Abstract
The function currently attributed to tetraspanins is to organize molecular complexes in the plasma membrane by using multiple cis-interactions. Additionally, the tetraspanin CD9 may be a receptor that binds the soluble ligand PSG17, a member of the immunoglobulin superfamily (IgSF)/CEA subfamily. However, previous data are also consistent with the PSG17 receptor being a CD9 cis-associated protein. In the current study, CD9 extracellular loop (EC2) specifically bound to PSG17-coated beads, indicating a direct interaction between the two proteins. However, CD9-EC2 did not bind to PSG17-coated beads if the CD9-EC2 had the mutation SFQ (173-175) to AAA, a previously studied mutation in egg CD9 that abolishes sperm-egg fusion. Also, PSG17 bound to 293 T cells transfected with wild-type CD9 but not the mutant CD9. By immunofluorescence, PSG17 bound to wild-type eggs but not to CD9 null eggs. The presence of ∼2 μM recombinant PSG17 produced a significant and reversible inhibition (60-80%) of sperm-egg fusion. Thus, we conclude that CD9 is a receptor for PSG17 and when the PSG17 binding site is mutated or occupied, sperm-egg fusion is impaired. These findings suggest that egg CD9 may function in gamete fusion by binding to a sperm IgSF/CEA subfamily member and such proteins have previously been identified on sperm.
INTRODUCTION
Mammalian fertilization involves a series of complex cellular and biochemical processes, culminating in sperm-egg fusion and subsequent formation of an embryo. Despite its biological importance, fusion between the plasma membranes of the sperm and the oocyte is not well understood. Although there is general agreement on the concept that gamete fusion may involve the interaction of multiple complementary molecules on the sperm and the oocyte, only a few potentially relevant proteins have been identified.
A protein found on the mammalian egg surface that is known to be required for gamete fusion is CD9. CD9 belongs to the tetraspanin family of proteins, which are integral membrane proteins with four transmembrane domains and two extracellular domains (one short, one long) (Maecker et al., 1997). In mammals there are >30 tetraspanin family members, implicated in a variety of cellular and physiological processes, such as cell motility, cell aggregation, signaling, and cell fusion (Boucheix and Rubinstein, 2001; Hemler, 2001). Tetraspanins are believed to act as “molecular facilitators,” grouping specific cell-surface proteins and thus increasing the formation and stability of functional protein complexes (Maecker et al., 1997). CD9 associates with a great variety of membrane proteins, such as membrane-anchored growth factors, integrins, members of the immunoglobulin superfamily (IgSF), and other tetraspanins (Boucheix and Rubinstein, 2001).
Conclusive evidence for a role of CD9 in gamete fusion was the finding that CD9 knockout females are infertile due to the inability of their oocytes to fuse with sperm (Kaji et al., 2000; Le Naour et al., 2000; Miyado et al., 2000), but how CD9 acts in fusion remains unknown. It has been suggested that CD9 acts in sperm-egg fusion via an association with a β1 integrin on the egg that binds to ADAM proteins on the sperm, specifically ADAM 2 (fertilin β) and ADAM 3 (cyritestin) (Chen et al., 1999; Takahashi et al., 2001). However, experiments using gametes deficient for either the sperm ADAMs or the egg integrins, question the validity of this model. Sperm lacking fertilin β, cyritestin, or both proteins are still able to fuse with eggs (Cho et al., 1998; Shamsadin et al., 1999; Nishimura et al., 2001). Furthermore, it was recently shown that oocytes lacking all β1 integrins are fully functional in fusing with sperm in vitro and in vivo. Also function-blocking antibodies against other egg integrins (β3 and αv), when incubated with eggs lacking β1 integrins, do not inhibit sperm-egg fusion (He et al., 2003). These results indicate that none of the integrins known to be present on the oocyte is essential for sperm-egg fusion and egg integrins do not have redundant functions in the fusion process.
Because the proposed mechanism for CD9 function in sperm-egg fusion, acting through integrins, does not agree with these current results, an alternative model is required. Recently, PSG17, a member of the pregnancy-specific glycoprotein (PSG) family, has been suggested to be a CD9-ligand (Waterhouse et al., 2002). Pregnancy-specific glycoproteins belong to the carcinoembryonic antigen (CEA) subfamily of the immunoglobulin superfamily (IgSF) (Beauchemin et al., 1999). PSGs are a group of proteins synthesized by the placenta and secreted into the maternal circulation. Several PSGs are known to stimulate secretion of cytokines by macrophages (Wessells et al., 2000; Snyder et al., 2001), and thus PSGs may contribute to preventing fetal rejection by the mother by activating the maternal innate immune system (Sacks et al., 1999). PSG17 binds to macrophages with high affinity and the binding is mediated by CD9 (Waterhouse et al., 2002). However, it is not known whether CD9 is the receptor itself or whether it functions as a coreceptor.
We report here that PSG17 binds directly to CD9 and that CD9 amino acid residue F 174 is essential for this interaction. As a CD9-ligand molecule, PSG17 interactions may give insights into the molecular mechanism underlying the role of CD9 in sperm-egg fusion. We found that PSG17 binds to eggs and inhibits gamete fusion, suggesting that CD9 may function in sperm-egg fusion by interacting with an IgSF protein, possibly a CEA subfamily protein. Thus, these results provide evidence for the potential involvement of an IgSF member in sperm-egg fusion and give rise to new ideas of how CD9 acts in this process.
