Abstract
Polarized cell growth requires the coupling of a defined spatial site on the cell cortex to the apparatus that directs the establishment of cell polarity. In the budding yeast Saccharomyces cerevisiae, the Ras-family GTPase Rsr1p/Bud1p and its regulators select the proper site for bud emergence on the cell cortex. The Rho-family GTPase Cdc42p and its associated proteins then establish an axis of polarized growth by triggering an asymmetric organization of the actin cytoskeleton and secretory apparatus at the selected bud site. We explored whether a direct linkage exists between the Rsr1p/Bud1p and Cdc42p GTPases. Here we show specific genetic interactions between RSR1/BUD1 and particular cdc42 mutants defective in polarity establishment. We also show that Cdc42p coimmunoprecipitated with Rsr1p/Bud1p from yeast extracts. In vitro studies indicated a direct interaction between Rsr1p/Bud1p and Cdc42p, which was enhanced by Cdc24p, a guanine nucleotide exchange factor for Cdc42p. Our findings suggest that Cdc42p interacts directly with Rsr1p/Bud1p in vivo, providing a novel mechanism by which direct contact between a Ras-family GTPase and a Rho-family GTPase links the selection of a growth site to polarity establishment.
INTRODUCTION
Within eukaryotic cells, an asymmetric reorganization of the cytoskeleton and secretory apparatus precedes and supports polarized cell growth at selected sites on the cell cortex (Drubin and Nelson, 1996). Many studies continue to identify the intra- and extracellular signals that bias growth at specific cortical locations. These cortical cues serve to position the axis of polarized growth but are usually not essential for the asymmetric organization of the specific proteins and organelles needed to reinforce the axis of polarity (reviewed in Drubin, 2000). Although central to processes in which function is dependent on polarized morphogenesis (e.g., neuronal growth, nutrient transport, cell migration, and asymmetric cell division), the linkage of a spatial cue to the molecules that regulate the establishment of cell polarity is not fully defined in any cell type.
Cells of the budding yeast Saccharomyces cerevisiae provide a unique opportunity to decipher the molecular mechanism of polarized morphogenesis. During the mitotic cell cycle of S. cerevisiae, cell surface growth mainly takes place in the bud. This polarized growth is supported by the orientation of the secretory apparatus and cytoskeleton toward the bud. The site chosen for polarization is dependent on cell type: haploid a and α cells exhibit an axial budding pattern, in which mother and daughter cells bud immediately adjacent to the site selected for budding in the previous cell cycle. In contrast, diploid a/α cells exhibit a bipolar pattern, in which mother and daughter cells bud at either pole of the cell (Hicks et al., 1977; Chant and Herskowitz, 1991). Selection of a bud site hence determines an axis of cell polarity and ultimately determines the plane of cell division.
A GTPase module comprised of the Ras-family GTPase Rsr1p/Bud1p (hereafter Rsr1p), its guanine nucleotide exchange factor (GEF) Bud5p, and its GTPase activating protein Bud2p is necessary for selecting the proper site for polarized growth in both haploid and diploid cells (Bender and Pringle, 1989; Chant and Herskowitz, 1991; Chant et al., 1991; Bender, 1993; Park et al., 1993). In the absence of the Rsr1p GTPase or its regulators, cortical cues such as Axl2p/Bud10p, which mark the proper site of polarized growth on the cell cortex, are no longer coupled to polarity establishment (Chant and Pringle, 1995; Park et al., 1997; Kang et al., 2001), resulting in random bud-site selection. The rsr1Δ, bud2Δ, or bud5Δ mutants still undergo polarized growth, indicating that the Rsr1p GTPase module plays an essential role not in establishing the axis of polarized growth, but in positioning it.
The establishment of cell polarity relies upon another GTPase, Cdc42p. First discovered in S. cerevisiae (Adams et al., 1990; Johnson and Pringle, 1990), this multifunctional GTPase of the Rho family has evolutionarily conserved functions critical for polarized morphogenesis (reviewed in Johnson, 1999). Specific mutations in yeast CDC42 and CDC24, which encodes the GEF of Cdc42p, prevent polarized organization of the actin structures and exocytotic landmarks (e.g., Sec3p) at the bud site (Sloat et al., 1981; Adams et al., 1990; Pringle et al., 1995; Pruyne and Bretscher, 2000; Zhang et al., 2001). Without this asymmetric distribution of the actin cytoskeleton and secretory apparatus to the bud site, an axis of polarized growth is not maintained and bud formation does not occur. Thus a key issue in understanding how yeast cells are committed to utilize a specific site for polarization is to identify functional linkages between the Cdc42p and Rsr1p GTPase modules.
Previous studies modeled Cdc24p as a link between the Rsr1p and Cdc42p GTPases (reviewed in Gulli and Peter, 2001). An interaction between Cdc24p and Rsr1p was first deduced from genetic analyses (Bender and Pringle, 1989) and was confirmed subsequently in vitro using recombinant proteins (Zheng et al., 1995; Park et al., 1997). The association of Cdc24p with Rsr1p is nucleotide-specific: GTP-bound Rsr1p preferentially interacts with Cdc24p in vitro (Zheng et al., 1995; Park et al., 1997). In contrast, GDP-bound Cdc42p preferentially interacts with Cdc24p (Zheng et al., 1995; Davis et al., 1998; Drees et al., 2001), as expected for a GTPase interacting with its GEF. Combined with the observation that Rsr1p is required to localize Cdc24p at the proper bud site (Park et al., 2002), these results favor a model in which the cycling of Rsr1p through its GTP bound state recruits Cdc24p to the proper bud site, where it activates Cdc42p for interaction with its downstream effectors (Park et al., 1997). A similar bridging of Ras and Rho GTPases by a RhoGEF has been observed in Schizosaccharomyces pombe (Chang et al., 1994).
Herein, we present both genetic and biochemical data indicating that Rsr1p directly interacts with Cdc42p, in addition to its association with Cdc24p. Our data support the idea that the mechanism that couples the selection of a polarized growth site to the establishment of cell polarity involves multiple cross-talks between the Ras and Rho GTPase modules, rather than a single communication across a GEF bridge as previously thought.
MATERIALS AND METHODS
Media and Transformations
Yeast strains were cultured in rich (YPD) medium (Sherman et al., 1986) at 25°C unless stated otherwise. Strains used in this study are listed in Table 1. To track the segregation of auxotrophic markers and to selectively maintain plasmids, strains were cultured in complete synthetic (SC) medium (Sherman et al., 1986) lacking the appropriate amino acid(s) (e.g., SC-Ura). Nourseothricin-resistant strains were selected on 100 μg/ml clonNAT (Werner Bioagents, Jena-Cospeda, Germany). All yeast transformations were performed according to Schiestl and Gietz (1989).
Table 1.
S. cerevisiae strains used in this study
| Strain | Relevant genotype | Source |
|---|---|---|
| DDY1102 | MATa/MATα his3Δ200/his3Δ200 leu2-3,112/leu2-3,112 ura3-52/ura3-52 ADE2/ade2-1 lys2-801am/LYS2 | Kozminski et al. (2000) |
| DDY1300 | CDC42:LEU2 | Kozminski et al. (2000) |
| DDY1304 | cdc42-101:LEU2 | Kozminski et al. (2000) |
| DDY1326 | cdc42-118:LEU2 | Kozminski et al. (2000) |
| DDY1336 | cdc42-123:LEU2 | Kozminski et al. (2000) |
| DDY1344 | cdc42-129:LEU2 | Kozminski et al. (2000) |
| KKY37 | MATarho1-104 leu2-3,112 ura3-52 lys2-801am | This study |
| KKY238 | CDC42:LEU2 [pKK1365, 2μ vector YEplac195] | This study |
| KKY239 | cdc42-101:LEU2 [pKK1365] | This study |
| KKY240 | cdc42-118:LEU2 [pKK1365] | This study |
| KKY286 | cdc42-123:LEU2 [pKK1365] | This study |
| KKY288 | cdc42-129:LEU2 [pKK1365] | This study |
| KKY290 | rho1-104 [pKK1365] | This study |
| KKY1010 | CDC42:LEU2 [pKK1369, 2μ YEp24 library clone] | This study |
| KKY1011 | cdc42-101:LEU2 [pKK1369] | This study |
| KKY1012 | cdc42-118:LEU2 [pKK1369] | This study |
| KKY1013 | CDC42:LEU2 [pKK980, 2μ YEp24 library clone rsr1Δ] | This study |
| KKY1014 | cdc42-118:LEU2 [pKK980] | This study |
| KKY190 | CDC42:LEU2 [pKK925, YEplac195 (RSR1)] | This study |
| KKY66 | cdc42-101:LEU2 [pKK925] | This study |
| KKY68 | cdc42-118:LEU2 [pKK925] | This study |
| KKY287 | cdc42-123:LEU2 [pKK925] | This study |
| KKY289 | cdc42-129:LEU2 [pKK925] | This study |
| KKY291 | rho1-104 [pKK925] | This study |
| KKY256 | CDC42:LEU2 [pKK1095, YEplac195 (rsr1T35A)] | This study |
| KKY60 | cdc42-118:LEU2 [pKK1095] | This study |
| KKY193 | CDC42:LEU2 [pKK1240, YEplac195 (rsr1G12V)] | This study |
| KKY200 | cdc42-118:LEU2 [pKK1240] | This study |
| KKY194 | CDC42:LEU2 [pKK1243, YEplac195 (rsr1K16N)] | This study |
| KKY201 | cdc42-118:LEU2 [pKK1243] | This study |
| KKY69 | cdc42-118:LEU2 [pHP569, YEp103 (CDC24)] | This study |
| Y147 | MATacdc24-4 ura3 leu2-3, 112 his3 | Bender and Pringle (1989) |
| KKY342 | CDC42:LEU2 rsr1::HIS3 | This study |
| KKY343 | cdc42-101:LEU2 rsr1::HIS3 | This study |
| KKY344 | cdc42-101:LEU2 RSR1 | This study |
| KKY345 | cdc42-118:LEU2 rsr1::HIS3 | This study |
| KKY346 | cdc42-118:LEU2 RSR1 | This study |
| KKY297 | cdc42-129:LEU2 rsr1::HIS3 | This study |
| KKY298 | cdc42-129:LEU2 RSR1 | This study |
| KKY85 | MATa/MATα rsr1::HIS3/RSR1 his3Δ200/his3Δ200 leu2-3,112/leu2-3,112 ura3-52/ura3-52 ADE2/ade2-1 lys2-801am/LYS2 | This study |
| KKY86 | MATα rsr1::HIS3 ura3-52 leu2-3,112 his3Δ200 lys2-801 am | This study |
| Y3611 | MATa/MATα can1Δ::MFA1pr-HIS3-MFα1pr-LEU2/CAN1 his3Δ1/his3Δ1 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 met15Δ0/MET15 lys2Δ0/LYS2 | This study |
| Y3598 | MATacan1Δ::MFA1pr-HIS3-MFα1pr-LEU2 his3Δ1 leu2Δ0 ura3Δ0 met15Δ0 | This study |
| Y2420 | MATamfa1Δ::MFA1pr-HIS3 ura3Δ0 leu2Δ0 his3Δ1 met15Δ0 | Tong et al. (2001) |
| Y2823 | MATα mfα1Δ::MFα1pr-LEU2 his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Tong et al. (2001) |
| BY4741 | MATahis3Δ1 leu2Δ0 ura3Δ0 met15Δ0 | Brachmann et al. (1998) |
| BY4742 | MATα his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | Brachmann et al. (1998) |
| KKY281 | MATα CDC42:natMX can1Δ::MFA1pr-HIS3-MFα1pr-LEU2 his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | This study |
| KKY283 | MATα cdc42-118:natMX can1Δ::MFA1pr-HIS3-MFα1pr-LEU2 his3Δ1 leu2Δ0 ura3Δ0 lys2Δ0 | This study |
| YD00000-YD09999 | MATaorfΔ::kanMX4 his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 | Winzeler et al. (1999) |
| HPY16 | MATaura3-52 his3-Δ1 leu2 trp1Δ63 prb1-1122 pep4-3 prc1-407 | Park et al. (1993) |
All cdc42:LEU2 and CDC42:LEU2 strains above are MATa ura3-52 leu2-3,112 his3Δ200 lys2-801am and are isogenic except at CDC42. The strain background is S288C. Ten back-crosses transferred rho1-104 (Yamochi et al., 1994) into the S288C DDY/KKY strain background. Plasmid bearing KKY strains were isolated as transformants of DDY1300, DDY1304, DDY1326, DDY1336, DDY1344 (Kozminski et al., 2000), and KKY37, respectively.
