Abstract
Electrocorticographic brain recordings from patients with surgically implanted electrodes have recently emerged as a powerful tool for examining the neural basis of human cognition. These recordings measure the brain's electrical activity directly, and thus provide data with a higher temporal and spatial resolution than other human neuroimaging techniques. Here, we review recent research in this area and, in particular, we explain how electrocorticographic recordings have informed the neural basis of human working memory, episodic memory, language, and spatial cognition. In some cases this research has identified patterns of human brain activity that were unexpected on the basis of studies in animals.
Brain oscillations and cognition
Neuronal oscillations are a fundamental component of normal brain function. In both humans and animals, neuronal oscillations exhibit specific spatiotemporal patterns that show active brain regions, indicate the types of neuronal computations that occur, and reveal how information flows through the brain. For ethical reasons researchers typically examine these phenomena only in animals. However, in the past decade researchers have increasingly examined electrocorticographic (ECoG) recordings of brain oscillations from patients with surgically implanted electrodes. These recordings measure human brain activity with a higher spatial and temporal resolution than other recording techniques. During ECoG monitoring, patients are typically conscious and capable of performing complex cognitive tasks in free time between clinical procedures. Thus, researchers can use these recordings to study electrophysiological correlates of a wide range of cognitive processes [1, 2].
Here we review recent research using ECoG recordings of brain oscillations to analyze the neural basis of cognition. First, we outline the patterns of oscillations that appear in human ECoG recordings and describe how these signals relate to neuronal spiking. Then, we explain how this research expands our understanding of the neural basis of four complex cognitive domains: working memory, episodic memory, language, and spatial cognition.
Human electrocorticographic recordings
Because they measure brain activity with high spatial and temporal resolution, surgically implanted electrodes help physicians diagnose and treat neurological conditions such as epilepsy, Parkinson's disease, and tumors. Here our focus is on ECoG recordings from patients undergoing invasive monitoring for drug-resistant epilepsy. In this procedure, surgeons implant ∼40–120 electrodes in widespread brain regions (Fig. 1A) to identify epileptic foci for potential surgical resection. Electrodes remain implanted throughout each patient's ∼1–3-week hospitalization. These electrodes include grid and strip electrodes (Fig. 1B,C), which record ECoG signals from the cortical surface, and depth electrodes (Fig. 1D), which penetrate the cortex to record field potentials from deep brain structures. In this review we use the term ‘ECoG’ to refer to both surface and depth recordings. On occasion surgeons implant microelectrodes, which record individual action potentials (Fig. 1E). Here, we discuss microelectrode recordings only briefly because this procedure is rare and has been reviewed recently [3].
Figure 1. Performing electrocorticographic recordings in humans.
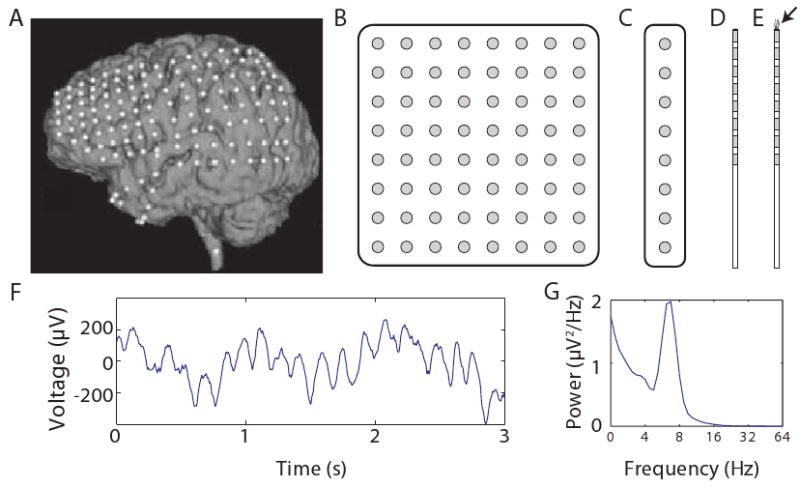
A. An MRI image of one patient's brain with the locations of implanted ECoG electrodes indicated with white dots. Modified, with permission, from Ref. [56]. B. An illustration of an 8×8 electrode grid; gray shading indicates electrodes' conductive surfaces. (Illustrations not to scale). C. A illustration of an 8-electrode strip. D. A depth electrode with eight contacts. E. A depth electrode with microwires extending from the tip to record action potentials (marked by the arrow). F. A recording of ECoG activity from the right temporal gyrus. G. The power spectrum of the recording from Panel F, which shows that this trace exhibits a robust theta oscillation.
ECoG recordings measure brain activity directly with a resolution of ∼4 mm2 [4]. This high spatial resolution is a unique feature of ECoG compared to noninvasive methods like scalp electroencephalography (EEG) or magnetoencephalography (MEG). Noninvasive recordings, even with advanced localization algorithms, sometimes miss signals that are clearly visible with ECoG [5]. Furthermore, noninvasive techniques have difficulty isolating activity from deep brain structures and are relatively susceptible to muscle artifacts [6]. Thus, ECoG is considered the clinical “gold standard” for accurately identifying seizure foci [7, 1]. For the same reasons that ECoG recordings are useful to doctors, these data are beneficial for researchers.
Each ECoG electrode measures the combined synaptic activity across the local population of neurons, rather than recording individual action potentials [8, 1]. Due to this aggregation, ECoG recordings measure the electrical activity that is synchronized across these neurons, which often includes oscillations. Neuronal oscillations appear as sinusoidal changes over time in the voltage observed from an electrode (Fig. 1F,G). They appear at frequencies from <0.1 Hz to 500 Hz and are visible at multiple spatial scales, from scalp EEG to intracellular recordings. Researchers believe that oscillations play a critical role in large-scale neuronal computations. When an individual neuron oscillates, it undergoes rhythmic variations in its level of excitability [9]. Animal recordings and computational models indicate that oscillations facilitate communication in large neuronal networks because they cause groups of neurons to become excited synchronously to form new functional networks [10, 11]. Generally, slower oscillations synchronize large neuron groups across broad brain regions and faster oscillations coordinate smaller, localized neuronal assemblies [12]. However sometimes relatively fast oscillations synchronize widely separated brain regions [13, 14]. Although oscillations at different frequencies and regions are often caused by distinct physiological mechanisms [12], interneurons typically play a critical role [15, 16]. Thus, the appearance of an oscillation in an ECoG recording generally indicates that nearby interneurons are especially active [8] and firing synchronously [17].
Studies in animals show that neuronal oscillations have a number of interesting functional properties. Generally, the presence of an oscillation indicates that neurons in a region have an increased level of spiking relative to baseline [9]. When groups of neurons oscillate together synchronously, they are more effectively able to communicate with each other [10, 11]. Furthermore, oscillations underlie phase coding, a phenomenon in which neurons encode information, such as spatial location [18], by varying the phase of an oscillation when they spike [9].
To characterize the oscillatory brain patterns that support human cognitive processes, researchers measured the amplitude of oscillations in ECoG recordings throughout cognitive tasks. This research revealed that oscillations at various frequencies change in amplitude according to task demands. For example, during memory tasks the amplitude of theta oscillations (see Glossary) increases in widespread cortical regions [19, 20]. This is consistent with research in animals that implicates theta in synaptic plasticity [21]. Behavior-related amplitude changes are also common in the gamma band. During motor and sensory processing, there is a focal increase in the amplitude of gamma activity in the neocortical region that corresponds to the body part that performs a movement or feels a percept [1]. Attention also plays a critical role in modulating the amplitude of brain oscillations. ECoG recordings from non-human primates, and subsequent work in humans, revealed that if a presented stimulus is attended, the resulting gamma oscillations have different properties—most notably, a larger amplitude—compared with the oscillations that appear after the presentation of stimuli that are ignored [22, 9, 23, 11, 24, 25, 23, 26]. As we describe below, attention also modulates the amplitude of oscillations related to other cognitive processes beyond perception.