MATERIALS AND METHODS
Cell Culture
Human embryonic kidney (HEK) 293T cells (Edge Biosystems, Gaithersburg, MD) were cultured in DMEM, 10% fetal bovine serum. BeWo cells (American Type Culture Collection, Manassas, VA) were cultured in F12K medium with 1.5 g/l sodium bicarbonate and 10% fetal bovine serum.
Plasmids
The construction of pGEX-CD9EC2, pCD9-eGFP, and pCD9-F174A-eGFP has been described previously (Zhu et al., 2002). For the expression of glutathione S-transferase (GST)-CD9EC2-SFQ to AAA, the mutated template (Zhu et al., 2002) was amplified by polymerase chain reaction and subcloned into EcoRI and BamHI restriction sites of pGEX-3× (Amersham Biosciences, Piscataway, NJ). pCD9-SFQ to AAA (173-175)-eGFP was generated after digestion of the cDNA in pBluescript KS (Zhu et al., 2002) with SalI and SacI and subcloned into the same sites in the pIRES2-eGFP vector (BD Biosciences Clontech, Palo Alto, CA).
Pull-Down Assays
The GST-mouse CD9 extracellular loop 2 (EC2) fusion protein was generated as described previously (Zhu et al., 2002). Recombinant PSG17N-Myc-His includes the N-terminal immunoglobulin domain of PSG17 and binds with high affinity to CD9-expressing cells (Waterhouse et al., 2002). Recombinant PSG17N-Myc-His purified as described in Waterhouse et al. (2002) was tested for its ability to bind to GST-CD9EC2 with the pull-down polyHis protein: protein interaction kit (Pierce Chemical, Rockford, IL) following the manufacturer's protocol. Briefly, 100 μg of purified PSG17N-Myc-His or two control proteins, recombinant green fluorescent protein (GFP)-His (Upstate Biotechnology, Lake Placid, NY) or CEACAM1a[1-4]-His (Zelus et al., 1998; Beauchemin et al., 1999), were incubated with the immobilized cobalt chelate gel overnight at 4°C. After five washes, 100 μg of GST-CD9EC2 (prey) or the same construct carrying the SFQ (173-175) to AAA mutation were added to the gel and incubated overnight with gentle rocking at 4°C. After seven washes, the proteins were eluted with 150 μl of 290 mM imidazole elution buffer. Twenty-five microliters of the eluted material was loaded on a 4-20% NuPAGE gel (Invitrogen, Carlsbad, CA), and the proteins were detected by immunoblotting with an anti-CD9 mAb, KMC8 (BD Pharmingen, Palo Alto, CA), or an anti-GST monoclonal antibody (mAb) (Santa Cruz Biotechnology, Santa Cruz, CA) followed by horseradish peroxidase-conjugated goat anti-rat antibody or goat anti-mouse antibody and the Super Signal chemiluminescent detection system (Pierce Chemical).
Detection of PSG17N-Myc-His Binding to Transfected 293T Cells by Enzyme-linked Immunosorbent Assay (ELISA)
The assays were performed as described previously (Waterhouse et al., 2002). Briefly, HEK 293T cells were seeded in poly-l-lysine-coated 96-well plates and were transiently transfected with plasmid DNA by using LipofectAMINE 2000 (Invitrogen). At 48 h posttransfection, the cells were washed with binding buffer containing 0.01% sodium azide after which PSG17N-Myc-His (10 μg/ml) or no ligand was added to each well. After 1-h incubation, the cells were washed five times and binding of PSG17N-Myc-His was detected with horseradish peroxidase-conjugated anti-Myc mAb (Invitrogen) followed by trimethylbenzyl-peroxidase substrate (KPL) and 2 N H2SO4. The color change was quantitated at 450 nm in an ELISA reader.
Flow Cytometry
For detection of PSG17N-Myc-His binding to 293T cells transfected with pCD9-eGFP, pCD9-F174A-eGFP, and pCD9-SFQ to AAA (173-175)-eGFP, the cells were sequentially incubated with 10 μg/ml PSG17N-Myc-His, anti-Myc mAb, and phycoerythrine (PE)-labeled rat anti-mouse IgG1 (BD Pharmingen). Expression of wild-type CD9, and the mutated forms of CD9 on the surface of the transfected cells, was confirmed by staining with biotin-labeled anti-CD9 mAb, KMC8, followed by Cy-Chrome-labeled streptavidin (BD Pharmingen).
Gamete Isolation
Mature, cumulus-free oocytes were collected from superovulated 6-8-wk-old ICR female mice as described previously (Yuan et al., 1997). To loosen the zona pellucida (ZP) the oocytes were treated with 30 μg/ml chymotrypsin (Sigma-Aldrich, St. Louis, MO) for 3 min at 37°C and 5% CO2 in medium M199 (Invitrogen) containing 3.5 mM sodium pyruvate, 1000 IU of penicillin-streptomycin, and 0.3% bovine serum albumin (BSA) (Sigma-Aldrich) (M199*). The zonae pellucidae were then removed mechanically using a narrow bore pipette. The oocytes were washed through three 100-μl drops of fresh M199* and then incubated in M199* at 37°C and 5% CO2 for 3 h before use. Sperm were collected from the cauda epididymis and vas deferens of 10-12-wk-old ICR males. Sperm were allowed to disperse in a 500-μl drop of M199* containing 3% BSA and then diluted 1:10 in 500 μl of M199* + 3% BSA and capacitated for 2 h at 37°C and 5% CO2.