Identification and Analyses of Synthetic Genetic Interactions
Synthetic genetic arrays (SGA) were screened three times at 33°C with KKY281 and KKY283 by the method of Tong et al. (2001). Synthetic interactions were confirmed by random spore and/or tetrad analysis. The function of interacting genes was defined by reference to the Yeast Proteome Database (YPD; Incyte Genomics, Beverly, MA). KKY281 and KKY 283 were derived from Y3611, using pKK1467 and pKK1471 as sources of integrating DNA following the method of Kozminski et al. (2000) for linking LEU2 to cdc42. Y3611 was created by crossing Y3598 to BY4742. In Y3598, MFA1pr-HIS3-MFα1pr-LEU2 was integrated at the CAN1 locus to generate can1Δ::MFA1pr-HIS3-MFα1pr-LEU2. The construction of Y3598 involved four steps. First, a 455-base pair fragment containing the upstream sequence of CAN1 was amplified from yeast genomic DNA with primers (TAGGGCGAACTTGAAGAATAACC) and (GCCACGTTGCACACTATCCTGTGCTATGCCTTTTTTTTTTTTTGTT), which contained a 21-base pair sequence (underlined) from the 5′ end of MFA1pr-HIS3. Second, MFA1pr-HIS3 was amplified from genomic DNA obtained from Y2420 with primers (CAGGATAGTGTGCAACGTGGC) and (CTCATTGAATCTTTGCATTCAGAGCGTCTACATAAGAACACCTT-TGGTGG), which contained a 27-base pair sequence (underlined) from the 5′ end of the MFα1-LEU2. Third, MFα1pr-LEU2 was amplified from genomic DNA obtained from Y2823 with primers (ACGCTCTGAATGCAAAGATTCAATGAG) and (ATCAAAGGTAATAAAACGTCATATTTAAGCAAGGATTTTCTTAACTTC), which contained a 24-base pair sequence (underlined) from the 5′ end of the CAN1 upstream sequence. Fourth, a 300-base pair fragment containing the CAN1 downstream sequence was amplified from yeast genomic DNA with primers (ATATGACGTTTTATTACCTTTGAT) and (ACGAAAAATGAGTAAAAATTATCTT). Finally, the set of PCR products above were used as templates to generate a fused product with primers (TAGGGCGAACTTGAAGAATAACC) and (ACGAAAAATGAGTAAAAATTATCTT). BY4741 transformants carrying can1Δ::MFA1pr-HIS-MFα1pr-LEU2 were selected on synthetic medium lacking histidine.
Isogenic rsr1Δ, cdc42, rsr1Δ cdc42 haploid strains were derived as meiotic products of a cross between KKY86 and KKY400, 404, 426, or 444. KKY86 is a meiotic product of KKY85, which was produced by the integration of an HIS3-containing PCR product (primers oKK47: CTCCACTGAACAATAATACTATTATTTAGTAACGATATAGAACATTTACATACAACGCAGATTGTACTGAGAGTGCACC and oKK48: CTTTAAAAACTTATACAACGTATGCTAATACTTTAAACTAAACCTTTTAGAACTATACTGTGCGGTATTTCACACCGC; RSR1 flanking sequence underlined; template pRS303) at the RSR1 locus of DDY1102, creating a complete deletion of RSR1.
Screen for cdc42 Dosage Suppressors
To identify dosage suppressors of the cdc42-118 temperature-sensitive growth defect, DDY1326 was transformed with a 2-μm plasmid-based S. cerevisiae genomic library (Carlson and Botstein, 1982), plated on SC-Ura, and then incubated immediately at 37°C for 4 d, selecting simultaneously for transformants and dosage suppressors. Approximately 8300 transformants were screened. Fifty-three transformants grew at 37°C. To test whether survival at 37°C was plasmid dependent, transformants were streaked onto SC plates containing 0.5 mg/ml 5-fluoroorotic acid (FOA) to counterselect the library plasmid. After incubation for 4 d at 25°C, single colonies were picked from SC+FOA plates, struck onto SC plates, and incubated for 3 d at 37°C. Eleven transformants displayed plasmid-dependent growth. PCR analysis using primers KK27 (GTACATCACCATTGTCCAGGTG) and D (Kozminski et al., 2000) eliminated transformants with plasmid-borne copies of CDC42 from further consideration. Library plasmids were rescued from these transformants, amplified in Escherichia coli, and tested for redundancy using RsaI restriction site mapping. To verify that each nonredundant library plasmid contained a plasmid-borne cdc42 suppressor, library plasmids were retransformed into DDY1326. Seven transformants grew on SC-Ura at 37°C. The ends of each genomic fragment in the seven nonredundant library plasmids were partially sequenced from the flanking vector sequence, using primers YEp24F (CCGCTTTGGCCGCCG) and YEp24R (GCCTATATCGCCGACATC). The resulting sequences were then used in a WU-BLAST search of the Saccharomyces Genome Database (http://www.yeastgenome.org/) to obtain the full sequence of each genomic fragment. Library plasmid pKK1369 contains a genomic fragment of chromosome VII (base pairs 794092–802090). The other six suppressors will be described elsewhere.
Identification of RSR1 as a Dosage Suppressor of cdc42-118
To determine whether RSR1 is an allele-specific high copy suppressor of cdc42-118 and the only suppressor of cdc42-118 in YEp24 library clone pKK1369, isogenic CDC42 (DDY1300), cdc42-101 (DDY1304), cdc42-118 (DDY1326), cdc42-123 (DDY1336), cdc42-129 (DDY1344), and rho1–104 (KKY37) strains were transformed with pKK1369, pKK1369 with RSR1 deleted (pKK980), or a 2-μm plasmid (YEplac195) with (pKK925) or without (pKK1365) a RSR1 insert. Transformants were streaked onto SC-Ura plates, and scored for growth after incubation at 25 and 36°C for 3 d. To determine whether CDC24 and alleles of RSR1 are dosage suppressors of cdc42-118, a cdc42-118 strain was transformed with 2-μm YEplac195 plasmids carrying rsr1T35A (pKK1095), rsr1G12V (pKK1240), rsr1K16N (pKK1243), rsr1K260-264S (pHP1123) (Park et al., 2002), or YEp103 (CDC24) (Ziman and Johnson, 1994).
Construction of Plasmids
To delete RSR1 from the YEp24 library clone, pKK1369 was digested with BssHII and SacI. The vector was then blunt-end ligated after Mung Bean nuclease digestion, forming pKK980. To clone RSR1 into YEplac195, a SacI-SalI RSR1-containing fragment was subcloned into the SacI and SalI sites of pKK1365 (YEplac195) from pHP674-1 (Park et al., 1997), forming pKK925. To clone rsr1T35A into YEplac195, a SacI-Bsu36I fragment from pHP675 (Park et al., 1997) was subcloned into the SacI and Bsu36I sites of pKK925, forming pKK1095. To clone rsr1G12V and rsr1K16N into YEplac195, a SacI-Bsu36I fragment from YEp13(rsr1G12V) and YEp13(rsr1K16N) (Ruggieri et al., 1992), was subcloned into the SacI and Bsu36I sites of pKK1095, forming pKK1240 and pKK1243, respectively. The rsr1 constructs were sequenced to verify the identity of each rsr1 allele. To clone rsr1-7 (rsr1K260-264S) into YEplac195, Bsu36I-SalI fragment of pKK925 was replaced with the Bsu36I-SalI fragment from pRS304-rsr1K260-264S-GFP (pHP1065) (Park et al., 2002).
