenesis (Haijun Xu, Melissa Caimano, Tao Lin, Ming He, Justin Radolf, Steven J. Norris, Frank Gheradini, Alan J. Wolfe, and X. Frank Yang, unpublished).
VicR and essentiality
Within the Firmicutes, the 2CST pathway VicKR (also known as YycGF or WalKR) is ubiquitous. For many family members, e.g. Bacillus subtilis, both the SK and the RR are essential. For others, i.e. S. pneumoniae (pneumococcus) and S. mutans, the only essential component appears to be the RR VicR [35]. For pneumococcal VicR, growth requires that the conserved residue D52 become phosphorylated. This SK-independent, phosphorylation-dependent behavior begs the question: does acP serve as the phosphoryl donor? The answer appears to be 'yes.'
For wild-type pneumococcus growing exponentially in Brain Heart Infusion (BHI), pyruvate oxidase (SpxB) synthesizes about 80% of the acP [36] (Malcolm Winkler, personal communication). The other 20% is produced by the Pta-AckA pathway. The spxB mutant is attenuated for virulence in murine pneumonia models of infection [37]. In contrast to an earlier report [36], new determinations show that cellular ATP levels are not reduced in the spxB mutant relative to its parent (Malcolm Winkler, personal communication). Thus, ATP deficiency likely does not cause attenuation.
To test the involvement of acP, a triple spxB ackA pta mutant was constructed, which lacked measurable levels of acP. When grown in BHI, this triple mutant exhibited a similar growth rate and yield as its parent. However, microarray studies revealed decreased transcript levels from genes regulated by three different pneumococcal 2CST systems (Malcolm Winkler, personal communication) that play roles in maintaining cell wall homeostasis, including VicRK, which can autophosphorylate in vitro using acP as its phosphoryl donor [38]. Epistasis experiments accompanied by determinations of cellular acP amounts indicated that decreased transcript levels largely correlated with acP amount. Furthermore, combinations of mutations that restored acP amounts also restored the expression of the 2CST regulons. It is noteworthy that these strains retained the cognate SKs. Thus, the effects could not result from aberrant cross-talk due to the lack of phosphatase activity (Malcolm Winkler, personal communication).
Together, the results described in the previous paragraph suggest that acP may contribute to the basal level of phosphorylation of VicR and the other two RRs that impact cell wall homeostasis. However, to detect effects on regulon transcription, acP levels must be reduced to below 20% of their normal amount because the spxB mutant did not exhibit an appreciable effect on 2CST target genes [37]. It remains to be determined whether metabolic conditions exist that lead to 80% reductions in the acP pool in wild-type pneumococcus. On the other hand, changes in acP amounts must be considered in studies of conditions that modulate the signaling and regulation performed by the three cell wall homeostasis-associated 2CST pathways (Malcolm Winkler, personal communication).
Emerging Pta-AckA stories: a cautionary tale
The Pta-AckA pathway does more than synthesize acP. During acetogenesis, this pathway recycles CoASH, generates ATP, and evolves acetate. During acetate activation, it synthesizes acCoA. Thus, disruption impacts more than acP and a mutant phenotype can result from the inability to utilize acetate as a carbon source, from the reduced ability to excrete acetate or efficiently generate ATP, or from some perturbation of central metabolism. The following studies provide evidence for pathway involvement but lack definitive proof that the central figure is acP. For some, further study may verify a key role for acP. For others, continued investigation may discover new mechanisms that do not involve acP or even 2CST.
DspA and pigment production
Under nitrogen-replete conditions, the photosynthetic cyanobacterium Synechocystis sp. PCC6803 assembles two photosystems (PSI and PSII). Loss of either the SK DspA (dspA) or acP (pta) decreases PSII. Loss of both DspA and acP (dspA pta) causes almost complete absence of PSII. When nitrogen-starved, dspA and pta mutants and their WT parent become almost completely bleached. In contrast, the double dspA pta mutant retains large amounts of PSI. Thus, DspA and acP both may contribute to the phosphorylation of an unknown RR that controls photosystem degradation [39]. However, the ackA mutant was not tested. If acP donates its phosphoryl group to a RR, then cells that accumulate acP (ackA) should exhibit behavior opposite that of cells that cannot synthesize acP (pta). In the absence of this test, the possibility remains that the observed behaviors result from some non-acP-associated consequence of Pta-AckA pathway disruption.