In addition to amplitude, researchers also examined the relation between the phase of ECoG oscillations and the timing of behavioral events. A common oscillatory phenomenon is a phase reset, in which an oscillation changes its timing to exhibit a particular phase (e.g., a peak) after an external event [27]. Besides measuring phase directly, a different technique for analyzing the temporal relation between ECoG activity and behavior is to compute an event-related potential (ERPs). This involves computing the mean ECoG voltage at each timepoint after a stimulus. Although the ERP technique is designed to measure evoked ECoG waveforms rather than true oscillations, ERPs also measure oscillatory phase resets and thus sometimes it is difficult to distinguish between these phenomena [28, 29]. In this review we emphasize research findings concerning oscillations, rather than ERPs, because more is known about how oscillations relate to the activities of individual neurons [8, 30, 17].
Early ECoG work focused on measures of amplitude and phase that were separately computed at each frequency and electrode. However, soon it became evident that the neural patterns that support cognition also involve complex interactions between oscillations at different frequencies and brain regions. One of these phenomena is phase–amplitude coupling [31, 32, 33], in which the amplitude of a fast oscillation varies with the phase of a slower oscillation (see Glossary). Additionally human ECoG data, like animal recordings, exhibit phase synchrony between oscillations at different sites [34, 35, 36]. Phase–amplitude coupling and phase synchrony have important roles in various cognitive processes, including working and episodic memory [37, 31, 38], as we review below.
In addition to oscillations that appear only at specific frequencies, researchers recently observed that ECoG recordings contain broadband signals that exhibit power changes at many frequencies simultaneously [39, 40, 1]. Miller et al. [41] suggested that these broadband power changes are caused by nearby neurons increasing their spiking rate. Consistent with this is the observation that neuronal spiking positively correlates with broadband power [42] and the prediction from computational models that spiking appears at various frequencies in ECoG recordings [4, 43]. Together, these findings raise the intriguing possibility that standard ECoG electrodes can measure the rate of neuronal spiking that is nonoscillatory, even though these electrodes are much larger than the ones typically used to record action potentials. To this end, an important area of future research is developing improved methods to distinguish broadband signals from narrowband oscillations.
Finally, a different set of studies examined the relation between ECoG data and other types of neural recordings. By examining simultaneous recordings of ECoG oscillations and neuronal spiking, it revealed that neuronal spiking is correlated with the phase and amplitude of ECoG oscillations [30, 17, 44, 42] (Box 1). Furthermore, research in humans and animals showed that ECoG gamma oscillations correlate with the blood-oxygenation signal observed with functional magnetic resonance imaging (fMRI) [8, 17, 45, 46, 47]. Scientists have shown that fMRI reveals how the brain encodes diverse types of perceptual and conceptual information [48]. Because fMRI data and ECoG gamma oscillations are correlated, it suggests that ECoG recordings can elucidate neural oscillations that underlie various cognitive representations (Box 2). Together, these findings indicate that ECoG is an important link between noninvasive human brain recordings (scalp EEG, MEG and fMRI) and the direct brain recordings commonly made in animals.
Box 1: Oscillatory activity and neuronal spiking
Some epilepsy patients undergoing intracranial monitoring are implanted with special depth electrodes that have microwires extending from their tips (Fig. 1E). These microwires record single-neuron action potentials, which allows researchers to examine the relation between neuronal spiking and simultaneous brain oscillations.
Research in animals shows that brain oscillations provide a neuronal timing signal that allows neurons to encode information by spiking at a particular phase of an oscillation—a phenomenon called phase coding [18, 9]. To examine the prevalence and properties of phase coding in humans, one recent study examined how neurons in widespread regions varied their instantaneous firing rate according to the phase of ongoing oscillations [30]. This work found that many neurons were phase locked to oscillations, a phenomenon in which they increased their firing rate at a particular phase of these oscillations. Figure 3A shows the activity of a neuron that exhibits this phenomenon by spiking just before the peak of the theta oscillation. The properties of neuronal phase locking varied between high- and low-frequency oscillations. Neurons phase locked to oscillations at frequencies slower than 10 Hz had various preferred phases, whereas neurons phase locked to oscillations faster than 10 Hz had preferred phases near the oscillation's trough. This indicates that oscillations faster than ∼10 Hz reveal specific times (the trough of the oscillation) when many neurons are active, whereas slower oscillations cannot predict population spike times with this level of precision.
In addition to examining the timing of individual action potentials, a different set of studies examined the relation between the rate of neuronal spiking and the amplitude of oscillatory activity. In some cases, neuronal firing rate is well predicted by the amplitude of simultaneous oscillations (Fig. 3B). However, the details of this relation dramatically vary according to the oscillation and brain region being examined. Oscillations at high frequencies (>10 Hz) in sensory cortex correlate positively with neuronal spiking [17] and a similar, but weaker, pattern appears in hippocampus [45]. In contrast, low-frequency oscillations exhibit varied correlations with single-neuron spiking: In neocortex, theta- and alpha-band oscillatory power is negatively correlated with neuronal spiking [17], but in hippocampus these oscillations do not correlate with spiking rate [45]. Overall, this work shows that ECoG recordings provide a temporally precise indication of neuronal spiking, which may complement techniques like fMRI that measure neuronal activity with less precision [8, 17, 47].
Box 2: Brain oscillations reveal neuronal correlates of specific cognitive representations
Research on human brain oscillations has generally sought to identify broad cognitive processes that are correlated with the properties of different neural signals. For example, research on the hippocampal theta oscillation has characterized oscillatory activity that increases in amplitude during memory and navigation [49, 88]. However, in addition, Freeman found that gamma oscillations could be used to reveal specific cortical network states [59]. Examining oscillatory activity in sensory cortices, Freeman observed that the identity of a percept was encoded in the landscape of gamma-band activity. This indicates that cortical recordings of brain oscillations can predict sensory inputs, because individual stimuli were associated with distributed patterns of gamma activity that had different spatial topographies. These stimulus-specific patterns are important theoretically because they suggest that gamma oscillations can identify neuronal patterns that underlie specific cortical-network states.
Following this line of work, a recent study examined stimulus-specific activity in human ECoG recordings [53]. This study measured gamma-band oscillatory brain activity from 37 patients memorizing lists of letters. After each letter was presented, the amplitude of oscillatory activity at many sites varied according to the identity of the viewed letter. As an example of this phenomenon, Figure 4 depicts the amplitude of high-gamma activity observed at a site from left temporal cortex. At this site, an overall increase in gamma activity occurred ∼100 ms after the letter appeared; and, subsequently, the amplitude of this signal varied with the viewed letter's identity. Significant numbers of electrodes exhibiting stimulus-specific activity appeared in occipital and temporal regions. Furthermore, at some sites in occipital cortex these patterns encoded visual features of the viewed letter's shape, which is consistent with previous observations that activity in sensory regions encoded perceptual features of stimuli [22, 17, 48]. This work shows that human ECoG recordings can reveal detailed information about the state of a cortical network. Because gamma-band activity appears in widespread brain regions [54, 9], going forward, stimulus-specific gamma patterns may be used for mapping the neural basis of various specific cognitive states.
Oscillations and cognition
Over the past decade, researchers examining ECoG recordings of human brain oscillations identified neural correlates of various perceptual, motor, and cognitive processes [1, 2]. Below, we review how ECoG data have informed our understanding of the neural basis of four cognitive domains: working memory, episodic memory, language, and spatial cognition.
Working memory
Working memory—the process of remembering a stimulus temporarily for immediate processing—is critical for many common tasks. Experimentally, researchers often examine working memory using a task where a participant views a short list of items and, after a short delay, is asked to indicate whether a probe item appeared in the list (Fig. 2A). Thus, a trial in this task has three phases: stimulus encoding, memory retention, and memory retrieval.