Egg Immunofluorescence
Wild-type or CD9-null zona-free oocytes were obtained as described above and incubated in 50 μg/ml PSG17N-Myc-His (∼26 kDa) or XylE-His (∼35 kDa) in M199* for 30 min at 37°C in a 5% CO2 incubator. After three washes in M199*, the cells were incubated in anti-Myc antibody (Invitrogen) (10 μg/ml in phosphate-buffered saline [PBS]-0.4% BSA) for 1 h at room temperature. Oocytes were washed through three drops of PBS-0.4% BSA and then exposed to Oregon green-conjugated anti-mouse IgG secondary antibody (Molecular Probes, Eugene, OR) diluted 1:100 in PBS-0.4% BSA for 1 h at room temperature. After washing, oocytes were mounted on glass slides and visualized using a Zeiss Axiophot microscope.
In Vitro Fertilization Assay
Zona-free eggs were loaded with 4′,6-diamidino-2-phenylindole dihydrochloride (Polysciences, Warrington, PA) at 10 μg/ml for 15 min at 37°C, 5% CO2. After washing out excess dye, the eggs were incubated for 30 min at 37°C and 5% CO2 in M199* alone (control) or M199* containing 50 μg/ml PSG17NMyc-His (∼2 μM). Oocytes were inseminated in droplets containing recombinant protein for the first set of in vitro fertilization experiments, or were washed in M199* alone, by using three successive 7-min incubations in 100-μl M199* drops at 37°C, 5% CO2 before insemination, for the in vitro fertilization experiments testing the reversibility of the inhibition. Sperm were added at a final concentration of 1-3 × 105 sperm/ml, and gametes were coincubated for 40 min at 37°C, 5% CO2. The oocytes were then washed to release loosely bound sperm, fixed in 4% paraformaldehyde in PBS for 10 min, and mounted onto microscope slides. Sperm-egg fusion was scored by the fluorescent labeling of sperm nuclei by 4′,6-diamidino-2-phenylindole transferred from the preloaded eggs. Two different parameters of fusion were calculated: fertilization rate is the percentage of oocytes with at least one fused sperm, and fertilization index is the mean number of fused sperm per egg.
Sperm Motility Assay
Capacitated sperm were incubated for 30 min at 37°C, 5% CO2 in M199* alone (control) or containing 50 μg/ml PSG17N-Myc-His. Samples were placed on warm glass slides and analyzed using a light microscope. Cells showing progressive motility were counted as “motile,” whereas cells with nonprogressive motility or no motility were considered as “immotile.”
RESULTS
Direct Binding of PSG17 to CD9
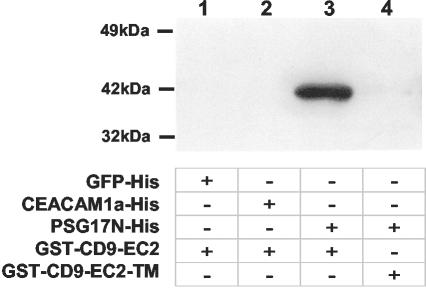
Previous results demonstrated that PSG17 binds specifically to CD9-expressing cells (Waterhouse et al., 2002), but it has been uncertain whether PSG17 binds to CD9 itself or to a CD9-associated protein. To test whether PSG17 binds directly to CD9, pull-down assays were performed. The recombinant N-terminal Ig domain of PSG17 containing both 6 × His and Myc epitope tags was bound through its His tag to cobalt beads. Two control proteins were used, eGFP-6 × His and CEACAM1a[1-4]-6 × His. CEACAM1a is closely related to PSG17; it is also an IgSF member and, like PSG17, belongs to the CEA subfamily. Recombinant CEACAM1a[1-4] has also been shown to have biological activity (Zelus et al., 1998). The beads with bound protein were incubated with a fusion protein, including GST fused to the CD9 long extracellular loop (EC2) (GST-CD9 EC2). After extensive washing, the presence of CD9 on the beads was detected by Western blot by using an anti-CD9 antibody. GST-CD9 EC2 bound to PSG17-coated beads but not to the GFP-coated beads or to the CEACAM1a[1-4]-coated beads (Figure 1, lanes 1-3), showing that PSG17 binds directly to CD9-EC2.
Figure 1.
PSG17N binds to the extracellular loop 2 of murine CD9. Cobalt chelate beads were incubated with GFP-His (lane 1), CEACAM1a[1-4]-His (lane 2) or PSG17N-Myc-His (lanes 3 and 4) used as baits. The beads were then incubated with GST-CD9EC2 (lanes 1-3) or the same construct carrying the triple mutation SFQ (173-175) to AAA (GST-CD9EC2-TM) (lane 4). After several washes, the proteins were eluted with a buffer containing 290 mM imidazole, separated on a 4-20% NuPAGE gel, and detected by Western blot by using an anti-CD9 antibody.