For the integration of CDC42:natMX or cdc42-118:natMX into Y3611, a NotI-NotI LEU2 fragment was removed, respectively, from pKK655 and pAC326 (Kozminski et al., 2000) and replaced with a NotI-NotI fragment containing natMX, forming pKK1467 and pKK1471. The natMX fragment was amplified by PCR from p4339 (Tong et al., 2001) using the primers (NotI sites underlined) oKK133 (AGTCTCTAGCGGCCGCACATGGAGGCCCAGAATACCC) and oKK134 (AGTCTCTAGCGGCCGCAGTATAGCGACCAGCATTCAC).
To fuse GST to the N-terminus of Cdc42p, CDC42 was subcloned as a BsgI-HindIII fragment from pKK944 into the BsgI and HindIII sites of vector pKK1017, forming pKK1025. To construct pKK1017, GST coding sequence (BglII-BamHI ends) was amplified by PCR from the template pGEX-2T (pKK858; Amersham Bioscience, Piscataway, NJ), using primers (restriction sites underlined) oKK42 (GCGCGAGATCTATGTCCCCTATACTAGGTTATTG) and oKK43 (ATATAAGGATCCACGCGGAACCAGATC) and subcloned into the BamHI site of pFastBac-1 (pKK855; GIBCO-BRL). To construct pKK944, a BsgI-HindIII fragment was subcloned from pKK661 into the BsgI and HindIII sites of pKK1017. pKK661 was constructed by subcloning a PCR fragment (BamHI-HindIII ends) that contains the CDC42 coding region amplified from pKK177 (Kozminski et al., 2000) into the same sites of vector pGAT2 (Peränen et al., 1996). The fusion constructs were sequenced to verify an in-frame fusion and the sequence of the GST and CDC42 coding sequences.
Preparation of Yeast Lysate and Immunoprecipitation
Yeast lysate preparation and immunoprecipitation were carried out at 4°C using an Eppendorf centrifuge. Yeast cell extracts were prepared from protease-deficient strain HPY16 carrying pRS425(HA-RSR1) (Park et al., 1997) and YEp103(CDC42) using the lysis buffer G (4.3 mM Na2HPO4.7H2O, 1.4 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl, 1 mM DTT, 10%, glycerol, pH 8.1) and various proteases as previously described (Park et al., 1997) with slight modifications. After centrifugation for 4 min at 1900 rpm, the whole cell extracts were centrifuged for 15 min at 14,000 rpm to obtain a membrane fraction. The membrane fraction was resuspended in Buffer G containing 1% Triton X-100 and rocked for 30 min at 4°C. A soluble protein fraction was recovered by centrifugation for 15 min at 14,000 rpm, diluted approximately threefold with Buffer G (with no detergent; typically ∼1 ml final), and precleared with anti-mouse IgG agarose beads (Sigma, St. Louis, MO) before incubating with 2 μl of anti-HA antibody (HA.11 from Covance Research Products, Denver, PA). After immunoprecipitation, association of Cdc42p with Rsr1p was determined by immunoblotting with affinity-purified rabbit polyclonal antibodies against Cdc42p (prepared according to the method of Ziman et al., 1991), and recovery of Rsr1p was determined with polyclonal antibodies against Rsr1p (a kind gift of A. Bender).
Protein Expression and Purification
The Bac-To-Bac Baculovirus Expression System (Invitrogen, Carlsbad, CA) was used to express GST-Cdc42 fusion proteins. Plasmid constructions are described above. GST-Cdc42p was purified from HIGH FIVE cells (Invitrogen) 48 h postinfection at an MOI of 10, using glutathione agarose beads as described by Cerione et al. (1995).
GST-Rsr1p, GST-Rsr1pT35A, MBP-Cdc24C, and His6-tagged Cdc42p were expressed and purified from a protease-deficient E. coli strain (BL21) essentially as previously described (Kellogg et al., 1995; Park et al., 1997). After harvesting cells, the cell pellet was frozen and ground under liquid nitrogen in a mortar. The fine powder was then rapidly resuspended in PBS containing 0.1% Triton X-100, 1 mM DTT and a cocktail of protease inhibitors. His6-tagged Cdc42p was purified using a column containing iminodiacetic acid immobilized on Sepharose 6B (Sigma) coupled to Co2+. When necessary, Rsr1p was released from the affinity matrix by cleaving GST-Rsr1p with thrombin (∼2000 NIH units/mg protein, Sigma) as follows: After resuspension of the Sepharose in PBS buffer containing 150 mM NaCl and 2.5 mM CaCl2, the protein concentration was adjusted to 0.3 mg/ml before thrombin was added to the bead slurry at a ratio of 1% (wt/wt). After incubation for 3.5 h at 25°C, the GST moiety that remained bound to the beads was removed by centrifugation and the supernatant was incubated 1 h at 4°C in presence of p-aminobenzamidine coupled to agarose beads (Sigma) to remove the thrombin.
In Vitro Binding Assays
In vitro binding assays were performed as described (Park et al., 1997) with slight modification. GST-fusion proteins, His6-Cdc42p, and Rsr1p (after the GST moiety was cleaved off) were dialyzed overnight at 4°C against a buffer (20 mM Tris-HCl, pH 7.5, 1 mM DTT, 5 mM MgCl2, 10% glycerol) containing 2.5 μM GDP after purification. Approximately 1 μg of protein was diluted to a final volume of 50 μl with 50% glutathione Sepharose bead slurry and incubated for 1 h at 4°C. The beads were collected by centrifugation and resuspended in Buffer I (20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 1 mM DTT, 10 mM EDTA, 10% glycerol, 0.1% Triton X-100, 10 μg/ml leupeptin, 10 μg/ml aprotinin). After incubation for 1 h at room temperature, Buffer I was substituted with Buffer I containing 5 mM MgCl2 plus 0.5 mM GTPγS or 0.5 mM GDP (Roche Diagnostics, Indianapolis, IN). After 30 min incubation at room temperature, the beads were resuspended in Buffer I containing 10 mM MgCl2 plus 0.5 mM GTPγS or 0.5 mM GDP instead of 10 mM EDTA and then incubated 20 min at room temperature to stabilize the nucleotide bound state of the GTPase.
Filter Binding Assays
Filter binding assays were carried out as described previously (Diekmann et al., 1994). The purified recombinant proteins were preloaded with nucleotides as described above except [8,5′-3H]GTP or [8,5′-3H]GDP (38.2 Ci/mmol, NEN Life Science Products) was loaded onto one of the GTPases. After in vitro binding reaction, the reaction mix was diluted to 1 ml with wash buffer (20 mM Tris-Cl, pH 7.5, 100 mM NaCl, 10 mM MgCl2, 1 mM DTT, 10% glycerol) and then filtered through a nitrocellulose filter (0.2-μm pore diameter, Millipore). After washing with 10 ml of Buffer I, the radioactivity retained on the filters was quantified by scintillation counting.
RESULTS
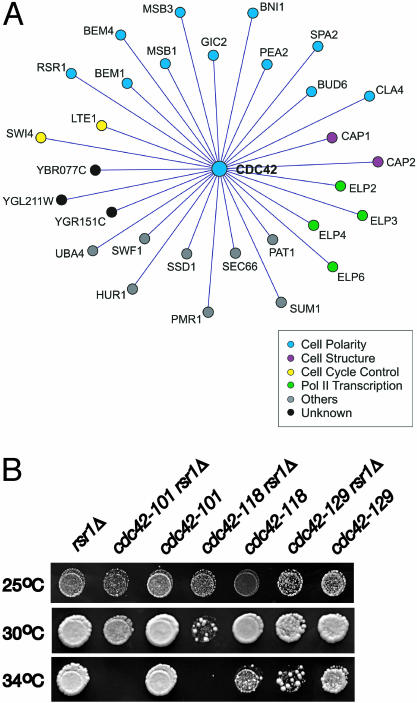
A CDC42 Allele Defective in Polarity Establishment Identifies a RSR1-CDC42 Interaction To identify genes that interact with CDC42 during the establishment of cell polarity in late G1, we applied synthetic genetic array (SGA) analysis (Tong et al., 2001) to screen for genes that confer a growth defect when deleted in combination with cdc42-118, an allele that is specifically and conditionally defective in polarity establishment (Kozminski et al., 2000). At restrictive temperatures, cdc42-118 cells arrest as large, round, unbudded, multinucleate cells, most likely due to an inability to organize the actin cytoskeleton at the bud site (Kozminski et al., 2000). We identified 30 CDC42-interacting genes (Figure 1A). Ten of these genes have a previously defined role in polarized bud growth (see the “Cell Polarity” category in Figure 1A), validating the screen. Of these, only six have shown either a synthetic genetic interaction (MSB3, GIC2, BNI1, CLA4; Caviston et al., 2002; Hofken and Schiebel, 2002) with CDC42 and/or a physical interaction (GIC2, BNI1, CLA4, BEM1, and BEM4) between Cdc42p and their gene product (Chen et al., 1997; Mack et al., 1996; Brown et al., 1997; Evangelista et al., 1997; Uetz et al., 2000; Bose et al., 2001; Drees et al., 2001). For the 20 remaining genes, especially those placed into functional categories other than “Cell Polarity,” their identification in this screen suggested that they have a function associated with the Cdc42p-dependent development of cell polarity in late G1. For the open reading frames classified as “Unknown,” this interaction with CDC42 was the first assignment of function.
Figure 1.
Synthetic genetic analyses identified RSR1 as a CDC42-interacting gene. (A) Genetic interaction network representing synthetic lethal/sick interactions of cdc42-118 determined by SGA analysis. Genes are colored according to their YPD-defined cellular function. (B) Equivalent dilutions of rsr1Δ, cdc42, and rsr1Δ cdc42 mutants grown at 25, 30, and 34°C for 2d on SC-URA. The strains shown (left to right) are KKY 342, 343, 344, 345, 346, 297, and 298.