DegU, biofilm development, and the virulence
It has been proposed that acP donates its phosphoryl donor to DegU, a RR that controls the transition from motile individual to sessile biofilm. In B. subtilis, DegU possesses a cognate SK called DegS. Intriguingly, loss of DegS has no effect on transcription from the DegU-dependent flgB promoter. Since activation of the flgB promoter requires the conserved D56 residue of DegU, two alternative phosphoryl donors were envisioned: a non-cognate SK and acP [40]. Support for the latter comes from studies of L. monocytogenes, which does not encode DegS. Hence, DegU is an orphan that can receive a phosphoryl group from acP if the conserved D55 residue remains intact. Loss of the Pta-AckA pathway results in behaviors similar to those of the D55N mutant, lending support for acP. Unfortunately, attempts to construct an ackA mutant failed [41], leaving open the possibility that mutant behavior results from some non-acP consequence of Pta-AckA pathway disruption. The approaches used to test acP involvement in the activation of B. burgdorferi Rrp2 (Haijun Xu, Melissa Caimano, Tao Lin, Ming He, Justin Radolf, Steven J. Norris, Frank Gheradini, Alan J. Wolfe, and X. Frank Yang, unpublished) might resolve the issue.
The Pta-AckA pathway and colonization
The Pta-AckA pathway is clearly involved in the ability of several pathogens to colonize their hosts, but the specific mechanism is not apparent. Two recent studies highlight the problem. Cells of S. enterica that lack Pta are attenuated for virulence in mice. Unfortunately, the authors were unable to determine the mechanism, although they presented solid evidence against loss of acetate activation [42]. Uropathogenic E. coli (UPEC) is adapted for growth in urine, which contains abundant d- and l-serine. Exposure to d-serine upregulates pta and ackA transcription. Disruption of either gene attenuates kidney colonization, while disruption of both genes attenuates colonization of both kidney and bladder. All three mutants grow more poorly than their WT parent in tryptone broth, whose composition resembles urine. However, the growth defect likely does not explain attenuation since the single mutants colonize the bladder while the double mutant does not. Although leaving open the possibility that acP is involved, the authors correctly recognize that attenuation could result from some other consequence of Pta-AckA pathway disruption [43].
The Pta-AckA pathway and protein quality control
Efficient ATP-dependent proteolysis, protein folding, and aggregation in E. coli requires the Pta-AckA pathway. Mutants that lack the entire pathway (pta ackA) exhibit a pleiotropic phenotype including temperature sensitive growth, slowed rate of unstable protein degradation, accumulation of protein aggregates, increased heat shock protein expression, and thermotolerance [44,45]. This phenotype might be due to the absence of acP, but also could be due to some other consequence of Pta-AckA pathway disruption. The observation that ackA mutants exhibit a temperature-sensitive growth defect similar to that of the pta ackA mutant suggests that the latter is quite likely (Sylvia A. Reimann and Alan J. Wolfe, unpublished).
Concluding remarks
In addition to acP, other small phosphorylated compounds can donate their phosphoryl group to purified RRs [46,47]. Of these, carbamoyl phosphate and γ-glutamyl phosphate may be the most relevant physiologically {Box 1). Currently, no evidence exists that carbamoyl phosphate can function as a phosphoryl donor in vivo and only one report proposes such a role for γ-glutamyl phosphate [48]. However, it would be enlightening to perform a rigorous test of the ability of these physiologically relevant phosphorylated compounds to function as phosphoryl donors in vivo. In E. coli, for example, one could screen for promoters that respond to either compound by comparing cells that accumulate them to those that do not synthesize them.
Box 1. Future directions.
How extensive is the impact of acP on the set of 2CST pathways in E. coli?
In other organisms, which 2CST pathways and thus cellular processes are influenced by acP?
Do other high-energy phosphorylated metabolites function like acP?
How does the acP pool change relative to the rest of the central metabolome?
In addition to CheY, are other RRs acetylated? If so, what is the impact of acetylation on RR function?