PSG17 Binds to a Specific Site on CD9
It has been previously shown that amino acid residues 173-175 in CD9 EC2 are critical for CD9 function in sperm-egg fusion. When CD9 EC2 residue F174 is replaced by A, CD9 activity in gamete fusion is greatly reduced; when the three residues (173-175) are altered to AAA, the mutant CD9 has no activity in fusion (Zhu et al., 2002). To ask whether this region of CD9 is also relevant for the binding of PSG17, a GST-CD9 EC2 construct with the triple mutation SFQ to AAA was expressed and used in the pull-down assay. In contrast to the wild-type construct, the mutated CD9 EC2 was not pulled down by PSG17-coupled beads (Figure 1, lane 4), suggesting that amino acid residues 173-175 are directly involved in binding to PSG17.
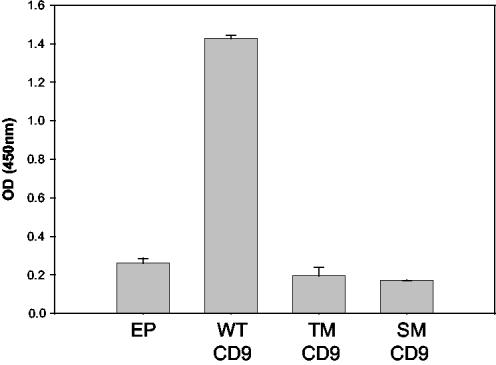
We also evaluated whether mutations in CD9 would affect PSG17 binding when CD9 was expressed in a cell membrane. We compared by ELISA the binding of PSG17 to HEK 293T cells that had been transfected with either wild-type CD9 or mutant CD9. Two CD9 mutations were used, the triple mutant, residues 173-175 SFQ to AAA, mentioned above, or the single mutant, residue F174 to A. Compared with control cells transfected with empty plasmid, wild-type CD9 transfected cells bound about five times higher levels of PSG17 (Figure 2). Cells transfected with CD9 with the triple mutation (SFQ 173-175 to AAA) or CD9 with the single mutation (F 174 to A) bound PSG17 at the same level as the control (empty plasmid) transfected cells. Cells transfected with any of the three constructs showed similar surface staining by immunofluorescence by using the anti-CD9 KMC8 antibody (our unpublished data).
Figure 2.
Binding of PSG17N-Myc-His to CD9-transfected 293T cells. HEK 293T cells were transfected with empty plasmid (EP), pCD9-eGFP (wild-type CD9:WT-CD9), pCD9-F174A-eGFP (single mutant CD9: SM-CD9), or pCD9-SFQ to AAA (173-175)-eGFP (triple mutant CD9: TM-CD9). Forty-eight hours posttransfection, the cells were incubated with 10 μg/ml PSG17N-Myc-His and bound PSG17N-Myc-His was detected after treatment with HRP-conjugated anti-Myc mAb and TMB/peroxidase substrate. The data are expressed as mean absorbance ± SE. Each data point represents four identical wells and the experiment was repeated two independent times with similar results.
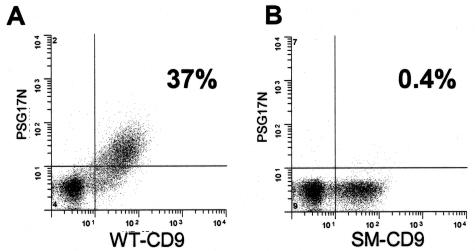
Similar results were obtained using fluorescence-activated cell sorting (FACS) analysis of PSG17 binding to CD9-transfected 293T cells. 293T cells were transfected with plasmids coding for wild-type or mutant CD9 fused to enhanced green fluorescent protein (eGFP). Transfected cells were incubated with PSG17-N-Myc-His and stained with an anti-Myc antibody followed by a secondary antibody coupled to PE. Expression levels of the CD9-eGFP fusion proteins were evaluated by the fluorescence of eGFP and PSG17 binding was quantified by PE fluorescence. Results indicated that eGFP and PE-labeled double positive cells constituted 37% of the forward versus side scatter-gated pCD9-eGFP transfected cells (Figure 3). In contrast, only 0.4% of the gated cells were eGFP and PE double positive when cells were transfected with pCD9-F174A-eGFP. Results similar to those with the F174A mutant were obtained upon transfection of cells with CD9-SFQ to AAA (173-175)-eGFP encoding plasmid (our unpublished data). Together, these results show that PSG17 binds to CD9 and that F174 is critical for the interaction with PSG17.
Figure 3.
FACS analysis of PSG17N-Myc-His binding to HEK 293T wild-type and mutated CD9-transfected cells. HEK 293T cells were transfected with pCD9-eGFP (wild-type CD9: WT-CD9) (A) or pCD9-F174A-eGFP (single mutant CD9: SM-CD9) (B), after which they were sequentially incubated with 10 μg/ml PSG17N-Myc-His, anti-Myc mAb, and PE-labeled rat anti-mouse IgG1.