Figure 2. Oscillatory brain activity in human working memory.
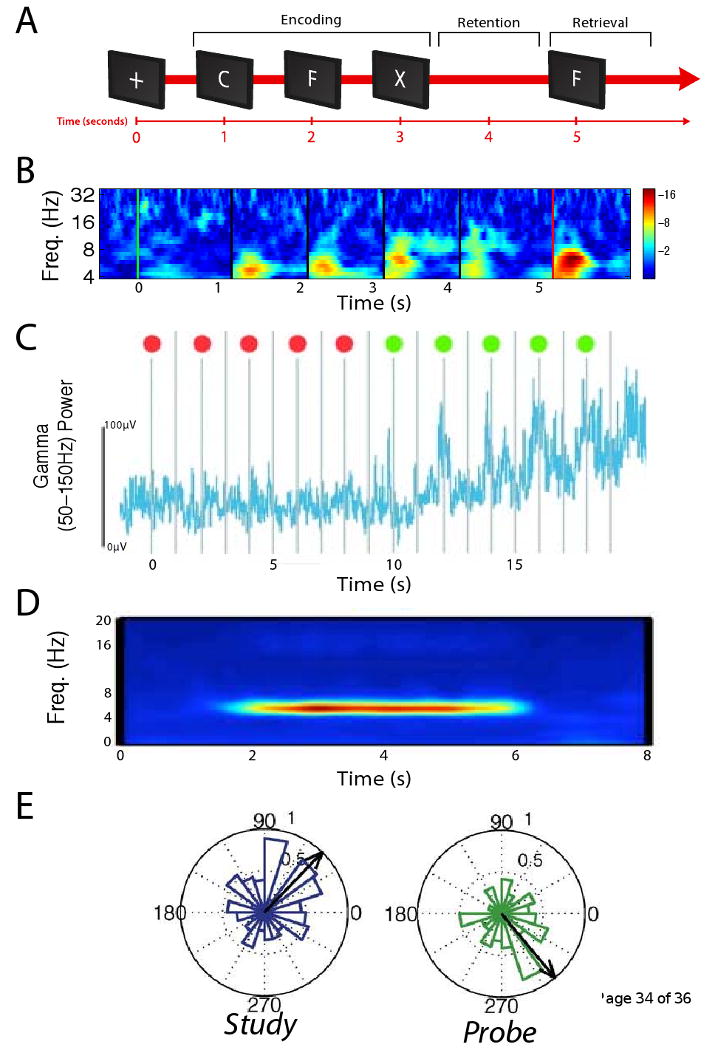
A. Schematic of a working-memory task. B. Phase-reset analysis of ECoG activity from an electrode in one patient's right subcallosal gyrus during this task. The color at each frequency and timepoint indicates the z score from a Raleigh test evaluating the uniformity of the ECoG phase distribution (computed across trials). Warm colors indicate significant phase resetting. Modified, with permission, from Ref. [27]. C. Gamma power from a Broca's Area electrode in a different patient performing a variant of this task where each stimulus is preceded by an indication of whether the item should be attended (green dot) or ignored (red dot). This electrode's gamma power is correlated with memory load, as this activity increases following stimuli that are remembered. Modified, with permission, from Ref. [62]. D. Normalized oscillatory power at a site from one patient's in the parahippocampal gyrus that exhibited elevated theta activity during memory retention. Red coloring indicates elevated oscillatory power relative to baseline. Modified, with permission, from Ref. [97]. E. An electrode from a right frontal cortex that resetted to different phases between viewing study items (left) and viewing cues (right). Each plot is a circular histogram that indicates the number of trials where different theta phases were observed 100 ms after stimulus onset (0°indicates the peak phase of theta, and 180°is the trough). Black arrow indicates the mean theta phase. Modified, with permission, from [66].
Examining ECoG activity during stimulus encoding, researchers observed patterns of theta and gamma oscillations that help illustrate how the brain encodes memories. After each stimulus presentation, theta oscillations at widespread regions undergo a phase reset (Fig. 2B) [27]. Because individual neurons spike at different theta phases (see Box 1) [30], these phase resets cause the spiking of widespread neurons to occur in precise temporal patterns. In addition to theta, after stimulus presentation there is an increase in the amplitude of gamma activity at many electrodes in temporal and occipital cortices [52, 40]. This indicates that temporal and occipital cortices are involved in stimulus encoding, because elevated gamma activity indicates that nearby neurons are especially active [9, 17]. Furthermore, this gamma activity has greater amplitude at the trough of simultaneous theta oscillations [37, 53], which supports the view that theta–gamma phase–amplitude coupling is important for memory encoding [50, 31]. One study described a site in ventral temporal cortex that exhibited elevated gamma activity after viewing images of animals compared to viewing images of tools [40]. Other ECoG studies also reported analogous patterns, in which gamma activations were specific to certain stimulus classes [54, 52, 55, 56, 57, 58]. These selective activations indicate that specific regions, especially in the ventral temporal lobe, encode different categories of cognitive representations [48]. Furthermore, recent work showed that gamma activity encodes the identities of specific stimuli (Box 2) [53]. Thus, ECoG gamma oscillations are a rich information source that reveal specific cortical network states [59, 60].
ECoG recordings of gamma activity can distinguish brain regions that process low-level visual percepts from the areas that encode richer representations of consciously attended stimuli. A study by Fisch et al. examined recordings while patients were shown stimuli for different durations [61]. When a stimulus was presented rapidly and could not be recognized or attended, gamma activity appeared only in primary sensory regions. In contrast, when stimuli were presented for longer durations, high-amplitude gamma activity appeared for a long duration throughout ventral temporal cortex. Thus, when a stimulus is recognized and consciously attended, it “ignites” neural assemblies in high-level regions. A different study provided complementary evidence of the effect of attention on human brain oscillations, by showing that gamma activity appears at many sites only when a patient actively attends to a stimulus (Fig. 2C; [62]). Together, these findings indicate that much of the brain's gamma activity is a correlate of consciously recognizing and attending to stimuli, rather than low-level perception.
After a person encodes a stimulus, their next task is to retain it in memory. ECoG recordings indicate that the set of brain regions that support working-memory maintenance is different from the areas involved in perception and involves some areas that were traditionally thought to support language [62]. During memory retention, one study observed oscillatory phase synchrony in the beta band (∼16–30 Hz) between sites in extrastriate cortex [34]. Notably, other human studies also reported phase synchrony in the beta range [35, 36], rather than in the gamma band where cortical phase synchrony typically appears in animals [22, 11]. The different frequencies of cortical phase synchrony is an important difference between the brains of humans and animals. Researchers have also examined the neural basis of memory retention by identifying electrophysiological patterns that correlate with memory load (the number of simultaneously remembered stimuli; Fig. 2C). In particular, the amplitude of gamma activity correlates with memory load in both neocortex [63, 62, 64] and hippocampus [65]. This widespread phenomenon indicates that various brain regions support memory maintenance and that gamma oscillations help maintain persistent neural activations [9, 23, 11]. In addition to gamma, theta oscillations are also linked to memory maintenance. During memory maintenance, widespread neocortical sites exhibit increased theta power (Fig. 2D; [19]) and the hippocampus exhibits phase–amplitude coupling between theta and gamma oscillations [38].