The possibility also exists for involvement by some non-phosphorylated central metabolite, e.g. acCoA. This high-energy molecule is well-known for its ability to donate acetyl groups to proteins [49]. The acetylome of E. coli appears to be extensive [28,50]. Although no RRs were among the proteins identified as acetylated, these high-throughput proteome studies were designed to query highly abundant proteins, which RRs are not. For example, the chemotaxis RR CheY is reported to be acetylated [51]; yet, CheY was not identified by either study. Could other RRs be acetylated? If so, how does acetylation influence their activity? Only time will tell (Box 1).
Acknowledgments
I wish to thank Ruth Silversmith and Robert Bourret for giving me the opportunity to write this review, Malcolm Winkler for communication of information prior to publication, the National Institutes of Health for funding (grant GM066130), and my collaborators and colleagues for both their skepticism and support. Finally, I want to thank all the past and present members of my laboratory who contributed directly or indirectly to this endeavor.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wolfe AJ. The acetate switch. Microbiol Mol Biol Rev. 2005;69:12–50. doi: 10.1128/MMBR.69.1.12-50.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawers RG, Clark DP. Chapter 3.5.3, Fermentative pyruvate and acetyl-coenzyme A metabolism. In: Böck RCI A, Kaper JB, Neidhardt FC, Nyström T, Rudd KE, Squires CL, editors. EcoSal–Escherichia coli and Salmonella: cellular and molecular biology. ASM Press; 2004. Jul 27, [Online.] http://www.ecosal.org, posting date. [Google Scholar]
- 3.El-Mansi M. Flux to acetate and lactate excretions in industrial fermentations: physiological and biochemical implications. J Ind Microbiol Biotechnol. 2004;31:295–300. doi: 10.1007/s10295-004-0149-2. [DOI] [PubMed] [Google Scholar]
- 4.Somerville GA, Said-Salim B, Wickman JM, Raffel SJ, Kreiswirth BN, Musser JM. Correlation of acetate catabolism and growth yield in Staphylococcus aureus: implications for host-pathogen. Infect Immun. 2003;71:4724–4732. doi: 10.1128/IAI.71.8.4724-4732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klein AH, Shulla A, Reimann SA, Keating DH, Wolfe AJ. The intracellular concentration of acetyl phosphate in Escherichia coli is sufficient for direct phosphorylation of two-component response regulators. J Bacteriol. 2007;189:5574–5581. doi: 10.1128/JB.00564-07. This reassessment of the intracellular acetyl phosphate concentration demonstrates that the acetyl phosphate pool can be at least as large as that of ATP and thus provides strong biochemical support for the hypothesis that acetyl phosphate can serve as a phosphoryl donor in vivo.
- 6.Keating DH, Shulla A, Klein AH, Wolfe AJ. Optimized two-dimensional thin layer chromatography to monitor the intracellular concentration of acetyl phosphate and other small phosphorylated molecules. Biological Procedures On-line. 2008;10:36046. doi: 10.1251/bpo141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metzler DE. Biochemistry: the Chemical Reactions of Living Cells. vol 1. San Diego: Academic Press; 2001. [Google Scholar]
- 8.Lehninger AL. Biochemistry. edn 2. New York, N. Y.: Worth Publishers, Inc.; 1975. [Google Scholar]
- 9.Voet D, Voet JG. Biochemistry. New York: John Wiley & Sons, Inc.; 1990. [DOI] [PubMed] [Google Scholar]
- 10.McCleary WR, Stock JB, Ninfa AJ. Is acetyl phosphate a global signal in Escherichia coli? J. Bacteriol. 1993;175:2793–2798. doi: 10.1128/jb.175.10.2793-2798.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wanner BL. Gene regulation by phosphate in enteric bacteria. J Cell Biochem. 1993;51:47–54. doi: 10.1002/jcb.240510110. [DOI] [PubMed] [Google Scholar]