Binding of PSG-17 to Eggs and Inhibition of Sperm-Egg Fusion
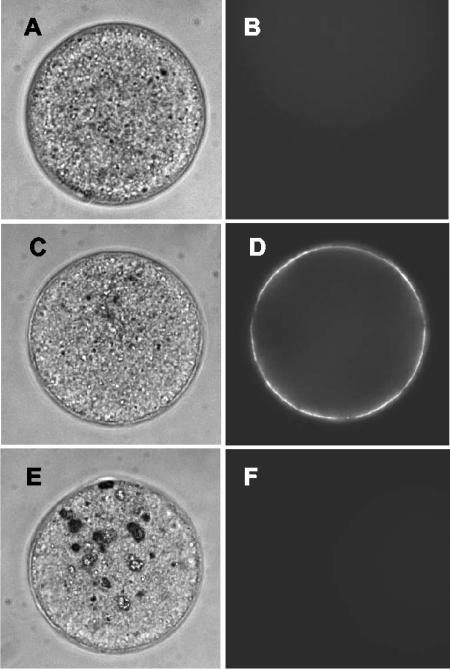
Considering the essential role CD9 has in sperm-egg fusion, and in light of the evidence above supporting a direct interaction between PSG-17 and CD9, we tested whether PSG17 binds to eggs and affects gamete fusion. The presence of CD9 on the eggs has been demonstrated (Chen et al., 1999). However, CD9 is known to have multiple cis-partners and the complement of CD9 cis-partners may be cell type specific, so the accessibility of the PSG17 binding site on the egg could be different from that on macrophages or HEK 293 cells. To explore whether PSG17 is able to bind to eggs, zona-free oocytes were incubated with PSG17N-Myc-His and then examined by indirect immunofluorescence by using an anti-Myc antibody. Oocytes incubated in the presence of a control His-tagged protein (XylE) showed no fluorescence (Figure 4, A and B), whereas oocytes incubated with PSG17 displayed a bright staining (Figure 4, C and D). To confirm that PSG17 binding is CD9-dependent, we examined whether CD9 null eggs were able to bind PSG17 and found no staining in CD9 KO eggs (Figure 4, E and F).
Figure 4.
Binding of PSG17N-Myc-His to the oocyte. Zona-free eggs from wild-type (A-D) or CD9 knockout animals (E and F) were incubated in 50 μg/ml XylE (a His-tagged control protein) (A and B) or PSG17N-Myc-His (C-F) for 30 min, and stained by indirect immunofluorescence by using an anti-Myc antibody, followed by Oregon Green-conjugated secondary antibody.
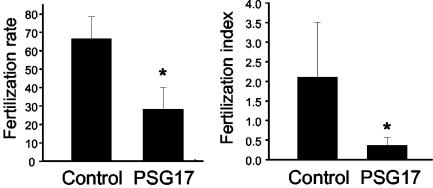
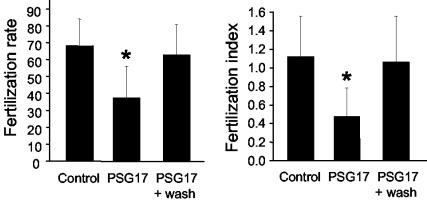
Next, we asked whether binding of PSG17 to CD9 would affect the ability of eggs to fuse with sperm. Zona-free eggs were preincubated with PSG17, and then inseminated with capacitated sperm. After a coincubation period of 40 min, the percentage of fertilized eggs and the mean number of fused sperm per egg were determined. Oocytes preincubated with PSG17 had a significantly lower fertilization rate (58% inhibition) and fertilization index (83% inhibition) than control oocytes (Figure 5). Sperm motility was unaffected by the presence of PSG17 (61% motility with PSG17, 58% motility in the absence of PSG17). Immunofluorescence experiments showed that sperm did not bind PSG17 during coincubation with the eggs, suggesting that the oocyte is the targeted gamete. To study whether the oocyte was blocked in a reversible or irreversible manner, oocytes were incubated with PSG17, washed, and then inseminated with capacitated sperm. Eggs, exposed to PSG17 and then washed, had a fertilization rate and a fertilization index significantly higher than those of the nonwashed oocytes, and not significantly different from the values obtained for control oocytes (Figure 6). These results indicate that the inhibitory effect could be reversed by PSG17 removal and argue against a toxic effect of the protein preparation on the oocytes.
Figure 5.
Inhibition of sperm-egg fusion in the presence of PSG17N-Myc-His. Zona-free eggs were incubated for 30 min in medium (Control) or in medium containing PSG17N-Myc-His (50 μg/ml), and then inseminated with capacitated sperm. After coincubating the gametes for 40 min, oocytes were washed and the fertilization rate (number of fertilized eggs/total number of eggs) and the fertilization index (mean number of sperm fused per egg) were scored. Data represents the mean value ± SE from four independent experiments. *p < 0.05 compared with control.
Figure 6.
Reversibility of sperm-egg fusion inhibition by PSG17NMyc-His. Zona-free eggs were incubated for 30 min in medium containing PSG17N-Myc-His (50 μg/ml), washed through 3 drops of medium, and then inseminated with capacitated sperm (PSG17 + wash). Control groups consisted of oocytes incubated in medium (Control), or in the presence of PSG17N-Myc-His without its subsequent removal by washing (PSG17). After coincubating the gametes for 40 min, oocytes were washed and the fertilization rate and the fertilization index were scored. Data represents the mean value ± SE from three independent experiments. *p < 0.05 compared with control and to PSG17 + wash.