The final phase of each trial is the retrieval interval, where the participant determines whether a probe stimulus matches one of the remembered list items. ECoG recordings during this interval are informative about the types of neural computations that support memory retrieval. After a probe is viewed, theta oscillations at many sites again exhibit phase resets (Fig. 2B,E). However, between the probe and the list items, some sites reset to different phases [66], with varying levels of precision [27]. These phase resets occur in an arrangement [66] that is consistent with the trough and peak phases of theta supporting memory encoding and retrieval, respectively [51]. ECoG recordings also reveal the nature of the neural computations involved in comparing a stimulus to the contents of memory: For example, van Vugt et al. found that the amplitude of frontal activity in the delta band (1–4 Hz) correlated with the similarity between the probe and the items held in memory [67]. This establishes electrophysiological support for psychological theories that humans recognize stimuli by computing a graded measure of the similarity between a percept and the contents of memory [68, 69].
Episodic memory
An issue of significant practical and theoretical interest is why people remember some events easily whereas they are unable to remember others despite much effort [70]. This issue is a core topic in the study of episodic memory (i.e., memory for autobiographical events). One technique for probing the neural basis of episodic memory is the free-recall task. In this task, a person is presented with a list of items and is later asked to recall the items in any order.
Analyzing ECoG recordings as patients performed the free-recall task,Sederberg et al. identified theta and gamma oscillations in widespread brain regions that varied in amplitude according to whether a viewed stimulus would be recalled successfully [20]. Later studies showed that these oscillations were especially prominent at gamma frequencies in the left inferior frontal gyrus [71], which is consistent with neuroimaging studies implicating this region in memory formation [72]. The high spatial resolution of ECoG was critical for identifying this phenomenon, which had not been observed previously with noninvasive recordings. Furthermore, during memory retrieval, theta and gamma activity appeared in the same set of brain regions as during encoding [73]. This supports the view that memory retrieval involves reinstating the pattern of brain activity that appeared during learning [70].
Since a broad literature shows that the hippocampus underlies long-term memory [74, 49], it is important to characterize the electrophysiological activity in this region during memory formation. This research revealed that humans and animals exhibit different patterns of memory-related hippocampal activity. In animals, hippocampal theta oscillations increase in amplitude during memory encoding [75, 49]. In contrast, human memory formation is associated with decreased hippocampal activity at many frequencies [71]. A subsequent study further illustrated the complex role of the hippocampus in human memory, showing that hippocampal activity (measured via slow ECoG voltage shifts) is positively correlated with successfully remembering stimuli that are retained for long durations and negatively correlated with remembering stimuli that are retained for short durations [76]. These differing patterns suggest that humans use different physiological processes to remember items that must be retained for different lengths of time [77].
ECoG data indicate that distinct patterns of cortical activity support memory formation during periods of high and low attention. Remembering the viewing of a common word requires more attention than memorizing a novel stimulus. Accordingly, memorizing common words elicited a larger “N400” ERP response compared with memorizing rare words. This pattern appeared in rhinal cortex but not hippocampus [78]. Scalp EEG also showed that successful memory formation when attention is high is associated with greater gamma activity in posterior cortices, compared with memory formation during low attention [77]. Importantly, this research indicates that attention-related differences in the neural correlates of memory formation are limited to neocortex rather than hippocampus [78].
A body of research indicates that episodic memories are initially encoded in hippocampus and later consolidated into neocortex [74]. This theory predicts that there is communication between hippocampus and neocortex during memory encoding [79]. Supporting this prediction, several ECoG studies report synchronous oscillations between hippocampus and neocortex. When a stimulus is successfully memorized, there is increased gamma-band coherence (a similar phenomenon to phase synchrony) between rhinal cortex and hippocampus [13]. Furthermore, during memory retrieval, there is increased gamma activity in the layers of entorhinal cortex that project to hippocampus [80]. Cortico–hippocampal communication also appears outside of controlled experiments, as demonstrated by the finding that increased rhinal–hippocampal coherence during sleep predicts that dreams would be remembered [81]. These findings show that cortico–hippocampal interactions play an important role in human memory and, more broadly, demonstrate that neuronal oscillations are not only informative about the activity within individual brain areas, but they also show how information is transferred between regions.
Language
ECoG recordings are especially useful for studying the neural basis of auditory linguistic processes because electrodes are frequently implanted in regions that are critical for listening and speaking. ECoG recordings during listening and speaking support the traditional view that language comprehension is supported by Wernicke's Area and that language production involves Broca's Area [1, 82, 56]. However, these studies also implicate more widespread cortical networks in language.
After hearing a word, there is a dramatic increase in the amplitude of gamma activity in regions near Wernicke's Area, including the superior temporal gyrus (STG) and the superior temporal sulcus (STS) [1, 82]. ECoG recordings revealed that language-related gamma oscillations flow from the posterior STG, to the middle STG, and then the STS. In the STG, gamma activity generally encodes low-level acoustic properties of a sound [82]. However, when this activity reaches the STS, its amplitude and duration encode lexical information [82, 56]. This progression of gamma activity is consistent with a model proposing the sequential processing of linguistic information along the STG–STS pathway [83]. Language-related activity also appears outside the temporal lobe, as demonstrated by the finding that there is increased gamma activity in parietal and frontal cortices when a perceived syllable is consciously recognized [84]. The amplitude of gamma oscillations is greater when a person listens to outside speech, compared to hearing their own words [56]. This indicates that speech-related neuronal activity correlates with attentional demands, because greater attention is required to comprehend a different person's speech than one's own words.
ECoG studies provided data that support theories that semantic information is represented throughout bilateral temporal regions [48], but that low-level linguistic information is represented only in the language-dominant hemisphere [83]. One study found that when a patient performed a lexical-decision task, gamma activity appeared only in the temporal cortex of the language-dominant hemisphere. In contrast, a picture-naming task, which required access to deeper semantic information, elicited gamma activity in bilateral temporal regions [40]. In addition to temporal cortex, semantic information is also represented in frontal regions, as demonstrated by a study that examined ECoG recordings from patients judging whether two words were semantically related. This study reported that some frontal sites exhibited gamma activity that appeared in a dynamic pattern that varied with a word pair's semantic properties. Here, processing two unrelated words immediately elicited brief high-amplitude gamma activity, whereas viewing two related words induced lower-amplitude gamma activity that appeared only after a delay [1]. The detailed structure of these patterns indicates that neural activity in human frontal cortex exhibits important temporal variations.
A recent study by Sahin et al. investigated ECoG recordings from Broca's Area to examine the role of this region in language comprehension and production [85]. Examining recordings while participants viewed words that they were asked to inflect and imagine speaking, the authors identified three ERP components whose amplitudes correlated with different linguistic processes: The ERP component at ∼200-ms encoded the word's lexical properties (whether it was rare or common), the ∼320-ms component reflected processes related to word inflection, and the ∼450-ms component correlated with the produced word's phonological properties (number of syllables). Because these ERP components appeared at different recording sites, it indicates that Broca's Area contains a series of spatially distinct neuronal networks that sequentially perform different linguistic computations.
ECoG recordings revealed that speech production is associated with gamma activity in various brain regions, including not only Broca's Area but also other prefrontal and temporal cortices [1, 56]. This extends the traditional view that Broca's Area is the focus of speech production. Furthermore, analyzing the temporal dynamics of ECoG activity during speech has led to additional insights. Frontal gamma activity becomes elevated ∼800 ms before speaking, which suggests that this area supports speech–motor planning in addition to direct motor output [56]. During speaking, gamma activity is synchronized between several cortical regions, including the mouth region of motor cortex, various frontal regions, and Wernicke's Area [86].
Spatial cognition
Spatial navigation is an essential behavior for nearly all humans and animals. Because navigation is such an innate function, understanding its neural basis can reveal important similarities and differences between human and animal neurophysiology. Although patients undergoing ECoG monitoring are confined to a hospital bed, researchers can examine neural correlates of spatial processing using computer-based virtual-navigation tasks [87, 88, 89].
During navigation, the human brain exhibits several patterns of theta and gamma oscillations related to spatial processing. When a human moves through an environment, there is a widespread increase in the amplitude of theta activity [87, 88]. This is similar to the movement-related theta oscillations observed in animals [49]. Furthermore, the amplitude of human theta oscillations positively correlates with navigation performance [90], adding to evidence that theta critically supports spatial processing [49].