- 12.Gao R, Stock AM. Biological insights from structures of two-component proteins. Ann Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Re SS, Deville-Bonne D, Tolstykh T, Veron M, Stock JB. Kinetics of CheY phosphorylation by small molecule phosphodonors. FEBS Lett. 1999;457:323–326. doi: 10.1016/s0014-5793(99)01057-1. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe AJ, Chang D-E, Walker JD, Seitz-Partridge JE, Vidaurri MD, Lange CF, Pruess BM, Henk MC, Larkin JC, Conway T. Evidence that acetyl phosphate functions as a global signal during biofilm development. Mol Microbiol. 2003;48:977–988. doi: 10.1046/j.1365-2958.2003.03457.x. [DOI] [PubMed] [Google Scholar]
- 15.Fredericks CE, Shibata S, Aizawa S-I, Reimann SA, Wolfe AJ. Acetyl phosphate-sensitive regulation of flagellar biogenesis and capsular biosynthesis depends on the Rcs phosphorelay. Mol Microbiol. 2006;61:734–747. doi: 10.1111/j.1365-2958.2006.05260.x. [DOI] [PubMed] [Google Scholar]
- 16.Pruss BM, Besemann C, Denton A, Wolfe AJ. A complex transcription network controls the early stages of biofilm development by Escherichia coli. J Bacteriol. 2006;188:3731–3739. doi: 10.1128/JB.01780-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Zhao Y, McClelland M, Harshey RM. The RcsCDB signaling system and swarming motility in Salmonella enterica serovar Typhimurium: dual regulation of flagellar and SPI-2 virulence genes. J Bacteriol. 2007;189:8447–8457. doi: 10.1128/JB.01198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Martin W, Russell MJ. On the origin of biochemistry at an alkaline hydrothermal vent. Phil Trans Royal Soc B: Biol Sci. 2007;362:1887–1926. doi: 10.1098/rstb.2006.1881. How did metabolism begin? The authors have built an extremely credible model in which small phosphorylated compounds provide energy to an ATP-less world. A must read for anyone who wants to understand how metabolic pathways and, by extension, signal transduction pathways evolved.
- 19.Ninfa AJ, Jiang P, Atkinson MR, Peliska JA. Integration of antagonistic signals in the regulation of nitrogen assimilation in Escherichia coli. Curr Top Cell Regul. 2000;36:31–75. doi: 10.1016/s0070-2137(01)80002-9. [DOI] [PubMed] [Google Scholar]
- 20.Cai SJ, Inouye M. EnvZ-OmpR interaction and smoregulation in Escherichia coli. J Biol Chem. 2002;277:24155–24161. doi: 10.1074/jbc.M110715200. [DOI] [PubMed] [Google Scholar]
- 21.Matsubara M, Mizuno T. EnvZ-independent phosphotransfer signaling pathway of the OmpR-mediated osmoregulatory expression of OmpC and OmpF in Escherichia coli. Biosci Biotechnol Biochem. 1999;63:408–414. doi: 10.1271/bbb.63.408. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Ferenci T. An analysis of multifactorial influences on the transcriptional control of ompF and ompC porin expression under nutrient limitation. Microbiol. 2001;147:2981–2989. doi: 10.1099/00221287-147-11-2981. [DOI] [PubMed] [Google Scholar]
- 23.Heyde M, Laloi P, Portalier R. Involvement of carbon source and acetyl phosphate in the external-pH-dependent expression of porin genes in Escherichia coli. J. Bacteriol. 2000;182:198–202. doi: 10.1128/jb.182.1.198-202.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouche S, Klauck E, Fischer D, Lucassen M, Jung K, Hengge-Aronis R. Regulation of RssB-dependent proteolysis in Escherichia coli: a role for acetyl phosphate in a response regulator-controlled process. Mol Microbiol. 1998;27:787–795. doi: 10.1046/j.1365-2958.1998.00725.x. [DOI] [PubMed] [Google Scholar]
- 25.MacRitchie DM, Buelow DR, Price NL, Raivio TL. Two-component signaling and Gram negative envelope stress response systems. In: Utsumi R, editor. Bacterial Signal Transduction: Networks and Drug Targets. Springer; 2008. pp. 80–110. Advances in Experimental Medicine and Biology, vol 631. [DOI] [PubMed] [Google Scholar]
- 26. Wolfe AJ, Parikh N, Lima BP, Zemaitaitis B. Signal integration by the two-component signal transduction response regulator CpxR. J Bacteriol. 2008;190:2314–2322. doi: 10.1128/JB.01906-07. How does one determine if acetyl phosphate or some other high-energy metabolite impacts a signal transduction pathway? This detailed genetic analysis provides an answer.