The identification of RSR1 as a CDC42-interacting gene was particularly intriguing. To date, Rsr1p has been thought only to position the axis of polarized growth before bud emergence and not to affect bud formation per se. To confirm that RSR1 affects the polarity establishment function of Cdc42p, we compared the growth of rsr1Δ cells containing different separation-of-function alleles of CDC42 (Kozminski et al., 2000). As expected, alleles of CDC42 that exhibit a growth defect due to a loss of polarity (cdc42-101, cdc42-118) in late G1 conferred synthetic sickness at 30°C and synthetic lethality at 34°C when combined with rsr1Δ (Figure 1B). In contrast, an allele of CDC42 that is defective in the switch from apical to isotropic bud growth in G2/M (cdc42-129) conferred only synthetic sickness at 34°C when combined with rsr1Δ (Figure 1B). Because the cdc42 alleles used in this study are temperature-sensitive for growth, it was important to verify that the sick or lethal phenotype displayed by a rsr1Δ cdc42 double mutant was due to the absence of RSR1. As shown in Figure 1B, the restrictive temperature range of the rsr1Δ cdc42 mutants did not correlate with the restrictive temperature range of each cdc42 allele, indicating a bona fide synthetic interaction between cdc42 and rsr1Δ in particular double mutants. These results suggest that RSR1 stimulates the polarity establishment function of CDC42.
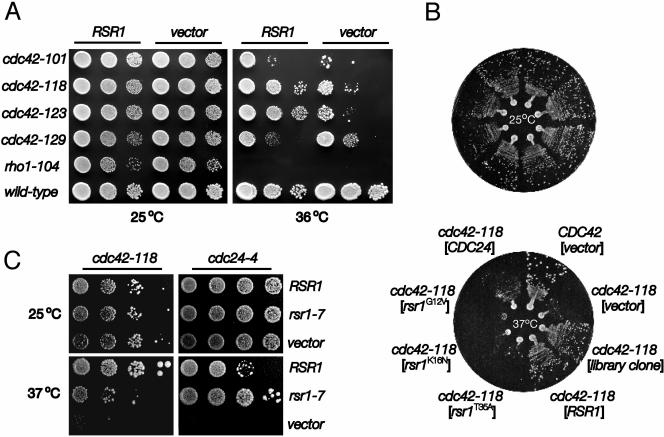
Although RSR1 has a known role in bud-site selection (Bender and Pringle, 1989; Chant and Herskowitz, 1991), a second independent line of evidence supported a role for RSR1 in CDC42-dependent polarity establishment. Concomitant with our synthetic lethality studies, RSR1 was identified in a second screen as a multicopy dosage suppressor of the cdc42-118 temperature-sensitive growth defect. In contrast to cdc42-118 cells containing a multicopy vector plasmid, cdc42-118 cells bearing a multicopy RSR1 plasmid did not undergo growth arrest at 36°C (Figure 2A), and appeared for the most part, even at 37°C, morphologically wild-type (≤4% of the population exhibited a large, unbudded morphology; our unpublished results). Multicopy RSR1 did not suppress the minor budding pattern defect found in the cdc42-118 mutant at 25°C (our unpublished results). As observed with the synthetic genetic interactions described above, the multicopy dosage suppression by RSR1 was also specific to the cdc42 alleles defective in polarity establishment. Overexpression of RSR1 neither suppressed a temperature-sensitive allele of RHO1 (Figure 2A), which encodes a Rho-family GTPase essential for bud formation (Yamochi et al., 1994), nor every CDC42 separation-of-function allele tested (Figure 2A). Like cdc42-118, these separation-of-function alleles are temperature-sensitive for growth at 36°C, even though they are expressed at the wild-type levels (Kozminski et al., 2000). At 36°C, multicopy RSR1 suppressed the growth defect of the three alleles defective in polarity establishment: cdc42-118 and cdc42-123, and to a moderate extent, cdc42-101 (Figure 2A). Suppression of cdc42-129, which is not defective in polarized growth, did not exceed that of the vector control (Figure 2A), indicating that RSR1 is not a general bypass suppressor of cdc42 mutations. Consistent with the synthetic lethality data, these results suggest that RSR1 specifically interacts with CDC42 to affect its polarity establishment function before bud emergence.
Figure 2.
A multicopy RSR1 plasmid, but not plasmids carrying other rsr1 alleles, suppressed the growth defect of cdc42-118. (A) Dilution series compared growth at 25 and 36°C (SC-URA, 4d) of wild-type and mutant yeast transformed with a high-copy (YEplac195) 2-μm plasmid containing RSR1 or no insert. Each spot of cells represented a 10-fold serial dilution from left to right. From top to bottom, RSR1, 2 μm-plasmid containing strains are KKY 66, 68, 287, 289, 291, and 190; strains containing only 2 μm-plasmid are KKY 239, 240, 286, 288, 290, and 238. (B) Wild-type and cdc42-118 strains were transformed with 2-μm plasmids (shown in brackets) containing no insert, a genomic fragment containing RSR1, alleles of RSR1, or CDC24 and grown at 25 (top panel) or 37°C (bottom panel) for 3 d on SC-URA. The strains shown (clockwise from 1 o'clock) are KKY 238, 240, 1012, 68, 60, 201, 200, and 69. Note: RSR1 suppressed cdc24-118 better than CDC24. (C) The cdc42-118 and cdc24-4 mutants, transformed with 2-μm plasmids containing RSR1, rsr1-7, or no insert, were grown at 25°C (for 3 d) or 37°C (for 4 d) on SC-URA (for cdc42-118) or SC-URA + 1 M sorbitol (for cdc24-4). Tenfold serial dilutions of each cdc42-118 transformant and 2.5-fold serial dilutions of each cdc24-4 transformant are shown.
Alleles of RSR1 Suggest a Direct Interaction between Rsr1p and Cdc42p In Vivo
RSR1 was previously identified as a multicopy dosage suppressor of a temperature-sensitive cdc24-4 polarized growth defect (Bender and Pringle, 1989). This interaction requires Rsr1p to be in the GTP-bound state and to possess an intact effector domain (Ruggieri et al., 1992; Park et al., 1997). To determine whether similar requirements exist for the RSR1-CDC42 interaction, we tested whether particular alleles of RSR1, expressed at about wild-type levels (our unpublished results), could suppress the cdc42-118 growth defect. As in the case of cdc24, we found that an effector domain mutation in RSR1 (rsr1T35A) inhibited the multicopy suppression of the temperature-sensitive cdc42-118 growth defect (Figure 2B), indicating that the effector domain of Rsr1p is necessary for efficient suppression. In contrast to the RSR1-CDC24 interaction, neither multicopy rsr1G12V nor rsr1K16N, which encode Rsr1p locked in the GTP- or GDP-bound state respectively (Ruggieri et al., 1992), could suppress the cdc42-118 growth defect at 37°C (Figure 2B), suggesting that cycling of Rsr1p between GTP- and GDP-bound states is important for suppression. Interestingly, CDC24 was a weaker multicopy suppressor of cdc42-118 than RSR1 (Figure 2B). Thus, the suppression of the cdc42 growth defect by multicopy RSR1 was not likely to be due to the enhancement of an interaction between crippled Cdc42p and Cdc24p by Rsr1p. Consistent with the idea, we found that multicopy rsr1-7 (rsr1K260-264S) (Park et al., 2002), expressed at about the same level as wild-type RSR1 (our unpublished results), poorly suppressed the cdc42-118 growth defect, though in contrast it suppressed the cdc24-4 growth defect similarly to (or slightly better than) wild-type RSR1 (Figure 2C). Taken together, this allele-specific suppression of cdc42-118 by RSR1 suggests that the interaction between Rsr1p and Cdc42p is direct in vivo and that the interaction is not bridged by Cdc24p.
Rsr1p Interacts with Cdc42p In Vivo and In Vitro
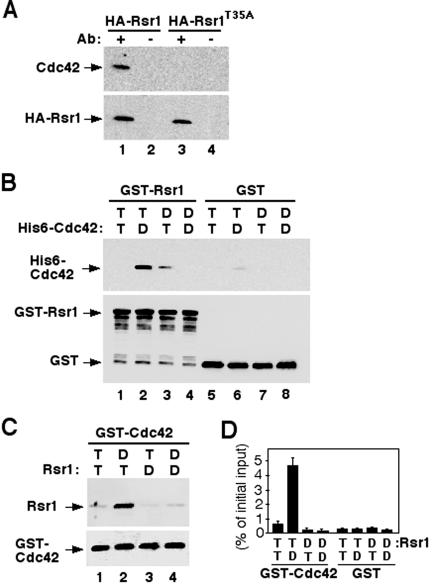
To test whether Rsr1p interacts with Cdc42p in vivo, we carried out immunoprecipitation experiments. We prepared a Triton X-100 soluble fraction of membrane-associated proteins from a yeast strain in which Cdc42p and a functional HA epitope-tagged Rsr1p were expressed from multicopy plasmids. As shown in Figure 3A, Cdc42p coimmunoprecipitated with HA-Rsr1p, but not with HA-Rsr1T35Ap. These data indicate that Cdc42p associates with Rsr1p in vivo and that the effector domain of Rsr1p is important for the association.
Figure 3.