The ability of human intracranial recordings to record from deep brain structures allowed researchers to compare the properties of hippocampal theta between humans and animals. As a result, researchers identified two important interspecies differences. In rodents, hippocampal theta oscillations reliably appear at 4–8 Hz [49]. However, in humans hippocampal oscillations usually appear instead at 1–4 Hz [91, 92, 88, 30, 93, 90, 94]. Furthermore, whereas rodent theta oscillations are routinely sustained for over ten seconds [18, 49], human hippocampal oscillations usually appear only transiently [87, 88] and sometimes not at all [95]. Despite these differences, it seems that human 1–4-Hz hippocampal oscillations are functionally analogous to rodent 4–8-Hz theta. During navigation, both of these oscillations increase in amplitude during movement [88, 49, 89] and the phase of both oscillations modulates neuronal spiking [18, 15, 30]. Unlike hippocampus, human neocortical theta oscillations usually appear at 4–8 Hz [87, 31, 30]. However, because a recent study reported significant 1–4-Hz coherence between hippocampus and neocortex [93], it suggests that the human neocortex also exhibits 1–4-Hz activity in addition to theta [32].
Beyond theta, navigation-related brain oscillations also appear in the gamma band [87, 88]. Neuroimaging and lesion studies have shown that the right hemisphere plays a unique role in spatial cognition [96]. In agreement with the hemisphere-lateralization literature, a recent ECoG study compared the prevalence of navigation-related oscillations throughout the brain and found that navigation-related gamma activity was especially prevalent in the right hemisphere [89]. This supports the view that the right hemisphere is important for spatial processing and supplements research indicating that gamma oscillations and fMRI activations identify similar neuronal patterns [8, 17].
Conclusions and future directions
Human ECoG recordings have implicated brain oscillations in various types of brain functions, including both cognitive and sensorimotor processes [1, 2]. Although oscillations at many frequencies correlate with different cognitive processes, the theta and gamma bands are most frequently found to correlate with task demands. Theta and gamma oscillations each have distinct physiological and computational properties (Box 1). Thus, these oscillations provide insight into the neuronal processes that underlie different human cognitive processes: Theta oscillations are more-closely associated with temporally precise neuronal spiking, rather than changes in firing rate [30, 17, 45]. This suggests that cognitive processes that correlate with theta activity, such as movement during navigation [87] and working-memory retention [19, 97], are supported neurally by temporally precise spiking, rather than by firing-rate changes. In contrast, gamma-band activity is correlated with both elevated neuronal firing rates [8, 41, 17, 11] and precisely timed spiking [9, 30]. Thus, cognitive variables that relate to gamma activity, such as memory load [63, 64], are likely associated with neuronal computations that involve both rate and temporal coding. Beyond theta and gamma, an emerging ECoG signal is broadband power. Broadband power changes are an indication of cognitive processes that involve firing-rate changes but not temporally precise oscillatory spiking [4]. More generally, these signals show that ECoG recordings can be used to elucidate the neuronal patterns that support different human cognitive processes. Because ECoG reveals this information with greater precision than noninvasive techniques, it allows researchers to probe human brain activity with a rare level of detail. Thus, ECoG data have revealed unique human electrophysiological phenomena that do not appear in animals [34, 97, 30, 89, 85], as well as similarities between human and animal brain activities [8, 87, 49, 88, 17, 5, 45].
There are several exciting developments underway in the study of cognitive electrophysiology with ECoG data. Perhaps the most important of these is the study of oscillatory communication across regions [97, 10, 35, 44, 98, 14] and of interactions between oscillations at different frequencies within the same region [37, 31, 33, 38]. To the extent that oscillations at different frequencies correlate with different functional processes, cross-frequency interactions appear to play a critical role in linking physically disparate neuronal networks [32]. Thus, an important area of future research is identifying how oscillatory interactions, across both regions and frequencies, relate to cognitive processes. Another emerging research trend is the use of real-time “closed loop” systems that vary the parameters of an experiment according to instantaneous brain activity [99, 100]. This research seeks to determine whether brain recordings can be used to alter human behavioral performance, and thus this work has the potential to distinguish the neural signals that have a causal role in behavior.
Figure 3. The relation between oscillatory brain activity and neuronal spiking.
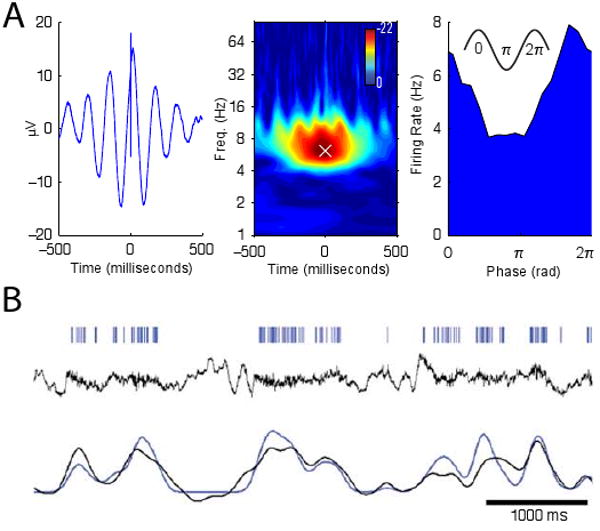
A. The activity of a neuron from the right superior temporal gyrus that spiked just before the peak of the theta oscillation. Left panel, average local-field potential (LFP) computed relative to each spike. Middle panel, z score from a Rayleigh test, which measured LFP phase uniformity at the time of each spike, as a function of frequency and time offset. White ‘×’ indicates the frequency of peak phase locking. Right panel, firing rate of this cell as a function of instantaneous theta phase at the frequency of peak phase locking. Adapted, with permission, from Ref. [30]. B. The activity of a neuron from one patient's auditory cortex whose spiking was tightly coupled to the amplitude of simultaneous gamma oscillations (r = 0.84). Ticks in top row indicate individual action potentials. Middle row depicts the LFP signal filtered to only include frequencies below 130 Hz. Bottom row indicates LFP gamma power (black) and neuronal firing rate (blue), showing that these two measures are closely related. Adapted, with permission, from Ref. [17].
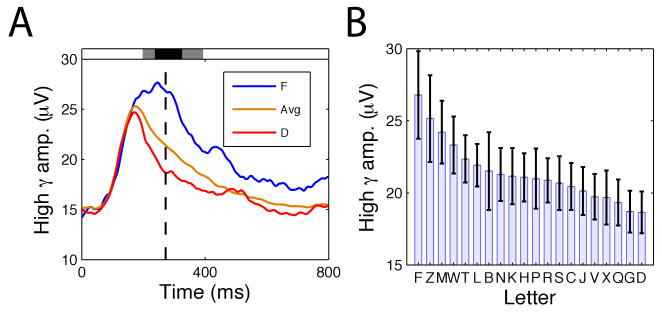
Figure 4. Gamma-band correlates of specific cognitive representations.
A. The activity of an electrode from left-temporal cortex that exhibited significant variations in high-gamma (65–128 Hz) amplitude according to the identity of the stimulus that was viewed. Blue line indicates the gamma amplitude after viewing ‘F,’ red indicates the gamma amplitude after viewing ‘D,’ and orange indicates the mean gamma power across all letters. Shaded rectangles indicate timepoints where this effect is significant (gray indicates p < 0.05, black indicates p < 10-5). B. Right panel indicates the mean high-gamma power for each letter at the timepoint of peak letter-related differences (indicated by the black dashed line in left panel). Modified, with permission, from Ref. [53].