- 27.Danese PN, Silhavy TJ. CpxP, a Stress-Combative Member of the Cpx Regulon. J. Bacteriol. 1998;180:831–839. doi: 10.1128/jb.180.4.831-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu BJ, Kim JA, Moon JH, Ryu SE, Pan JG. The diversity of lysine-acetylated proteins in Escherichia coli. J Microbiol Biotechnol. 2008;18:1529–1536. [PubMed] [Google Scholar]
- 29.Carlsson KE, Liu J, Edqvist PJ, Francis MS. Influence of the Cpx extracytoplasmic-stress-responsive pathway on Yersinia sp.-eukaryotic cell contact. Infect Immun. 2007;75:4386–4399. doi: 10.1128/IAI.01450-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutchings MI, Hong H-J, Buttner MJ. The vancomycin resistance VanRS two-component signal transduction system of Streptomyces coelicolor. Mol Microbiol. 2006;59:923–935. doi: 10.1111/j.1365-2958.2005.04953.x. [DOI] [PubMed] [Google Scholar]
- 31.Arthur M, Depardieu F, Gerbaud G, Galimand M, Leclercq R, Courvalin P. The VanS sensor negatively controls VanR-mediated transcriptional activation of glycopeptide resistance genes of Tn1546 and related elements in the absence of induction. J Bacteriol. 1997;179:97–106. doi: 10.1128/jb.179.1.97-106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haldimann A, Fisher S, Daniels L, Walsh C, Wanner B. Transcriptional regulation of the Enterococcus faecium BM4147 vancomycin resistance gene cluster by the VanS-VanR two-component regulatory system in Escherichia coli K-12. J Bacteriol. 1997;179:5903–5913. doi: 10.1128/jb.179.18.5903-5913.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park D, Forst S. Co-regulation of motility, exoenzyme and antibiotic production by the EnvZ-OmpR-FlhDC-FliA pathway in Xenorhabdus nematophila. Mol Microbiol. 2006;61:1397–1412. doi: 10.1111/j.1365-2958.2006.05320.x. [DOI] [PubMed] [Google Scholar]
- 34. Park D, Ciezki K, Hoeven Rvd, Singh S, Reimer D, Bode HB, Forst S. Genetic analysis of xenocoumacin antibiotic production in the mutualistic bacterium Xenorhabdus nematophila. Mol Microbiol. 2009;73:938–949. doi: 10.1111/j.1365-2958.2009.06817.x. A good example of how genetics should be used to test for the involvement of acetyl phosphate (or any other metabolite) in a signaling pathway.
- 35. Winkler ME, Hoch JA. Essentiality, bypass, and targeting of the YycFG (VicRK) two-component regulatory system in Gram-positive bacteria. J Bacteriol. 2008;190:2645–2648. doi: 10.1128/JB.01682-07. How does one determine if a signal transduction pathway is essential? This short review provides an answer that includes the possibility that some organisms can use a high-energy metabolite, e.g. acetyl phosphate, in lieu of the cognate sensor kinase.