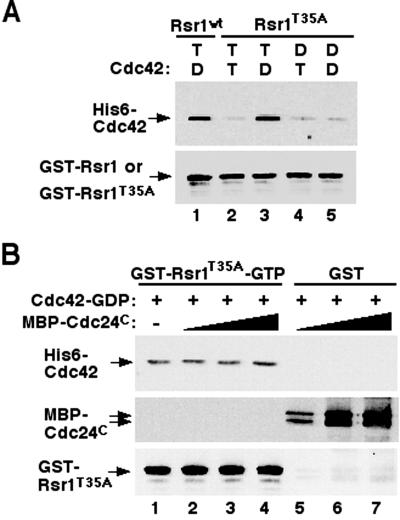
Rsr1p interacts with Cdc42p. (A) Cdc42p coimmunoprecipitated with HARsr1p from yeast extracts. CDC42, HA-RSR1, and HA-rsr1T35A were expressed from multicopy plasmids in yeast. Association of Cdc42p and HA-Rsr1p was not detectable when HA-Rsr1p and Cdc42p were expressed at the endogenous level. After immunoprecipitation of HA-Rsr1p (lane 1) or HARsr1T35Ap (lane 3) with an antibody against the HA epitope, association of Cdc42p was determined by immunoblotting with polyclonal antibodies against Cdc42p (top panel). Approximately equal amounts of HA-Rsr1p and HA-Rsr1T35Ap were recovered for each reaction as judged by immunoblotting with polyclonal antibodies against Rsr1p (bottom panel). Control reactions without HA antibody were shown in lanes 2 and 4. (B) Association of GST-Rsr1p and His6-Cdc42p in vitro. GST-Rsr1p (∼400 nM) preloaded with GTPγS (T) or GDP (D) was incubated with His6-tagged Cdc42p (∼400 nM) preloaded with GTPγS or GDP. Rsr1p-GTPγS + Cdc42p-GTPγS (lane 1); Rsr1p-GTPγS + Cdc42p-GDP (lane 2); Rsr1p-GDP + Cdc42p-GTPγS (lane 3); Rsr1p-GDP + Cdc42p-GDP (lane 4). GST that had been preincubated with GTPγS (lanes 5 and 6) or GDP (lanes 7 and 8) was used as a control. Association of Cdc42p with Rsr1p was determined by immunoblotting with antibodies against Cdc42p (top panel). Approximately equal amounts of GSTRsr1 and GST proteins were recovered for each reaction as judged by immunoblotting with antibodies against GST (bottom panel). (C) Association of Rsr1p and GST-Cdc42p in vitro. Rsr1p (∼400 nM) purified after removal of GST was preloaded with GTPγS (T) or GDP (D) and incubated with GST-Cdc42p (∼400 nM) preloaded with GTPγS or GDP. Rsr1p-GTPγS + Cdc42p-GTPγS (lane 1); Rsr1p-GTPγS + Cdc42p-GDP (lane 2); Rsr1p-GDP + Cdc42p-GTPγS (lane 3); Rsr1p-GDP + Cdc42p-GDP (lane 4). Association of Rsr1p with GST-Cdc42p was determined by immunoblotting with antibodies against Rsr1p (top panel). Approximately equal amounts of GST-Cdc42p were recovered for each reaction as judged by immunoblotting with antibodies against Cdc42p (lower panel). (D) Quantification of the amounts of Rsr1p associated with GST-Cdc42p. Rsr1p preloaded with [3H]GTPγS (T) or [3H]GDP (D) was incubated with GST-Cdc42p preloaded with GTPγS or GDP. The amount of [3H]GTPγS- or [3H]GDP-bound Rsr1p that associated with GST-Cdc42p was determined by a filter binding assay. An average of five independent assays is shown as percentage of counts (cpm) divided by the initial input to each reaction.
To test whether Rsr1p can bind to Cdc42p in the absence of Cdc24p, we carried out in vitro binding experiments using Rsr1p fused to glutathione S-transferase (GST) and six-histidine-tagged (His6-) Cdc42p purified from E. coli. After preloading GST-Rsr1p and His6-Cdc42p with GTPγS, a nonhydrolyzable GTP analogue, or with GDP, the GTPases were incubated together. In control experiments, GST preincubated with GTPγS or GDP was mixed with nucleotide-bound His6-Cdc42p. As shown in Figure 3B, Cdc42p-GDP associated preferentially with Rsr1p-GTPγS. In a complementary analysis, Rsr1p (GST removed) was incubated with GST-Cdc42p purified from baculovirus-infected cells. As in the previous experiment, Rsr1p-GTPγS interacted preferentially with Cdc42p-GDP (Figure 3C) but not with the GST control (unpublished data).
To quantify the amount of Rsr1p associated with Cdc42p and to confirm the nucleotide-bound state of each associated GTPase, we performed a filter-binding assay (Park et al., 1993; Diekmann et al., 1994) after the in vitro binding reaction using Rsr1p preloaded with [3H]GTP or [3H]GDP. Approximately 5% of the total [3H]GTP-Rsr1p added to the reaction associated with Cdc42p-GDP (Figure 3D). A smaller amount of [3H]GTP-Rsr1p also associated with Cdc42p-GTPγS. Similar results were obtained in a complementary experiment using His6-Cdc42p preloaded with [3H]GDP or [3H]GTP and GST-Rsr1p. Taken together, these results indicate that Rsr1p interacts directly with Cdc42p in a nucleotide-dependent manner.
The nucleotide-specific association of Rsr1p and Cdc42p was observed at a relatively low concentration (<430 nM) and equimolar ratio of Rsr1p and Cdc42p. At the higher concentrations we tested (0.8 ∼ 2 μM of each GTPase), the interaction between the two GTPases was not specific to each nucleotide-bound state. For example, we observed a strong interaction between Rsr1p-GTP and Cdc42p-GTP (our unpublished results), which might be due to the formation of heterodimers—similar to the formation of homodimers in vitro by these (Beven and Park, unpublished data) and mammalian Rho GTPases at high concentrations (Zhang and Zheng, 1998; Inouye et al., 2000). The physiological significance of homodimer formation of small GTPases is not clear at the present time.
The Cdc24p Fragment Associated with Rsr1p-GTP Enhances Interaction Between Rsr1p-GTP and Cdc42p-GDP In Vitro
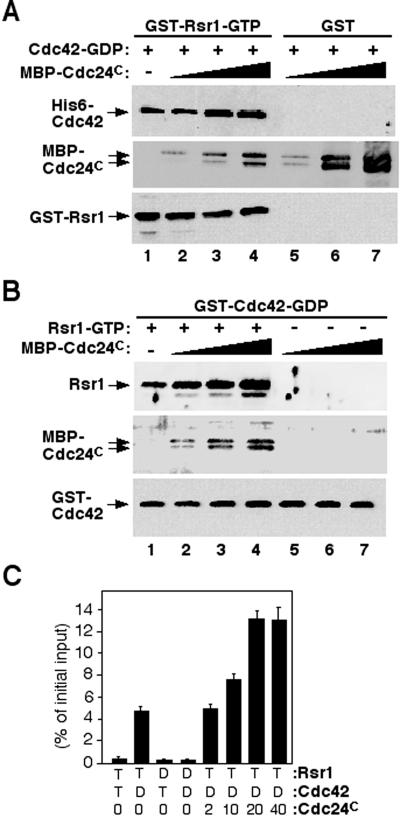
Because both Cdc24p (Zheng et al., 1995; Park et al., 1997) and Cdc42p (this study) appear to interact specifically with the GTP-bound form of Rsr1p, we wanted to know whether these associations were cooperative or competitive. To address this question, we performed in vitro binding reactions using GST-Rsr1p-GTPγS, His6-Cdc42p-GDP, and the C-terminal half (residues 472–854) of Cdc24p (Cdc24C) fused to a maltose-binding protein (MBP). This truncated form of Cdc24p interacts with Rsr1p (Park et al., 1997), but lacks the GEF domain that would exchange the nucleotide on Cdc42p. The amount of Cdc42p associated with Rsr1p increased proportionally with an increase of Cdc24C in the reaction (Figure 4A, lanes 1–4). When MBP-Cdc24C was cosedimented with amylose resin, Cdc42p was not associated with Cdc24C in the absence of Rsr1p (Figure 4A, lanes 5–7), confirming that Cdc42p binds to Rsr1p and not to Cdc24C. Similarly, when we used GST-Cdc42p and Rsr1p (GST removed), a proportionally higher amount of Rsr1p was associated with GST-Cdc42p with an increase of Cdc24C (Figure 4B; lanes 1–4). In the absence of Rsr1p, no Cdc24C was recovered with GST-Cdc42p (Figure 4B; lanes 5–7), as expected. To quantify the enhancement of the Rsr1p-Cdc42p interaction by Cdc24C, the binding reaction was carried out using His6-Cdc42p preloaded with [3H]GTP or [3H]GDP and GST-Rsr1p preloaded with GTPγS in the presence of Cdc24C, and the amount of Cdc42p associated with GSTRsr1p was determined by a filter-binding assay. Association of [3H]GDP-Cdc42p with Rsr1p-GTPγS increased about threefold when 20 nM Cdc24C (∼ 5% of the concentration of Rsr1p or Cdc42p) was added to the reaction (Figure 4C). Taken together, these data show that Rsr1p interacts with both Cdc24C and Cdc42p, and that Cdc24C enhances the interaction between Rsr1p-GTP and Cdc42p-GDP.
Figure 4.
The C-terminal half of Cdc24p enhances interaction between Rsr1p-GTP and Cdc42p-GDP. (A) GST-Rsr1p (∼400 nM) preloaded with GTPγS was incubated with His6-tagged Cdc42p (∼400 nM) preloaded with GDP in the absence (lane 1) or in the presence of MBP-Cdc24C (lane 2: 2 nM; lane 3: 10 nM; lane 4: 20 nM). GST-Rsr1p was collected with glutathione Sepharose. As a control, GST was incubated with MBP-Cdc24 C and Cdc42p-GDP, and MBPCdc24 C was brought down with amylose resin (lanes 5–7). Approximately equal amounts of GST-Rsr1p were recovered for each reaction as judged by immunoblotting with Rsr1p-specific antibodies (bottom panel: lanes 1–4). Association of Cdc42p and MBP-Cdc24 C with GST-Rsr1p or GST was determined by immunoblotting with Cdc42p-specific antibodies (top panel) and with polyclonal antibodies against Cdc24p (middle panel), respectively. (B) GST-Cdc42p (∼400 nM) preloaded with GDP was incubated with Rsr1p (after cleavage of GST; ∼400 nM) preloaded with GTPγS in the absence (lane 1) or in the presence of MBP-Cdc24C (lane 2: 2 nM; lane 3: 10 nM; lane 4: 20 nM). As a control, GST-Cdc42p preloaded with GDP was incubated with MBP-Cdc24C (2–20 nM) without Rsr1p (lanes 5–7). Approximately equal amounts of GST-Cdc42p were recovered for each reaction as judged by immunoblotting with GST-specific antibodies (bottom panel). Association of Rsr1p with Cdc42p was determined by immunoblotting with Rsr1p-specific antibodies (top panel). Presence of MBP-Cdc24C in the eluents was determined by immunoblotting with Cdc24p-specific antibodies (middle panel). (C) His6-Cdc42p preloaded with [3H]GTP (T) or [3H]GDP (D) was used in a filter binding assay with GST-Rsr1p preloaded with GTPγS (T) or GDP (D) in the presence of various amount of Cdc24C (0–40 nM). The data are averages of three independent assays.