Acknowledgments
We are thankful to Jeremy Caplan, Delphine Dahan, Arne Ekstrom, Brad Lega, Jeremy Manning, Sean Polyn, Ashwin Ramayya, Per Sederberg, Mijail Serruya, Marieke van Vugt, Christoph Weidemann, and Kareem Zaghloul for helpful feedback. This work was sponsored by National Institutes of Health research grants MH61975, MH062196, NS054575, and NS50067; and National Science Foundation grant SBE0354378.
Glossary
- Broadband power
The overall energy, or variance, of a time series. Whereas changes in broadband power appear at many or all frequencies, changes in narrowband power are often specific to a given frequency band
- Gamma oscillation
Rhythmic neural activity in the ∼30–200 Hz frequency range. Gamma oscillations have been implicated in a wide range of cognitive processes including perception, attention, and memory [23, 11]
- Phase synchrony
Two or more neural assemblies oscillating together with a consistent phase relationship
- Phase reset
An oscillation exhibiting an altered phase as the result of an external event
- Phase–amplitude coupling
A pattern where one oscillation's amplitude varies with the phase of a slower oscillation. Phase–amplitude coupling is prevalent in human neocortex, where gamma oscillations have greater amplitude at the trough of theta oscillations [31]
- Theta oscillation
Rhythmic neural activity at ∼3–10 Hz. Theta oscillations have been implicated in memory both at the behavioral [19, 49] and cellular level [50, 51]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Progress in Brain Research. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- 2.Jerbi K, Ossandon T, Hamame C, Senova S, Dalal S, Jung J, Minotti L, Bertrand O, Berthoz A, Kahane P, et al. Task-related gamma-band dynamics from an intracerebral perspective: Review and implications for surface EEG and MEG. Human Brain Mapping. 2009;30(6) doi: 10.1002/hbm.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engel AK, Moll CKE, Fried I, Ojemann GA. Invasive recordings from the human brain–clinical insights and beyond. Nature Reviews Neuroscience. 2005;6:35–47. doi: 10.1038/nrn1585. [DOI] [PubMed] [Google Scholar]
- 4.Miller KJ, Sorensen L, Ojemann J, den Nijs M. ECoG observations of power-law scaling in the human cortex. Public Library of Science: Computational Biology. In press. [Google Scholar]
- 5.Dalal S, Baillet S, Adam C, Ducorps A, Schwartz D, Jerbi K, Bertrand O, Garnero L, Martinerie J, Lachaux J. Simultaneous MEG and intracranial EEG recordings during attentive reading. Neuroimage. 2009;45(4):1289–1304. doi: 10.1016/j.neuroimage.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, Lachaux J. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topography. 2009;22(1):18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- 7.Lachaux JP, Rudrauf D, Kahane P. Intracranial EEG and human brain mapping. Journal of Physiology-Paris. 2003;97(4–6):613–628. doi: 10.1016/j.jphysparis.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. Journal of Neuroscience. 2003;23(10):3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fries P, Nikolić D, Singer W. The gamma cycle. Trends in Neurosciences. 2007;30(7):309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Womelsdorf T, Schoffelen J, Oostenveld R, Singer W, Desimone R, Engel A, Fries P. Modulation of Neuronal Interactions Through Neuronal Synchronization. Science. 2007;316(5831):1609. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 11.Fries P. Neuronal Gamma-band Synchronization as a Fundamental Process in Cortical Computation. Annual Review of Neuroscience. 2009;32(1) doi: 10.1146/annurev.neuro.051508.135603. [DOI] [PubMed] [Google Scholar]
- 12.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proceedings of the National Academy of Sciences, USA. 2000;97(4):1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fell J, Klaver P, Lehnertz K, Grunwald T, Schaller C, Elger CE, Fernandez G. Human memory formation is accompanied by rhinal-hippocampal coupling and decoupling. Nature Neuroscience. 2001;4(12):1259–1264. doi: 10.1038/nn759. [DOI] [PubMed] [Google Scholar]
- 14.Gregoriou G, Gotts S, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klausberger T, Magill PJ, Marton LF, Roberts JD, Cobden PM, Buzsáki G, Somogyi P. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 16.Cardin J, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, Tsai L, Moore C. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009 doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nir Y, Fisch L, Mukamel R, Gelbard-Sagiv H, Arieli A, Fried I, Malach R. Coupling between Neuronal Firing Rate, Gamma LFP, and BOLD fMRI Is Related to Interneuronal Correlations. Current Biology. 2007;17(15):1275–1285. doi: 10.1016/j.cub.2007.06.066. [DOI] [PubMed] [Google Scholar]
- 18.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3:317–30. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 19.Raghavachari S, Rizzuto DS, Caplan JB, Kirschen MP, Bourgeois B, Madsen JR, Kahana MJ, Lisman JE. Gating of human theta oscillations by a working memory task. Journal of Neuroscience. 2001;21:3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. Journal of Neuroscience. 2003;23(34):10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 22.Singer W, Gray CM. Visual feature integration and the temporal correlation hypothesis. Annu Rev Neurosci. 1995;18:555–586. doi: 10.1146/annurev.ne.18.030195.003011. [DOI] [PubMed] [Google Scholar]
- 23.Jensen O, Kaiser J, Lachaux J. Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences. 2007;30(7):317–324. doi: 10.1016/j.tins.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 24.Rotermund D, Taylor K, Ernst U, Kreiter A, Pawelzik K. Attention Improves Object Representation in Visual Cortical Field Potentials. Journal of Neuroscience. 2009;29(32):10120. doi: 10.1523/JNEUROSCI.5508-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cerebral Cortex. 2005;15(5):654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- 26.Ray S, Niebur E, Hsiao S, Sinai A, Crone N. High-frequency gamma activity (80–150Hz) is increased in human cortex during selective attention. Clinical Neurophysiology. 2008;119(1):116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizzuto D, Madsen JR, Bromfield EB, Schulze-Bonhage A, Seelig D, Aschenbrenner-Scheibe R, Kahana MJ. Reset of human neocortical oscillations during a working memory task. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:7931–7936. doi: 10.1073/pnas.0732061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah A, Bressler S, Knuth K, Ding M, Mehta A, Ulbert I, Schroeder C. Neural dynamics and the fundamental mechanisms of event-related brain potentials. Cerebral Cortex. 2004;14(5):476. doi: 10.1093/cercor/bhh009. [DOI] [PubMed] [Google Scholar]
- 29.Yeung N, Bogacz R, Holroyd CB, Cohen JD. Detection of synchronized oscillations in the encephalogram: an evaluation of methods. Psychophysiology. 2004;41(6):822–832. doi: 10.1111/j.1469-8986.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. Journal of Neuroscience. 2007;27(14):3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canolty RT, Edwards E, Dalal SS, Soltani M, Nagarajan SS, Kirsch HE, Berger MS, Barbaro NM, Knight RT. High gamma power is phase-locked to theta oscillations in human neocortex. Science. 2006;313(5793):1626–1628. doi: 10.1126/science.1128115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schroeder C, Lakatos P, Kajikawa Y, Partan S, Puce A. Neuronal oscillations and visual amplification of speech. Trends in Cognitive Sciences. 2008;12(3):106–113. doi: 10.1016/j.tics.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tort A, Kramer M, Thorn C, Gibson D, Kubota Y, Graybiel A, Kopell N. Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proceedings of the National Academy of Sciences. 2008;105(51):20517. doi: 10.1073/pnas.0810524105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tallon-Baudry C, Bertrand O, Fischer C. Oscillatory synchrony between human extrastriate areas during visual short-term memory maintenance. Journal of Neuroscience. 2001;21:RC177:1–5. doi: 10.1523/JNEUROSCI.21-20-j0008.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sehatpour P, Molholm S, Schwartz T, Mahoney J, Mehta A, Javitt D, Stanton P, Foxe J. A human intracranial study of long-range oscillatory coherence across a frontal–occipital–hippocampal brain network during visual object processing. Proceedings of the National Academy of Sciences. 2008;105(11):4399. doi: 10.1073/pnas.0708418105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaillard R, Dehaene S, Adam C, Clémenceau S, Hasboun D, Baulac M, Cohen L, Naccache L. Converging intracranial markers of conscious access. PLoS Biology. 2009;7(3):1–21. doi: 10.1371/journal.pbio.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mormann F, Fell J, Axmacher N, Weber B, Lehnertz K, Elger C, Fernández G. Phase/amplitude reset and theta–gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus. 2005;15(7):890–900. doi: 10.1002/hipo.20117. [DOI] [PubMed] [Google Scholar]