DISCUSSION
Tetraspanins are known to associate with a variety of different molecules, such as membrane-anchored growth factors, integrins, other tetraspanins, and members of the IgSF. A key feature of these reported molecular interactions is that all of them occur in cis, i.e., between the tetraspanin and other transmembrane proteins anchored in the same lipid bilayer (Maecker et al., 1997; Boucheix and Rubinstein, 2001). One trans-interaction has been reported for a tetraspanin, the association between CD81 and the Hepatitis C virus envelope glycoprotein E2. There is evidence indicating that the tetraspanin CD81 interacts in vitro with the virus protein (Flint et al., 1999), and key amino acids in CD81 for this interaction have been identified within the EC2 loop (Flint et al., 1999; Drummer et al., 2002). Nevertheless, the interaction between CD81 and E2 does not seem to mediate virus entry (Petracca et al., 2000; Takikawa et al., 2000) and there are other molecules proposed to function as Hepatitis C virus receptors (Germi et al., 2002; Scarselli et al., 2002), so the biological significance of the CD81-E2 interaction is uncertain.
Previous work implicated CD9 in the binding of an external ligand, PSG17, to macrophages (Waterhouse et al., 2002). One of the aims of the present study was to determine whether CD9 mediates the binding of PSG17 directly or, on the contrary, whether it functions in an indirect way, enhancing the binding of PSG17 to a CD9-associated protein. Results of pull-down experiments, carried out with the purified recombinant proteins, indicate PSG17 binds directly to the EC2 loop of CD9, thus confirming that CD9 is the actual receptor for PSG17. Because PSG17 is the first molecule known to be a biological ligand for a tetraspanin, the association between PSG17 and CD9 represents an unexpected, novel interaction in which the tetraspanin is a receptor.
The EC2 loop of tetraspanins, particularly the stretch of residues preceding the most C-terminal cysteine, is a region in which tetraspanin-associated proteins bind (Stipp et al., 2003). This region in CD9 is also the location of CD9 mutations that affect gamete fusion. A striking feature of our data is that the same CD9 mutations that affect CD9 activity in gamete fusion abolish CD9-PSG17 binding. In previous work, we found that CD9 with F (174) mutated to A has greatly reduced activity in gamete fusion and CD9 with SFQ (173-175) mutated to AAA is no longer active in gamete fusion (Zhu et al., 2002). In our current results, use of the F to A or SFQ to AAA mutants indicate that these residues are also essential for CD9 binding to PSG17. A possible trivial explanation for these results could be that misfolding of CD9 is produced by the amino acid changes. However, substantial data indicate that the mutated protein (SFQ to AAA) has correct folding (Zhu et al., 2002), and therefore it is likely that CD9 residues SFQ 173-175 represent part of a PSG17 binding site.
Alternative explanations exist for how soluble PSG17 might inhibit gamete fusion. Because PSG17 binding initiates signal transduction in macrophages, it is possible that PSG17 binding to egg CD9 produces a similar effect that might trigger egg activation. However, oocytes exposed to PSG17 and then washed, showed a fertilization rate and index similar to control oocytes, indicating that the inhibition is reversible, and therefore arguing against an induction of egg activation by PSG17. Another possibility is that the binding of PSG17 inhibits gamete fusion by displacing a CD9 cis-partner from its normal association with CD9. The two most abundant and tightly associated CD9 cis-partners in tissue culture cells are both members of the EWI subfamily of the IgSF, EWI-F, and EWI-2 (Charrin et al., 2001; Stipp et al., 2001a,b). Given the fact that CD9 associates in cis with these IgSF members and that PSG17 is also an IgSF member, one can speculate that PSG17 disrupts the cis-association between CD9 and egg EWI-F or EWI-2. However, the EWI subfamily and the CEA subfamily (of which PSG17 is a member) have relatively little sequence relationship aside from both possessing Ig domains, making this hypothesis less attractive. Further exploration of this possibility will require the identification of CD9-associated proteins in the oocyte and their binding site(s) in CD9.
Another explanation of our findings is that egg CD9 may bind in trans to a PSG17-related ligand present on the sperm surface. When the CD9 SFQ site is mutated or already occupied by soluble PSG17, the sperm surface ligand cannot bind to egg CD9 and this essential step in gamete fusion is blocked. Relevant to this interpretation are two separate findings in our previous article (Zhu et al., 2002). One was that soluble CD9-EC2 when preincubated with eggs, inhibits gamete fusion, indicating that CD9-EC2 interacts with another egg surface protein. A second finding was that the SFQ sequence in CD9 is required for fusion, but the issue of SFQ's acting in trans or cis was not addressed. If CD9 has a trans-interaction, we would propose it is in addition to the cis-interaction indicated by the previous finding.
Although CD9-EC2 inhibits gamete fusion when preincubated with eggs, it has no effect on fusion when preincubated with sperm. We previously suggested this could mean that egg CD9 does not bind to sperm (Zhu et al., 2002), but other explanations of this result are possible. For instance, a sperm trans-ligand for CD9 may be inactive or inaccessible until after initial steps in sperm-egg adhesion occur and CD9 is positioned to interact with the trans-ligand. Once these adhesion steps occur, the trans-ligand becomes activated or accessible to bind the egg surface CD9 in preference to the soluble CD9-EC2.