The association of Cdc24p and Cdc42p with Rsr1p led to the idea that Cdc24p and Cdc42p bind to different regions of Rsr1p. Consistent with this prediction, we found that the effector domain mutant Rsr1T35Ap, although defective in interaction with Cdc24p (Park et al., 1997), interacted with Cdc42p similarly to the wild-type Rsr1p in vitro (Figure 5A). Thus the effector domain of Rsr1p is unlikely to be directly involved in the Rsr1p-Cdc42p interaction. To address an apparent contradiction with our coimmunoprecipitation data, which indicated that the effector domain of Rsr1p is necessary for a Rsr1p-Cdc42p interaction, we examined the association of Rsr1T35Ap with Cdc42p in the presence of Cdc24C in vitro. Unlike Rsr1p, which displayed a proportional increase in Cdc42p binding as the amount of Cdc24C was increased (Figure 4), GST-Rsr1T35Ap associated with approximately equal amounts of Cdc42p in the presence or absence of MBP-Cdc24C (Figure 5B, lanes 1–4; top panel). MBP-Cdc24C did not associate with GST-Rsr1T35Ap (Figure 5B, lanes 1–4; middle panel), and thus was unlikely to enhance GST-Rsr1T35Ap binding to His6-Cdc42p. Thus, the effector domain of Rsr1p is required for enhanced association of Rsr1p with Cdc42p. Taken with our coimmunoprecipitation and multicopy suppression data, the efficient association between Cdc42p and Rsr1p in vivo is likely to require interaction between Cdc24p and Rsr1p through the effector domain of Rsr1p.
Figure 5.
The effector domain of Rsr1p is not directly involved in interaction with Cdc42p. (A) The Rsr1pT35A protein interacts with His6-tagged Cdc42p similarly to the wild-type Rsr1p in vitro. Rsr1p-GTPγS + Cdc42p-GDP (lane 1); Rsr1pT35A-GTPγS + Cdc42p-GTPγS (lane 2); Rsr1pT35A-GTPγS + Cdc42p-GDP (lane 3); Rsr1pT35A-GDP + Cdc42p-GTPγS (lane 4); Rsr1pT35A-GDP + Cdc42p-GDP (lane 5). Proteins were analyzed as in Figure 3B. (B) Interaction between GST-Rsr1pT35A-GTP and His6-Cdc42p-GDP in the presence of C-terminal half of Cdc24p. GST-Rsr1pT35Ap (∼400 nM) preloaded with GTPγS was incubated with His6-Cdc42p (∼400 nM) preloaded with GDP in the absence (lane 1) or in the presence of MBP-Cdc24C (lane 2: 2 nM; lane 3: 10 nM; lane 4: 20 nM). GST was used as a control instead of GST-Rsr1p in the presence of MBP-Cdc24 C and Cdc42p-GDP, and MBP-Cdc24 C was brought down with amylose resin (lanes 5–7). Proteins were analyzed as in Figure 4A.
DISCUSSION
Two different genetic screens for genes important for Cdc42p-dependent polarity establishment identified RSR1, a gene known to be necessary for proper bud-site selection in S. cerevisiae (Bender and Pringle, 1989; Chant and Herskowitz, 1991). Rsr1p interacted with Cdc42p in vivo and in vitro: Cdc42p coimmunoprecipitated with Rsr1p from yeast cell extract and also directly interacted with Rsr1p in vitro. Previous work suggested that bud site selection is coupled to polarity establishment through direct interaction between Rsr1p-GTP and Cdc24p, a GEF for Cdc42p (Zheng et al., 1995; Park et al., 1997). Based on our new data, we propose that the GTPase modules that govern bud site selection and polarity establishment are bridged not only by the GEF Cdc24p but also through direct interactions of GTPases. The interaction between Rsr1p and Cdc42p may contribute to guiding Cdc42p to the proper bud site as well as to stabilizing a bud-site assembly complex.
Association of the Ras-family GTPase Rsr1p with the Rho Family GTPase Cdc42p
Three lines of evidence presented herein suggest that Rsr1p directly interacts with Cdc42p rather than being bridged by other binding partners such as Cdc24p or Bem1p in vivo. First, RSR1 displayed specific genetic interactions with CDC42, similarly to genes that encode proteins known to interact directly with Cdc42p such as GIC2 and CLA4. These interactions were allele-specific: rsr1Δ exhibited synthetic lethality with cdc42 mutants specifically defective in polarity establishment but not with another cdc42 mutant or rho1. In addition, RSR1 on a multicopy plasmid suppressed only cdc42 mutants with a polarity establishment defect. Furthermore, an allele of RSR1, rsr1K260-264S, that could suppress cdc24-4 similarly to the wild-type RSR1, poorly suppressed the cdc42 polarized growth defect. The allele specificity of these interactions suggested strongly that RSR1 affects a specific CDC42 function, the establishment of cell polarity in late G1. Second, Cdc42p coimmunoprecipitated with Rsr1p from yeast cell extract in a condition where we did not detect Cdc24p associated with Rsr1p, suggesting in vivo association of the two GTPases. However, we cannot rule out the possibility that Cdc24p was subject to detection limits in our experiments due to low avidity of our antibodies against Cdc24p and/or very transient association of Cdc24p with Rsr1p or Cdc42p. Third, recombinant Rsr1p and Cdc42p associated in vitro in the absence of other proteins. This interaction was nucleotide-dependent and was enhanced in the presence of a truncated form of Cdc24p that contains the Rsr1p-binding domain. One caveat of these in vitro binding assays is that the truncated form of Cdc24p rather than the full length Cdc24p was used. This truncated Cdc24p, which lacks the GEF domain, was used to avoid the nucleotide exchange of Cdc42p and also to determine direct association of Cdc42p with Rsr1p. It remains to be tested whether the full length Cdc24p functions similarly and if so, how association of Cdc24p with Rsr1p affects the interaction between Rsr1p and Cdc42p.
Although both Cdc24p and Cdc42p appear to interact with Rsr1p, the binding regions are likely to be different. It was shown previously that rsr1T35A on a multicopy plasmid failed to suppress cdc24-4, unlike wild-type RSR1, and that Rsr1T35Ap also failed to interact with Cdc24p in vitro (Park et al., 1997). In contrast, Rsr1T35Ap was not defective in interaction with Cdc42p in vitro, even though rsr1T35A on a multicopy plasmid failed to suppress cdc42-118 (this study). Interestingly, the fragment of Cdc24p that was shown to interact with Rsr1p failed to enhance the interaction between Rsr1T35Ap and Cdc42p. The same Cdc24p fragment does not bind to Rsr1T35Ap (Park et al., 1997) and thus likely fails to enhance the interaction between Rsr1T35Ap and Cdc42p. A similar failure of binding enhancement may explain why rsr1T35A failed to suppress cdc42-118. Thus, it is likely that the association of Cdc24p and Rsr1p facilitates the interaction between Rsr1p and Cdc42p in vivo.
Recent pieces of data hint at the possibility that Rsr1p may associate with Cdc42p to enhance the association of Cdc42p with its effectors. In vitro studies with Cdc42pD76A, which is encoded by cdc42-118, exhibited little defect in binding to GTP-bound Rsr1p, in comparison to the wild-type Cdc42p (Beven and Park, unpublished observations). Thus the cdc42-118 growth defect might be due to a defective interaction of Cdc42pD76A with a downstream target that can be ameliorated by high levels of Rsr1p. It has been proposed that D76 of Cdc42p forms an intramolecular hydrogen bond with K187 to stabilize the interaction of the C-terminus of Cdc42p with regulators or effectors of Cdc42p (Kozminski et al., 2000), consistent with the idea that distant mutations within small GTPases have allosteric effects on the selectivity of effector binding (Heo and Meyer, 2003). By interacting with residues of Cdc42p other than D76, Rsr1p may help stabilize of the C-terminus of Cdc42p to improve the selectivity of effector binding. As suggested by genetic data, Gic1p and Gic2p may be two such effectors. cdc42-118 exhibits synthetic lethality with gic2Δ (this study) and rsr1Δ is synthetically lethal with gic1 gic2 mutations (Kawasaki et al., 2003). This web of genetic interactions further supports the idea that Rsr1p has a role both in bud site selection and in polarity establishment.
A Model for Bud Site Assembly at a Proper Bud Site
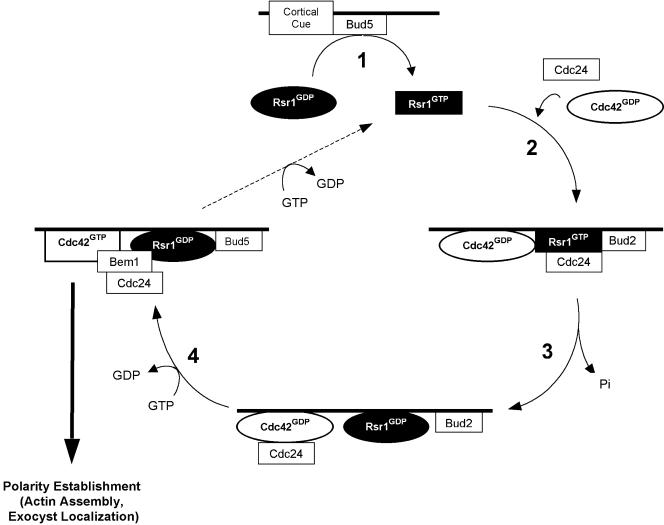
We previously proposed a scheme by which the Rsr1p GTPase cycle plays a role in localizing proteins necessary for cell polarity to a cellular landmark (Park et al., 1997). Based on the model, multiple proteins interact with each other and with the bud-site landmark to establish a site for new assembly of the actin cytoskeleton. Subsequently, we showed that the regulators of Rsr1p, Bud2p, and Bud5p, localize independently to the presumptive bud site (Park et al., 1999; Kang et al., 2001), although the maintenance of each at the bud site depends upon the presence of the other proteins of the GTPase module. Moreover, localization of Cdc24p to the presumptive bud site in late G1 is dependent on Rsr1p (Park et al., 2002). Here we present a revised model of how the Rsr1p and Cdc42p GTPase modules couple the identification of a specific growth site to the establishment of polarized growth at that site (Figure 6). We have incorporated the following new information into this scheme: Rsr1p-GTP binds to Cdc42p-GDP, the interaction is enhanced by the association of Rsr1p with Cdc24p (this study), and Cdc42p-GTP binds to Bem1p (Butty et al., 2002). Bem1p is thought to be a scaffold that interacts with Cdc24p, Cdc42p, Rsr1p, and Cla4p (Bender et al., 1996; Park et al., 1997; Butty et al., 1998, 2002; Gulli et al., 2000; Bose et al., 2001), all of which have been shown to localize to the proper bud site (Ziman et al., 1993; Ayscough et al., 1997; Toenjes et al., 1999; Nern and Arkowitz, 2000; Shimada et al., 2000; Richman et al., 2002). A recent study also indicates that localization of Bem1p to the incipient bud site requires activated Cdc42p (Butty et al., 2002).