- 36.Pericone CD, Park S, Imlay JA, Weiser JN. Factors contributing to hydrogen peroxide resistance in Streptococcus pneumoniae include pyruvate oxidase (SpxB) and avoidance of the toxic effects of the Fenton reaction. J Bacteriol. 2003;185:6815–6825. doi: 10.1128/JB.185.23.6815-6825.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramos-Montañez S, Tsui H-CT, Wayne KJ, Morris JL, Peters LE, Zhang F, Kazmierczak KM, Sham L-T, Winkler ME. Polymorphism and regulation of the spxB (pyruvate oxidase) virulence factor gene by a CBS-HotDog domain protein (SpxR) in serotype 2 Streptococcus pneumoniae. Mol Microbiol. 2008;67:729–746. doi: 10.1111/j.1365-2958.2007.06082.x. [DOI] [PubMed] [Google Scholar]
- 38.Ng W-L, Tsui H-CT, Winkler ME. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J Bacteriol. 2005;187:7444–7459. doi: 10.1128/JB.187.21.7444-7459.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morrison SS, Mullineaux C, Ashby M. The influence of acetyl phosphate on DspA signalling in the Cyanobacterium Synechocystis sp. PCC6803. BMC Microbiol. 2005;5:47. doi: 10.1186/1471-2180-5-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi K. Gradual activation of the response regulator DegU controls serial expression of genes for flagellum formation and biofilm formation in Bacillus subtilis. Mol Microbiol. 2007;66:395–409. doi: 10.1111/j.1365-2958.2007.05923.x. [DOI] [PubMed] [Google Scholar]
- 41.Gueriri I, Bay S, Dubrac S, Cyncynatus C, Msadek T. The Pta-AckA pathway controlling acetyl phosphate levels and the phosphorylation state of the DegU orphan response regulator both play a role in regulating Listeria monocytogenes motility and chemotaxis. Mol Microbiol. 2008;70:1342–1357. doi: 10.1111/j.1365-2958.2008.06496.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim YR, Brinsmade SR, Yang Z, Escalante-Semerena J, Fierer J. Mutation of phosphotransacetylase but not isocitrate lyase reduces the virulence of Salmonella enterica serovar Typhimurium in mice. Infect Immun. 2006;74:2498–2502. doi: 10.1128/IAI.74.4.2498-2502.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anfora AT, Halladin DK, Haugen BJ, Welch RA. Uropathogenic Escherichia coli CFT073 is adapted to acetatogenic growth but does not require acetate during murine urinary tract infection. Infect Immun. 2008;76:5760–5767. doi: 10.1128/IAI.00618-08. Understanding attenuation can be quite difficult. Disruption of the Pta-AckA pathway has multiple consequences, including but not limited to the loss or accumulation of acetyl phosphate. Which of these consequences causes the observed attenuation of Pta-AckA pathway mutants?
- 44.Mizrahi I, Biran D, Ron EZ. Involvement of the Pta-AckA pathway in protein folding and aggregation. Res Microbiol. 2009;160:80–84. doi: 10.1016/j.resmic.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 45.Mizrahi I, Biran D, Ron EZ. Requirement for the acetyl phosphate pathway in Escherichia coli ATP-dependent proteolysis. Mol Microbiol. 2006;62:201–211. doi: 10.1111/j.1365-2958.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- 46.Feng J, Atkinson MR, McCleary W, Stock JB, Wanner BL, Ninfa AJ. Role of phosphorylated metabolic intermediates in the regulation of glutamine synthetase synthesis in Escherichia coli. J Bacteriol. 1992;174:6061–6070. doi: 10.1128/jb.174.19.6061-6070.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukat GS, McCleary WR, Stock AM, Stock JB. Phosphorylation of bacterial response regulator proteins by low molecular weight phospho-donors. Proc Natl Acad Sci USA. 1992;89:718–722. doi: 10.1073/pnas.89.2.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogura M, Kawata-Mukai M, Itaya M, Takio K, Tanaka T. Multiple copies of the proB gene enhance degS-dependent extracellular protease production in Bacillus subtilis. J Bacteriol. 1994;176:5673–5680. doi: 10.1128/jb.176.18.5673-5680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yang X-J, Seto E. Lysine acetylation: codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31:449–461. doi: 10.1016/j.molcel.2008.07.002. Is the histone code simply a subset of a much broader protein modification code? This stellar review summarizes the current understanding of the process and consequences of protein acetylation in eukaryotes. With the recent discovery of the bacterial acetylome, this review is a must read.
- 50.Zhang J, Sprung R, Pei J, Tan X, Kim S, Zhu H, Liu C-F, Grishin NV, Zhao Y. Lysine acetylation is a highly abundant and evolutionarily conserved modification in Escherichia coli. Mol Cell Proteomics. 2009;8:215–225. doi: 10.1074/mcp.M800187-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barak R, Yan J, Shainskaya A, Eisenbach M. The chemotaxis response regulator CheY can catalyze its own acetylation. J Mol Biol. 2006;359:251–265. doi: 10.1016/j.jmb.2006.03.033. [DOI] [PubMed] [Google Scholar]