A sperm trans-ligand for CD9 might be a membrane-associated form of PSG17 or a related CEA member. A CEA protein has been identified on the sperm surface and named “sperad.” Sperad, initially called AH-20 (Primakoff and Myles, 1983), has been described in guinea pig sperm (Quill and Garbers, 1996). Relevant to sperad's biological function, monoclonal antibodies G3 and G11 stained the equatorial region of acrosome-reacted guinea pig sperm and were able to completely inhibit the fusion of guinea pig sperm with hamster oocytes (Allen and Green, 1995). Sperad was recently reported to be the protein recognized by antibodies G3 and G11 (Ilayperuma, 2002, 2003). Thus, current findings include 1) CD9 is required for sperm-egg fusion; 2) CD9 binds PSG17, a member of the CEA subfamily, and PSG17 inhibits sperm-egg fusion; and 3) there is a CEA protein on sperm that has been implicated in sperm-egg fusion. Together, these results support the idea that CD9 may function in gamete fusion by binding to a sperm CEA protein.
During recent years models for gamete fusion have focused on an adhesion role of a sperm ADAM(s) binding to an egg integrin(s) and CD9 was implicated as facilitator of this interaction (Takahashi et al., 2001; Evans, 2002). Recent data have raised doubts about the participation of ADAMs and integrins in sperm-egg fusion (Primakoff and Myles, 2002; He et al., 2003), although CD9 is clearly required. Our current findings suggest the participation in gamete fusion of IgSF proteins that bind to CD9. In this study we found that CD9 is a receptor for an IgSF/CEA subfamily ligand, PSG17, which binds to a CD9 site, including residues SFQ 173-175, known to be an active site for gamete fusion. Further work should reveal whether during gamete fusion the egg SFQ site binds an IgSF/CEA ligand on the sperm surface and/or is essential for CD9 cis-interactions in the egg plasma membrane.
Acknowledgments
We are grateful to K. Wolcott for technical assistance in the FACS analysis and to Dr. Kathryn V. Holmes (Department of Microbiology, University of Colorado Health Sciences Center) for supplying the recombinant CEACAM1a[1-4]-His protein. This work was supported by National Institutes of Health grants HD35832 (to G.D.) and HD16850 (to D.G.M.).
References
- Allen, C.A., and Green, D.P. (1995). Monoclonal antibodies which recognize equatorial segment epitopes presented de novo following the A23187-induced acrosome reaction of guinea pig sperm. J. Cell Sci. 108, 767-777. [DOI] [PubMed] [Google Scholar]
- Beauchemin, N., et al. (1999). Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252, 243-249. [DOI] [PubMed] [Google Scholar]
- Boucheix, C., and Rubinstein, E. (2001). Tetraspanins. Cell Mol. Life Sci. 58, 1189-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrin, S., Le Naour, F., Oualid, M., Billard, M., Faure, G., Hanash, S.M., Boucheix, C., and Rubinstein, E. (2001). The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J. Biol. Chem. 276, 14329-14337. [DOI] [PubMed] [Google Scholar]
- Chen, M.S., Tung, K.S., Coonrod, S.A., Takahashi, Y., Bigler, D., Chang, A., Yamashita, Y., Kincade, P.W., Herr, J.C., and White, J.M. (1999). Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc. Natl. Acad. Sci. USA 96, 11830-11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho, C., Bunch, D.O., Faure, J.E., Goulding, E.H., Eddy, E.M., Primakoff, P., and Myles, D.G. (1998). Fertilization defects in sperm from mice lacking fertilin beta. Science 281, 1857-1859. [DOI] [PubMed] [Google Scholar]
- Drummer, H.E., Wilson, K.A., and Poumbourios, P. (2002). Identification of the hepatitis C virus E2 glycoprotein binding site on the large extracellular loop of CD81. J. Virol. 76, 11143-11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, J.P. (2002). The molecular basis of sperm-oocyte membrane interactions during mammalian fertilization. Hum. Reprod. Update 8, 297-311. [DOI] [PubMed] [Google Scholar]
- Flint, M., Maidens, C., Loomis-Price, L.D., Shotton, C., Dubuisson, J., Monk, P., Higginbottom, A., Levy, S., and McKeating, J.A. (1999). Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 73, 6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germi, R., Crance, J.M., Garin, D., Guimet, J., Lortat-Jacob, H., Ruigrok, R.W., Zarski, J.P., and Drouet, E. (2002). Cellular glycosaminoglycans and low density lipoprotein receptor are involved in hepatitis C virus adsorption. J. Med. Virol. 68, 206-215. [DOI] [PubMed] [Google Scholar]
- He, Z.Y., Brakebusch, C., Fassler, R., Kreidberg, J.A., Primakoff, P., and Myles, D.G. (2003). None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm-egg binding and fusion. Dev. Biol. 254, 226-237. [DOI] [PubMed] [Google Scholar]
- Hemler, M.E. (2001). Specific tetraspanin functions. J. Cell Biol. 155, 1103-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilayperuma, I. (2002). Identification of the 48-kDa G11 protein from guinea pig testes as sperad. J. Exp. Zool. 293, 617-623. [DOI] [PubMed] [Google Scholar]