- 38.Axmacher N, Henseler M, Jensen O, Weinreich I, Elger C, Fell J. Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proceedings of the National Academy of Sciences, USA. 2010 doi: 10.1073/pnas.0911531107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lachaux JP, Rodriguez E, Martinerie J, Adam C, Hasboun D, Varela FJ. A quantitative study of gamma-band activity in human intracranial recordings triggered by visual stimuli. Eur J Neurosci. 2000 Jul;12:2608–22. doi: 10.1046/j.1460-9568.2000.00163.x. [DOI] [PubMed] [Google Scholar]
- 40.Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency γ-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. Journal of Neuroscience. 2005;25(13):3287–3293. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller KJ, Leuthardt EC, Schalk G, Rao RPN, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral Changes in Cortical Surface Potentials during Motor Movement. Journal of Neuroscience. 2007;27(9):2424. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Manning J, Jacobs J, Fried I, Kahana M. Broadband shifts in LFP power spectra are correlated with single-neuron spiking in humans. Journal of Neuroscience. 2009 October;29:13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milstein J, Mormann F, Fried I, Koch C. Neuronal shot noise and Brownian 1/f2 behavior in the local field potential. PLoS One. 2009;4(2):e4338. doi: 10.1371/journal.pone.0004338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of Neocortical Neurons and Gamma Oscillations by the Hippocampal Theta Rhythm. Neuron. 2008;60(4):683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ekstrom A, Suthana N, Millett D, Fried I, Bookheimer S. Correlation Between BOLD fMRI and Theta-Band Local Field Potentials in the Human Hippocampal Area. Journal of Neurophysiology. 2009;101(5):2668. doi: 10.1152/jn.91252.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lachaux JP, Jung J, Mainy N, Dreher JC, Bertrand O, Baciu M, Minotti L, Hoffmann D, Kahane P. Silence is golden: Transient neural deactivation in the prefrontal cortex during attentive reading. Cerebral Cortex. 2008;18(2):443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- 47.Ojemann G, Corina D, Corrigan N, Schoenfield-McNeill J, Poliakov A, Zamora L, Zanos S. Neuronal correlates of functional magnetic resonance imaging in human temporal cortex. Brain. 2009 doi: 10.1093/brain/awp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mitchell T, Shinkareva S, Carlson A, Chang K, Malave V, Mason R, Just M. Predicting Human Brain Activity Associated with the Meanings of Nouns. Science. 2008;320(5880):1191. doi: 10.1126/science.1152876. [DOI] [PubMed] [Google Scholar]
- 49.Buzsáki G. Theta rhythm of navigation: Link between path integration and landmark navigation, episodic and semantic memory. Hippocampus. 2005;15:827–840. doi: 10.1002/hipo.20113. [DOI] [PubMed] [Google Scholar]
- 50.Lisman J, Idiart MA. Storage of 7 ± 2 short-term memories in oscillatory subcycles. Science. 1995;267:1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 51.Hasselmo ME, Bodelon C, Wyble BP. A proposed function for hippocampal theta rhythm: Separate phases of encoding and retrieval enhance reversal of prior learning. Neural Computation. 2002;14:793–817. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- 52.Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L, Kahane P, Renault B. The many faces of the gamma band response to complex visual stimuli. NeuroImage. 2005;25:491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs J, Kahana MJ. Neural representations of individual stimuli in humans revealed by gamma-band ECoG activity. Journal of Neuroscience. 2009;29(33):10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121(12):2301. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- 55.Kraskov A, Quiroga R, Reddy L, Fried I, Koch C. Local Field Potentials and Spikes in the Human Medial Temporal Lobe are Selective to Image Category. Journal of Cognitive Neuroscience. 2007;19(3):479–492. doi: 10.1162/jocn.2007.19.3.479. [DOI] [PubMed] [Google Scholar]
- 56.Towle V, Yoon H, Castelle M, Edgar J, Biassou N, Frim D, Spire J, Kohrman M. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131(8):2013. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mainy N, Jung J, Baciu M, Kahane P, Schoendorff B, Minotti L, Hoffmann D, Bertrand O, Lachaux J. Cortical dynamics of word recognition. Human brain mapping. 2008;29(11):1215. doi: 10.1002/hbm.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edwards E, Soltani M, Kim W, Dalal S, Berger M, Nagarajan S, Knight R. Comparison of time-frequency responses and the event-related potential to auditory speech stimuli in human cortex. Journal of Neurophysiology. 2009:90954–2008. doi: 10.1152/jn.90954.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freeman W. The physiology of perception. Scientific American. 1991;264(2):78–85. doi: 10.1038/scientificamerican0291-78. [DOI] [PubMed] [Google Scholar]
- 60.Andersen R, Musallam S, Pesaran B. Selecting the signals for a brain–machine interface. Current Opinion in Neurobiology. 2004;14(6):720–726. doi: 10.1016/j.conb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 61.Fisch L, Privman E, Ramot M, Harel M, Nir Y, Kipervasser S, Andelman F, Neufeld M, Kramer U, Fried I, Malach R. Neural “Ignition”: Enhanced Activation Linked to Perceptual Awareness in Human Ventral Stream Visual Cortex. Neuron. 2009;64(4):562–574. doi: 10.1016/j.neuron.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux J. Neural correlates of consolidation in working memory. Human brain mapping. 2007;28(3):183–193. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Howard MW, Rizzuto DS, Caplan JC, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, Schultze-Bonhage A, Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cerebral Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 64.Meltzer JA, Zaveri HP, Goncharova II, Distasio MM, Papademetris X, Spencer SS, Spencer DD, Constable RT. Effects of working memory load on oscillatory power in human intracranial EEG. Cerebral Cortex. 2008;18(8):1843–1855. doi: 10.1093/cercor/bhm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A, Kahana MJ. Hippocampal gamma oscillations increase with working memory load. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.0567-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rizzuto D, Madsen JR, Bromfield EB, Schulze-Bonhage A, Kahana MJ. Human neocortical oscillations exhibit theta phase differences between encoding and retrieval. NeuroImage. 2006;31(3):1352–1358. doi: 10.1016/j.neuroimage.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 67.van Vugt MK, Schulze-Bonhage A, Sekuler R, Litt B, Brandt A, Baltuch G, Kahana MJ. Intracranial electroencephalography reveals two distinct two similarity effects during item recognition. Brain Research. doi: 10.1016/j.brainres.2009.07.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Estes WK. Classification and Cognition Oxford. U. K.: Oxford University Press; 1994. [Google Scholar]