Figure 6.
A model of how bud site selection couples to polarity establishment in late G1. Step 1: Bud5p exchanges GDP for GTP on Rsr1p. Step 2: Cdc24p and Cdc42p associate with GTP-bound Rsr1p at the bud site. Step 3: Bud2p activates Rsr1p to hydrolyze GTP bound to Rsr1p. Step 4: Dissociation of GDP-bound Rsr1p from Cdc24p may activate Cdc24p. Activated Cdc24p exchanges GDP for GTP on Cdc42p. GTP-bound Cdc42 then triggers actin assembly and exocyst localization to establish an axis of polarity. Bud5p at the bud site would then return Rsr1p to a GTP bound state (dashed line), allowing for another cycle of signal transduction. See DISCUSSION for details of the model.
In Step 1 of our model (Figure 6), Bud5p, which localizes to the proper bud site through the interaction with a cortical cue (Kang et al., 2001), exchanges GDP bound to Rsr1p for GTP. In Step 2, association of Rsr1p-GTP and its binding partners yields a complex of Rsr1p-GTP, Cdc24p, and Cdc42p-GDP at the presumptive bud site where Bud2p also localizes (Park et al., 1999). We propose that association of Rsr1p-GTP with Cdc24p enhances the interaction between Rsr1p-GTP and Cdc42p-GDP. We further speculate that this ordered assembly of the complex allows the activation of Cdc42p by Cdc24p only at the proper bud site. In Step 3, Rsr1p-GTP is converted to Rsr1p-GDP through the action of its GAP, Bud2p (Park et al., 1993). Conversion of Rsr1p-GTP to Rsr1p-GDP by Bud2p may allow targeted release of budsite assembly proteins at the proper bud site (Benton et al., 1993; Park et al., 1997). The final critical event occurs in Step 4 where dissociation of Rsr1p from Cdc24p may activate the GEF activity of Cdc24p, converting Cdc42p-GDP to Cdc42-GTP. Bem1p may then join and maintain the bud-site assembly complex through the interaction with Cdc42p-GTP (Butty et al., 2002) or with Rsr1p-GDP (Park et al., 1997). Alternatively, activation of Cdc24p, without a concomitant stimulation of its GEF activity (Zheng et al., 1995), may result from an interaction with Rsr1p. Conversion of Rsr1p-GDP to Rsr1p-GTP by Bud5p would then allow recycling of Rsr1p and further shuttling of Cdc24p, Cdc42p, and Bem1p to the proper bud site. The cycle is thus proposed to result in localization of a critical level of Cdc42p-GTP and polarity establishment proteins (e.g., Bem1p) to the bud site, triggering polarized assembly/organization of the actin cytoskeleton and secretory apparatus.
This model provides a simplified view for selection of a proper growth site, and the details of the model remain to be tested. Nonetheless, we think that the ordered assembly of a complex through multiple protein-protein interactions proposed here may ensure the establishment of polarity at a correct location. In the absence of the Rsr1p GTPase module, localization of Cdc24p and Cdc42p to a random bud site may occur through a distinct default pathway yet to be identified or by the stochastic accumulation of bud-site assembly proteins on the plasma membrane.
This study leads us to consider whether other Rasfamily proteins are interacting directly with Cdc42p to foster the establishment of cell polarity. Ras2p, for example, appears to have a Cdc42p-dependent role in polarized growth. Ras2p is required for the polarized distribution of Cdc42p and maintenance of cytoskeletal polarity during mild temperature stresses (Ho and Bretscher, 2001). Furthermore, Ras2p signals via a Cdc42p/MAP kinase module to induce the polarized morphogenesis required for filamentous growth (Mosch et al., 1996, 1999). In either case, the mechanism by which Ras2p signals to Cdc42p is unknown. It is tempting to speculate that Ras2p may interact directly with Cdc42p in a manner similar to that observed with Rsr1p. Similar interactions between Ras and Rho proteins may also occur in other organisms. A prime candidate is S. pombe, in which polarized morphogenesis is regulated by an interaction of Ras1p with Scd1p, a GEF of Cdc42p (Papadaki et al., 2002). It will be interesting to see whether this morphogenetic pathway, analogous to the Rsr1p-Cdc24p-Cdc42p pathway in budding yeast, also contains a direct Ras-Rho interaction.
Acknowledgments
This article is dedicated to I. Herskowitz in whose laboratory part of this work was initiated. We thank I. Herskowitz and D. Drubin for their generous support and encouragement. We also thank J. Pringle, A. Fischer, D. Siekhaus, M. Duncan, A. Groen, M. Carlson, D. Kellogg, and A. Bender for helpful advice and reagents. This work was supported by research grants from the National Institutes of Health to H.-O.P. (GM56997) and I.H. (GM48052), and from the Canadian Institute of Health Research and the National Cancer Institute of Canada to C.B. K.K. was supported by The University of Virginia and The Leukemia and Lymphoma Society.
References
- Adams, A.E., Johnson, D.I., Longnecker, R.M., Sloat, B.F., and Pringle, J.R. (1990). CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111, 131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough, K.R., Stryker, J., Pokala, N., Sanders, M., Crews, P., and Drubin, D.G. (1997). High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A., and Pringle, J.R. (1989). Multicopy suppression of the cdc24 budding defect in yeast by CDC42 and three newly identified genes including the ras-related gene RSR1. Proc. Natl. Acad. Sci. USA 86, 9976-9980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A. (1993). Genetic evidence for the roles of the bud-site-selection genes BUD5 and BUD2 in control of the Rsr1p (Bud1p) GTPase in yeast. Proc. Natl. Acad. Sci., USA 90, 9926-9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, L., Lo, H.S., Lee, H., Kokojan, V., Peterson, V., and Bender, A. (1996). Associations among PH and SH3 domain-containing proteins and Rho-type GTPases in yeast. J. Cell Biol. 133, 879-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton, B., Tinkelenberg, A.H., Jean, D., Plump, S.D., and Cross, F.R. (1993). Genetic analysis of CLN/CDC28 regulation of cell morphogenesis in budding yeast. EMBO J. 12, 5267-5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose, I., Irazoqui, J.E., Moskow, J.J., Bardes, E.S., Zyla, T.R., and Lew, D.J. (2001). Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276, 7176-7186. [DOI] [PubMed] [Google Scholar]
- Brachmann, C.B., Davies, A., Cost, G.J., Caputo, E., Li, J., Hieter, P., and Boeke, J.D. (1998). Designer deletion strains derived from Saccharomyces cerevisiaes S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115-132. [DOI] [PubMed] [Google Scholar]
- Brown, J.L., Jaquenoud, M., Gulli, M.P., Chant, J., and Peter, M. (1997). Novel Cdc42-binding proteins Gic1 and Gic2 control cell polarity in yeast. Genes Dev. 11, 2972-2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butty, A.-C., Pryciak, P.M., Huang, L.S., Herskowitz, I., and Peter, M. (1998). The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 282, 1511-1516. [DOI] [PubMed] [Google Scholar]
- Butty, A.-C., Perrinjaquet, N., Petit, A., Jaquenoud, M., Segall, J.E., Hofmann, K., Zwahlen, C., and Peter, M. (2002). A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 21, 1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, M., and Botstein, D. (1982). Two differentially regulated mRNAs with different 5′ ends encode secreted with intracellular forms of yeast invertase. Cell 28, 145-154. [DOI] [PubMed] [Google Scholar]
- Caviston, J.P., Tcheperegine, S.E., and Bi, E. (2002). Singularity in budding: A role for the evolutionarily conserved small GTPase Cdc42p. Proc. Natl. Acad. Sci. USA 99, 12185-12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione, R.A., Leonard, D., and Zheng, Y. (1995). Purification of baculovirusexpressed Cdc42hs. Methods Enzymol. 256, 11-15. [DOI] [PubMed] [Google Scholar]
- Chang, E.C., Barr, M., Wang, Y., Jung, V., Xu, H.-P., and Wigler, M.H. (1994). Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79, 131-141. [DOI] [PubMed] [Google Scholar]
- Chant, J., Corrado, K., Pringle, J.R., and Herskowitz, I. (1991). Yeast BUD5, encoding a putative GDP-GTP exchange factor, is necessary for bud site selection and interacts with bud formation gene BEM1. Cell 65, 1213-1224. [DOI] [PubMed] [Google Scholar]
- Chant, J., and Herskowitz, I. (1991). Genetic control of bud site selection in yeast by a set of gene products that constitute a morphogenetic pathway. Cell 65, 1203-1212. [DOI] [PubMed] [Google Scholar]
- Chant, J., and Pringle, J.R. (1995). Patterns of bud-site selection in the yeast Saccharomyces cerevisiae. J. Cell Biol. 129, 751-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, G.C., Kim, Y.J., and Chan, C.S. (1997). The Cdc42 GTPase-associated proteins Gic1 and Gic2 are required for polarized cell growth in Saccharomyces cerevisiae. Genes Dev. 11, 2958-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis, C.R., Richman, T.J., Deliduka, S.B., Blaisdell, J.O., Collins, C.C., and Johnson, D.I. (1998). Analysis of the mechanisms of action of the Saccharomyces cerevisiae dominant lethal cdc42G12V and dominant negative cdc42D118A mutations. J. Biol. Chem. 273, 849-858. [DOI] [PubMed] [Google Scholar]
- Diekmann, D., Abo, A., Johnston, C., Segal, A.W., and Hall, A. (1994). Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science 265, 531-533. [DOI] [PubMed] [Google Scholar]
- Drees, B.L. et al. (2001). A protein interaction map for cell polarity development. J. Cell Biol. 154, 549-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin, D.G., and Nelson, W.J. (1996). Origins of cell polarity. Cell 84, 335-344. [DOI] [PubMed] [Google Scholar]
- Drubin, D.G. (2000). Cell polarity. In: Frontiers in Molecular Biology. New York: Oxford University Press.