- Ilayperuma, I. (2003). Monoclonal antibody G3 epitope location on Guinea pig sperm membrane protein, sperad. J. Exp. Zool. 295A, 92-98. [DOI] [PubMed] [Google Scholar]
- Kaji, K., Oda, S., Shikano, T., Ohnuki, T., Uematsu, Y., Sakagami, J., Tada, N., Miyazaki, S., and Kudo, A. (2000). The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 24, 279-282. [DOI] [PubMed] [Google Scholar]
- Le Naour, F., Rubinstein, E., Jasmin, C., Prenant, M., and Boucheix, C. (2000). Severely reduced female fertility in CD9-deficient mice. Science 287, 319-321. [DOI] [PubMed] [Google Scholar]
- Maecker, H.T., Todd, S.C., and Levy, S. (1997). The tetraspanin superfamily: molecular facilitators. FASEB J. 11, 428-442. [PubMed] [Google Scholar]
- Miyado, K., et al. (2000). Requirement of CD9 on the egg plasma membrane for fertilization. Science 287, 321-324. [DOI] [PubMed] [Google Scholar]
- Nishimura, H., Cho, C., Branciforte, D.R., Myles, D.G., and Primakoff, P. (2001). Analysis of loss of adhesive function in sperm lacking cyritestin or fertilin beta. Dev. Biol. 233, 204-213. [DOI] [PubMed] [Google Scholar]
- Petracca, R., et al. (2000). Structure-function analysis of hepatitis C virus envelope-CD81 binding. J. Virol. 74, 4824-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primakoff, P., and Myles, D.G. (1983). A map of the guinea pig sperm surface constructed with monoclonal antibodies. Dev. Biol. 98, 417-428. [DOI] [PubMed] [Google Scholar]
- Primakoff, P., and Myles, D.G. (2002). Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 296, 2183-2185. [DOI] [PubMed] [Google Scholar]
- Quill, T.A., and Garbers, D.L. (1996). Sperad is a novel sperm-specific plasma membrane protein homologous to a family of cell adhesion proteins. J. Biol. Chem. 271, 33509-33514. [DOI] [PubMed] [Google Scholar]
- Sacks, G., Sargent, I., and Redman, C. (1999). An innate view of human pregnancy. Immunol. Today 20, 114-118. [DOI] [PubMed] [Google Scholar]
- Scarselli, E., Ansuini, H., Cerino, R., Roccasecca, R.M., Acali, S., Filocamo, G., Traboni, C., Nicosia, A., Cortese, R., and Vitelli, A. (2002). The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 21, 5017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsadin, R., Adham, I.M., Nayernia, K., Heinlein, U.A., Oberwinkler, H., and Engel, W. (1999). Male mice deficient for germ-cell cyritestin are infertile. Biol. Reprod. 61, 1445-1451. [DOI] [PubMed] [Google Scholar]
- Snyder, S.K., Wessner, D.H., Wessells, J.L., Waterhouse, R.M., Wahl, L.M., Zimmermann, W., and Dveksler, G.S. (2001). Pregnancy-specific glycoproteins function as immunomodulators by inducing secretion of IL-10, IL-6 and TGF-beta1 by human monocytes. Am. J. Reprod. Immunol. 45, 205-216. [DOI] [PubMed] [Google Scholar]
- Stipp, C.S., Kolesnikova, T.V., and Hemler, M.E. (2001a). EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J. Biol. Chem. 276, 40545-40554. [DOI] [PubMed] [Google Scholar]
- Stipp, C.S., Kolesnikova, T.V., and Hemler, M.E. (2003). Functional domains in tetraspanin proteins. Trends Biochem. Sci. 28, 106-112. [DOI] [PubMed] [Google Scholar]
- Stipp, C.S., Orlicky, D., and Hemler, M.E. (2001b). FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J. Biol. Chem. 276, 4853-4862. [DOI] [PubMed] [Google Scholar]
- Takahashi, Y., Bigler, D., Ito, Y., and White, J.M. (2001). Sequence-specific interaction between the disintegrin domain of mouse ADAM 3 and murine eggs: role of beta1 integrin-associated proteins CD9, CD81, and CD98. Mol. Biol. Cell 12, 809-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa, S., Ishii, K., Aizaki, H., Suzuki, T., Asakura, H., Matsuura, Y., and Miyamura, T. (2000). Cell fusion activity of hepatitis C virus envelope proteins. J. Virol. 74, 5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse, R., Ha, C., and Dveksler, G.S. (2002). Murine CD9 is the receptor for pregnancy-specific glycoprotein 17. J. Exp. Med. 195, 277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells, J., Wessner, D., Parsells, R., White, K., Finkenzeller, D., Zimmermann, W., and Dveksler, G. (2000). Pregnancy specific glycoprotein 18 induces IL-10 expression in murine macrophages. Eur. J. Immunol. 30, 1830-1840. [DOI] [PubMed] [Google Scholar]
- Yuan, R., Primakoff, P., and Myles, D.G. (1997). A role for the disintegrin domain of cyritestin, a sperm surface protein belonging to the ADAM family, in mouse sperm-egg plasma membrane adhesion and fusion. J. Cell Biol. 137, 105-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelus, B.D., Wessner, D.R., Williams, R.K., Pensiero, M.N., Phibbs, F.T., deSouza, M., Dveksler, G.S., and Holmes, K.V. (1998). Purified, soluble recombinant mouse hepatitis virus receptor, Bgp1(b), and Bgp2 murine coronavirus receptors differ in mouse hepatitis virus binding and neutralizing activities. J. Virol. 72, 7237-7244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, G.Z., Miller, B.J., Boucheix, C., Rubinstein, E., Liu, C.C., Hynes, R.O., Myles, D.G., and Primakoff, P. (2002). Residues SFQ (173-175) in the large extracellular loop of CD9 are required for gamete fusion. Development 129, 1995-2002. [DOI] [PubMed] [Google Scholar]