- 69.Sekuler R, Kahana MJ. A stimulus-oriented approach to memory. Current Directions in Psychological Science. 2008;16:305–310. doi: 10.1111/j.1467-8721.2007.00526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polyn SM, Kahana MJ. Memory search and the neural representation of context. Trends in Cognitive Sciences. 2008;12:24–30. doi: 10.1016/j.tics.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, McCarthy DC, Brandt A, Tully MS, Kahana MJ. Hippocampal and neocortical gamma oscillations predict memory formation in humans. Cerebral Cortex. 2007;17(5):1190–1196. doi: 10.1093/cercor/bhl030. [DOI] [PubMed] [Google Scholar]
- 72.Fernández G, Weyerts H, Schrader-Bölsche M, Tendolkar I, Smid HGOM, Tempelmann C, Hinrichs H, Scheich H, Elger CE, Mangun GR, Jochen Heinze H. Successful verbal encoding into episodic memory engages the posterior hippocampus: A parametrically analyzed functional magnetic resonance imaging study. Journal of Neuroscience. 1998;18(5):1841–1847. doi: 10.1523/JNEUROSCI.18-05-01841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sederberg PB, Schulze-Bonhage A, Madsen JR, Bromfield EB, Litt B, Brandt A, Kahana MJ. Gamma oscillations distinguish true from false memories. Psychological Science. 2007;18(11):927–932. doi: 10.1111/j.1467-9280.2007.02003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7(2):217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 75.Berry SD, Thompson RF. Prediction of learning rate from the hippocampal electroenephalogram. Science. 1978;200:1298–300. doi: 10.1126/science.663612. [DOI] [PubMed] [Google Scholar]
- 76.Axmacher N, Elger CE, Fell J. Working memory-related hippocampal deactivations interferes with long-term memory formation. Journal of Neuroscience. 2009;29(4):1052–1060. doi: 10.1523/JNEUROSCI.5277-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sederberg PB, Gauthier LV, Terushkin V, Miller JF, Barnathan JA, Kahana MJ. Oscillatory correlates of the primacy effect in episodic memory. NeuroImage. 2006;32(3):1422–1431. doi: 10.1016/j.neuroimage.2006.04.223. [DOI] [PubMed] [Google Scholar]
- 78.Fernandez G, Klaver P, F J, Grunwald Thomas, Elger CE. Human declarative memory formation: segregating rhinal and hippocampal contributions. Hippocampus. 2002;12:514–519. doi: 10.1002/hipo.10050. [DOI] [PubMed] [Google Scholar]
- 79.Miller R. Cortico-hippocampal interplay and the representation of contexts in the brain. Springer-Verlag; 1991. [Google Scholar]
- 80.Steinvorth S, Wang C, Ulbert I, Schomer D, Halgren E. Human entorhinal gamma and theta oscillations selective for remote autobiographical memory. Hippocampus. 2009 doi: 10.1002/hipo.20597. [DOI] [PubMed] [Google Scholar]
- 81.Fell J, Fernandez G, Lutz M, Kockelmann E, Burr W, Schaller C, Elger C, Helmstaedter C. Rhinal-hippocampal connectivity determines memory formation during sleep. Brain. 2006;129(1):108. doi: 10.1093/brain/awh647. [DOI] [PubMed] [Google Scholar]
- 82.Canolty RT, Soltani M, Dalal S, Edwards E, Dronkers N, Nagarajan S, Kirsch H, Barbaro N, Knight R. Spatiotemporal dynamics of word processing in the human brain. Frontiers in Neuroscience. 2007;1(1):185. doi: 10.3389/neuro.01.1.1.014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hickok G, Poeppel D. The cortical organization of speech processing. Nature Reviews Neuroscience. 2007;8(5):393–402. doi: 10.1038/nrn2113. [DOI] [PubMed] [Google Scholar]
- 84.Basirat A, Sato M, Schwartz J, Kahane P, Lachaux J. Parieto-frontal gamma band activity during the perceptual emergence of speech forms. Neuroimage. 2008;42(1):404–413. doi: 10.1016/j.neuroimage.2008.03.063. [DOI] [PubMed] [Google Scholar]
- 85.Sahin N, Pinker S, Cash S, Schomer D, Halgren E. Sequential Processing of Lexical, Grammatical, and Phonological Information Within Broca's Area. Science. 2009;326(5951):445. doi: 10.1126/science.1174481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korzeniewska A, Crainiceanu C, Kus R, Franaszczuk P, Crone N. Dynamics of event-related causality in brain electrical activity. Human Brain Mapping. 2008;29(10) doi: 10.1002/hbm.20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caplan JB, Madsen JR, Schulze-Bonhage A, Aschenbrenner-Scheibe R, Newman EL, Kahana MJ. Human theta oscillations related to sensorimotor integration and spatial learning. Journal of Neuroscience. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ekstrom AD, Caplan J, Ho E, Shattuck K, Fried I, Kahana M. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- 89.Jacobs J, Korolev I, Caplan J, Ekstrom A, Litt B, Baltuch G, Fried I, Schulze-Bonhage A, Madsen J, Kahana M. Right-lateralized Brain Oscillations in Human Spatial Navigation. Journal of Cognitive Neuroscience. :1–13. doi: 10.1162/jocn.2009.21240. no Early Access. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cornwell B, Johnson L, Holroyd T, Carver F, Grillon C. Human Hippocampal and Parahippocampal Theta during Goal-Directed Spatial Navigation Predicts Performance on a Virtual Morris Water Maze. Journal of Neuroscience. 2008;28(23):5983. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bódizs R, Kántor S, Szabó G, Szũcs A, Erõss L, Halász P. Rhythmic hippocampal slow oscillation characterizes REM sleep in humans. Hippocampus. 2001:747–753. doi: 10.1002/hipo.1090. [DOI] [PubMed] [Google Scholar]
- 92.de Araujo DB, Baffa O, Wakai RT. Theta oscillations and human navigation: A magnetoencephalography study. Journal of Cognitive Neuroscience. 2002;14(1):70–78. doi: 10.1162/089892902317205339. [DOI] [PubMed] [Google Scholar]
- 93.Babiloni C, Vecchio F, Mirabella G, Buttiglione M, Sebastiano F, Picardi A, Di Gennaro G, Quarato P, Grammaldo L, Buffo P, et al. Hippocampal, amygdala, and neocortical synchronization of theta rhythms is related to an immediate recall during Rey auditory verbal learning test. Human Brain Mapping. 2008 doi: 10.1002/hbm.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clemens Z, Weiss B, Szu˝cs A, Ero˝ss L, Rásonyi G, Halász P. Phase coupling between rhythmic slow activity and gamma characterizes mesiotemporal rapid-eye-movement sleep in humans. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 95.Niedermeyer E. Hippocampic theta rhythm. Clinical EEG and neuroscience: official journal of the EEG and Clinical Neuroscience Society (ENCS) 2008;39(4):191. doi: 10.1177/155005940803900408. [DOI] [PubMed] [Google Scholar]
- 96.van Asselen M, Kessels R, Neggers S, Kappelle L, Frijns C, Postma A. Brain areas involved in spatial working memory. Neuropsychologia. 2006;44(7):1185–1194. doi: 10.1016/j.neuropsychologia.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Raghavachari S, Lisman JE, Tully M, Madsen JR, Bromfield EB, Kahana MJ. Theta oscillations in human cortex during a working memory task: Evidence for local generators. Journal of Neurophysiology. 2006;95(3):1630–1638. doi: 10.1152/jn.00409.2005. [DOI] [PubMed] [Google Scholar]
- 98.Darvas F, Miller K, Rao R, Ojemann J. Nonlinear Phase-Phase Cross-Frequency Coupling Mediates Communication between Distant Sites in Human Neocortex. Journal of Neuroscience. 2009;29(2):426. doi: 10.1523/JNEUROSCI.3688-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Leuthardt E, Schalk G, Wolpaw J, Ojemann J, Moran D. A brain–computer interface using electrocorticographic signals in humans. Journal of Neural Engineering. 2004;1:63–71. doi: 10.1088/1741-2560/1/2/001. [DOI] [PubMed] [Google Scholar]
- 100.Lachaux JP, Jerbi K, Bertrand O, Minotti L, Hoffman D, Schoendorff B, Kahane P. A blueprint for real-time functional mapping via human intracranial recordings. PLoS One. 2007;2(10):e1094. doi: 10.1371/journal.pone.0001094. [DOI] [PMC free article] [PubMed] [Google Scholar]