- Evangelista, M., Blundell, K., Longtine, M.S., Chow, C.J., Adames, N., Pringle, J.R., Peter, M., and Boone, C. (1997). Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276, 118-122. [DOI] [PubMed] [Google Scholar]
- Gulli, M., Jaquenoud, M., Shimada, Y., Niederhauser, G., Wiget, P., and Peter, M. (2000). Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell 6, 1155-1167. [DOI] [PubMed] [Google Scholar]
- Gulli, M.P., and Peter, M. (2001). Temporal and spatial regulation of Rho-type guanine-nucleotide exchange factors: the yeast perspective. Genes Dev. 15, 365-379. [DOI] [PubMed] [Google Scholar]
- Heo, W.D., and Meyer, T. (2003). Switch-of-function mutants based on morphology classification of Ras superfamily small GTPases. Cell 113, 315-328. [DOI] [PubMed] [Google Scholar]
- Hicks, J.B., Strathern, J.N., and Herskowitz, I. (1977). Interconversion of yeast mating types. ii. Action of the homothallism (HO) gene in cells homozygous for the mating type locus. Genetics 85, 373-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho. J., and Bretscher, A. (2001). Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 12, 1541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofken, T., and Schiebel, E. (2002). A role for cell polarity proteins in mitotic exit. EMBO J. 21, 4851-4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye, K., Mizutani, S., Koide, H., and Kaziro, Y. (2000). Formation of the Ras dimer is essential for Raf-1 activation. J. Biol. Chem. 275, 3737-3740. [DOI] [PubMed] [Google Scholar]
- Johnson, D.I., and Pringle, J.R. (1990). Molecular characterization of CDC42, a Saccharomyces cerevisiae gene involved in the development of cell polarity. J. Cell Biol. 111, 143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, D.I. (1999). Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol. Mol. Biol. Rev. 63, 54-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, P.J., Sanson, A., Lee, B., and Park, H.-O. (2001). A GDP/GTP exchange factor involved in linking a spatial landmark to cell polarity. Science 292, 1376-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki, R., Fujimura-Kamada, K., Toi, H., Kato, H., and Tanaka, K. (2003). The upstream regulator, Rsr1p, and downstream effector, Gic1p and Gic2p, of the Cdc42p small GTPase coordinately regulate initiation of budding in Saccharomyces cerevisiae. Genes Cells 8, 235-250. [DOI] [PubMed] [Google Scholar]
- Kellogg, D.R., Kikuchi, A., Fujii-Nakata, T., Turck, C., and Murray, A.W. (1995). Members of the NAP/SET family of proteins interact specifically with B-type cyclins. J. Cell Biol. 130, 661-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski, K.G., Chen, A.J., Rodal, A.A., and Drubin, D.G. (2000). Functions and functional domains of the GTPase Cdc42p. Mol. Biol. Cell 11, 339-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack, D., Nishimura, K., Dennehey, B.K., Arbogast, T., Parkinson, J., Toh-e, A., Pringle, J.R., Bender, A., and Matsui, Y. (1996). Identification of the bud emergence gene Bem4 and its interactions with Rho-type GTPases in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 4387-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch, H.U., Kubler, E., Krappmann, S., Fink, G.R., and Braus, G.H. (1999). Cross-talk between the Ras2p-controlled mitogen-activated protein kinase and cAMP pathways during invasive growth of Saccharomyces cerevisiae. Mol. Biol. Cell 10, 1325-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosch, H.U., Roberts, R.L., and Fink, G.R. (1996). Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 93, 5352-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nern, A., and Arkowitz, R.A. (2000). Nucleocytoplasmic shuttling of the Cdc42p exchange factor Cdc24p. J. Cell Biol. 148, 1115-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadaki, P., Pizon, V., Onken, B., and Chang, E.C. (2002). Two ras pathways in fission yeast are differentially regulated by two ras guanine nucleotide exchange factors. Mol. Cell. Biol. 2002, 4598-4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.-O., Chant, J., and Herskowitz, I. (1993). BUD2 encodes a GTPase-activating protein for Bud1/Rsr1 necessary for proper bud-site selection in yeast. Nature 365, 269-274. [DOI] [PubMed] [Google Scholar]
- Park, H.-O., Bi, E., Pringle, J., and Herskowitz, I. (1997). Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc. Natl. Acad. Sci. USA 94, 4463-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.-O., Sanson, A., and Herskowitz, I. (1999). Localization of Bud2p, a GTPase-activating protein necessary for programming cell polarity in yeast, to the presumptive bud site. Genes Dev. 13, 1912-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H.-O., Kang, P.J., and Rachfal, A.W. (2002). Localization of the Rsr1/Bud1 GTPase involved in selection of a proper growth site in yeast. J. Biol. Chem. 277, 26721-26724. [DOI] [PubMed] [Google Scholar]
- Peränen, J., Rikkonen, M., Hyvonen, M., and Kaariainen, L. (1996). T7 vectors with modified T7lac promoter for expression of proteins in Escherichia coli. Anal. Biochem. 236, 371-373. [DOI] [PubMed] [Google Scholar]
- Pringle, J.R., Bi, E., Harkiins, H.A., Zahner, J.E., De Virgilio, C., Chant, J., Corrado, K., and Fares, H. (1995). Establishment of cell polarity in yeast. In: CSH Symp. Quant. Biol. 60, 729-744. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and Bretscher, A. (2000). Polarization of cell growth in yeast. I. Establishment and maintenance of polarity states. J. Cell Sci. 113, 365-375. [DOI] [PubMed] [Google Scholar]
- Richman, T.J., Sawyer, M.M., and Johnson, D.I. (2002). Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot. Cell 1, 458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri, R., Bender, A., Matsui, Y., Powers, S., Takai, Y., Pringle, J.R., and Matsumoto, K. (1992). Rsr1, a Ras-like gene homologous to krev-1 (smg21a/rap1a): Role in the development of cell polarity and interactions with the Ras pathway in Saccharomyces cerevisiae. Mol. Cell. Biol. 12, 758-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl, R.H., and Gietz, R.D. (1989). High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16, 339-346. [DOI] [PubMed] [Google Scholar]
- Sherman, F., Fink, G.R., and Hicks, J.B. (1986). Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Shimada, Y., Gulli, M.-P., and Peter, M. (2000). Nuclear sequestration of the exchange factor Cdc24p by Far1 regulates cell polarity during mating. Nat. Cell Biol. 2, 117-124. [DOI] [PubMed] [Google Scholar]
- Sloat, B., Adams, A., and Pringle, J.R. (1981). Roles of the CDC24 gene product in cellular morphogenesis during the Saccharomyces cerevisiae cell cycle. J. Cell Biol. 89, 395-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toenjes, K.A., Sawyer, M.M., and Johnson, D.I. (1999). The guanine-nucleotide-exchange factor Cdc24p is targeted to the nucleus and polarized growth sites. Curr. Biol. 9, 1183-1186. [DOI] [PubMed] [Google Scholar]
- Tong, A.H.Y. et al. (2001). Systematic genetic analysis with ordered arrays of yeast deletion mutants. Science 294, 2364-2368. [DOI] [PubMed] [Google Scholar]
- Uetz, P. et al. (2000). A comprehensive analysis of protein–protein interactions in Saccharomyces cerevisiae. Nature 403, 623-627. [DOI] [PubMed] [Google Scholar]
- Winzeler, E.A. et al. (1999). Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901-906. [DOI] [PubMed] [Google Scholar]
- Yamochi, W., Tanaka, K., Nonaka, H., Maeda, A., Musha, T., and Takai, Y. (1994). Growth site localization of Rho1 small GTP-binding protein and its involvement in bud formation in Saccharomyces cerevisiae. J. Cell Biol. 125, 1077-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, B., and Zheng, Y. (1998). Negative regulation of rho family GTPases Cdc42 and Rac2 by homodimer formation. J. Biol. Chem. 273, 25728-25733. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Bi, E., Novick, P., Du, L., Kozminski, K.G., Lipschutz, J.H., and Guo, W. (2001). Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276, 46745-46750. [DOI] [PubMed] [Google Scholar]
- Zheng, Y., Bender, A., and Cerione, R.A. (1995). Interactions among proteins involved in bud-site selection and bud-site assembly in Saccharomyces cerevisiae. J. Biol. Chem. 270, 626-630. [DOI] [PubMed] [Google Scholar]
- Ziman, M., O'Brien, J.M., Ouellette, L.A., Church, W.R., and Johnson, D.I. (1991). Mutational analysis of CDC42Sc, Saccharomyces cerevisiae gene that encodes a putative GTP-binding protein involved in the control of cell polarity. Mol. Cell. Biol. 11, 3537-3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman, M., Preuss, D., Mulholland, J., O'Brien, J.M., Botstein, D., and Johnson, D.I. (1993). Subcellular localization of Cdc42p, a Sacchromyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4, 1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman, M., and Johnson, D.I. (1994). Genetic evidence for a functional interaction between Saccharomces cerevisiae CDC24 and CDC42. Yeast 10, 463-474. [DOI] [PubMed] [Google Scholar]